Development, Validation and Application of a Bridging ELISA for Detection of Antibodies against GQ1001 in Cynomolgus Monkey Serum
Abstract
:1. Introduction
2. Results
2.1. Method Validation
2.1.1. Screening Cut Point
2.1.2. Confirmatory Cut Point
2.1.3. Titration Cut Point
2.1.4. QC Ranges
2.1.5. Sensitivity of the Screening Assay and Precision of the Titration Assay
2.1.6. Intra- and Inter-Assay Precision for the Screening Assay and Confirmation Assay
2.1.7. Effect of Hemolysis and Specificity
2.1.8. Drug Tolerance
2.1.9. Hook Effect
2.1.10. Stability
2.2. Method Application
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. ADA Samples
4.3. Principle of Acid-Dissociation Bridging ELISA
4.3.1. Screening Assay
4.3.2. Confirmatory Assay
4.3.3. Titration Assay
4.3.4. Selectivity
4.3.5. Sensitivity and Precision of the Titration Assay
4.3.6. Effect of Hemolysis and Specificity
4.3.7. Hook Effect
4.3.8. Drug Tolerance
4.3.9. Stability
4.4. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Zuo, P. Capturing the Magic Bullet: Pharmacokinetic Principles and Modeling of Antibody-Drug Conjugates. AAPS J. 2020, 22, 105. [Google Scholar] [CrossRef] [PubMed]
- Qin, Q.; Gong, L. Current Analytical Strategies for Antibody–Drug Conjugates in Biomatrices. Molecules 2022, 27, 6299. [Google Scholar] [CrossRef] [PubMed]
- Drago, J.Z.; Modi, S.; Chandarlapaty, S. Unlocking the potential of antibody–drug conjugates for cancer therapy. Nat. Rev. Clin. Oncol. 2021, 18, 327–344. [Google Scholar] [CrossRef] [PubMed]
- Kostova, V.; Désos, P.; Starck, J.-B.; Kotschy, A. The Chemistry Behind ADCs. Pharmaceuticals 2021, 14, 442. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Li, S.; Han, S.; Shi, C.; Zhang, Y. Antibody drug conjugate: The “biological missile” for targeted cancer therapy. Signal Transduct. Target. Ther. 2022, 7, 93. [Google Scholar] [CrossRef] [PubMed]
- Carrasco-Triguero, M.; Dere, R.C.; Milojic-Blair, M.; Saad, O.; Nazzal, D.; Hong, K.; Kaur, S. Immunogenicity of antibody–drug conjugates: Observations across 8 molecules in 11 clinical trials. Bioanalysis 2019, 11, 1555–1568. [Google Scholar] [CrossRef] [PubMed]
- Mou, S.; Huang, Y.; Rosenbaum, A.I. ADME Considerations and Bioanalytical Strategies for Pharmacokinetic Assessments of Antibody-Drug Conjugates. Antibodies 2018, 7, 41. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Chen, X.; Vicini, P.; Rup, B.; Hickling, T.P. Therapeutic outcomes, assessments, risk factors and mitigation efforts of immunogenicity of therapeutic protein products. Cell. Immunol. 2015, 295, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Fiorotti, C.K. Immunogenicity considerations for antibody–drug conjugates: A focus on neutralizing antibody assays. Bioanalysis 2018, 10, 65–70. [Google Scholar] [CrossRef] [PubMed]
- US FDA. Immunogenicity Testing of Therapeutic Protein Products—Developing and Validating Assays for Anti-Drug Antibody Detection; The United States Food and Drug Administration: Silver Spring, MD, USA, 2019. [Google Scholar]
- EMA. Guideline on Immunogenicity Assessment of Biotechnology-Derived Therapeutic Proteins (Draft); The European Medicines Agency: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Ferraro, E.; Drago, J.Z.; Modi, S. Implementing antibody-drug conjugates (ADCs) in HER2-positive breast cancer: State of the art and future directions. Breast Cancer Res. 2021, 23, 84. [Google Scholar] [CrossRef] [PubMed]
- Lambert, J.M.; Chari, R.V.J. Ado-trastuzumab Emtansine (T-DM1): An Antibody–Drug Conjugate (ADC) for HER2-Positive Breast Cancer. J. Med. Chem. 2014, 57, 6949–6964. [Google Scholar] [CrossRef] [PubMed]
- Beck, A.; Goetsch, L.; Dumontet, C.; Corvaïa, N. Strategies and challenges for the next generation of antibody–drug conjugates. Nat. Rev. Drug Discov. 2017, 16, 315–337. [Google Scholar] [CrossRef] [PubMed]
- Jabbour, E.; Paul, S.; Kantarjian, H. The clinical development of antibody–drug conjugates—Lessons from leukaemia. Nat. Rev. Clin. Oncol. 2021, 18, 418–433. [Google Scholar] [CrossRef] [PubMed]
- Coats, S.; Williams, M.; Kebble, B.; Dixit, R.; Tseng, L.; Yao, N.-S.; Tice, D.A.; Soria, J.-C. Antibody–Drug Conjugates: Future Directions in Clinical and Translational Strategies to Improve the Therapeutic Index. Clin. Cancer Res. 2019, 25, 5441–5448. [Google Scholar] [CrossRef] [PubMed]
- GeneQuantum Healthcare. GQ1001. Available online: http://www.genequantum.com/#/common/product?pid=12&id=13&type=7 (accessed on 24 January 2023).
- Baert, K.; De Backer, P. Comparative pharmacokinetics of three non-steroidal anti-inflammatory drugs in five bird species. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2003, 134, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Werthén, M.; Nygren, H. Cooperativity in the antibody binding to surface-adsorbed antigen. Biochim. Biophys. Acta 1993, 1162, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Patton, A.; Mullenix, M.C.; Swanson, S.J.; Koren, E. An acid dissociation bridging ELISA for detection of antibodies directed against therapeutic proteins in the presence of antigen. J. Immunol. Methods 2005, 304, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Q.; Pottanat, T.G.; Carter, Q.L.; Troutt, J.S.; Konrad, R.J.; Sloan, J.H. Affinity capture elution bridging assay: A novel immunoassay format for detection of anti-therapeutic protein antibodies. J. Immunol. Methods 2016, 431, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Smith, H.W.; Butterfield, A.; Sun, D. Detection of antibodies against therapeutic proteins in the presence of residual therapeutic protein using a solid-phase extraction with acid dissociation (SPEAD) sample treatment prior to ELISA. Regul. Toxicol. Pharmacol. 2007, 49, 230–237. [Google Scholar] [CrossRef] [PubMed]
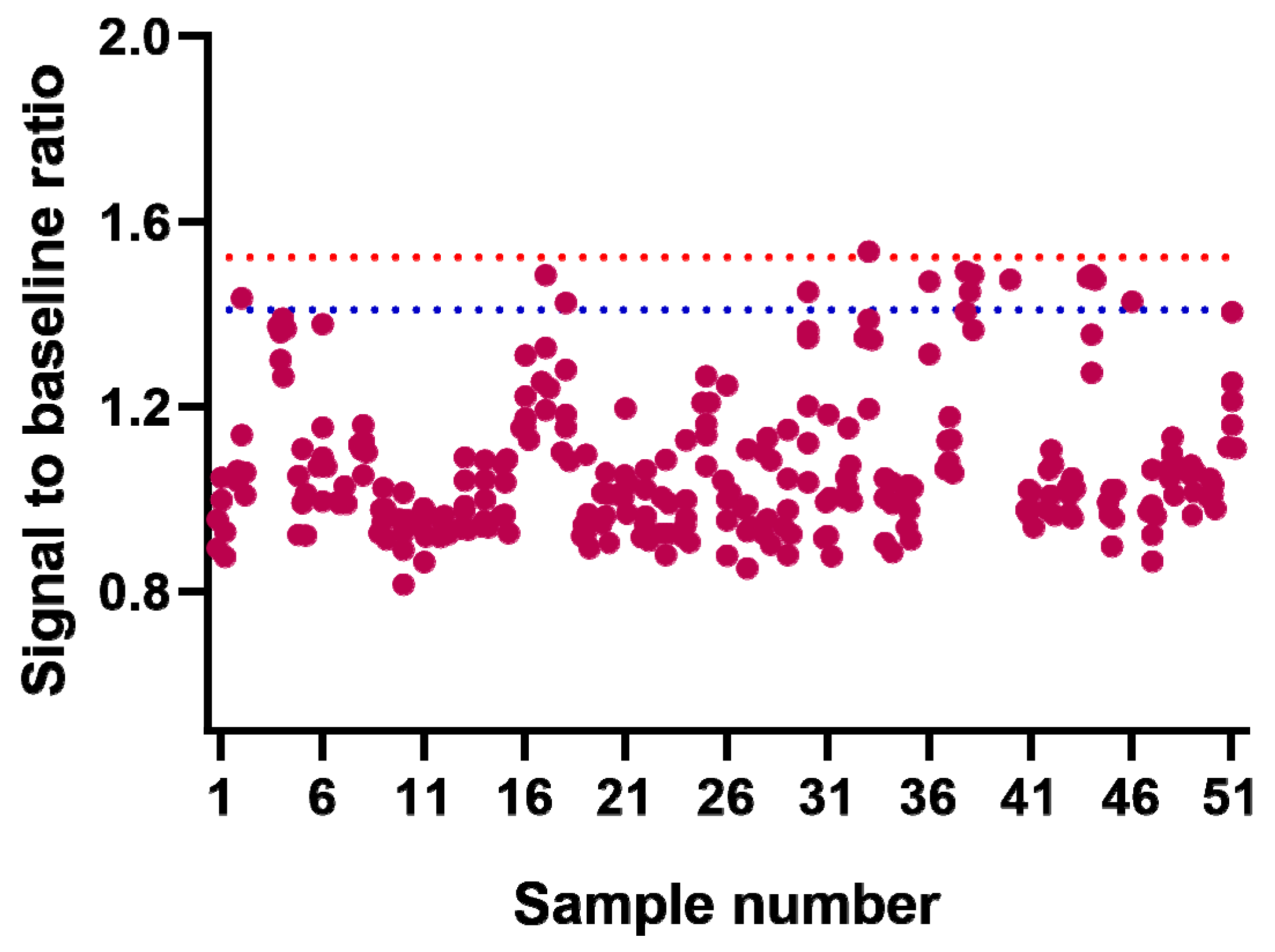

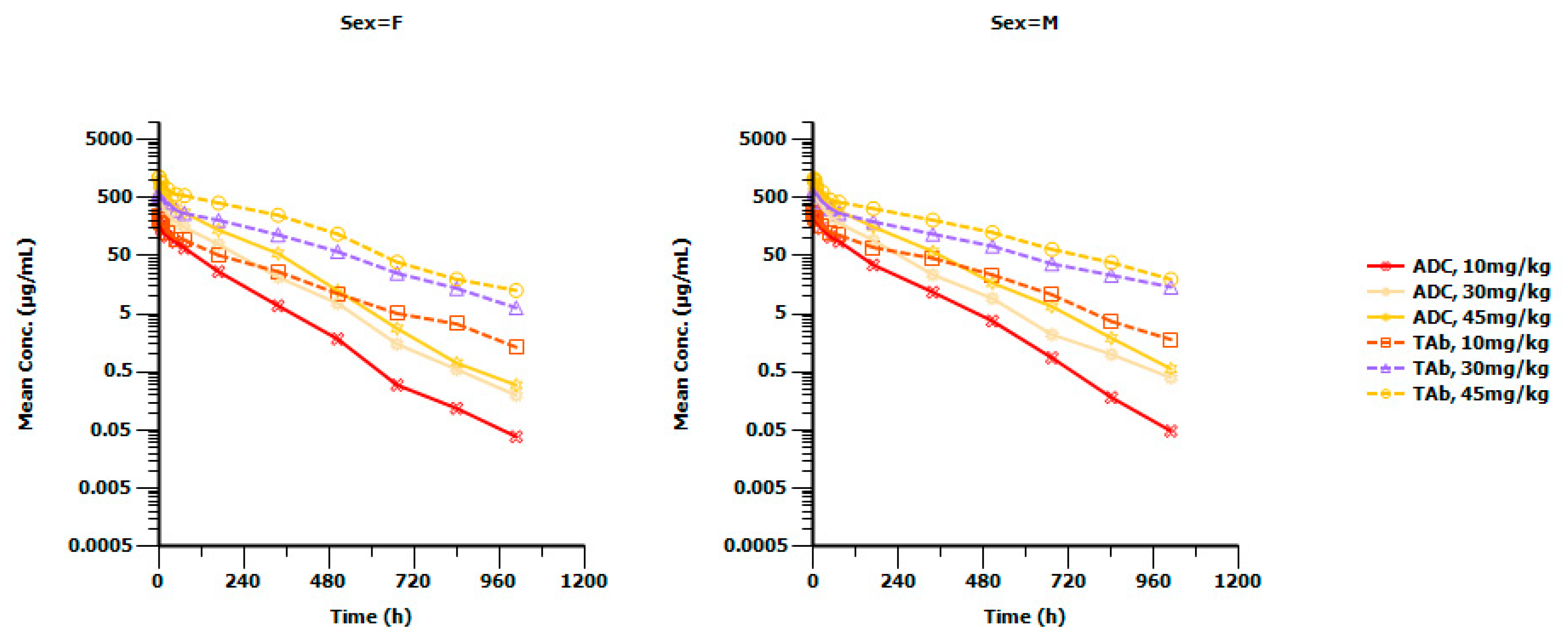
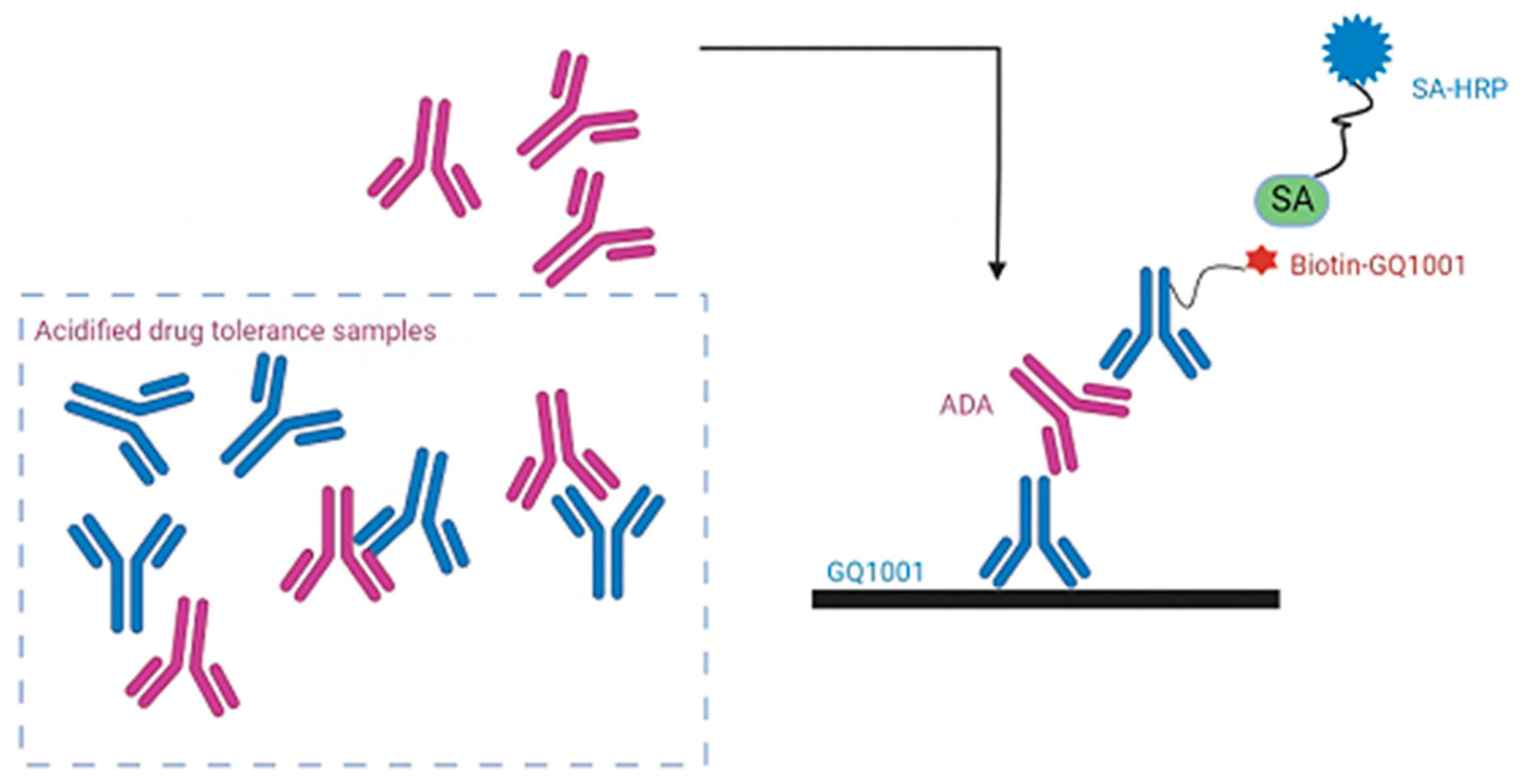
| Test Samples | High-Level Testing Sample (8000 ng/mL) | Low-Level Testing Sample (200 ng/mL) | Negative Control Sample | ||
|---|---|---|---|---|---|
| SB | IR% | SB | IR% | OD Value | |
| Mean | 52.657 | 98.1 | 1.957 | 50.144 | 0.059 |
| Intra-assay precision (%CV) | 2.5 | 0.1 | 1.5 | 2.3 | 1.4 |
| Inter-assay precision (%CV) | 15.5 | 0.4 | 10.3 | 20.3 | 15.6 |
| Number of replicates | 6 | 6 | 6 | 6 | 6 |
| Number of runs | 6 | 6 | 6 | 6 | 6 |
| Test Samples | High-Level Testing Sample (8000 ng/mL) | Low-Level Testing Sample (200 ng/mL) | Negative Control Sample | ||||
|---|---|---|---|---|---|---|---|
| SB | IR% | SB | IR% | SB | IR% | ||
| 2% Hemolysis | 44.736 | 97.8 | 1.892 | 48.1 | 1.072 | 6.2 | |
| 44.627 | 97.8 | 1.887 | 41.2 | 1.078 | 0.1 | ||
| 41.733 | 97.5 | 1.851 | 44.5 | 1.127 | 12.4 | ||
| 5% Hemolysis | 41.958 | 97.3 | 2.002 | 39.0 | 1.222 | 2.4 | |
| 41.902 | 97.3 | 2.056 | 44.1 | 1.253 | 9.7 | ||
| 40.556 | 97.0 | 2.060 | 41.6 | 1.287 | 0.3 | ||
| Human IgG1 | 2500 ng/mL | 38.905 | 97.7 | 1.745 | 47.8 | ||
| 500 ng/mL | 40.914 | 97.8 | 1.715 | 49.2 | |||
| 100 ng/mL | 39.212 | 97.8 | 1.687 | 48.5 | |||
| 20 ng/mL | 37.705 | 97.6 | 1.722 | 49.4 | |||
| 0 ng/mL | 39.368 | 97.7 | 1.782 | 49.0 | |||
| Monkey Anti-GQ1001 Antibodies (ng/mL) | GQ1001 (μg/mL) | Mean OD | CV% | SB |
|---|---|---|---|---|
| 8000 | 300 | 0.059 | 0.7 | 1.068 |
| 200 | 0.069 | 0.1 | 1.247 | |
| 100 * | 0.098 | 5.9 | 1.755 * | |
| 80 | 0.110 | 2.0 | 1.968 | |
| 50 | 0.164 | 2.3 | 2.941 | |
| 25 | 0.303 | 0.6 | 5.440 | |
| 0 | 2.969 | 5.0 | 53.284 | |
| 150 | 12.5 | 0.062 | 2.4 | 1.119 |
| 6.25 | 0.074 | 1.1 | 1.322 | |
| 3.125 * | 0.086 | 6.7 | 1.543 * | |
| 1.563 | 0.091 | 0.1 | 1.632 | |
| 0.781 | 0.100 | 2.7 | 1.800 | |
| 0.391 | 0.106 | 6.1 | 1.900 | |
| 0 | 0.106 | 2.1 | 1.906 | |
| 0 | 0 | 0.056 | 6.3 |
| Monkey Anti-GQ1001 Antibodies (μg/mL) | Mean OD | CV% | SB |
|---|---|---|---|
| 64 | Overflow | NA | NA |
| 32 | Overflow | NA | NA |
| 16 | Overflow | NA | NA |
| 8 | 2.695 | 2.8 | 48.379 |
| 0 | 0.056 | 6.3 |
| Stability | High-Level Stability Sample (8000 ng/mL) | Low-Level Stability Sample (200 ng/mL) | ||||
|---|---|---|---|---|---|---|
| Mean OD | %CV | SB | Mean OD | %CV | SB | |
| RT 0 h | 2.994 | 8.6 | 55.925 | 0.116 | 3.4 | 2.169 |
| RT 24 h | 3.198 | 2.7 | 56.646 | 0.121 | 1.8 | 2.134 |
| 3.244 | 0.3 | 57.455 | 0.121 | 3.3 | 2.150 | |
| 3.123 | 2.4 | 55.303 | 0.117 | 0.2 | 2.063 | |
| 2–8 °C 24 h | 2.961 | 4.7 | 52.444 | 0.114 | 0.9 | 2.023 |
| 3.091 | 2.2 | 54.736 | 0.116 | 0.1 | 2.050 | |
| 2.959 | 1.5 | 52.407 | 0.115 | 0.4 | 2.037 | |
| 96 days −80 °C | 2.453 | 4.1 | 49.611 | 0.092 | 2.1 | 1.866 |
| 2.424 | 4.2 | 49.030 | 0.091 | 0.8 | 1.841 | |
| 2.365 | 1.8 | 47.840 | 0.092 | 0.5 | 1.870 | |
| 5 F/T cycles | 3.225 | 3.7 | 53.480 | 0.119 | 2.0 | 1.966 |
| 2.961 | 1.5 | 49.107 | 0.116 | 0.2 | 1.928 | |
| 3.020 | 2.4 | 50.079 | 0.132 | 23.0 | 2.196 | |
| Dose Level (mg/kg) | Sex | Time Point | |||
|---|---|---|---|---|---|
| Day 1 (Pre-Dose) | Day 8 | Day 15 | Day 43 | ||
| 0 | M | − | − | − | − |
| M | − | − | − | − | |
| M | − | − | − | − | |
| F | − | − | − | − | |
| F | − | − | − | − | |
| F | − | − | − | − | |
| 10 | M | − | − | − | − |
| M | − | − | − | − | |
| M | − | − | − | 80 | |
| F | − | − | − | − | |
| F | − | − | − | − | |
| F | − | − | − | − | |
| 30 | M | − | − | − | − |
| M | − | − | − | − | |
| M | − | − | − | − | |
| F | − | − | − | − | |
| F | − | − | − | − | |
| F | − | − | − | − | |
| 45 | M | − | − | − | − |
| M | − | − | − | − | |
| M | − | − | − | − | |
| F | − | − | − | 160 | |
| F | − | − | − | − | |
| F | − | − | − | 640 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, T.; Sun, Y.; Deng, X.; Shi, L.; Chen, W.; Fang, W.; Wu, J.; Fan, X.; Chen, X.; Sun, J.; et al. Development, Validation and Application of a Bridging ELISA for Detection of Antibodies against GQ1001 in Cynomolgus Monkey Serum. Molecules 2023, 28, 1684. https://doi.org/10.3390/molecules28041684
Liu T, Sun Y, Deng X, Shi L, Chen W, Fang W, Wu J, Fan X, Chen X, Sun J, et al. Development, Validation and Application of a Bridging ELISA for Detection of Antibodies against GQ1001 in Cynomolgus Monkey Serum. Molecules. 2023; 28(4):1684. https://doi.org/10.3390/molecules28041684
Chicago/Turabian StyleLiu, Tingting, Yajun Sun, Xiaojie Deng, Lili Shi, Wenyi Chen, Wenjing Fang, Junliang Wu, Xiaotian Fan, Xiaoqiang Chen, Jianhua Sun, and et al. 2023. "Development, Validation and Application of a Bridging ELISA for Detection of Antibodies against GQ1001 in Cynomolgus Monkey Serum" Molecules 28, no. 4: 1684. https://doi.org/10.3390/molecules28041684
APA StyleLiu, T., Sun, Y., Deng, X., Shi, L., Chen, W., Fang, W., Wu, J., Fan, X., Chen, X., Sun, J., Qin, G., Gong, L., & Qin, Q. (2023). Development, Validation and Application of a Bridging ELISA for Detection of Antibodies against GQ1001 in Cynomolgus Monkey Serum. Molecules, 28(4), 1684. https://doi.org/10.3390/molecules28041684







