Abstract
Emerging chromium (Cr) species have attracted increasing concern. A majority of Cr species, especially hexavalent chromium (Cr(VI)), could lead to lethal effects on human beings, animals, and aquatic lives even at low concentrations. One of the conventional water-treatment methodologies, adsorption, could remove these toxic Cr species efficiently. Additionally, adsorption possesses many advantages, such as being cost-saving, easy to implement, highly efficient and facile to design. Previous research has shown that the application of different adsorbents, such as carbon nanotubes (carbon nanotubes (CNTs) and graphene oxide (GO) and its derivatives), activated carbons (ACs), biochars (BCs), metal-based composites, polymers and others, is being used for Cr species removal from contaminated water and wastewater. The research progress and application of adsorption for Cr removal in recent years are reviewed, the mechanisms of adsorption are also discussed and the development trend of Cr treatment by adsorption is proposed.
1. Introduction
The oxidation of chromium (Cr) ranks from Cr(II) to Cr(VI), and Cr could exist as chromous ion [Cr(II) ion, Cr2+], chromic ion [Cr(III) ion, Cr3+], chromate ion [Cr(VI) or CrO42−] and dichromate ion [Cr2O72−] [1]. Many Cr compounds are relatively water insoluble, and Cr(II) ion is readily oxidized to Cr(III) in aqueous solutions. Therefore, in aqueous solutions, Cr would be present as Cr(III) or Cr(VI). Except for Cr(III) oxide (Cr2O3) and Cr(III) hydroxide [Cr(OH)3], most Cr(III) compounds are usually water insoluble because they are largely bound to the floating particles in water. As a consequence of the abovementioned physicochemical properties of Cr, the existence of three forms, namely Cr2O3, Cr(OH)3 and Cr(VI), in aqueous solutions has been reported and detected (Figure 1).
Figure 1.
The schematic diagrams of aqueous solutions for Cr species with various oxidation states.
Normally, the toxicity of Cr has some relation with its oxidation states. No toxicokinetic data are available on Cr metal and Cr(II) compounds. An oral-dose-finding study indicated that water-soluble Cr(III) compounds only exhibited moderate toxicity due to the relatively higher LD50 values (40–422 mg/kg) of Cr(III) for rats and mice, whereas Cr(VI) was evaluated as a carcinogenic species [2], and Cr(VI) compounds are thought to be toxic for both plants and animals at low concentrations [3].
The major Cr discharged from the metal industry is Cr(III), and Cr(VI) in industrial wastewaters mainly originates from tanning and painting. As one of the toxic heavy metals which exists stably in nature, Cr(VI) is more toxic than Cr(III). In an aqueous medium, Cr(VI) is presented as CrO42− or Cr2O72−. Up to now, Cr(VI) species originating from Cr-containing electroplating wastewater have become a typical primary water pollutant. River water was estimated to contain only approximately 1 ppb of Cr, but it was a long time ago. The Cr content has dramatically increased in recent years, for example, 6–12 ppb in the Godavari River in 2014 [4] and 10.5–113 ppb in the Yangtze River in 2009 [5] have been reported. The consistent diffusion of Cr(VI) into groundwater was also detected [6]. Undoubtedly, Cr species would have a great influence on the drinking-water quality and public health.
Due to the different oxidation states of Cr, the selective removal of Cr species from sewage water is therefore difficult to operate. The conventional physical and chemical treatment techniques are usually costly and consume a high amount of energy, they produce harmful by-products, and they can cause secondary pollution. Usually, Cr(III) could be precipitated as hydroxide; however, coagulation is not a very practical method for Cr(VI) removal due to the problems aroused by the disposal of Cr(VI)-containing solid waste. The employment of iron sulphate (FeSO4) is also a good choice to reduce Cr(VI) to Cr(III); however, the practical application of this method in drinking-water production seems infeasible.
Currently, the Cr removal from water is necessary. Ion exchange [7,8] and adsorption [9] can be applied for this purpose. As a well-known equilibrium separation process, adsorption has been an effective method for water-decontamination applications and is superior to other technologies. Moreover, adsorption also does not produce any additional harmful substances. The present review article hopes to analyze and discuss the research progress and perspectives of Cr species removal from wastewater by adsorption. The main aim of this review is to provide a summary of the most recent information on the research and development of multifunctional and efficient sorbents. For this purpose, a wide range of lists for the reported adsorbents were compiled. The adsorbents’ properties, maximum adsorption capacities and effects of condition parameters (solution pH, initial adsorbate concentration, adsorbent dosage, contact time and temperature) on the adsorption procedures were critically discussed in detail.
2. Cr Removal: The Developed Adsorbents
To overcome problems associated with Cr species pollution, lots of new functional materials have been developed and widely used (Table 1). The practical application of versatile adsorbents has also been carried out, providing water security for people of different regions.
2.1. Carbon Materials
Carbon materials, including active carbons (ACs), carbon microsphere (CMS), carbon nanotubes (CNTs), graphene (Gr) and graphene oxide (GO), are of great importance in adsorption fields. In particular, ACs and CMS have been extensively used in various industrial adsorption and separation processes for the treatment of metal ions, such as lead (Pb), copper (Cu), mercury (Hg) and so on, due to their cost-saving and highly efficient performance. Additionally, the excellent stability and the multifunctionality of carbon-material shave guaranteed their wider and practical applications in Cr removal.
2.1.1. ACs
Due to its excellent physicochemical properties and the availability of providing abundant adsorption sites, ACs have been used for the adsorption removal of Cr species. Specially, the massive production of ACs by activation of particular agricultural wastes, such as peanut shells [10], and hard-to-dispose wastes, such as waste rubber tires [11], has been successfully carried out, opening up some new opportunities for AC based adsorbents. Usually, the micropores in ACs could adsorb Cr(VI) via physical interactions such as the Van de Waals force [12,13]. To fulfill the highly efficient removal of Cr species, physical adsorption was not enough. Thereupon, the further modification of ACs was carried out. The introduction of various functional groups onto ACs could provide additional adsorption sites for more efficient adsorption [14,15]. The existence of different adsorption sites at ACs which were suitable for positively charged metal ions or negatively charged Cr(VI) [16]. Normally, positively charged adsorbents usually possess relatively higher adsorption capacity toward Cr(VI). For example, quaternary amine-anchored AC (AC-NH2) exhibited a much higher adsorption capacity of 112.36 mg/g for Cr(VI) than that (26.25 mg/g) of as-received AC [17].
The coexistence of Cr(VI) and humic acid (HA) in wastewater is obvious. The concurrent adsorption of Cr(VI) and HA by powdered AC was evaluated, and the adsorption of both HA and Cr(VI) could be enhanced with increasing levels of either HA or Cr(VI) [18]. Recently, more and more biomass-based ACs have been fabricated for Cr species removal, providing new thoughts for developing novel low-toxicity, cost-saving and easy-to-operate bio-adsorbents [19,20,21].
2.1.2. CMS
The small pore sizes and low pore volume of ACs usually limit their wider applications due to the slower mass transport kinetics. Mesoporous carbons, such as CMS, were therefore developed and believed to be a kind of promising adsorption material. Due to its relatively bigger pores, well-developed mesoporous channels and larger pore volumes which are beneficial to accommodating more or larger ions, the diffusion of Cr(VI) in CMS would be greatly accelerated. Due to its larger surface area, the adsorption capacity (Qm) of mesoporous carbon microsphere (MPCMS) for Cr(VI) reached 165.3 mg/g [22]. The combination of CMS with aminated sodium lignosulfonate could produce novel layered N-containing a carbon–lignin-based adsorbent, which exhibited 99% Cr(VI) removal under neutral conditions [23]. Magnetic carbon microspheres (MCMSs) showed 100% Cr(VI) removal from a Se(IV)-Cr(VI) coexisting system in 120 min [24].
Novel mesoporous composites of goethite/carbon microspheres (α-FeOOH/CMSs) and akaganeite/carbon microspheres (β-FeOOH/CMSs) were employed to selectively remove Cr(VI); the coexistence of Cl−, Cu2+ and Ni2+ would not cause significant negative effect, whereas SO42− and PO43− would hinder the effective removal [25]. CMS prepared from powdered sodium lignosulfonate and polystyrene could be used for Cr(VI) removal without adding any binder, and its high adsorption capacity toward Cr(VI) (227.7 mg/g at an initial pH value of 2) made it very attractive [26].
2.1.3. CNTs-Based Composites
Owing to its merit of having a very high specific surface area, CNTs have displayed a high adsorption capacity and fast adsorption rate toward various organic contaminants (phenols, dyes, antibiotics, and so on) and metal ions (Pb, Cu, Cr, etc.). Interestingly, the CNTs systems after adsorption of Cr(VI) exhibited less toxicity and carcinogenic activity [27]. Hydroxylated and carboxylated CNTs possess different surface functional groups, such as hydroxyl or carboxyl groups, which exhibited a higher Cr removal efficiency than pristine CNTs, confirming that the modification of CNTs is beneficial [28,29]. To quickly remove Cr(VI), polyamine-modified carbon nanotubes (PA-CNTs) were developed, and a high Cr(VI) adsorption capacity of 168.54 mg/g, as well as ultrafast removal, could be detected due to the introduced amine groups on the surface. Interestingly, its adsorption capacity increased to 392.78 mg/g in the presence of Rhodamine B [30]. To facilitate the recovery of CNT-based composites, a high-ferromagnetism Fe3O4/CNT composite possessing adsorption capacities of 47.98~83.54 mg/g for Cr(VI) and magnetic separation ability was developed [31].
The utilization of CNTs-based adsorbents for future practical applications should be evaluated. Therefore, the separation behaviors of a chitosan–multiple-walled CNTs–iron (CS–MWCNTs–Fe)-based continuous-flow column was tested, and around 54% Cr(VI) removal could be achieved (water flow rate = 1 mL/min, feed Cr-VI concentration = 30 mg/L, packed bed height = 8 cm) [32]. The vertical and uniform growing of CNTs on carbon nanofibers (CNFs) could enhance the specific surface area (398.127 m2/g) and create a higher adsorption capacity toward Cr(VI) (150 mg/g) within an hour, and 227 mg/g at equilibrium could be observed [33]. However, the recyclability of CNTs–CNFs needs to be improved due to the fact that the vertical form of CNTs would be easily damaged. MWCNTs modified by iron–manganese binary oxide (FeMnOx/MWCNTs) showed superior adsorbability toward Cr(VI) in a binary system of Cr(VI) and As(III) due to the electrostatic attraction, the redox and ligand exchange [34]. The regeneration studies showed that FeMnOx/MWCNTs had good reproducibility and a good recycling performance, which is also critical for the possible practical application. The further introduction of mono-tosyl-β-cyclodextrin (MT-β-CDTs) onto MWCNTs/Fe3O4 would increase its adsorption capacity toward Cr(III) [35]. A natural organic polymer, chitin-modified MWCNTs/Fe3O4, was also developed, which possessed a removal efficiency of 99.1% toward Cr(VI) [36], again indicating that the introduction of organic components onto CNTs was beneficial to their adsorbability toward Cr(VI). In addition, the modification of CNTs with special molecules would enhance the adsorption selectivity. For example, the sensitive and highly selective removal of 90% of Cr(VI) by magnetic CNTs functionalized with imidazolium ionic liquid could be carried out, even at a level of ppb and in the presence of high concentrations of other cations, such as Hg2+ and Cd2+, or anions such as NO3− and SO42− [37].
2.1.4. Gr and GO-Based Composites
Significantly great attraction was shown for the design and development of efficient and robust adsorbents with outstanding stability, namely Gr and GO-based versatile adsorbents, for the removal of environmental pollutants. Pristine Gr and GO themselves could be used as highly efficient adsorbents; for example, GO exhibited a high adsorption capacity (366.3 mg/g) toward Cr(III) [38]. However, it is very difficult to separate/recover Gr and GO after adsorption, leading to secondary pollution. Fortunately, Gr/GO compounded with other materials could output versatile nanocomposites, which might be used for the selective adsorption of different contaminants (metal ions, organic dyes, pesticides, medicines, etc.) due to the introduced various surface functionalities. In addition, these nanocomposites are usually more easily recovered, which might show more promising practical applications in wastewater treatment [39]. The performance of GO/Gr-based composites depends on the active sites and functionalities on the surface [40]. To exploit the maximal efficacy of these composites, different components have been introduced onto GO/Gr via multifarious approaches, such as covalent coupling, self-assembly, electroless plating and so on.
Metal-based adsorbents possess lower adsorption capacities and slower adsorption kinetics for Cr(VI). However, the combination of a metallic compound with GO/Gr could effectively overcome these deficiencies, as well as improve the recovery properties of Gr/GO-based composites. The immobilization of CaO nanoparticles (CaO NPs) from Lala clamshells onto GO was carried out, and the obtained biogenic CaO-GO exhibited an adsorption capacity (38.04 mg/g) for Cr(VI) removal (Figure 2). Obviously, it could be used as a sensitive sensor for Cr(VI) sensing besides high adsorption capacity (38.04 mg/g) and removal efficiency (~90%) for Cr(VI). A combination of external mass transfer and chemisorption might devote to the adsorption [41]. When zinc oxide (ZnO) NPs were decorated onto GO, the mobility of Cr(VI) in the composite was improved, facilitating Cr(VI) adsorption kinetics and enhancing removal efficiency [42]. The simultaneous combination of Ca and Zn with GO was also conducted, and the GO@CZ nanocomposite demonstrated high adsorption capacity of 285.71 mg/g for Cr(III) at pH = 7 [43]. It is worth noting that when organic components were incorporated into GO-metal-based materials, the removal efficiency and adsorption capacity would be significantly improved [44,45]. Poly(dimethyl diallyl ammonium chloride) (PDMDAAC)-modified magnetic GO (GO/Fe3O4) exhibited a high adsorption capacity (95.2 mg/g) toward Cr(VI) due to the electrostatic attraction by quaternary amine [46]. Polypyrrole (PPy)-anchored Gr-silica nanosheets (Gr-Si-PPy) possessed a high specific surface area, and they exhibited a much higher adsorption capacity for Cr(VI), i.e., 429.2 mg/g at 298 K (pH = 2) [47].
The assembly of GO with other nanosheets, such as MoS2, would produce novel composites with a higher specific surface area and higher removal efficiency. MoS2/rGO with an adsorption capacity of 80.8 mg/g toward Cr(VI) was therefore fabricated [48]. Although a relatively lower maximum adsorption capacity of 43.95 mg/g for Cr(VI) at a rod-like nano-MoS2/GO nanocomposite could be obtained, the adsorption selectivity toward Cr(VI) in the coexistence of organic contaminants was significantly enhanced [49]. To improve the surface area of metal and Gr/GO-based adsorbents, novel sandwich-like GO@SiO2@C@Ni composites were developed, and GO@SiO2@C@Ni-400 sintered at a carbonization temperature of 400 °C exhibited excellent dispersibility, a large surface area (229.88 m2/g) and high saturation magnetization, revealing a high adsorption capacity (299.20 mg/g) toward Cr(VI) [50]. If GO- and NiFe-layered double hydroxide were hydrothermally treated, a novel three-dimensional (3D) hierarchical GO-NiFe LDH composite with a sandwich-like, highly porous and well-ordered structure would be fabricated, and it would be more stable and easier to recycle [51]. The hybridization of nZVI with LDH decorated rGO, ternary (Fe@LDH/rGO) composite with better dispersibility, improved hydrophilicity and more positive surfaces would be produced, which possessed higher removal efficiency and capacity for Cr(VI) oxyanions [52], which was superior to nZVI-rGO [53], indicating the introduction of LDH was beneficial. The idea and practical operation of 3D composites continued to evolve in recent years, which offered a lot of possibilities for design and fabrication of novel useful adsorbents [54].
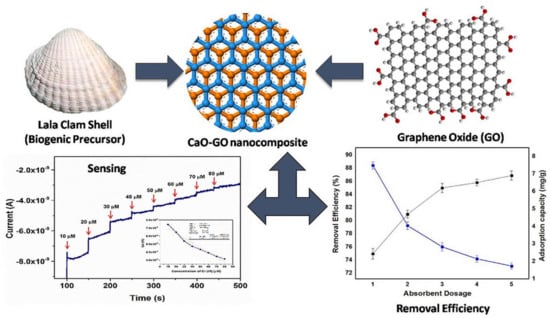
Figure 2.
CaO NPs cross linked GO for Cr(VI) removal and sensing [41]. Copyright belongs to Elsevier B.V.
Fe3O4/GO nanocomposite was also employed for the effective adsorption of Cr(VI) from aqueous solution, and the magnetic separation of nanocomposite from solutions after adsorption was easily realized [55]. When CS was introduced onto Fe3O4/GO nanocomposite, its adsorption performance would be greatly changed, indicating the combination of organic components with GO or GO-based composites might be a very exciting research area [56]. More and more researchers have focused on the development of novel multifunctional GO/Gr-based adsorbents by introducing various organic components. GO/CS composite was crosslinked with 3-glycidoxypropyltrimethoxysilane (KH-560) to form a three-dimensional (3D) GO/CS/KH-560 aerogel with porous network structure, which possessed an equilibrium adsorption capacity of 146 mg/g toward Cr(VI) in within 120 min [57]. The removal of Cr(VI) in the presence of Hg(II) from aqueous solutions by 4-amino-3-hydrazino-5-mercapto-1,2,4-triazole covalently bonded GO was evaluated; the tremendous adsorption capacities toward Cr(VI) (734.2 mg/g) and Hg(II) (1091.1 mg/g) were even higher than pure GO, indicating the introduced multidentate ligands was really conducive [58]. Similar research was carried out by grafting poly(allylamine hydrochloride) (PAH) onto amino-modified GO, and the resulting PAH-ASGO also exhibited high adsorption capacity of 373.1 mg/g for Cr(VI) [59]. PAH-ASGO could especially effectively reduce Cr(VI) from a high concentration level (9.9 mg/L) to an extremely low level of 0.004 mg/L in a very short time of 10 s, and its excellent reusability was also significant [59].
The electrons’ transfer ability of different composites based on rGO, GO and Gr sheets could be promoted; therefore, their photocatalytic ability would be greatly enhanced, which could be employed to increase the Cr-removal effectiveness through the synergistic effect. By employing reduced graphene oxide (rGO) as a charge-transfer mediator, a three dimensional (3D) photocatalyst [TiO2-ZnxCd1−xS rGO aerogel (TSGA)] fabricated by combining TiO2 and ZnxCd1−xS with Gr aerogel could aqueously remove Cr(VI) (R = 100%) via adsorption, along with visible light (wavelength > 420 nm) photocatalysis in 30 min (Figure 3) [60].
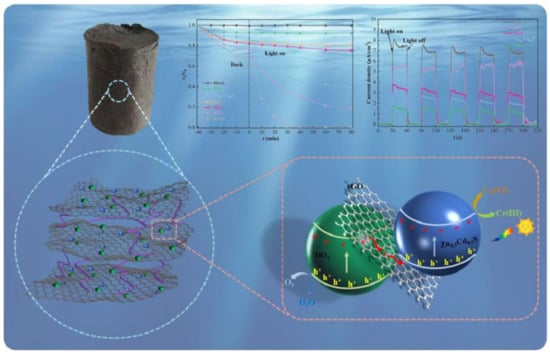
Figure 3.
Efficient removal of Cr(VI) by TSGA via the synergy of adsorption and photocatalysis [60]. Copyright belongs to Elsevier B.V.
2.2. Silicon-Based Materials
Various silicon-based porous materials, including crystalline metallosilicates (zeolites and zeotype materials), silica (microporous, mesoporous and microporous), silica gels and so on, have been of tremendous importance for the adsorption methodology besides ACs and CMS. However, the large-sized silicon-based adsorbents always exhibit a relatively lower adsorption capacity, which greatly limits their possible wider applications. The nano-crystallization of materials is helpful to improve their specific surface area, thus improving the adsorption efficiencies. Compared with as-prepared mesoporous silica–carbon (MSC), nanoscale zero-valent iron (nZVI)-particles-embedded MSC (nZVI-MSC) exhibited a more ordered structure, larger surface area and much higher Cr(VI) removal efficiency [61]. The treatment of attapulgite with HCl or HF of different concentrations could regulate the morphology and composition [62]. Further characterization of silicon-rich biochar-supported nanoscale zero-valent iron (nZVI) was performed, and ferrous chromite (FeCr2O4) on the surface could be detected, indicating that the reduction and adsorption both contributed to the removal of Cr(VI) [63]. By immobilizing the nZVI composite onto modified attapulgite, the enhanced Si-O-Fe coupling mediated by silicon would occur, resulting in the reduction of Cr(VI) and higher Cr(VI) removal efficiency. The nanosilver-assisted chemical etching (Ag-ACE) of kerf loss silicon waste was performed, which was functionalized with 3-aminopropylethoxysilane (APTES) to obtain a hybrid material, APTES-NPSi, for the highly effective removal of Cr(VI) [64]. Interestingly, its adsorption capability was highly pH dependent.
The introduction of specific functional groups onto silicon-based materials was also conductive to regulate their adsorption performances. For example, the introducing alkylamine chains onto porous silica beads could enhance its adsorption capacity toward Cr(VI) due to the abundant interfacial amino groups [65]. Thereafter, calcium silicate hydrate prepared by coal gangue was used to adsorb Cr(VI), and a relatively higher adsorption capacity, i.e., 68.03 mg/g, could be obtained [66].
2.3. Resins
Resins are not only used as adsorbents for the removal of contaminants, but they are also widely applied to recover valuable substances, such as Cr(VI), from electroplating wastewater. To eliminate toxic Cr species, the rational selection of resins with specific functional groups (for example, C=S, C-S, C-N, -NH2, -CHO, -OH, etc.) and suitable pores is very important [67]. For example, the resins with hydrophobic properties exhibited low adsorption selectivity, which could be improved by surface modifications [68,69].
By combining ion-exchange and precipitation-reduction mechanisms, a strong-base silica-supported pyridine resin (SiPyR-N4) was developed to remove/recover Cr(VI) from electroplating wastewater. Rapid Cr(VI) removal (99.3% removal within 5 min from a 100 mg/L Cr(VI) solution) could be realized, demonstrating that the ion exchange by SiPyR-N4 was really efficient [70]. By in situ polymerization of different monomers, including epichlorohydrin (ECH), dimethylamine (DMA) and ethylenediamine (EDA), with the weakly alkaline anion-exchange resin D301, the modified anion-exchange resin (EDE-D301) possessed enhanced surface area, positive charge and high contents of N and Cl, facilitating the highly efficient adsorption of Cr(VI) (capacity of 298 mg/g, and 93% removal after four consecutive adsorption–desorption cycles) [71]. Similarly, a styrene-based ion-exchange D201 resin-hydrated iron oxide (HFO) nanocomposites for Cr(VI) removal was investigated, and the efficient removal of Cr(VI) from 10 mg/L to below 50 ppb could be carried out [72]. Macroporous strong acidic cation-exchange resin (D001) modified by nanosized goethite (nFeOOH@D001) also exhibited much better ability (80.2% removal and capacity of 7.4 mg/g) to remove Cr(VI) than D001 [73]. Further modification of sulfide onto nZVI supported porous D201 could improve its removal performance (89.67% removal and capacity of 137.35 mg/g for 100 mg/L of Cr(VI) solution), recycling performance and longevity [74]. The possible adsorption mechanisms of nZVI-modified resins, including Amberlyst 15 or resin D201, were investigated, and the simultaneous reduction of Cr(VI) to Cr(III) might be implemented for the efficient cation-exchange-based Cr removal [75,76]. The evaluation of quaternary amine functional groups of anion-exchange resins for Cr(VI) removal was further implemented, and different ion-exchange capacities of 280.25 mg/g, 147.67 mg/g and 163.67 mg/g for Eichrom 1-X4, Lewatit M+ M800 and Lewatit A8071 could be observed, respectively [77].
The removal of trace Cr species is really a huge challenge. To overcome the shortages of conversional resins which only possess high removal efficiencies for Cr species of high concentrations, a weak base anion-exchange resin, Duolite A7, was developed for trace Cr(VI) removal. Extensive investigations revealed that redox reactions occurred besides ion exchange, and the produced Cr(III) could be precipitated inside the resin or bound to the carboxylic groups outside due to the high-affinity interaction [78].
2.4. Iron-Based Adsorbents
Recently, the reaction of Cr(VI) with ferric and ferrous compounds has received as much attention due to the assumption of strong interactions between Cr(VI) and the oxide surface [79]. Because of the slow redox transformation of Cr(VI), it is important to understand the reactions of Cr(VI) and iron oxides in various systems. For example, ferrihydrite (Fh) was used as an adsorbent for Cr(VI) removal from organic ligands and Cr(VI)-containing wastewater, and the removal efficiency could be improved by adding aluminum (Al) to increase its specific surface area, indicating that the surface complexation and electrostatic attraction played an important role in the adsorption [80]. Moreover, sulfonated nanoscale zero-valent iron (S-nZVI), together with organic acids, including citric acid and oxalic acid, exhibited a significant promotion of Cr(VI) removal due to the synergistic effects of adsorption and reduction [81]. Other iron-based materials, such as ferrous sulfide (FeS), could be used to effectively remediate Cr pollution [82]. To improve the adsorption capacity of iron-based adsorbents, many tentative works have been performed in the past few years.
2.4.1. Iron Oxyhydroxide (FeOOH) and Its Derivatives
Owing to the high specific surface area and abundant surface hydroxyl groups, iron oxyhydroxide (FeOOH)-polymorphs-based nanoparticles, including goethite (α-FeOOH), akaganeite (β-FeOOH), lepidocrocite (γ-FeOOH) and feroxyhyte (δ-FeOOH), showed strong electrostatic interactions toward Cr(VI) [83]. The δ-FeOOH might be used more widely in the treatment of wastewater due to its ferromagnetic properties and ability to easily separate/regenerate [84]. A ferrate K2FeO4 could be decomposed to γ-FeOOH/γ-Fe2O3 in the presence of Mn(II), and the reduction of Cr(VI) could be carried out, facilitating the more efficient removal (97.7%, C0 = 10.0 mg/L) of Cr(VI) [85].
Nano iron oxides (Fe2O3) and ferrites have also been intensively used for wastewater treatment [86]. The newly developed very thin and porous α-Fe2O3 nanofibers exhibited a maximum adsorption capacity of 16.17 mg/g for Cr(VI) removal, and it can be practically utilized due to its highly efficient and low-cost properties [87]. The cost-saving adsorbents are always expected, and industrial wastes, such as foundry sands from the iron foundry industry, could be used for the removal of Cr (VI) [88], providing some references for developing cheaper sorbents.
2.4.2. Magnetic Adsorbents
It is difficult to regenerate traditional adsorbents due to their sinking properties or excellent dispersibility, inevitably causing secondary pollution. To overcome these shortcomings, magnetic materials were developed as novel recyclable adsorbents. The adsorption capacities of magnetite nano-Fe3O4 particles toward Cr(VI) are usually very low (usually below 5 mg/g) due to the fact that the uptake was mainly composed of physicochemical processes, which greatly limits their widely practical uses [89]. The adsorption capacity of an adsorbent would increase as its specific surface area (SSA) increases [90]. The monodispersed Fe3O4 hollow microspheres showed a high adsorption capacity of 180 mg/g toward Cr(VI) [91]. The increase in the adsorption capacity could also be expected through the modification or introduction of various new components onto nano-Fe3O4. For example, the NH2-functionalized nano-Fe3O4 composites exhibited a maximum sorption capacity of 25.58 mg/g for Cr(VI) [92], and the ethylenediamine (EDA)-functionalized magnetic composite showed an improved capacity of 32.15 toward Cr(VI) [93].
Furthermore, the increase in surface area is beneficial to the adsorption and reduction ability of magnetic nano-adsorbents. As-prepared MnFe2O4 microspheres with myrica rubra-like structures exhibited an excellent catalytic reduction ability for Cr(VI) removal from wastewater that was much better than commercial MnFe2O4 due to its smaller sizes (~17 nm) and highly promoted catalytic reduction performance [94].
The basic adsorption principles for Cr(VI) expand from ion exchanges to electrostatic gravitations and coordination interactions [95]. Based on these findings, diethylenetriamine (DETA), triethylentetramine (TETA) and tetraethylenepentamine (TEPA) were also used to functionalize nanomagnetic adsorbents, and their adsorption capacities were increased to impressive levels of 149.25, 204.08 and 370.37 mg/g, respectively [96]. The polymerization of glycidylmethacrylate (GMA) onto Fe3O4 nanoparticles would produce magnetic polymer microspheres (MPMs), which were further modified with EDA (EDA-MPMs) to reach a high maximum adsorption capacity of 253.2 mg/g for Cr(VI), indicating that the introduced multiple functional groups were useful [97].
The combination of magnetic particles with various sorbents would slightly decrease their adsorption capacities due to the low adsorption capacity of the inorganic magnetic particles. However, a good regeneration ability would be achieved as a compensation. For example, Fe3O4-loaded MCMs could be rapidly separated and regenerated in contrast with pristine MCMs, and a preferable capacity (123.9 mg/g) would be maintained even after five adsorption–desorption cycles compared with its initial capacity of 156.3 mg/g [22]. Polypyrrole (PPy)-coated secondary fly-ash–iron (PPy-MSFA) composites were fabricated; they were floatable and exhibited adsorption capacities of 119.33 mg/g for Cr(VI) [98]. To more conveniently recover the adsorbents, novel magnetic carbon microspheres, which are carbon nanofibers (CNFs) decorated with iron nanoparticles (nanofurry microspheres), were developed, and they exhibited easy magnetic separation capability [99].
To improve its biocompatibility, versatile biodegradable components could be incorporated with a sorbent. Meanwhile, the multifunctional groups of any biodegradable component are beneficial to improving its adsorption capacity. Biomaterials such as corn straw [100], CS [101,102] and so on were grafted onto nano-Fe3O4, and the obtained magnetic biocomposites exhibited both higher adsorption capacities and better biocompatibility. In addition, the adsorption capacity of magnetic CS composites (Fe3O4@CS) toward Cr(VI) gradually increased with an increase in loading proportion of CS, indicating that the organic component of the magnetic sorbent dominated its adsorption effectiveness [103].
2.5. Polymers
Various adsorbents have been extensively investigated and utilized for Cr(VI) removal. Due to the lack of specific Cr-adsorptive functional groups or heaviness, their adsorption capacities are relatively lower, which greatly limits their practical applications. To obtain new adsorbents with larger Cr(VI) adsorption capacities, versatile polymers or macromolecules have been further developed, and the Cr removal efficiency has been improved. For example, the cationic group contents in polyacrylamide (PAM) was found to benefit the adsorption of Cr(III) [104]. A copolymer hydrogel prepared by 2-hydroxyethyl acrylate (HEA) and 2-acrylamido-2-methylpropane sulfonic acid (AMPS) exhibited a priority adsorption order of Cr(III) > Fe(III) > Cu(II) > Cd(II) > Pb(II) in a multicomponent solution [105].
The combination of polymers with other base materials, such as the introduction of inorganic components into polymers, is beneficial. Highly porous hydrophilic polyacrylonitrile (PAN)-membrane-supported nano zero-valent iron (NZVI) was fabricated, and the further graft of glutathione (GSH) could promote its adsorption capacity for Cr(VI); its removal efficiency could maintain over 86% after six adsorption–desorption cycles [106]. To relieve the serious agglomeration of FeS, carboxymethyl cellulose stabilized FeS@extracellular polymeric substance (CMC-FeS@EPS) was developed [82]. The high Cr(VI) removal efficiency (98.00%) in a wide pH range (4–9), as well as the strong anti-interference in the coexistence of ions, guaranteed its potential practical applications. In addition, sulfidated nanoscale zero-valent iron@EPS (SnZVI@EPS) also exhibited a higher adsorption capacity toward Cr(VI) (64.90 mg/g) in a wide pH range, under various conditions (anaerobic or aerobic), and with the coexistence of a high concentration of other ions [107]. Polyethyleneimine (PEI)-functionalized magnetic hydrochar adsorbent exhibits an excellent adsorption capacity of 287.7 mg/g for Cr(VI). L-cysteine-doped polypyrrole-modified bentonite (L-cys/PPy/BT) showed higher adsorption capacities for the removal of Cr(VI) (318.5 mg/g) [108], whereas crosslinked PEI exhibited a high removal efficiency (89~99%) and excellent selectivity and reusability toward Cr(III), even if it was applied to real wastewater samples [109], indicating that the chemisorption usually dominated the adsorption processes. To enhance the adsorption selectivity, the combination of metal–organic framework (MOF) with polyvinylpyrrolidone (PVP) was implemented, and a more significant adsorption effectiveness, as well as higher selectivity, could be obtained due to the improved hydrophilicity and porosity [110]. Through a surface-initiated atom-transfer radical polymerization process, silica-di-block polymer hybrids were prepared by using a hydrophobic monomer (butyl methacrylate, BA) and a hydrophilic monomer (2-(dimethylamino)ethylmethacrylate, DMAEMA) [111]. The hybrids with larger DMAEMA-to-BA mass ratios possessed higher adsorption capacities toward Cr(VI), indicating that N-containing groups contributed a lot to the efficient adsorption. Recyclable calcined CoFe-LDH/g-C3N4 [112], 3D hierarchical GO-NiFe LDH, hierarchical porous Ni/Co-LDH hollow dodecahedron [113] and so on have been developed based on structural synergistic effects and interpenetrating networks, facilitating their future potential utilization in practical applications.
Microplastics have been recognized as one of the pollutants, but aged microplastics could also be used for the removal of heavy metals to achieve waste utilization and recyclable development. To evaluate the adsorption properties of aged microplastics toward heavy metals, the adsorption of Cu(II) and Cr(VI) by aged polystyrene (PS) and polyvinyl chloride (PVC) was investigated, providing some references for future trends in sustainable development [114]. Green strategies have been increasingly used in developing novel adsorbents. A sodium polyacrylate hydrogel (PAAS) loaded with Fe(III) could generate Fe(II) to reduce Cr(VI) by 93.90–98.25%, which is efficient and eco-friendly for Cr(VI) removal [115].
2.6. Layered Double Hydroxides (LDHs)
The high adsorption capacity of an adsorbent is also one of the most important issues for water treatment. A class of two-dimensional materials containing positively charged layers (Al3+, Mg2+, Zn2+, etc.) and counter-anions (Cl−, NO3−, CO32−, and SO42−, etc.), layered double hydroxides (LDHs), has been extensively utilized as useful adsorbents due to large specific surface area, unique layer structure with abundant bonding sites. For example, the adsorption capacity of Zn/Al-LDHs toward Cr(VI) was over 120 mg/g [116]. Taking advantage of laboratory wastewater containing Cr(III), a novel Mg/Al/Cr layered compound (Mg/Al/Cr(III)-LDH) was developed for the removal of Cr(VI) from laboratory wastewater, and it had an adsorption capacity of 237.80 mg/g [117]. By in situ grown polyaniline (PANI) on Mg/Al LDHs, the organic–inorganic hybrid material exhibited an outstanding removal capacity toward Cr(VI) (393.70 mg/g) from aqueous solution in contrast with that (198.67 mg/g) of PANI nanotubes, providing an ideal material for the treatment of Cr(VI) wastewater [118]. From the point of view of a classic ion-exchange reaction, a single-phase material of Mg0.66Al0.34(OH)2(Mo3S13)0.03(NO3)0.14(CO3)0.07∙H2O (Mo3S13-LDH) was rationally designed by intercalating Mo3S132− into the MgAl-LDH gallery for specifical trapping Cr(VI) in the coexistence of multiple interfering anions [119]. The excellent structural stability of Mo3S13-LDH guaranteed its possible practical applications in the future.
To realize the synergetic effect of adsorption and photocatalysis, a novel CuBi2O4/calcined ZnAlBi LDHs (CBO/CLDHs) composite was fabricated for Cr(VI) removal, and a removal efficiency of above 95% toward Cr(VI) (40 mg/L) could be observed [120]. Meanwhile, Cr(VI) was gradually reduced to Cr(III) under visible-light irradiation. An iron–manganese LDH (MnFe-LDH)- and MnFe2O3-anchored three-dimensional porous carbon nanofiber (MnFe-LDH/MnFe2O3@3DNF) could effectively reduce high levels of Cr(VI) to meet the guideline concentrations for potable water supplies [121]. The newly developed nanostructures, inner transition metal ions doped LDHs embedded CNTs, could selectively adsorb Cr(VI) at ultra-low trace levels due to the improved synergistic catalytic properties, which could be employed for highly efficient sensing Cr(VI) [122]. A robust sorbent, Co-Al-LDH@CS/Fe3O4, was fabricated by the combination of Co-Al-LDH with magnetically interspersed CS and cystamine, and its higher adsorption capacity toward Cr(VI) (710.79 mg/g) confirmed the existence of positive synergistic effects and abundant affinity sites (-SH and -NH2) on the surface [123].
Recently, an effective approach based on defect engineering was developed for the fabrication of lignin-derived carbon and Ca/Fe/Al-trimetallic LDH (LDH@LDC) composite [124]. Due to the defects on the LDH layer from the H2 plasma treatment, LDH@LDC exhibited outstanding removal efficiency for Cr(VI) and U(VI) by enhanced ion exchange [124].
2.7. Titanates
Due to the high ion-exchange capacities and excellent stability, titanate-based materials have been utilized as efficient adsorbents to remove various contaminants from wastewater. Similarly, a better adsorption performance would be obtained when other multifunctional organic components are introduced onto titanates [125]. A core–shell structure made from polyaniline/hydrogen-titanate nanobelt (PANI/H-TNB) composite showed an excellent adsorption capacity of 156.94 mg/g toward Cr(VI) under various environmental conditions [126]. Additionally, the satisfactory reusability of PANI/H-TNB composite would greatly reduce the expenses of wastewater treatment. CS-based hydrogel was further incorporated with titanate- and carbon-dots-modified cellulose nanofibers, which could effectively remove Cr(VI) with higher adsorption capacity of 228.2 mg/g [127]. Definitely, the fabrication procedure and the composite were relatively too complicated.
The complete Cr(VI) removal from contaminated water could be implemented by simultaneous reduction of Cr(VI) and immobilization of Cr(III), using sulfonated carbon hemisphere (CHS)-supported mesoporous TiO2 nanoparticles, and the exceptional reduction efficiency, as well as the ion exchange by -SO3H+ on CHS, contributed to the removal of reduction of Cr(VI) and the sequestration of Cr(III), respectively [128]. The binary composites of PEI (an interior core)-anchored titanate nanotubes (TNTs, an exterior shell) also exhibited high Cr(VI) adsorption capabilities due to the electrostatic attraction, redox reaction and chelation [129].
The combination of inorganic components onto titanates is also beneficial. Abundant oxygen vacancies could be formed on the surface of lanthanum-doped bismuth titanate nanosheets; thus, the activated nanosheets exhibited a stronger photocatalytic ability, and the efficient synchronous removal of Cr(VI) and methyl orange could be successfully performed [130]. The encapsulation and homogenous dispersion of FeS NPs into the interlayers of titanate nanotubes were carried out, and highly efficient removal of total Cr was obtained [131]. The reduction of Cr(VI) to Cr(III) by FeS NPs and the adsorption of Cr(III) by titanate nanotubes both contributed a lot to the Cr(VI) removal.
2.8. Reductive Adsorbents
To reduce heavy metal ions to low levels, reductive materials have shown some advantages, such as high adsorption effectiveness, the use of inexpensive adsorbent materials, reducing the toxicity of metal ions and so on [132]. Lignite, brown coal and kerogen could reduce Cr(VI) to Cr(III), and various oxygen-containing functional groups, such as C=O and C-O, would be introduced onto lignite by the Cr(VI) oxidization; hence, Cr species could be removed from aqueous solutions due to the emerging intermolecular forces between Cr(III) ions and the O atoms of C=O and C-O groups in the oxidized lignite [132,133].
Additionally, an adsorption–reduction synergistic effect could be achieved by some adsorbents [79,134]. A ternary magnetic composite containing graphene oxide (GO), diethylenetriamine and Fe3O4 nanoparticles was developed, and the maximum adsorption capacity of 123.4 mg/g toward Cr(VI) was much higher than that of magnetic GO. The reduction of Cr(VI) to Cr(III) during the adsorption was confirmed by X-ray photoelectron spectroscopy (XPS) analysis, indicating that the Cr(VI) adsorption and the reduction both contributed to the Cr(VI) removal [135].
The Cr(VI)-removal performance could be promoted by combining the excellent adsorption effects and the reduction properties. Fe-ethylene glycol complex microspheres [136], mesoporous amino-group-functionalized iron/silica hollow spheres (Fe/SiO2-NH2 HSs) [137] and 3D porous CoFe2O4@SiO2-NH2 [138] have been applied in Cr(VI)-contaminated-water remediation. To realize the resourceful utilization of industrial waste, the Fe-P slag was employed as a reductive agent for Cr(VI) removal by a simplified industrial procedure, providing some new and feasible thoughts for the resourceful utilization of industrial waste in Cr wastewater treatment [139]. Industrial lignin (pulping black liquor lignin (BLN), enzymolytic lignin (ELN) and SPORL pretreatment spent liquor (FS)) showed a maximum adsorption amount of 864.30, 801.57 and 642.26 mg/g toward Cr(VI), respectively, where Cr(VI) was also reduced to low-toxicity Cr(III) [140].
Ultra-thin g-C3N4 itself especially possesses the ability of adsorption and photodegradation because it can act as electron donors (hole sacrificial agents) to accelerate the separation of photo-generated electron–hole pairs and allow more electrons/holes to participate in the reduction of Cr(VI) [141]. The reasonable design of multifunctional materials to enhance the synergistic efficiency proved to be feasible (Figure 4). Furthermore, TiO2/g-C3N4 heterojunctions were developed by evenly grounding and subsequently heat-treating acid-treated H2Ti3O7 nanobelts and superior thin g-C3N4 nanosheets, and the in situ growth of rhombic TiO2 on g-C3N4 nanosheets effectively decreased the bandgap by adjusting the surface and electronic structures [142]. Notably, a more efficient photoreduction of Cr(VI) could be observed, which was 22 times that of pristine g-C3N4 nanosheets.
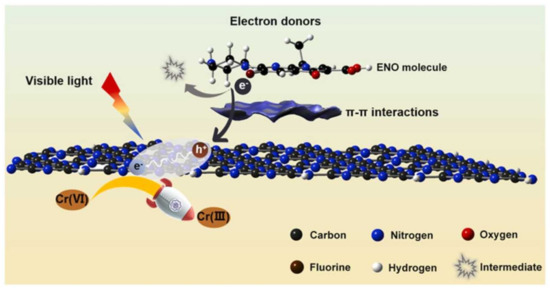
Figure 4.
The synergistic removal of pollutants (antibiotics and Cr (Ⅵ)) by g−C3N4 [141]. Copyright belongs to Elsevier B.V.
2.9. Montmorillonite
Montmorillonite, a clay, has been widely used to remove heavy-metal contaminations by cation exchange in the interlayers and formation of inner-sphere complexes through Si–O− and Al–O− groups. To enhance its adsorption capability, the combination of montmorillonite with other components containing abundant, accessible sorption is essential. For example, CS/montmorillonite nanocomposites exhibited a higher adsorption efficiency, i.e., 45% removal, toward Cr(III) [143]. Various Al-13 cations, dodecyl trimethyl ammonium chloride (DTAC) or dodecyl amine (DA) were used to modify montmorillonite nanoparticles; the as-prepared nanocomposites possessed specific surface areas and high adsorption capacities for Cr(VI) due to the large basal spacing and good porous structure [144].
Taking advantage of the agricultural wastes, a calcined composite from dolomite, montmorillonite and corn stover was fabricated for the aqueous removal of Cr(VI), with removal efficiencies of 84–86% [145].
2.10. Biological Adsorbents
The aqueous removal of Cr(VI) by biosorbents has shown some advantageous over the existing conventional physicochemical techniques, therefore it has attracted great attraction in recent years [146,147]. The removal of Cr species from the industrial effluents of electroplating units by the biomass of cyanobacterium was investigated, which showed a rapid rate for Cr adsorption within the first 15 min [148]. Resting cells of fusarium solani [149], traditional Chinese medicine residual (TCMR) [150], chromate-resistant bacterial strain [151], Yarrowia lipolytica cells [152] and living cells of chryseomonas luteola TEM 05 [153], Canadian peat and coconut fiber, Serratia proteamaculans [154], mussel-shell ash or a sludge/ashes waste mixture [155], activated dry flowers (ADF) of Alstonia Scholaris [156], Araucaria leaves [157], rice husk [158], thermal decomposition of wheat straw (BCS) and wicker (BCW) [159], UV-mutant Bacillus subtilis [160] and sludge-derived biochar (SDBC) [161] also showed a high adsorption capacity toward Cr(VI). The calcium hydroxide modified mangosteen peel especially exhibited highly selective adsorption for Cr(VI) over other metal ions, such as Pb(II), Fe(III), Zn(II), Cd(II) and Cr(III), and the cost-effective and ecofriendly functionalization process made it useful as a green adsorbent [162]. To remove Cr(VI) from groundwater, Fe-modified BC (FeBC) was used as permeable reactive barriers (PRBs) [163]. The long-period (563 days) tests confirmed its excellent performance in terms of Cr(VI) removal with a relatively low cost. To reduce the costs and increase the efficiency, cheaper biomasses will be utilized, or pyrolysis compounding of different biomasses should be carried out.
Heteroatom-containing compounds can form coordination bonds with Cr species, so heteroatom (nitrogen (N), sulfur (S), phosphorus (P), etc.)-modified materials would be efficient adsorbents [164]. Sulfide-modified nanoscale zero-valent iron (SnZVI) was further anchored onto BC (BC-SnZVI), which showed an outstanding performance for Cr(VI) removal from a Cr(VI)-Cd(II) binary system (Figure 5) [165]. The amine and hydroxyl groups of CS are predominantly responsible for binding interactions, and chemisorption was therefore involved in the removal of Cr(VI) by Fe3O4@CS [103]. The adsorption of Cr(VI) by disodium ethylene-diaminetetraacetate (EDTA-2Na)-modified GO/CS (EDTA/GO/CS) composite was evaluated; its adsorption capacity of 86.17 mg/g was relatively lower. However, the regeneration of the EDTA/GO/CS composite was feasible; the adsorbent exhibited a lowered capacity by 5% for Cr(VI) after seven times of reuse [166].
Tetraethylenepentamine (TEPA)-modified wheat straw (TEPA-WS) showed a sorption capacity of up to 454 mg/g for Cr(VI) due to the abundant grafted N- groups [167]. The grafting/polymerization of GMA onto bamboo hydrochar was performed, which was then aminated with diethylenetriamine and subsequently treated by hydrochloric acid [168]. Due to the bearing N+H2R group, it exhibited a superior adsorption capacity of 424.09 mg/g for Cr(VI). A chemical/physical modification of various adsorbents, using heteroatom-containing reagents, could significantly improve their ability to remove Cr(VI) from wastewater, providing some references for the construction and design of novel sorbents in the future.
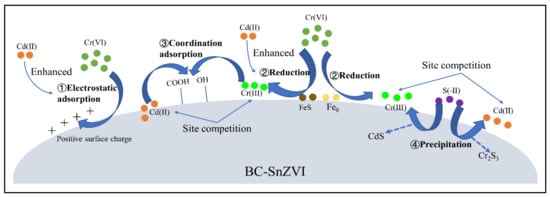
Figure 5.
The co-removal of Cd(II) and Cr(VI) by BC-SnZVI [165]. Copyright belongs to MDPI (Basel, Switzerland).
Novel spongeous adsorbents showed a high performance and are cost-effective for the removal of various toxic metal ions from aqueous solutions. A polypyrrole (PPy)-modified natural core of corncob (PPy-NCC) was rationally fabricated for Cr(VI) removal from aqueous solution, and the adsorption capacity of PPy-NCC was several times higher than other bulk-style PPy composites due to its unique macroporous spongeous microstructure [169]. The co-pyrolysis and decomposition of cotton and polyester could obtain new biochar for the adsorption of Cr(VI), and the synergy effect contributed a lot to the efficient removal [170].
The positive surface charge of an adsorbent could improve its capacity and adsorption speed toward Cr(VI) [171]. The positively charged biochar made from pyrolyzed ramie residues could interact electrostatically with Cr(VI) ions, providing a low-cost and effective sorbent for Cr(VI) removal [172].
Dithiocarbamate (DTC)-modified starch derivatives, such as DTC functionalized starch (DTCS), DTC functionalized enzymolysis starch (DTCES) and DTC functionalized mesoporous starch (DTCMS), showed a significant adsorption performance for Cr(VI), with a sequence of DTCMS > DTCES > DTCS [173]. Recently, ACs prepared by industrial black liquor (BL) were activated with K2CO3 to obtain a higher surface area and larger pore volume, and relatively higher adsorption capacities of ~670 mg/g for Cr(VI) could be observed [174]. The combination of ZnO with biochar (ZBC) could produce novel adsorbents with an enhanced adsorption capacity, owing to the introduced electrostatic attraction, surface complexation and ion-exchange interactions [175].
2.11. Three-Dimensional (3D) Nanocomposites
Three-dimensional network structures possess larger surface areas, more abundant functional groups and highly exposed active sites and higher pore volumes, as well as good elasticity and excellent reusability. The favorable properties of 3D network structures make them become an active focus in the area of adsorption toward Cr species [176].
2.12. Hydrogels and Aerogels
Porous materials with size-adjustable pores and relatively higher surface areas could be used as efficient adsorbents. However, common adsorbents suffer from lower removal efficiencies due to their smaller surface area. To seek more effective adsorbents, recently, ice-segregation-induced self-assembly (ISISA), also called the unidirectional freeze-drying method (UFDM), was developed to produce porous aerogel materials with oriented structures. It is worth noting that the preparation procedure itself is very facile and environmentally friendly.
A N+(CH3)3-containing cationic polyacrylamide aerogel (CPAMA) was intercalated into MoS2 (MoS2@C-PAMA), and the active sites of MoS2 were well exposed for facilitating the adsorption and reduction of Cr(VI) (Figure 6). The high removal efficiency of 95% for Cr(VI) within 50 min and the maximum adsorption capacity of 800.0 mg/g demonstrated its robust removal ability [177]. A 3D honeycomb-like eco-friendly PEI/sodium alginate (SA) aerogel can be fabricated via a one-step freeze-drying procedure. Due to the excellent compressibility and plentiful active adsorption sites, the fast and highly efficient adsorption of Cr(VI) (678.67 mg/g) could be obtained [178,179]. Based on a similar principle, a design of functionalized cellulose-based aerogel beads (CGP) was performed, and the simultaneous and adsorption, reduction and elimination of Cr(VI) could be efficiently implemented [180]. If PEI and amino-functionalized Ti3C2Tx (a transition metal carbide, MXene) were incorporated into the SA aerogel matrix, the robust adsorption capacity of 538.97 mg/g toward Cr(VI) could be observed due to the abundant active groups and the in situ reduction ability of MXene [181]. For the carbon-based aerogels, the introduction of such multifunctional molecules also seems beneficial [182,183].
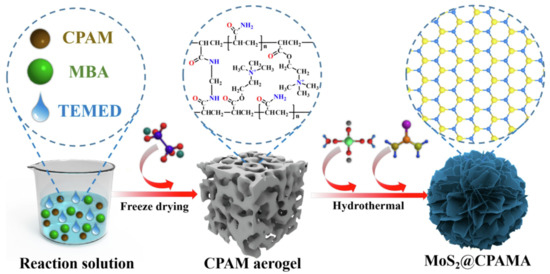
Figure 6.
Preparation route of MoS2@CPAMA [177]. Copyright belongs to Elsevier B.V.
2.13. Frameworks
As potential emerging environmental materials, novel frameworks have received extensive attention in Cr removal. Due to the high nitrogen (N) content, covalent triazine frameworks (CTFs) were developed for the efficient adsorption of Cr(VI), and the strong ion-exchange interaction of quaternary N on CTF toward Cr(VI) was confirmed [184]. The metal–organic frameworks (MOFs), which are hybrid materials of organic ligands and metal ions or clusters, are another ideal material for Cr removal [185]. Due to the adjustable metal center, MOFs with an ordered porous structure could be further modified with specific ligands for more efficient and selective Cr(VI) removal [186]. AC modified with iron terephthalate (Fe-BDC), an Fe-based MOF, exhibited a high monolayer adsorption capacity of 100 mg/g and fast adsorption rate toward Cr(VI) [187]. A bimetallic organic framework, (Fe/Co)-BDC, was further developed, which exhibited a notable capacity of 588 mg/g toward Cr(VI) [188]. The pectin-hydrogel-crosslinked Fe-based MOFs by calcium chloride was developed, which a showed higher adsorption capacity toward Cr(VI) (825.97 mg/g), indicating that the rational introduction of multifunctional groups onto MOFs was necessary [189].
Zeolitic imidazolate frameworks (ZIFs), a subgroup of MOFs, possess more excellent physicochemical properties and are more advantageous for adsorption applications [190]. A Co/Zn-based ZIF adsorbent was developed for Cr(VI) removal, and a homogeneous monolayer adsorption with maximum capacities of 69.4 mg/g could be obtained [191]. ZIFs could be utilized for capacitive deionization (CDI) desalination to simultaneously remove both Cr(VI) ions and other salts, and the radial distribution function (RDF) and ion distribution were involved in the adsorption process, which provided a new application prospect for ZIFs [189].

Table 1.
Adsorption of Cr species by different adsorbents.
Table 1.
Adsorption of Cr species by different adsorbents.
| Cr Species | Type of Adsorbent | Adsorption Conditions | Adsorption Capacities | Ref. |
|---|---|---|---|---|
| Cr(VI) | FeMnOx/MWCNTs | C0 = 50 mg/L; adsorbent dose: 1 g/L; contact time: 60 min; pH: 2.0 | 47.25 mg/g | [34] |
| Cr(III) | GO@CZ | C0 = 30 mg/L; adsorbent dose: 300 mg/L; contact time: 60 min; pH = 7.0 | 285.71 mg/g | [43] |
| Cr(VI) | Gr-Si-PPy | C0 = 100 mg/L; adsorbent dose: 400 mg/L; T = 25 °C; contact time: 60 min; pH: 2.0 | 429.2 mg/g | [47] |
| Cr(VI) | GO@SiO2@C@Ni-400 | C0 = 20 mg/L; T = 25 °C; adsorbent dose: 0.15 g/L; pH = 3.0 | 299.20 mg/g | [50] |
| Cr(VI) | GO-NiFe LDH | C0 = 20 mg/L; T = 30 °C; contact time: 280 min; adsorbent dose: 80 mg/L | 53.6 mg/g | [51] |
| Cr(VI) | Fe3O4-GO | C0 = 600 mg/L; T = 25 °C; adsorbent dose: 125 mg/L; pH = 6.0 | 280.6 mg/g | [55] |
| Cr(VI) | TSGA | C0 = 50 mg/L; T = 25 °C; adsorbent dose: 800 mg/L; contact time: 40 min; pH = 2.0 | 100% removal | [60] |
| Cr(VI) | APTES-NPSi | C0 = 200 mg/L; T = 25 °C; adsorbent dose: 5 mg; contact time: 180 min; pH = 2.0 | 103.75 mg/g | [64] |
| Cr(VI) | MnFe-LDH/MnFe2O3@3DNF | C0 = 300 mg/L; T = 25 °C; contact time: 120 min; adsorbent dose: 5 mg; pH = 2.0 | 564.88 mg/g | [121] |
| Cr(VI) | 3D porous CoFe2O4@SiO2-NH2 | C0 = 150 mg/L; contact time: 600 min; adsorbent dose: 1 g/L; T = 25 °C; pH = 2.0 | 126.8 mg/g | [138] |
| Cr(VI) | TCMR | C0 = 150 mg/L; contact time: 360 min; adsorbent dose: 2 g/L; T = 25 °C; pH = 5.0 | 27.04 mg/g | [150] |
| Cr(VI) | Rice husk | C0 = 100 mg/L; contact time: 60 min; adsorbent dose: 50 g/L; T = 25 °C; pH = 5.0–6.0 | 30 mg/g | [158] |
| Cr(VI) | BCS and BCW | C0 = 320 mg/L; contact time: 24 h; adsorbent dose: 4 g/L; T = 25 °C; pH = 2.0 | 24.6 mg/g for BCS, 23.6 mg/g for BCW | [159] |
| Cr(VI) | SDBC | C0 = 100 mg/L; contact time: 24 h; adsorbent dose: 1 g/L; T = 25 °C; pH = 5.0 | 688~738 μmol/g | [161] |
| Cr(VI) | ZBC | C0 = 100 mg/L; contact time: 600 min; adsorbent dose: 4 g/L; T = 25 °C; pH = 1.0 | 33.87 mg/g | [175] |
| Cr(VI) | PEI/SA | C0 = 240 mg/L; contact time: 300 min; adsorbent dose: 400 mg/L; T = 25 °C; pH = 2.0 | 678.67 mg/g | [178] |
| Cr(VI) | CGP | C0 = 100 mg/L; contact time: 800 min; adsorbent dose: 2 g/L; T = 25 °C; pH = 2.0 | 386.40 mg/g | [180] |
| Cr(VI) | Fe-BDC | C0 = 50 mg/L; contact time: 60 min; adsorbent dose: 50 mg; T = 25 °C; pH = 5.5 | 100 mg/g | [187] |
| Cr(VI) | (Fe/Co)-BDC | C0 = 50 mg/L; contact time: 60 min; adsorbent dose: 50 mg; T = 20 °C; pH = 5.3 | 588 mg/g | [188] |
| Cr(VI) | Pectin-hydrogel-crosslinked Fe-based MOFs | C0 = 50 mg/L; contact time: 60 min; adsorbent dose: 20 mg; T = 50 °C; pH = 3.0 | 825.97 mg/g | [189] |
| Cr(VI) | Co/Zn-based ZIF | C0 = 15.0 mg/L; contact time: 30 min; adsorbent dose: 33 mg; T = 25 °C; pH = 6.5 | 69.4 mg/g | [191] |
| Cr(VI) | NH2-SBA-15 | C0 = 25.0 mg/L; contact time: 4 h; adsorbent dose: 100 mg; T = 30 °C; pH = 2.0 | Removal efficiency of 88% | [192] |
| Cr(VI) | Ficus carica bast fiber | C0 = 350.0 mg/L; contact time: 210 min; adsorbent dose: 0.5 g; T = 25 °C; pH = 3.0 | 19.68 mg/g | [193] |
| Cr(VI) and Cr(III) | Canadian peat and coconut fiber | C0 = 250.0 mg/L; contact time: 20 h; adsorbent dose: 1.0 g; T = 25 °C; pH = 1.5 | 19.21 mg/g for Cr(III) and 9.54 mg/g for Cr(VI), respectively | [194] |
3. Novel Adsorption Technologies
Based on the basic principle of adsorption, novel technologies such as continuous fluidized bed process, membrane separation, capacitive deionization (CDI) and so on have also been developed and attracted more and more attention.
3.1. Continuous Fluidized Bed Process
The removal of Cr species from wastewater via the continuous fluidized bed process has shown a good application prospect. A circulated fluidized bed based on a non-hazardous residue, petroleum coke fly ash (FA), was used to remove Cr(VI). Due to the reduction of Cr(VI) to Cr(III) by FA and the neutralization effect, the efficient removal of Cr(VI) from acidic wastewaters could be carried out (eluting agent = acetic acid, liquid/solid ratio = 20 L/kg, agitation time = 18 h, pH = 4 and temperature = 25 °C) [195]. The use of an Intermittent Bubbling Fluidized Bed (IBFB) based on self-assembled modified Pleurotus cornucopiae material (SMPM) could remove Cr(VI) more efficiently (inner diameter = 0.04 m, height = 0.6 m, Cr(VI) volume = 500 mL, 100–500 mg/L, pH = 2–5 and temperature = 20–30 °C) [196].
3.2. Membrane Technology
Membrane separation was used as a cost-effective technology for the treatment of wastewater due to its relatively higher reutilization ratios. The removal capacity of a novel membrane made from polyaniline/poly(ethylene-co-vinyl alcohol) (PANI/EVOH) nanofiber composite toward Cr(VI) was 93.09 mg/g. In addition, the removal efficiency could be maintained up to 92.8% in the first 5 adsorption–desorption cycles and to 76.7% even after 25 cycles [197]. Catalytic membrane technology is highly efficient and feasible for wastewater remediation. The controllable immobilization of ultrasmall palladium nanoparticles (PdNPs) on the filter-paper-based botryoidal nanolignin channel (Pd@LNP/FP) membrane was performed, and the complete removal of Cr(VI) within 2.5 min via the synergetic filtration enrichment and catalysis reduction confirmed its high efficiency and feasibility [198].
3.3. Capacitive Deionization (CDI)
The conventional adsorption method suffers from some problems, such as limited efficiencies, potential organic matter fouling, high cost or low regeneration rates. Capacitive deionization (CDI) has developed as a highly efficient and low-cost methodology for metal ions removal due to its lower life-cycle cost, higher recovery rate, lower energy consumption and more eco-friendly in use [199]. Recently, CDI technology has been preliminarily investigated for desalination treatment of Cr-contaminated water. The viability and preference of capacitive deionization (CDI) for removing Cr(III) were evaluated, and the removal efficiency was positively correlated with the applied voltage (Figure 7) [200].
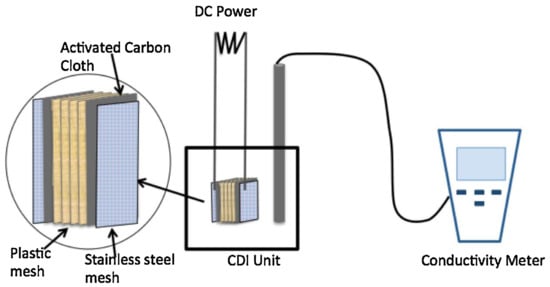
Figure 7.
A schematic of CDI for metal removal [200]. Copyright belongs to Elsevier B.V.
An activated carbon (AC) electrode was fabricated and further used for the electro-sorption of Cr(VI) or low-concentration Cr(VI) (10 mg/L) with relative higher efficiency (97.1%) [201]. Acid-treated AC from rice husk was used as a CDI electrode for the electro-sorption of Cr(VI), and a relatively lower maximum capacity of 2.8316 mg/g was obtained [202]. Highly porous AC from a Limonia acidissima shell-based electrode contributed a lot to the membrane capacitive deionization (MCDI) of Cr(VI) with higher efficiency [203]. In the presence of bovine serum albumin (BSA), the amount for the adsorption of Cr(VI) by MCDI increased to 190.8 mg/g due to the stronger driving forces [204]. Taking advantage of synergistic effects, the combination of photocatalyst (MIL-53(Fe)) and CDI was performed, and an enhanced removal of total Cr with a high removal ratio (72.2%) could be achieved.
Recently, AC from Bael fruit shell (BS) with a macroporous and mesoporous structure exhibited a relatively larger specific surface area (617.72 m2/g). The removal of Cr(VI) by CDI with a BS-based electrode was performed, and an extremely high removal efficiency of ~100% at an applied potential of 15 V could be obtained due to its high adsorption capacity [205]. Obviously, the results are getting better and better. However, it is worth mentioning that the application of CDI in removing Cr ions is still in its infancy. More efforts and scientific research are needed to push the technology forward.
4. Mechanism Studies
4.1. Adsorbent–Adsorbate Interactions
The electrostatic (physical) and ionic (chemical) interactions would be involved in the Cr removal due to the higher aromatic structure of most carbon-based adsorbents [172]. For ACs, the removal of Cr(VI) might occur through intraparticle diffusion, electrostatic attraction and adsorption-coupled reduction [174]. The abundant carboxyl and hydroxyl groups on the oxidized carbons were found to be beneficial to the enhanced adsorption efficiencies [172]. Sorption only occurred at localized sites of PEI functionalized magnetic hydrochar, and the interaction between Cr(VI) and -NH2 could be confirmed [206].
Endowing the adsorbents with some unique properties, such as reduction and catalytic performance, would effectively improve the removal efficiencies. The chemical sorption between PANI/H-TNB and Cr(VI) occurred due to the combined effect of reduction of Cr(VI) to Cr(III), which contributed a lot to the higher adsorption efficiency [126].
Taking full advantage of the surface electrostatic adsorption, chelation, ion-exchanging and reduction effects, newly developed adsorbents would possess relatively higher adsorption capacities and a faster adsorption rate toward Cr species [178]. The enhanced removal efficiency for Cr(VI) by MCDI might be attributed to ion adsorption, redox reaction of Cr(VI) into Cr(III) and precipitation effects [204].
4.2. Analyses of Adsorption Process
To properly understand an adsorption process, the migration of adsorbate(s) from solutions onto adsorbents should be further clarified. In recent years, linear and nonlinear regression analyses of adsorption equilibrium data, including dynamics and isotherms, have been utilized to elucidate the adsorption process. To define the best fitting adsorption models that could quantify the distribution of adsorbates, analyze the adsorption process and verify the theoretical assumptions, various efforts have been made to elucidate the nature of the adsorbate–adsorbent interactions.
As shown in Table 2, almost all adsorption kinetics could be best fitted by the pseudo-first or pseudo-second order rate laws. If an adsorption is diffusion controlled, the process will follow the pseudo-first order sorption rate. Obviously, the pseudo-second order is the superior model, as it can represent many adsorption systems, thus indicating that the physicochemical interactions between the two phases, involving surface adsorption and chemisorption, dominated the rate-limiting step. Other kinetic models, such as the Elovich model, might describe some adsorption processes, indicating the adsorption behaviors concurred with the nature of chemical adsorption [206]. To construct more comprehensive equilibrium and dynamic models for predicting the competitive adsorption and the dynamic behaviors of coexisting metal ions, the adsorption of Ni(II), Co(II), Cr(VI) and P(V) by goethite was simulated by an equilibrium model, CD-MUSIC, and further analyzed by in situ ATR–FTIR spectroscopy [207]. The successful prediction of the binary competitive adsorption of Ni(II)-Co (II) and Cr(VI)-P(V) indicated that the developed model might be used as a robust and scalable solver to elucidate the adsorption procedure.
The adsorption equilibrium could be illustrated by the Freundlich, Langmuir, Tempkin and Redlich–Peterson isotherms. Among them, the Langmuir isotherm was the most widely suitable to simulate the monolayer adsorption of Cr species onto the homogenous adsorbents’ surfaces. The Freundlich isotherm could be applicable to an adsorption process that occurs on the heterogonous surface, defining the surface heterogeneity and the exponential distribution of active sites and their energies. Obviously, most adsorption processes obey the Langmuir model, thus confirming the homogenous surfaces obtained due to the well-developed fabrication technologies and the monolayer adsorption of Cr species on the adsorbents’ surface with specific functional groups.
Unfortunately, only most-used empirical models (such as the Elovich, intraparticle diffusion, pseudo-first-order and pseudo-second-order kinetic models, and Langmuir and Freundlich isotherm models) were used to understand the Cr species adsorption processes. Other theoretical adsorption models (kinetic models such as Langmuir rate equation, pseudo-n-th-order (PNO) equation, mixed 1,2-order equation (MOE), fractal-like kinetic models, Vermeulen and fractal-like Vermeulen models, Weber–Morris model, Bangham–Sever model, Furusawa–Smith model, Haerifar–Azizian model and other empirical models; isotherm models such as linear models (Redlichepeterson (ReP) isotherm, Sips isotherm model and Toth isotherm model); models based on Polanyi’s potential theory (Dubinin–Radushkevich (D–R) model); the Dubinin–Astakhov (D–A) model; chemical adsorption models (Volmer isotherm model); and physical adsorption models (BET model, Aranovich model and ion-exchange isotherm model)) are expected to propose the adsorption mechanisms in the future.

Table 2.
Adsorption isotherm and kinetic studies of Cr removal from aqueous solution.
Table 2.
Adsorption isotherm and kinetic studies of Cr removal from aqueous solution.
| Type of Cr | Type of Adsorbent | Applicable Kinetic Model | Applicable Isotherm Model | Reference |
|---|---|---|---|---|
| Cr(VI) | MCMs | The pseudo-second-order model | The Langmuir model | [22] |
| Cr(VI) | FeMnOx/MWCNTs | The pseudo-second-order model | The Langmuir model | [34] |
| Cr(VI) | Gr-Si-PPy | The pseudo-second-order model | The Langmuir model | [47] |
| Cr(VI) | nZVI-MSC | The pseudo-first-order model | Not provided | [61] |
| Cr(VI) | nFeOOH@D001 | The pseudo-second-order model | The Langmuir model | [73] |
| Cr(VI) | FeBC | The intra-particle diffusion model | Not provided | [79] |
| Cr(VI) | Fe2(SO4)3@Egeria najas based biochar | The pseudo-second-order model | The Langmuir model | [86] |
| Cr(VI) | PPy-MSFA | The pseudo-second-order model | The Langmuir model | [98] |
| Cr(VI) | SnZVI@EPS | The pseudo-second-order model | Not provided | [107] |
| Cr(VI) | Zn/Al-LDHs | The pseudo-second-order model | The Langmuir model | [116] |
| Cr(VI) | PANI-Mg/Al LDHs | The pseudo-second-order model | The Langmuir model | [118] |
| Cr(VI) | Crayfish shell biochar–Fe composite | The pseudo-second-order model | The Langmuir model | [134] |
| Cr(VI) | EDTA/GO/CS | The pseudo-second-order model | The Freundlich model | [166] |
| Cr(VI) | ZBC | The pseudo-second-order model | The Langmuir model | [175] |
| Cr(VI) | PEI-modified Juncus effuses | The pseudo-second-order model | The Freundlich model | [176] |
| Cr(VI) | Co/Zn based ZIF | Not provided | The Langmuir model | [191] |
| Cr(VI) | PANI/EVOH | The pseudo-second-order model | The Freundlich model | [197] |
| Cr(VI) | Pd@LNP/FP | The pseudo-first-order model | Not provided | [198] |
| Cr(VI) | PEI functionalized magnetic hydrochar | The Elovich model | The Freundlich model | [206] |
| Cr(VI) | CVN | The pseudo-second-order model | The Langmuir model | [208] |
5. Conclusions and Future Perspectives
As an efficient method for wastewater treatment with relatively lower cost, better flexibility and simplicity of operation, higher ease of design and sensitivity to various contaminants, adsorption has attracted continuous attention in recent years. The current adsorbents for the decontamination of Cr species from wastewater still exhibit some limitations, such as high cost, low reproducibility, potential adverse effects and the possibility of producing secondary wastes. The development of reusable adsorbents will be helpful to correct this deficiency.
As discussed before, defect engineering could greatly enhance the removal efficiency of an adsorbent; further investigations could be performed to introduce defects, rather than compositing with other substances, which might be more eco-friendly, sustainable and practical.
Adsorbents with multifunctional properties, such as catalytic characteristics, are expected. The reduction of Cr(VI) to Cr(III) would greatly decrease the toxicity of Cr-species-based contaminations.
Unfortunately, only most-used empirical models (such as the Elovich, the intraparticle diffusion, the pseudo-first-order and pseudo-second-order kinetic models, and the Langmuir and Freundlich isotherm models) were used to understand the Cr species adsorption processes. Other theoretical adsorption models are expected to propose adsorption mechanisms in the future.
New technologies need to be developed for the more efficient and facile removal of Cr species. Membrane technology, CDI and the continuous fluidized bed process have shown excellent decontamination properties, and the environmentally friendly features, economic effectiveness and recycling features undoubtedly guarantee their wide and practical applications in the future. Some general limitations associated with the adsorbent in a powder media include surface area loss, process control and production time/cost. To control the packing density and pressure drop of the media while immobilizing the adsorbent, future research on adsorption technologies should primarily focus on the practical applications of fixed columns with the packing adsorbent in wastewater treatment plants.
Author Contributions
Conceptualization, J.-G.Y.; methodology, J.-G.Y.; software, J.-G.Y.; resources, J.-G.Y.; data curation, J.-G.Y.; writing—original draft preparation, J.-G.Y.; writing—review and editing, B.L., Y.-N.X., J.Z., Y.-P.L., S.P. and J.-G.Y.; visualization, B.L., Y.-N.X., J.Z., F.M.K., Y.-P.L., X.-Y.J., S.P. and J.-G.Y.; supervision, J.-G.Y.; project administration, J.-G.Y.; funding acquisition, J.-G.Y. and X.-Y.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Open Project Program of State Key Laboratory of Vanadium and Titanium Resources Comprehensive Utilization (No. 2022P4FZG07A) and National Natural Science Foundation of China (grant number: 51674292).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
3D, three-dimensional; Ag-ACE, nanosilver-assisted chemical etching; ASGO, amino-modified graphene oxide; α-FeOOH, goethite; β-FeOOH, akageneite; γ-FeOOH, lepidocrocite; δ-FeOOH feroxyhyte; AC-NH2, quaternary amine-anchored AC; ACs, active carbons; BC, biochar; BC-SnZVI, sulfide-modified nanoscale zero-valent iron (SnZVI) anchored BC; BL, black liquor; BLN, pulping black liquor lignin; CDI, capacitive deionization; CG-RS, coal gangue–rape straw biochar; CGP, cellulose-based aerogel beads; CHS, sulfonated carbon hemisphere; CMC, carboxymethyl cellulose; CMS, carbon microsphere; CNFs, carbon nano fibers; CNTs, carbon nanotubes; CPAMA, cationic polyacrylamide aerogel; Cr, chromium; Cr(II), chromous ion; Cr(III), chromic ion; Cr(VI) or or CrO42−, chromate ion; (Cr2O3), Cr(III) oxide; Cr(OH)3, Cr(III) hydroxide; Cr2O72−, dichromate ion; CTFs, covalent triazine frameworks; DETA, diethylenetriamine; ELN, enzymolytic lignin; EPS, extracellular polymeric substance; FeBC, Fe-modified BC; FeOOH, iron oxyhydroxide; FeS, ferrous sulfide; FeSO4, iron sulphate; FS, SPORL pretreatment spent liquor; GMA, glycidylmethacrylate; Gr, graphene; GO, graphene oxide; HA, humic acid; HFO, hydrated iron oxide; KH-560, 3-glycidoxypropyltrimethoxysilane; L-cys, L-cysteine; LDH, layered double hydroxide; MCMSs, magnetic carbon microspheres; MOFs, metal–organic frameworks; MPCMS, mesoporous carbon microsphere; MPMs, magnetic polymer microspheres; MSC, mesoporous silica–carbon; NPs, nanoparticles; nZVI, nanoscale zero-valent iron; NH2-SBA-15, amino-functionalized SBA-15; PA, polyamine; PAH, poly(allylamine hydrochloride); PDMDAAC, poly(dimethyl diallyl ammonium chloride); PEI, polyethyleneimine; PPy, polypyrrole; PRBs, permeable reactive barriers; PS, polystyrene; PVC, polyvinyl chloride; PVP, polyvinylpyrrolidone; Qm, adsorption capacity; R, removal percentage; RDF, radial distribution function; rGO, reduced graphene oxide; SA, sodium alginate; SnZVI, sulfidated nanoscale zero-valent iron; SnZVI@EPS, sulfidated nanoscale zero-valent iron@EPS; SSA, specific surface area; TCMR, traditional Chinese medicine residual; TEPA, tetraethylenepentamine; TETA, triethylentetramine; TSGA, TiO2-ZnxCd1-xS rGO aerogel; ZBC, ZnO modified biochar; ZIFs, Zeolitic imidazolate frameworks.
References
- Zhang, X.H.; Koropchak, J.A. Thermospray methods for rapid, sensitive, and nonchromatographic speciation of chromium oxidation states. Anal. Chem. 1999, 71, 3046–3053. [Google Scholar] [CrossRef] [PubMed]
- Proctor, D.M.; Thompson, C.M.; Suh, M.; Harris, M.A. A response to “A quantitative assessment of the carcinogenicity of hexavalent chromium by the oral route and its relevance to human exposure”. Environ. Res. 2011, 111, 468–470. [Google Scholar] [CrossRef] [PubMed]
- Zheng, F.Y.; Lin, X.F.; Yu, H.W.; Li, S.X.; Huang, X.G. Visible-light photoreduction, adsorption, matrix conversion and membrane separation for ultrasensitive chromium determination in natural water by X-ray fluorescence. Sens. Actuators B Chem. 2016, 226, 500–505. [Google Scholar] [CrossRef]
- Ghorade, I.B.; Thakur, V.R.; Patil, S.S. Assessment of heavy metal content in sediment of Godavari River basin. Eur. Acad. Res. 2014, 2, 3560–3584. [Google Scholar]
- An, Q.; Wu, Y.; Wang, J.; Li, Z. Heavy metals and polychlorinated biphenyls in sediments of the Yangtze river estuary, China. Environ. Earth Sci. 2009, 59, 363–370. [Google Scholar] [CrossRef]
- Zhao, W.; Huang, X.; Gong, J.; Ma, L.; Qian, J. Influence of aquifer heterogeneity on Cr(VI) diffusion and removal from groundwater. Environ. Sci. Pollut. Res. 2022, 29, 3918–3929. [Google Scholar] [CrossRef]
- Liu, X.; Li, Y.; Wang, C.; Ji, M. Cr (VI) removal by a new type of anion exchange resin DEX-Cr: Adsorption affecting factors, isotherms, kinetics, and desorption regeneration. Environ. Prog. Sustain. Energy 2015, 34, 387–393. [Google Scholar] [CrossRef]
- Kusku, O.; Rivas, B.L.; Urbano, B.F.; Arda, M.; Kabay, N.; Bryjak, M. A comparative study of removal of Cr(VI) by ion exchange resins bearing quaternary ammonium groups. J. Chem. Technol. Biotechnol. 2014, 89, 851–857. [Google Scholar] [CrossRef]
- Yao, Z.; Li, Y.; Cui, Y.; Zheng, M.; Zhu, B.; Xu, H.; Zhu, L. Tertiary amine block copolymer containing ultrafiltration membrane with pH-dependent macromolecule sieving and Cr(VI) removal properties. Desalination 2015, 355, 91–98. [Google Scholar] [CrossRef]
- Alothman, Z.A.; Naushad, M.; Ali, R. Kinetic, equilibrium isotherm and thermodynamic studies of Cr(VI) adsorption onto low-cost adsorbent developed from peanut shell activated with phosphoric acid. Environ. Sci. Pollut. Res. 2013, 20, 3351–3365. [Google Scholar] [CrossRef]
- Gupta, V.K.; Ali, I.; Saleh, T.A.; Siddiqui, M.N.; Agarwal, S. Chromium removal from water by activated carbon developed from waste rubber tires. Environ. Sci. Pollut. Res. 2013, 20, 1261–1268. [Google Scholar] [CrossRef]
- Rangabhashiyam, S.; Suganya, E.; Selvaraju, N. Packed bed column investigation on hexavalent chromium adsorption activated carbon prepared from Swietenia Mahogani fruit shells. Desalination Water Treat. 2016, 57, 13048–13055. [Google Scholar] [CrossRef]
- Rajesh, Y.; Namrata, G.; Ramgopal, U. Ni(II) adsorption characteristics of commercial activated carbon from synthetic electroless plating solutions. Desalination Water Treat. 2016, 57, 13807–13817. [Google Scholar] [CrossRef]
- Pang, M.L.; Xu, L.; Kano, N.; Imaizumi, H. Adsorption of heavy metal onto activated carbon modified with potassium permanganate. J. Chem. Eng. Jpn. 2014, 47, 386–391. [Google Scholar] [CrossRef]
- Kundu, A.; Redzwan, G.; Sahu, J.N.; Mukherjee, S.; Sen Gupta, B.; Hashim, M.A. Hexavalent chromium adsorption by a novel activated carbon prepared by microwave activation. Bioresources 2014, 9, 1498–1518. [Google Scholar] [CrossRef]
- Boustila, H.; Boutillara, Y.; Velasco, L.F.; Djellali, A.; Tazibet, S. Tailoring activated carbon properties for Pb(II) and Cr(VI) removal from water in continuous mode. Chem. Eng. Technol. 2022, 45, 258–265. [Google Scholar] [CrossRef]
- Wang, X.G.; Zhang, Y.L.; Li, J.; Zhang, G.Z.; Li, X.M. Enhance Cr(VI) removal by quaternary amine-anchoring activated carbons. J. Taiwan Inst. Chem. Eng. 2016, 58, 434–440. [Google Scholar] [CrossRef]
- Chen, Y.; Qian, Y.; Ma, J.; Mao, M.; Qian, L.; An, D. New insights into the cooperative adsorption behavior of Cr(VI) and humic acid in water by powdered activated carbon. Sci. Total Environ. 2022, 817, 153081. [Google Scholar] [CrossRef]
- Beyan, S.M.; Prabhu, S.V.; Ambio, T.A.; Gomadurai, C. A statistical modeling and optimization for Cr(VI) adsorption from aqueous media via teff straw-based activated carbon: Isotherm, kinetics, and thermodynamic studies. Adsorpt. Sci. Technol. 2022, 2022, 7998069. [Google Scholar] [CrossRef]
- Tu, B.; Chen, H.; Xue, S.; Deng, J.; Tao, H. Ultrafast and efficient removal of aqueous Cr(VI) using iron oxide nanoparticles supported on Bermuda grass-based activated carbon. J. Mol. Liq. 2021, 334, 116026. [Google Scholar] [CrossRef]
- Nasseh, N.; Khosravi, R.; Rumman, G.A.; Ghadirian, M.; Eslami, H.; Khoshnamvand, M.; Al-Musawi, T.J.; Khosravi, A. Adsorption of Cr(VI) ions onto powdered activated carbon synthesized from Peganum harmala seeds by ultrasonic waves activation. Environ. Technol. Innov. 2021, 21, 101277. [Google Scholar] [CrossRef]
- Zhou, J.G.; Wang, Y.F.; Wang, J.T.; Qiao, W.M.; Long, D.H.; Ling, L.C. Effective removal of hexavalent chromium from aqueous solutions by adsorption on mesoporous carbon microspheres. J. Colloid Interface Sci. 2016, 462, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Cai, J.; Aikelaimu, A.; Li, Y. Removal of Cr (III) from aqueous solutions by carbon lignin-based composite. Sep. Sci. Technol. 2022, 57, 523–531. [Google Scholar] [CrossRef]
- Lu, J.; Fu, F.; Zhang, L.; Tang, B. Insight into efficient co-removal of Se(IV) and Cr(VI) by magnetic mesoporous carbon microspheres: Performance and mechanism. Chem. Eng. J. 2018, 346, 590–599. [Google Scholar] [CrossRef]
- Zhang, L.; Fu, F.; Tang, B. Adsorption and redox conversion behaviors of Cr(VI) on goethite/carbon microspheres and akaganeite/carbon microspheres composites. Chem. Eng. J. 2019, 356, 151–160. [Google Scholar] [CrossRef]
- Yang, K.; Xing, J.; Xu, P.; Chang, J.; Zhang, Q.; Usman, K.M. Activated carbon microsphere from sodium lignosulfonate for Cr(VI) adsorption evaluation in wastewater treatment. Polymers 2020, 12, 236. [Google Scholar] [CrossRef]
- Schwarz, A.P.; Bergmann, C.P.; Fagan, S.B. Carbon nanotubes functionalized with titanium complexes for hexavalent chromium adsorption: An ab initio approach. Comput. Theor. Chem. 2017, 1113, 110–119. [Google Scholar] [CrossRef]
- Ding, H.; Li, X.; Wang, J.; Zhang, X.J.; Chen, C. Adsorption of chlorophenols from aqueous solutions by pristine and surface functionalized single-walled carbon nanotubes. J. Environ. Sci. 2016, 43, 187–198. [Google Scholar] [CrossRef]
- Huang, Y.; Song, K.; Luo, W.; Yang, J. Adsorption and reduction of Cr(VI) by hydroxylated multiwalled carbon nanotubes: Effects of humic acid and surfactants. Environ. Sci. Pollut. Res. 2020, 27, 12746–12754. [Google Scholar] [CrossRef]
- Jia, D.; Jing, Z.; Duan, Y.; Li, J. Ultrafast removal of Cr(VI) ions using polyamine modified carbon nanotubes. J. Taiwan Inst. Chem. Eng. 2022, 133, 104265. [Google Scholar] [CrossRef]
- Chen, R.H.; Chai, L.Y.; Li, Q.Z.; Shi, Y.; Wang, Y.Y.; Mohammad, A. Preparation and characterization of magnetic Fe3O4/CNT nanoparticles by RPO method to enhance the efficient removal of Cr(VI). Environ. Sci. Pollut. Res. 2013, 20, 7175–7185. [Google Scholar] [CrossRef]
- Aslam, M.M.A.; Den, W.; Kuo, H.-W. Elucidating the mass transfer mechanism of Cr-VI adsorption by encapsulated chitosan-carbon nanotubes-iron beads in packed-bed columns. J. Water Process Eng. 2022, 46, 102586. [Google Scholar] [CrossRef]
- Chen, L.; Chen, N.; Wu, H.; Li, W.; Fang, Z.; Xu, Z.; Qian, X. Flexible design of carbon nanotubes grown on carbon nanofibers by PECVD Chock for enhanced Cr(VI) adsorption capacity. Sep. Purif. Technol. 2018, 207, 406–415. [Google Scholar] [CrossRef]
- Ma, T.; Wu, Y.; Liu, N.; Yan, C. Adsorption behavior of Cr(VI) and As(III) on multiwall carbon nanotubes modified by iron-manganese binary oxide (FeMnOx/MWCNTs) from aqueous solution. Sep. Sci. Technol. 2022, 57, 192–208. [Google Scholar] [CrossRef]
- Abbasi, Z.; Farrokhnia, A.; Lemraski, E.G.; Moafi, Z.; Sabzezari, M. Performance evaluation of nanocomposite magnetic mono-tosyl-b-cyclodextrin conjugated carbon nanotubes-iron oxide in removal of Cr(III) from aqueous solutions using Taguchi method. Russ. J. Phys. Chem. A 2022, 96, 163–170. [Google Scholar] [CrossRef]
- Salam, M.A. Preparation and characterization of chitin/magnetite/multiwalled carbon nanotubes magnetic nanocomposite for toxic hexavalent chromium removal from solution. J. Mol. Liq. 2017, 233, 197–202. [Google Scholar] [CrossRef]
- Wu, C.L.; Fan, J.; Jiang, J.H.; Wang, J.J. pH/temperature dependent selective removal of trace Cr(VI) from aqueous solution by imidazolium ionic liquid functionalized magnetic carbon nanotubes. RSC Adv. 2015, 5, 47165–47173. [Google Scholar] [CrossRef]
- Ahmed, S.; Fatema Tuj, Z.; Mahdi, M.M.; Mahmudunnabi, D.M.; Choudhury, T.R.; Alam, M.Z.; Nurnabi, M. Synthesis and characterization of graphene oxide for removal of Cr(III) from tannery effluent. Desalination Water Treat. 2021, 244, 201–211. [Google Scholar] [CrossRef]
- Ren, H.; Cao, Z.-F.; Chen, Y.-Y.; Jiang, X.-Y.; Yu, J.-G. Graphene oxide-Bicine composite as a novel adsorbent for removal of various contaminants from aqueous solutions. J. Environ. Chem. Eng. 2021, 9, 106769. [Google Scholar] [CrossRef]
- Balaji, K.; Hardian, R.; Kumar, V.; Viswanatha, R.; Kumar, S.; Singh, A.; Santosh, M.; Szekely, G. Composite nanofiltration membrane comprising one-dimensional erdite, two-dimensional reduced graphene oxide, and silkworm pupae binder. Mater. Today Chem. 2021, 22, 100602. [Google Scholar] [CrossRef]
- Singh, S.; Naik, T.S.S.K.; Anil, A.G.; Khasnabis, S.; Nath, B.; Basavaraju, U.; Kumar, V.; Garg, V.K.; Subramanian, S.; Singh, J.; et al. A novel CaO nanocomposite cross linked graphene oxide for Cr(VI) removal and sensing from wastewater. Chemosphere 2022, 301, 134714. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Anil, A.G.; Khasnabis, S.; Kumar, V.; Nath, B.; Adiga, V.; Naik, T.S.S.K.; Subramanian, S.; Kumar, V.; Singh, J.; et al. Sustainable removal of Cr(VI) using graphene oxide-zinc oxide nanohybrid: Adsorption kinetics, isotherms and thermodynamics. Environ. Res. 2022, 203, 111891. [Google Scholar] [CrossRef]
- Sharma, R.; Kumar, D. Adsorption of Cr(III) and Cu(II) on hydrothermally synthesized graphene oxide-calcium-zinc nanocomposite. J. Chem. Eng. Data 2018, 63, 4560–4572. [Google Scholar] [CrossRef]
- Sun, X.J.; Chen, F.N.; Wei, J.Z.; Zhang, F.M.; Pang, S.Y. Preparation of magnetic triethylene tetramine-graphene oxide ternary nanocomposite and application for Cr (VI) removal. J. Taiwan Inst. Chem. Eng. 2016, 66, 328–335. [Google Scholar] [CrossRef]
- Lv, X.; Zhang, Y.; Fu, W.; Cao, J.; Zhang, J.; Ma, H.; Jiang, G. Zero-valent iron nanoparticles embedded into reduced graphene oxide-alginate beads for efficient chromium (VI) removal. J. Colloid Interface Sci. 2017, 506, 633–643. [Google Scholar] [CrossRef]
- Li, N.; Yue, Q.; Gao, B.; Xu, X.; Kan, Y.; Zhao, P. Magnetic graphene oxide functionalized by poly dimethyl diallyl ammonium chloride for efficient removal of Cr(VI). J. Taiwan Inst. Chem. Eng. 2018, 91, 499–506. [Google Scholar] [CrossRef]
- Fang, W.; Jiang, X.; Luo, H.; Geng, J. Synthesis of graphene/SiO2@polypyrrole nanocomposites and their application for Cr(VI) removal in aqueous solution. Chemosphere 2018, 197, 594–602. [Google Scholar] [CrossRef]
- Zhou, S.; Gao, J.; Wang, S.; Fan, H.; Huang, J.; Liu, Y. Highly efficient removal of Cr(VI) from water based on graphene oxide incorporated flower-like MoS2 nanocomposite prepared in situ hydrothermal synthesis. Environ. Sci. Pollut. Res. 2020, 27, 13882–13894. [Google Scholar] [CrossRef]
- Zhou, J.; Si, C.; Gao, H. The adsorption performance of MoS2 nano-rod by combined with graphene oxide for Cr(VI) removal from aqueous solution. Mater. Sci.-Medzg. 2022, 28, 20–29. [Google Scholar] [CrossRef]
- Wang, J.; Li, Y.; Song, G.; Xie, Y.; Zhu, K.; Alsaedi, A.; Hayat, T.; Chen, C. Construction of novel graphene-based materials GO@SiO2@C@Ni for Cr(VI) removal from aqueous solution. J. Colloid Interface Sci. 2019, 557, 254–265. [Google Scholar] [CrossRef]
- Zheng, Y.; Cheng, B.; You, W.; Yu, J.; Ho, W. 3D hierarchical graphene oxide-NiFe LDH composite with enhanced adsorption affinity to Congo red, methyl orange and Cr(VI) ions. J. Hazard. Mater. 2019, 369, 214–225. [Google Scholar] [CrossRef]
- Lv, X.; Qin, X.; Wang, K.; Peng, Y.; Wang, P.; Jiang, G. Nanoscale zero valent iron supported on MgAl-LDH-decorated reduced graphene oxide: Enhanced performance in Cr(VI) removal, mechanism and regeneration. J. Hazard. Mater. 2019, 373, 176–186. [Google Scholar] [CrossRef]
- Ren, L.; Dong, J.; Chi, Z.; Huang, H. Reduced graphene oxide-nano zero value iron (rGO-nZVI) micro-electrolysis accelerating Cr(VI) removal in aquifer. J. Environ. Sci. 2018, 73, 96–106. [Google Scholar] [CrossRef]
- Liu, Y.-P.; Lv, Y.-T.; Guan, J.-F.; Khoso, F.M.; Jiang, X.-Y.; Chen, J.; Li, W.-J.; Yu, J.-G. Rational design of three-dimensional graphene/graphene oxide-based architectures for the efficient adsorption of contaminants from aqueous solutions. J. Mol. Liq. 2021, 343, 117709. [Google Scholar] [CrossRef]
- Wang, X.; Lu, J.; Cao, B.; Liu, X.; Lin, Z.; Yang, C.; Wu, R.; Su, X.; Wang, X. Facile synthesis of recycling Fe3O4/graphene adsorbents with potassium humate for Cr(VI) removal. Colloids Surf. A Physicochem. Eng. Asp. 2019, 560, 384–392. [Google Scholar] [CrossRef]
- Subedi, N.; Lande, A.; Abu-Danso, E.; Iqbal, J.; Bhatnagar, A. A comparative study of magnetic chitosan (Chi@Fe3O4) and graphene oxide modified magnetic chitosan (Chi@Fe3O4GO) nanocomposites for efficient removal of Cr(VI) from water. Int. J. Biol. Macromol. 2019, 137, 948–959. [Google Scholar] [CrossRef]
- Kong, D.; He, L.; Li, H.; Zhang, F.; Song, Z. Preparation and characterization of graphene oxide/chitosan composite aerogel with high adsorption performance for Cr(VI) by a new crosslinking route. Colloids Surf. A Physicochem. Eng. Asp. 2021, 625, 126832. [Google Scholar] [CrossRef]
- Bao, S.; Wang, Y.; Wei, Z.; Yang, W.; Yu, Y.; Sun, Y. Amino-assisted AHMT anchored on graphene oxide as high performance adsorbent for efficient removal of Cr(VI) and Hg(II) from aqueous solutions under wide pH range. J. Hazard. Mater. 2021, 416, 125825. [Google Scholar] [CrossRef]
- Bao, S.; Yang, W.; Wang, Y.; Yu, Y.; Sun, Y. Highly efficient and ultrafast removal of Cr(VI) in aqueous solution to ppb level by poly(allylamine hydrochloride) covalently cross-linked amino-modified graphene oxide. J. Hazard. Mater. 2021, 409, 124470. [Google Scholar] [CrossRef]
- Liang, Q.; Chen, X.; Liu, R.; Xu, K.; Luo, H. Efficient removal of Cr(VI) by a 3D Z-scheme TiO2-ZnxCd1-xS graphene aerogel via synergy of adsorption and photocatalysis under visible light. J. Environ. Sci. 2023, 124, 360–370. [Google Scholar] [CrossRef]
- Xiong, K.; Gao, Y.; Zhou, L.; Zhang, X.M. Zero-valent iron particles embedded on the mesoporous silica-carbon for chromium (VI) removal from aqueous solution. J. Nanoparticle Res. 2016, 18, 267. [Google Scholar] [CrossRef]
- Zhang, W.; Qian, L.; Chen, Y.; Ouyang, D.; Han, L.; Shang, X.; Li, J.; Gu, M.; Chen, M. Nanoscale zero-valent iron supported by attapulgite produced at different acid modification: Synthesis mechanism and the role of silicon on Cr(VI) removal. Chemosphere 2021, 267, 129183. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; Shang, X.; Zhang, B.; Zhang, W.; Su, A.; Chen, Y.; Ouyang, D.; Han, L.; Yan, J.; Chen, M. Enhanced removal of Cr(VI) by silicon rich biochar-supported nanoscale zero-valent iron. Chemosphere 2019, 215, 739–745. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Chen, X.; Li, S.; Ma, W.; Li, Y.; He, Z.; Hu, H. Effective removal of Cr(VI) from aqueous solution based on APTES modified nanoporous silicon prepared from kerf loss silicon waste. Environ. Sci. Pollut. Res. 2020, 27, 10899–10909. [Google Scholar] [CrossRef] [PubMed]
- Nishino, A.; Taki, A.; Asamoto, H.; Minamisawa, H.; Yamada, K. Kinetic, isotherm, and equilibrium investigation of Cr(VI) Ion adsorption on amine-functionalized porous silica beads. Polymers 2022, 14, 2104. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, G.; Peng, S.; Zhou, C. Synthesis of calcium silicate hydrate from coal gangue for Cr(VI) and Cu(II) removal from aqueous solution. Molecules 2021, 26, 6192. [Google Scholar] [CrossRef]
- Zhang, Y.; Bian, T.; Zhang, Y.; Zheng, X.; Li, Z. Chelation resin efficient removal of Cu(II), Cr(III), Ni(II) in electroplating wastewater. Fuller. Nanotub. Carbon Nanostruct. 2018, 26, 765–776. [Google Scholar] [CrossRef]
- Zang, Y.; Yue, Q.; Kan, Y.; Zhang, L.; Gao, B. Research on adsorption of Cr(VI) by Poly-epichlorohydrin-dimethylamine (EPIDMA) modified weakly basic anion exchange resin D301. Ecotoxicol. Environ. Saf. 2018, 161, 467–473. [Google Scholar] [CrossRef]
- Coskun, R.; Er, E.; Delibas, A. Synthesis of novel resin containing carbamothiolylimidamide group and application for Cr(VI) removal. Polym. Bull. 2018, 75, 963–983. [Google Scholar] [CrossRef]
- Ye, Z.; Yin, X.; Chen, L.; He, X.; Lin, Z.; Liu, C.; Ning, S.; Wang, X.; Wei, Y. An integrated process for removal and recovery of Cr(VI) from electroplating wastewater by ion exchange and reduction-precipitation based on a silica-supported pyridine resin. J. Clean. Prod. 2019, 236, 117631. [Google Scholar] [CrossRef]
- Han, S.; Zang, Y.; Gao, Y.; Yue, Q.; Zhang, P.; Kong, W.; Jin, B.; Xu, X.; Gao, B. Co-monomer polymer anion exchange resin for removing Cr(VI) contaminants: Adsorption kinetics, mechanism and performance. Sci. Total Environ. 2020, 709, 136002. [Google Scholar] [CrossRef]
- Song, Y.; Li, Z.; Shao, S.; Jiao, W.; Liu, Y. High-gravity intensified preparation of D201 resin-hydrated iron oxide nanocomposites for Cr(VI) removal. Adv. Powder Technol. 2021, 32, 1584–1593. [Google Scholar] [CrossRef]
- Wang, S.; Yin, W.; Bu, H.; Zeng, W.; Li, P.; Zheng, X.; Chiang, P.; Wu, J. A facile modification of cation exchange resin by nano-sized goethite for enhanced Cr(VI) removal from water. Environ. Technol. 2022, 43, 1833–1842. [Google Scholar] [CrossRef]
- Zeng, S.; Zhong, D.; Xu, Y.; Zhong, N. A novel sulfide-modified nanoscale zero valent iron supported on porous anion exchange resin composite for Cr(VI) effective removal from waste. Chem. Phys. Lett. 2022, 794, 139494. [Google Scholar] [CrossRef]
- Toli, A.; Mystrioti, C.; Xenidis, A.; Papassiopi, N. Continuous Flow Process for Cr(VI) removal from aqueous solutions using resin supported zero-valent iron. Bull. Environ. Contam. Toxicol. 2021, 106, 409–414. [Google Scholar] [CrossRef]
- Gao, W.; Zhong, D.; Xu, Y.; Luo, H.; Zeng, S. Nano zero-valent iron supported by macroporous styrene ion exchange resin for enhanced Cr(VI) removal from aqueous solution. J. Dispers. Sci. Technol. 2022, 43, 1197–1207. [Google Scholar] [CrossRef]
- Kahraman, H.T.; Pehlivan, E. Evaluation of anion-exchange resins on the removal of Cr(VI) polluted water: Batch ion-exchange modeling. Arab. J. Geosci. 2019, 12, 532. [Google Scholar] [CrossRef]
- Verma, R.; Maji, P.K.; Sarkar, S. Detailed investigation of effective trace Cr(VI) removal mechanism by anion exchange resin with phenol-formaldehyde matrix. J. Ind. Eng. Chem. 2022, 111, 147–154. [Google Scholar] [CrossRef]
- Yang, F.; Jiang, Y.; Dai, M.; Hou, X.; Peng, C. Active biochar-supported iron oxides for Cr(VI) removal from groundwater: Kinetics, stability and the key role of FeO in electron-transfer mechanism. J. Hazard. Mater. 2022, 424, 127542. [Google Scholar] [CrossRef]
- Zhang, W.; Li, Q.; Li, R.; Shen, N.; Li, J.; Shen, J.; Sun, X.; Han, W. Enhanced sequestration of chelated Cr(III) from aqueous by Al-containing ferrihydrite: New expectation of overall removal of various heavy metal complexes. Sep. Purif. Technol. 2022, 282, 120151. [Google Scholar] [CrossRef]
- Yuan, Y.; Wei, X.; Yin, H.; Zhu, M.; Luo, H.; Dang, Z. Synergistic removal of Cr(VI) by S-nZVI and organic acids: The enhanced electron selectivity and pH-dependent promotion mechanisms. J. Hazard. Mater. 2022, 423, 127240. [Google Scholar] [CrossRef] [PubMed]
- Xi, Y.; Xie, T.; Liu, Y.; Wu, Y.; Liu, H.; Su, Z.; Huang, Y.; Yuan, X.; Zhang, C.; Li, X. Carboxymethyl cellulose stabilized ferrous sulfide@extracellular polymeric substance for Cr(VI) removal: Characterization, performance, and mechanism. J. Hazard. Mater. 2022, 425, 127837. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.J.; Lu, J.W.; Ding, Z.C.; Li, N.; Fu, F.L.; Tang, B. Cr(VI) removal by mesoporous FeOOH polymorphs: Performance and mechanism. RSC Adv. 2016, 6, 82118–82130. [Google Scholar] [CrossRef]
- Wu, S.J.; Fu, F.L.; Cheng, Z.H.; Tang, B. Removal of Cr(VI) from wastewater by FeOOH supported on Amberlite IR120 resin. Desalination Water Treat. 2016, 57, 17767–17773. [Google Scholar] [CrossRef]
- Xu, L.; Fu, F.; Yu, P.; Sun, G. Properties and mechanism of Cr(VI) adsorption and reduction by K2FeO4 in presence of Mn(II). Environ. Technol. 2022, 43, 918–926. [Google Scholar] [CrossRef]
- Yi, Y.; Wang, X.; Zhang, Y.; Ma, J.; Ning, P. Adsorption properties and mechanism of Cr(VI) by Fe2(SO4)3 modified biochar derived from Egeria najas. Colloids Surf. A Physicochem. Eng. Asp. 2022, 645, 128938. [Google Scholar] [CrossRef]
- Ren, T.Y.; He, P.; Niu, W.L.; Wu, Y.J.; Ai, L.H.; Gou, X.L. Synthesis of a-Fe2O3 nanofibers for applications in removal and recovery of Cr(VI) from wastewater. Environ. Sci. Pollut. Res. 2013, 20, 155–162. [Google Scholar] [CrossRef]
- Campos, I.; Alvarez, J.A.; Villar, P.; Pascual, A.; Herrero, L. Foundry sands as low-cost adsorbent material for Cr (VI) removal. Environ. Technol. 2013, 34, 1267–1281. [Google Scholar] [CrossRef]
- Begum, K.; Anantharaman, N. Removal of chromium(VI) ions from aqueous solutions and industrial effluents using magnetic Fe3O4 nano-particles. Adsorpt. Sci. Technol. 2009, 27, 701–722. [Google Scholar] [CrossRef]
- Liu, G.; Deng, Q.; Wang, H.M.; Kang, S.H.; Yang, Y.; Ng, D.H.L.; Cai, W.P.; Wang, G.Z. Synthesis and characterization of nanostructured Fe3O4 micron-spheres and their application in removing toxic Cr ions from polluted water. Chem. A Eur. J. 2012, 18, 13418–13426. [Google Scholar] [CrossRef]
- Liu, Y.B.; Wang, Y.Q.; Zhou, S.M.; Lou, S.Y.; Yuan, L.; Gao, T.; Wu, X.P.; Shi, X.J.; Wang, K. Synthesis of high saturation magnetization superparamagnetic Fe3O4 hollow microspheres for swift chromium removal. ACS Appl. Mater. Interfaces 2012, 4, 4913–4920. [Google Scholar] [CrossRef]
- Zhao, Y.G.; Shen, H.Y.; Li, Q.; Xia, Q.H. Adsorption studies on Cr(VI) in wastewater by NH2-functionalized nano-Fe3O4 magnetic composites. Acta Chim. Sin. 2009, 67, 1509–1514. [Google Scholar]
- Zhao, Y.G.; Shen, H.Y.; Pan, S.D.; Hu, M.Q. Synthesis, characterization and properties of ethylenediamine-functionalized Fe3O4 magnetic polymers for removal of Cr(VI) in wastewater. J. Hazard. Mater. 2010, 182, 295–302. [Google Scholar] [CrossRef]
- Yuan, G.; Li, K.; Zhang, J.; Wang, H.; Huang, Z.; Li, F.; Zhang, H.; Jia, Q.; Zhang, S. Rubra-like MnFe2O4 Microsphere: A high efficiency microwave reduction catalyst for Cr(VI) removal from water. Sep. Purif. Technol. 2022, 286, 120434. [Google Scholar] [CrossRef]
- Gong, H.X.; Hu, P.Z.; Jiang, M.Y.; Huang, X.L.; Shen, T.; Ge, W.W.; Pan, S.D.; Shen, H.Y. Adsorption mechanism investigation of tetraethylenepentamine-functionalized nano-Fe3O4 magnetic polymers in Cu(II), Cr(VI) co-existing water system. Acta Chim. Sin. 2011, 69, 2673–2681. [Google Scholar]
- Zhao, Y.G.; Shen, H.Y.; Pan, S.D.; Hu, M.Q.; Xia, Q.H. Preparation and characterization of amino-functionalized nano-Fe3O4 magnetic polymer adsorbents for removal of chromium(VI) ions. J. Mater. Sci. 2010, 45, 5291–5301. [Google Scholar] [CrossRef]
- Wang, K.; Qiu, G.M.; Cao, H.Y.; Jin, R.F. Removal of chromium(VI) from aqueous solutions using Fe3O4 magnetic polymer microspheres functionalized with amino groups. Materials 2015, 8, 8378–8391. [Google Scholar] [CrossRef]
- Zhou, Q.; Yan, C.J.; Luo, W.J. Polypyrrole coated secondary fly ash-iron composites: Novel floatable magnetic adsorbents for the removal of chromium (VI) from wastewater. Mater. Des. 2016, 92, 701–709. [Google Scholar] [CrossRef]
- Pasinszki, T.; Krebsz, M.; Kotai, L.; Sajo, I.E.; Homonnay, Z.; Kuzmann, E.; Kiss, L.F.; Vaczi, T.; Kovacs, I. Nanofurry magnetic carbon microspheres for separation processes and catalysis: Synthesis, phase composition, and properties. J. Mater. Sci. 2015, 50, 7353–7363. [Google Scholar] [CrossRef]
- Wang, H.T.; Xu, X.; Ren, Z.F.; Gao, B.Y. Removal of phosphate and chromium(VI) from liquids by an amine-crosslinked nano-Fe3O4 biosorbent derived from corn straw. RSC Adv. 2016, 6, 47237–47248. [Google Scholar] [CrossRef]
- Sun, X.T.; Yang, L.R.; Dong, T.T.; Liu, Z.N.; Liu, H.Z. Removal of Cr(VI) from aqueous solution using amino-modified Fe3O4-SiO2-chitosan magnetic microspheres with high acid resistance and adsorption capacity. J. Appl. Polym. Sci. 2016, 133, 43078. [Google Scholar] [CrossRef]
- Beheshti, H.; Irani, M.; Hosseini, L.; Rahimi, A.; Aliabadi, M. Removal of Cr (VI) from aqueous solutions using chitosan/MWCNT/Fe3O4 composite nanofibers-batch and column studies. Chem. Eng. J. 2016, 284, 557–564. [Google Scholar] [CrossRef]
- Yang, W.C.; Tang, Q.Z.; Dong, S.Y.; Chai, L.Y.; Wang, H.Y. Single-step synthesis of magnetic chitosan composites and application for chromate (Cr(VI)) removal. J. Cent. South Univ. 2016, 23, 317–323. [Google Scholar] [CrossRef]
- Wisniewska, M.; Chibowski, S.; Urban, T. Synthetic polyacrylamide as a potential flocculent to remove commercial chromium(III) oxide from aqueous suspension. Int. J. Environ. Sci. Technol. 2016, 13, 679–690. [Google Scholar] [CrossRef]
- Li, Z.K.; Wang, Y.M.; Wu, N.M.; Chen, Q.C.; Wu, K. Removal of heavy metal ions from wastewater by a novel HEA/AMPS copolymer hydrogel: Preparation, characterization, and mechanism. Environ. Sci. Pollut. Res. 2013, 20, 1511–1525. [Google Scholar] [CrossRef]
- Yang, K.; Wang, X.; Yi, Y.; Ma, J.; Ning, P. Formulation of NZVI-supported lactic acid/PAN membrane with glutathione for enhanced dynamic Cr(VI) removal. J. Clean. Prod. 2022, 363, 132350. [Google Scholar] [CrossRef]
- Xi, Y.; Wu, Y.; Liu, Y.; Xie, T.; Liu, H.; Su, Z.; Huanga, Y.; Zhang, C.; Yuan, X.; Li, X. Covering extracellular polymeric substances to enhance the reactivity of sulfidated nanoscale zerovalent iron toward Cr(VI) removal. Chem. Eng. J. 2022, 448, 137610. [Google Scholar] [CrossRef]
- Wang, L.; Muhammad, H.; Laipan, M.; Fan, X.; Guo, J.; Li, Y. Enhanced removal of Cr(VI) and Mo(VI) from polluted water using L-cysteine doped polypyrrole/bentonite composite. Appl. Clay Sci. 2022, 217, 106387. [Google Scholar] [CrossRef]
- Saad, D. Selective removal of Cr(III) from aqueous solution using cross-linked polyethylenimine: Experimental optimization and modeling. Polym. Bull. 2022, 79, 1583–1595. [Google Scholar] [CrossRef]
- Rad, B.F.; Mahdavi, H.; Rad, M.F.; Baghdadi, M. Using design-expert to optimize the properties of a polyethersulfone ultrafiltration membrane through the incorporation of NH2-MIL-53(Fe) and PVP for maximum Cr(VI) removal and flux. J. Polym. Environ. 2022, 30, 3875–3889. [Google Scholar]
- Qu, J.; Yang, Q.; Gong, W.; Li, M.; Cao, B. Simultaneous removal of Cr(VI) and phenol from water using silica-di-block polymer hybrids: Adsorption kinetics and thermodynamics. Polymers 2022, 14, 2894. [Google Scholar] [CrossRef]
- Ou, B.; Wang, J.; Wu, Y.; Zhao, S.; Wang, Z. Efficient removal of Cr (VI) by magnetic and recyclable calcined CoFe-LDH/g-C3N4 via the synergy of adsorption and photocatalysis under visible light. Chem. Eng. J. 2020, 380, 122600. [Google Scholar] [CrossRef]
- Hu, H.; Liu, J.; Xu, Z.; Zhang, L.; Cheng, B.; Ho, W. Hierarchical porous Ni/Co-LDH hollow dodecahedron with excellent adsorption property for Congo red and Cr(VI) ions. Appl. Surf. Sci. 2019, 478, 981–990. [Google Scholar] [CrossRef]
- Zhou, Z.; Sun, Y.; Wang, Y.; Yu, F.; Ma, J. Adsorption behavior of Cu(II) and Cr(VI) on aged microplastics in antibiotics-heavy metals coexisting system. Chemosphere 2022, 291, 132794. [Google Scholar] [CrossRef]
- Song, L.; Qiu, Y.; Zhang, X.; Liu, F.; Li, A. Green strategy with high iron utilization for Cr(VI) removal via sodium polyacrylate-based hydrogel. Chem. Eng. J. 2022, 442, 136162. [Google Scholar] [CrossRef]
- Yang, H.D.; Zhao, Y.P.; Li, S.F.; Fan, X.; Wei, X.Y.; Zong, Z.M. Removal of hexavalent chromium from aqueous solution by calcined Zn/Al-LDHs. Water Sci. Technol. 2016, 74, 229–235. [Google Scholar] [CrossRef]
- Wang, X.J.; Zhu, X.P.; Lan, L.M.; Zuo, H.B. Removal of chromium from laboratory wastewater using preparation-adsorption technology with a Mg/Al/Cr layered compound. RSC Adv. 2016, 6, 85595–85602. [Google Scholar] [CrossRef]
- Zhu, K.R.; Gao, Y.; Tan, X.L.; Chen, C.L. Polyaniline-modified Mg/Al layered double hydroxide composites and their application in efficient removal of Cr(VI). ACS Sustain. Chem. Eng. 2016, 4, 4361–4369. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, H.; Zhang, Q.; Wang, W.; Yang, C.; Du, T.; Yue, T.; Zhu, M.; Wang, J. Demand-oriented construction of Mo3S13-LDH: A versatile scavenger for highly selective and efficient removal of toxic Ag(I), Hg(II), As(III), and Cr(VI) from water. Sci. Total Environ. 2022, 820, 153334. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, Y.; Zhang, W.; Huang, X.; Wang, X.; Huang, Y.; Zhang, P. CuBi2O4/calcined ZnAlBi-LDHs heterojunction: Simultaneous removal of Cr (VI) and tetracycline through effective adsorption and photocatalytic redox. J. Clean. Prod. 2022, 365, 132810. [Google Scholar] [CrossRef]
- Poudel, M.B.; Shin, M.; Kim, H.J. Interface engineering of MIL-88 derived MnFe-LDH and MnFe2O3 on three-dimensional carbon nanofibers for the efficient adsorption of Cr(VI), Pb(II), and As(III) ions. Sep. Purif. Technol. 2022, 287, 120463. [Google Scholar] [CrossRef]
- Manea, Y.K.; Khan, A.M.; Wani, A.A.; Saleh, M.A.S.; Qashqoosh, M.T.A.; Shahadat, M.; Rezakazemi, M. In-grown flower like Al-Li/Th-LDH@CNT nanocomposite for enhanced photocatalytic degradation of MG dye and selective adsorption of Cr (VI). J. Environ. Chem. Eng. 2022, 10, 106848. [Google Scholar] [CrossRef]
- Huang, Z.; Xiong, C.; Ying, L.; Wang, W.; Wang, S.; Ding, J.; Lu, J. Facile synthesis of a MOF-derived magnetic CoAl-LDH@chitosan composite for Pb (II) and Cr (VI) adsorption. Chem. Eng. J. 2022, 449, 137722. [Google Scholar] [CrossRef]
- Chen, H.; Gong, Z.; Zhuo, Z.; Zhong, X.; Zhou, M.; Xiang, X.; Zhang, Z.; Liu, Y.; Chen, Y. Tunning the defects in lignin-derived-carbon and trimetallic layered double hydroxides composites (LDH@LDC) for efficient removal of U(VI) and Cr (VI) in aquatic environment. Chem. Eng. J. 2022, 428, 132113. [Google Scholar] [CrossRef]
- Ma, R.; Wang, T.; Huang, T.; Sun, W.; Qiao, S.; Liu, W. Insights into interactions of Cr(III) and organic matters during adsorption onto titanate nanotubes: Differential absorbance and DFT study. J. Mol. Liq. 2020, 312, 113432. [Google Scholar] [CrossRef]
- Wen, T.; Fan, Q.H.; Tan, X.L.; Chen, Y.T.; Chen, C.L.; Xu, A.W.; Wang, X.K. A core-shell structure of polyaniline coated protonic titanate nanobelt composites for both Cr(VI) and humic acid removal. Polym. Chem. 2016, 7, 785–794. [Google Scholar] [CrossRef]
- Luo, Q.; Huang, X.; Luo, Y.; Yuan, H.; Ren, T.; Li, X.; Xu, D.; Guo, X.; Wu, Y. Fluorescent chitosan-based hydrogel incorporating titanate and cellulose nanofibers modified with carbon dots for adsorption and detection of Cr(VI). Chem. Eng. J. 2021, 407, 127050. [Google Scholar] [CrossRef]
- Zheng, L.; Chen, Z.; Han, J.; Wei, Z.; Yang, L.; Lu, M.; Ma, T.; Yang, L. An all-in-one photocatalyst: Photocatalytic reduction of Cr(VI) and anchored adsorption of Cr(III) over mesoporous titanium@sulfonated carbon hollow hemispheres. J. Environ. Chem. Eng. 2022, 10, 107864. [Google Scholar] [CrossRef]
- Li, H.; Huang, Y.; Luo, Q.; Liu, J. The simultaneous reduction and adsorption for V(V) and Cr(VI) anionic species in aqueous solution by polyethyleneimine cross-linked titanate nanotubes. Sep. Purif. Technol. 2022, 299, 121682. [Google Scholar] [CrossRef]
- Yao, L.; Chen, Z.; Li, J.; Shi, C. Creation of oxygen vacancies to activate lanthanum-doped bismuth titanate nanosheets for efficient synchronous photocatalytic removal of Cr(VI) and methyl orange. J. Mol. Liq. 2020, 314, 113613. [Google Scholar] [CrossRef]
- Kang, L.; Yang, H.; Wang, L.; Chai, S.; Zhang, R.; Wu, J.; Liu, X. Facile integration of FeS and titanate nanotubes for efficient removal of total Cr from aqueous solution: Synergy in simultaneous reduction of Cr(VI) and adsorption of Cr(III). J. Hazard. Mater. 2020, 398, 122834. [Google Scholar]
- Zhao, T.T.; Ge, W.Z.; Nie, Y.X.; Wang, Y.X.; Zeng, F.G.; Qiao, Y. Highly efficient detoxification of Cr(VI) by brown coal and kerogen: Process and structure studies. Fuel Process. Technol. 2016, 150, 71–77. [Google Scholar] [CrossRef]
- Zhao, T.T.; Ge, W.Z.; Yue, F.; Wang, Y.X.; Pedersen, C.M.; Zeng, F.G.; Qiao, Y. Mechanism study of Cr(III) immobilization in the process of Cr(VI) removal by Huolinhe lignite. Fuel Process. Technol. 2016, 152, 375–380. [Google Scholar] [CrossRef]
- Yan, L.; Dong, F.-X.; Li, Y.; Guo, P.-R.; Kong, L.-J.; Chu, W.; Diao, Z.-H. Synchronous removal of Cr(VI) and phosphates by a novel crayfish shell biochar-Fe composite from aqueous solution: Reactivity and mechanism. J. Environ. Chem. Eng. 2022, 10, 107396. [Google Scholar] [CrossRef]
- Zhao, D.L.; Gao, X.; Wu, C.N.; Xie, R.; Feng, S.J.; Chen, C.L. Facile preparation of amino functionalized graphene oxide decorated with Fe3O4 nanoparticles for the adsorption of Cr(VI). Appl. Surf. Sci. 2016, 384, 1–9. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Jia, Y. Removal combined with reduction of hexavalent chromium from aqueous solution by Fe-ethylene glycol complex microspheres. Appl. Surf. Sci. 2016, 389, 696–704. [Google Scholar] [CrossRef]
- Yao, H.L.; Ding, Q.Q.; Zhou, H.J.; Zhao, Z.F.; Liu, G.; Wang, G.Z. An adsorption-reduction synergistic effect of mesoporous Fe/SiO2-NH2 hollow spheres for the removal of Cr(VI) ions. RSC Adv. 2016, 6, 27039–27046. [Google Scholar] [CrossRef]
- Zhou, D.; Wang, J.; Chen, H.; Ge, X.; Wang, X. Enhanced Cr(VI) removal by hierarchical CoFe2O4@SiO2-NH2 via reduction and adsorption processes. New J. Chem. 2022, 46, 13686–13692. [Google Scholar] [CrossRef]
- Zhao, Q.; Yang, B.; Ren, H.; Chen, S.; Luo, C.; Li, Q.; Yang, W.; Yan, K. Removal of Cr(VI) from aqueous systems using Fe-P slag as a reducing agent. Hydrometallurgy 2022, 211, 105875. [Google Scholar] [CrossRef]
- Zhang, H.; Zhou, H. Industrial lignins: The potential for efficient removal of Cr(VI) from wastewater. Environ. Sci. Pollut. Res. 2022, 29, 10467–10481. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, J.; Gao, X.; Che, H.; Wang, P.; Ao, Y. In-depth insight into the mechanism on photocatalytic synergistic removal of antibiotics and Cr (VI): The decisive effect of antibiotic molecular structure. Appl. Catal. B Environ. 2022, 313, 121443. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, L.; Xu, B.; Yang, P. Rhombic TiO2 grown on g-C3N4 nanosheets towards fast charge transfer and enhanced Cr(VI) and NO removal. J. Ind. Eng. Chem. 2022, 111, 226–235. [Google Scholar] [CrossRef]
- Eladlani, N.; Dahmane, E.M.; Ablouh, E.-H.; Ouahrouch, A.; Rhazi, M.; Taourirte, M.; Neffa, M. “Chitosan/montmorillonite” nanocomposites: Adsorption of Cr(III). J. Water Chem. Technol. 2019, 41, 175–181. [Google Scholar] [CrossRef]
- Wang, G.F.; Hua, Y.Y.; Su, X.; Komarneni, S.; Ma, S.J.; Wang, Y.J. Cr(VI) adsorption by montmorillonite nanocomposites. Appl. Clay Sci. 2016, 124, 111–118. [Google Scholar] [CrossRef]
- Wang, Q.Y.; Chen, N.; Yu, Y.; Feng, C.P.; Ning, Q.; Hu, W.W. Chromium(VI) removal from aqueous solution using a new synthesized adsorbent. Desalination Water Treat. 2016, 57, 4537–4547. [Google Scholar] [CrossRef]
- Zhao, M.H.; Xu, Y.; Zhang, C.S.; Rong, H.W.; Zeng, G.M. New trends in removing heavy metals from wastewater. Appl. Microbiol. Biotechnol. 2016, 100, 6509–6518. [Google Scholar] [CrossRef]
- Liu, C.; Fiol, N.; Villaescusa, I.; Poch, J. New approach in modeling Cr(VI) sorption onto biomass from metal binary mixtures solutions. Sci. Total Environ. 2016, 541, 101–108. [Google Scholar] [CrossRef]
- Zinicovscaia, I.; Cepoi, L.; Chiriac, T.; Culicov, O.A.; Frontasyeva, M.; Pavlov, S.; Kirkesali, E.; Akshintsev, A.; Rodlovskaya, E. Spirulina platensis as biosorbent of chromium and nickel from industrial effluents. Desalination Water Treat. 2016, 57, 11103–11110. [Google Scholar] [CrossRef]
- Sen, M.; Dastidar, M.G. Biosorption of Cr (VI) by resting cells of fusarium solani. Iran. J. Environ. Health Sci. Eng. 2011, 8, 153–158. [Google Scholar]
- Zheng, Z.; Duan, X.; Tie, J. One-pot synthesis of a magnetic Zn/iron-based sludge/biochar composite for aqueous Cr(VI) adsorption. Environ. Technol. Innov. 2022, 28, 102661. [Google Scholar] [CrossRef]
- Rawat, M.; Rawat, A.P.; Giri, K.; Rai, J.P.N. Cr(VI) sorption by free and immobilised chromate-reducing bacterial cells in PVA-alginate matrix: Equilibrium isotherms and kinetic studies. Environ. Sci. Pollut. Res. 2013, 20, 5198–5211. [Google Scholar] [CrossRef]
- Rao, A.; Bankar, A.; Kumar, A.R.; Gosavi, S.; Zinjarde, S. Removal of hexavalent chromium ions by Yarrowia lipolytica cells modified with phyto-inspired Fe0/Fe3O4 nanoparticles. J. Contam. Hydrol. 2013, 146, 63–73. [Google Scholar] [CrossRef]
- Baysal, S.H.; Onal, S.; Ozdemir, G. Biosorption of chromium, cadmium, and cobalt from aqueous solution by immobilized living cells of chryseomonas luteola TEM 05. Prep. Biochem. Biotechnol. 2009, 39, 419–428. [Google Scholar] [CrossRef]
- Joutey, N.T.; Bahafid, W.; Sayel, H.; Ananou, S.; El Ghachtouli, N. Hexavalent chromium removal by a novel Serratia proteamaculans isolated from the bank of Sebou River (Morocco). Environ. Sci. Pollut. Res. 2014, 21, 3060–3072. [Google Scholar] [CrossRef]
- Seco-Reigosa, N.; Pena-Rodriguez, S.; Novoa-Munoz, J.C.; Arias-Estevez, M.; Fernandez-Sanjurjo, M.J.; Alvarez-Rodriguez, E.; Nunez-Delgado, A. Arsenic, chromium and mercury removal using mussel shell ash or a sludge/ashes waste mixture. Environ. Sci. Pollut. Res. 2013, 20, 2670–2678. [Google Scholar] [CrossRef]
- Sharma, S.; Kothiyal, N.C. Use of activated dry flowers (ADF) of Alstonia Scholaris for chromium (Vl) removal: Equilibrium, kinetics and thermodynamics studies. Environ. Sci. Pollut. Res. 2013, 20, 8986–8995. [Google Scholar] [CrossRef]
- Shukla, D.; Vankar, P.S. Efficient biosorption of chromium(VI) ion by dry Araucaria leaves. Environ. Sci. Pollut. Res. 2012, 19, 2321–2328. [Google Scholar] [CrossRef]
- Sobhanardakani, S.; Parvizimosaed, H.; Olyaie, E. Heavy metals removal from wastewaters using organic solid waste-rice husk. Environ. Sci. Pollut. Res. 2013, 20, 5265–5271. [Google Scholar] [CrossRef]
- Tytak, A.; Oleszczuk, P.; Dobrowolski, R. Sorption and desorption of Cr(VI) ions from water by biochars in different environmental conditions. Environ. Sci. Pollut. Res. 2015, 22, 5985–5994. [Google Scholar] [CrossRef]
- Wang, T.; Sun, H.W. Biosorption of heavy metals from aqueous solution by UV-mutant Bacillus subtilis. Environ. Sci. Pollut. Res. 2013, 20, 7450–7463. [Google Scholar] [CrossRef]
- Zhou, F.S.; Wang, H.; Fang, S.E.; Zhang, W.H.; Qiu, R.L. Pb(II), Cr(VI) and atrazine sorption behavior on sludge-derived biochar: Role of humic acids. Environ. Sci. Pollut. Res. 2015, 22, 16031–16039. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Xiu, Y.F.; Zhu, H.M. Selective removal of Cr(VI) from aqueous solution by adsorption on mangosteen peel. Environ. Sci. Pollut. Res. 2013, 20, 5930–5938. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Liu, P.; Wang, S.; Finfrock, Y.Z.; Ye, Z.; Feng, Y.; Li, X. Iron-modified biochar-based bilayer permeable reactive barrier for Cr(VI) removal. J. Hazard. Mater. 2022, 439, 129636. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Xu, Z.; You, S.; Komarek, M.; Alessi, D.S.; Yuan, X.; Palansooriya, K.N.; Ok, Y.S.; Tsang, D.C.W. Machine learning exploration of the direct and indirect roles of Fe impregnation on Cr(VI) removal by engineered biochar. Chem. Eng. J. 2022, 428, 131967. [Google Scholar] [CrossRef]
- Zhao, R.; Cao, X.; Li, T.; Cui, X.; Cui, Z. Co-removal effect and mechanism of Cr(VI) and Cd(II) by biochar-supported sulfide-modified nanoscale zero-valent iron in a binary system. Molecules 2022, 27, 4742. [Google Scholar] [CrossRef]
- Zhang, L.; Luo, H.J.; Liu, P.P.; Fang, W.; Geng, J.J. A novel modified graphene oxide/chitosan composite used as an adsorbent for Cr(VI) in aqueous solutions. Int. J. Biol. Macromol. 2016, 87, 586–596. [Google Scholar] [CrossRef]
- Yao, X.L.; Deng, S.B.; Wu, R.; Hong, S.Q.; Wang, B.; Huang, J.; Wang, Y.J.; Yu, G. Highly efficient removal of hexavalent chromium from electroplating wastewater using aminated wheat straw. RSC Adv. 2016, 6, 8797–8805. [Google Scholar] [CrossRef]
- Zhang, Y.-N.; Guo, J.-Z.; Wu, C.; Huan, W.-W.; Chen, L.; Li, B. Enhanced removal of Cr(VI) by cation functionalized bamboo hydrochar. Bioresour. Technol. 2022, 347, 126703. [Google Scholar] [CrossRef]
- Zhang, J.Q.; Chen, H.; Chen, Z.; He, J.J.; Shi, W.X.; Liu, D.M.; Chi, H.Z.; Cui, F.Y.; Wang, W. Microstructured macroporous adsorbent composed of polypyrrole modified natural corncob-core sponge for Cr(VI) removal. RSC Adv. 2016, 6, 59292–59298. [Google Scholar] [CrossRef]
- Zhong, M.; Chen, S.; Wang, T.; Liu, J.; Mei, M.; Li, J. Co-pyrolysis of polyester and cotton via thermogravimetric analysis and adsorption mechanism of Cr(VI) removal by carbon in aqueous solution. J. Mol. Liq. 2022, 354, 118902. [Google Scholar] [CrossRef]
- Zheng, W.H.; Hu, J.T.; Han, Z.S.; Diesel, E.; Wang, Z.X.; Zheng, Z.; Ba, C.Y.; Langer, J.; Economy, J. Interactions of Cr(VI) with hybrid anion exchange/porous carbon fibers in aqueous solution at natural pH. Chem. Eng. J. 2016, 287, 54–61. [Google Scholar] [CrossRef]
- Zhou, L.; Liu, Y.G.; Liu, S.B.; Yin, Y.C.; Zeng, G.M.; Tan, X.F.; Hu, X.; Hu, X.J.; Jiang, L.H.; Ding, Y.; et al. Investigation of the adsorption-reduction mechanisms of hexavalent chromium by ramie biochars of different pyrolytic temperatures. Bioresour. Technol. 2016, 218, 351–359. [Google Scholar] [CrossRef]
- Xiang, B.; Fan, W.; Yi, X.W.; Wang, Z.H.; Gao, F.; Li, Y.J.; Gu, H.B. Dithiocarbamate-modified starch derivatives with high heavy metal adsorption performance. Carbohydr. Polym. 2016, 136, 30–37. [Google Scholar] [CrossRef]
- Zhao, Y.; Lu, C.; Yu, F.; Zhou, H. Investigation of black liquor-derived carbon for removal of Cr(VI): Comparison with lignin-derived carbon. Colloids Surf. A Physicochem. Eng. Asp. 2022, 642, 128730. [Google Scholar] [CrossRef]
- Zhao, X.; Feng, H.; Jia, P.; An, Q.; Ma, M. Removal of Cr(VI) from aqueous solution by a novel ZnO-sludge biochar composite. Environ. Sci. Pollut. Res. 2022, 29, 83045–83059. [Google Scholar] [CrossRef]
- Zhang, S.; Li, W.; Li, M.; Lin, T.; Su, K.; Yang, H.; Chen, J. Efficient removal and detoxification of Cr(VI) by PEI-modified Juncus effuses with a natural 3D network structure. Sep. Purif. Technol. 2022, 297, 121543. [Google Scholar] [CrossRef]
- Yu, F.; Song, W.; Wu, Z.; Chen, W.; Cui, J.; Yao, Y. Cationic polyacrylamide aerogel intercalated molybdenum disulfide for enhanced removal of Cr(VI) and organic contaminants. Sep. Purif. Technol. 2022, 294, 121188. [Google Scholar] [CrossRef]
- Wang, Q.; Li, L.; Tian, Y.; Kong, L.; Cai, G.; Zhang, H.; Zhang, J.; Zuo, W.; Wen, B. Shapeable amino-functionalized sodium alginate aerogel for high-performance adsorption of Cr(VI) and Cd(II): Experimental and theoretical investigations. Chem. Eng. J. 2022, 446, 137430. [Google Scholar] [CrossRef]
- Wang, Q.; Tian, Y.; Kong, L.; Zhang, J.; Zuo, W.; Li, Y.; Cai, G. A novel 3D superelastic polyethyleneimine functionalized chitosan aerogels for selective removal of Cr(VI) from aqueous solution: Performance and mechanisms. Chem. Eng. J. 2021, 425, 131722. [Google Scholar] [CrossRef]
- Li, M.; Tang, C.; Fu, S.; Tam, K.C.; Zong, Y. Cellulose-based aerogel beads for efficient adsorption-reduction-sequestration of Cr(VI). Int. J. Biol. Macromol. 2022, 216, 860–870. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, H.; Xu, J.; Du, X.; Cheng, X.; Du, Z.; Wang, H. Fabrication of MXene/PEI functionalized sodium alginate aerogel and its excellent adsorption behavior for Cr(VI) and Congo Red from aqueous solution. J. Hazard. Mater. 2021, 416, 125777. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.; Yuan, W.; Zheng, Z.; Zhang, J.; Ao, C.; Zhao, J.; Wang, Q.; Zhang, W.; Lu, C. Iron-loaded carbon aerogels derived from bamboo cellulose fibers as efficient adsorbents for Cr(VI) removal. Polymers 2021, 13, 4338. [Google Scholar] [CrossRef]
- Li, J.; Cheng, R.; Chen, J.; Lan, J.; Li, S.; Zhou, M.; Zeng, T.; Hou, H. Microscopic mechanism about the selective adsorption of Cr(VI) from salt solution on nitrogen-doped carbon aerogel microsphere pyrolysis products. Sci. Total Environ. 2021, 798, 149331. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Zhou, Z.; Zhu, D.; Jiang, H.; Qi, Y.; Wang, S.; Jia, Y.; Wang, W. Preparation of covalent triazine-based framework for efficient Cr(VI) removal from water. Colloids Surf. A Physicochem. Eng. Asp. 2022, 632, 127757. [Google Scholar] [CrossRef]
- Hong, T.Z.X.; Dahanayaka, M.; Liu, B.; Law, A.W.-K.; Zhou, K. Zeolitic imidazolate frameworks as capacitive deionization electrodes for water desalination and Cr(VI) adsorption: A molecular simulation study. Appl. Surf. Sci. 2021, 546, 149080. [Google Scholar] [CrossRef]
- Li, Z.; Wang, L.; Qin, L.; Lai, C.; Wang, Z.; Zhou, M.; Xiao, L.; Liu, S.; Zhang, M. Recent advances in the application of water-stable metal-organic frameworks: Adsorption and photocatalytic reduction of heavy metal in water. Chemosphere 2021, 285, 131432. [Google Scholar] [CrossRef]
- Abuzalat, O.; Wong, D.; Elsayed, M.A. Nano-porous composites of activated carbon-metal organic frameworks (Fe-BDC@AC) for rapid removal of Cr (VI): Synthesis, adsorption, mechanism, and kinetics studies. J. Inorg. Organomet. Polym. Mater. 2022, 32, 1924–1934. [Google Scholar] [CrossRef]
- Abuzalat, O.; Tantawy, H.; Mokhtar, M.; Baraka, A. Nano-porous bimetallic organic frameworks (Fe/Co)-BDC, a breathing MOF for rapid and capacitive removal of Cr-oxyanions from water. J. Water Process Eng. 2022, 46, 102537. [Google Scholar] [CrossRef]
- Mahmoud, M.E.; Mohamed, A.K. Novel derived pectin hydrogel from mandarin peel based metal-organic frameworks composite for enhanced Cr(VI) and Pb(II) ions removal. Int. J. Biol. Macromol. 2020, 164, 920–931. [Google Scholar] [CrossRef]
- Liu, Y.; Pang, H.; Wang, X.; Yu, S.; Chen, Z.; Zhang, P.; Chen, L.; Song, G.; Alharbi, N.S.; Rabah, S.O. Zeolitic imidazolate framework-based nanomaterials for the capture of heavy metal ions and radionuclides: A review. Chem. Eng. J. 2021, 406, 127139. [Google Scholar] [CrossRef]
- Nqombolo, A.; Munonde, T.S.; Makhetha, T.A.; Moutloali, R.M.; Nomngongo, P.N. Cobalt/zinc based metal organic frameworks as an effective adsorbent for improved removal of As(V) and Cr(VI) in a wide pH range. J. Mater. Res. Technol. 2021, 12, 1845–1855. [Google Scholar] [CrossRef]
- Wu, Y.H.; Zhou, J.X.; Jin, Y.P.; Cao, J.L.; Yilihan, P.; Wen, Y.J.; Wu, Y.Y. Mechanisms of chromium and arsenite adsorption by amino-functionalized SBA-15. Environ. Sci. Pollut. Res. 2014, 21, 1859–1874. [Google Scholar] [CrossRef]
- Gupta, V.K.; Pathania, D.; Agarwal, S.; Sharma, S. Removal of Cr(VI) onto Ficus carica biosorbent from water. Environ. Sci. Pollut. Res. 2013, 20, 2632–2644. [Google Scholar] [CrossRef]
- Henryk, K.; Jaroslaw, C.; Witold, Z. Peat and coconut fiber as biofilters for chromium adsorption from contaminated wastewaters. Environ. Sci. Pollut. Res. 2016, 23, 527–534. [Google Scholar] [CrossRef]
- Gonzalez, A.; Moreno, N.; Navia, R.; Querol, X. Study of a Chilean petroleum coke fluidized bed combustion fly ash and its potential application in copper, lead and hexavalent chromium removal. Fuel 2010, 89, 3012–3021. [Google Scholar] [CrossRef]
- Xu, F.; Liu, X.; Chen, Y.J.; Zhang, K.; Xu, H. Self-assembly modified-mushroom nanocomposite for rapid removal of hexavalent chromium from aqueous solution with bubbling fluidized bed. Sci. Rep. 2016, 6, 26201. [Google Scholar] [CrossRef]
- Xu, D.D.; Yan, S.; Weng, W.; Xiao, R. Cost effective nanofiber composite membranes for Cr(VI) adsorption with high durability. RSC Adv. 2016, 6, 44723–44731. [Google Scholar] [CrossRef]
- Zhang, L.; An, B.; Chen, H.; Chu, J.; Ma, J.; Fan, Y.; Wang, Z. Botryoidal nanolignin channel stabilized ultrasmall PdNP incorporating with filter membrane for enhanced removal of Cr(VI) via synergetic filtration and catalysis. Sep. Purif. Technol. 2022, 296, 121409. [Google Scholar] [CrossRef]
- Dong, Y.; Xing, W.; Luo, K.; Zhang, J.; Yu, J.; Jin, W.; Wang, J.; Tang, W. Effective and continuous removal of Cr(VI) from brackish wastewater by flow-electrode capacitive deionization (FCDI). J. Clean. Prod. 2021, 326, 129417. [Google Scholar] [CrossRef]
- Huang, Z.; Lu, L.; Cai, Z.X.; Ren, Z.J. Individual and competitive removal of heavy metals using capacitive deionization. J. Hazard. Mater. 2016, 302, 323–331. [Google Scholar] [CrossRef]
- Gaikwad, M.S.; Balomajumder, C. Simultaneous electrosorptive removal of chromium(VI) and fluoride ions by capacitive deionization (CDI): Multicomponent isotherm modeling and kinetic study. Sep. Purif. Technol. 2017, 186, 272–281. [Google Scholar] [CrossRef]
- Gaikwad, M.S.; Balomajumder, C.; Tiwari, A.K. Acid treated RHWBAC electrode performance for Cr(VI) removal by capacitive deionization and CFD analysis study. Chemosphere 2020, 254, 126781. [Google Scholar] [CrossRef]
- Gaikwad, M.S.; Balomajumder, C. Removal of Cr(VI) and fluoride by membrane capacitive deionization with nanoporous and microporous Limonia acidissima (wood apple) shell activated carbon electrode. Sep. Purif. Technol. 2018, 195, 305–313. [Google Scholar] [CrossRef]
- Chen, L.; Wang, C.; Liu, S.; Zhu, L. Investigation of adsorption/desorption behavior of Cr(VI) at the presence of inorganic and organic substance in membrane capacitive deionization (MCDI). J. Environ. Sci. 2019, 78, 303–314. [Google Scholar] [CrossRef] [PubMed]
- Mohanraj, P.; AllwinEbinesar, J.S.S.; Amala, J.; Bhuvaneshwari, S. Biocomposite based electrode for effective removal of Cr (VI) heavy metal via capacitive deionization. Chem. Eng. Commun. 2020, 207, 775–789. [Google Scholar]
- Wu, Z.; Zhao, C.; Zeng, W.; Wang, X.; Liu, C.; Yu, Z.; Zhang, J.; Qiu, Z. Ultra-high selective removal of CR and Cr(VI) from aqueous solutions using polyethyleneimine functionalized magnetic hydrochar: Application strategy and mechanisms insight. Chem. Eng. J. 2022, 448, 137464. [Google Scholar] [CrossRef]
- Zhao, X.; Li, Z.; Tang, W.; Gu, X. Competitive kinetics of Ni(II)/Co(II) and Cr(VI)/P(V) adsorption and desorption on goethite: A unified thermodynamically based model. J. Hazard. Mater. 2022, 423, 127028. [Google Scholar] [CrossRef]
- Yu, T.L.; Liu, S.Q.; Xu, M.; Peng, J.; Li, J.Q.; Zhai, M.L. Synthesis of novel aminated cellulose microsphere adsorbent for efficient Cr(VI) removal. Radiat. Phys. Chem. 2016, 125, 94–101. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).