Abstract
Two new napyradiomycins derivatives, napyradiomycin A4 (1) and A80915 H (2), along with five known ones, were isolated from the ethyl acetate extract of fermentation culture of Streptomyces kebangsaanensis WS-68302. Their structures were elucidated by extensive spectroscopic analysis, including HR-MS, 1D and 2D NMR, CD spectrum, as well as comparison with literature data. Compound 1 exhibited significant antiviral activity against PRV (Pseudorabies virus) with an IC50 value of 2.056 μM and therapeutic ratio at 14.98, suggesting that it might have potential for development of an antiviral agent. Moreover, compound 1 displayed the strongest inhibition against PRV protein among the tested napyradiomycins in the indirect immunofuorescence assay. Compounds 3 and 4 showed higher activities against swine pathogenic Streptococcus suis than the positive control penicillin G sodium salt, with MIC values of 3.125 and 6.25 μg/mL, respectively. Compounds 1 and 3–6 exhibited moderate antibacterial activity against the swine pathogenic Erysipelothrix rhusiopathiae, with MIC values ranging from 25 to 50 μg/mL.
1. Introduction
In recent decades, infection with pathogens has caused huge economic losses to the swine industry. Pseudorabies virus (PRV), a herpes virus, is one of the most important pathogens in swine [1,2]. The swine pseudorabies caused by the pseudorabies virus is characterized by reproductive disturbance, dyspnea, and neurological symptoms, and different-aged pigs are susceptible to infection with the disease [3,4]. Vaccination is one of the most important methods to prevent and control PRV; however, with the emergence of PRV mutant strains in recent years, it is difficult to completely prevent and control them using conventional vaccines [5,6,7,8]. Moreover, no effective anti-PRV drugs have been approved [9]. Therefore, it is essential to discover antiviral drugs.
In addition to the virus, some bacteria, such as Streptococcus suis and Erysipelothrix rhusiopathiae, can also cause economic losses and zoonotic diseases. S. suis is an important zoonotic pathogen that causes several pathological conditions such as septicemia, meningitis, arthritis and pneumonia [10]. S. suis can cause bacterial encephalitis or toxic-shock-like syndrome in humans [11]. E. rhusiopathiae, a small Gram-positive bacterium, is also a zoonotic pathogen [12]. The swine erysipelas caused by E. rhusiopathiae mainly has acute, subacute and chronic clinical forms, and acute infection can even cause sudden death or general signs of septicemia to swine [13]. E. rhusiopathiae can also cause a skin disease, erysipeloid, in humans [13]. Although many antibiotic drugs can treat bacterial diseases, with the emergence bacterial resistance, the development of new drugs to prevent and treat these diseases is urgent [14].
Napyradiomycins are a large class of unique meroterpenoids including a semi-naphthoquinone nucleus and an isoprene unit or a monoterpenoid subunit. Napyradiomycins initially attracted attention owing to their strong antimicrobial properties toward Gram-positive bacteria [15,16,17,18,19]. Subsequently, napyradiomycins were reported to exhibit a wide array of biological activities, including gastric (H+-K+) ATPase-inhibiting activities [20], estrogen receptor antagonist activities [21], apoptosis-inducing activities [22], and cytotoxicity [23,24]. In recent years, the napyradiomycins produced by marine-derived actinomycetes have been found to have good antifouling activities [25]. In addition, a new regioisomer of napyradiomycin A1, 16Z-19-hydroxynapyradiomycin A1, has been confirmed to possess antioxidant and anti-inflammatory properties [26]. However, there have been no reports on inhibitory virus activities of napyradiomycins.
In our ongoing research on the bioactive secondary metabolites from actinomycetes, a soil-derived strain WS-68302 was found to show interesting antiviral activity against PRV and antibacterial activity. The actinomycete strain was identified as S. kebangsaanensis WS-68302. Further chemical investigation of the extract from the strain led to the discovery of two new derivatives of napyradiomycins, named napyradiomycin A4 (1) and A80915H (2), as well as five known ones (3–7) (Figure 1). The napyradiomycins (1 and 3–6) showed strong anti-PRV activity in vitro. In this study, we report the taxonomy of the strain, the isolation, the structural elucidation, and the antiviral and antibacterial activities of these compounds from the strain.
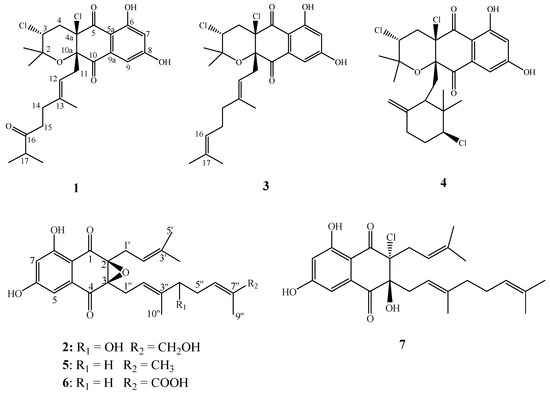
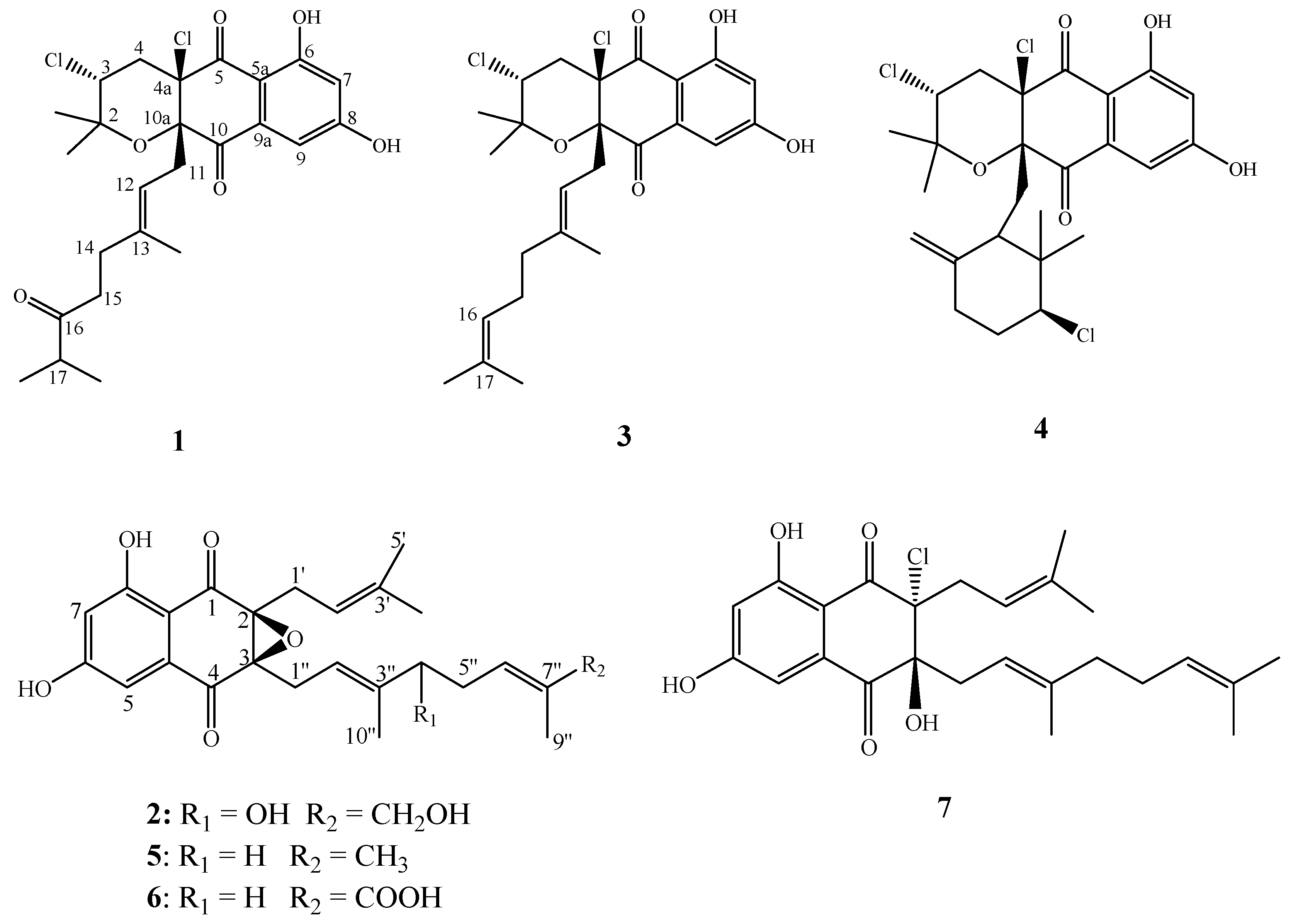
Figure 1.
Chemical structures of compounds 1–7.
2. Results and Discussion
2.1. Taxonomy of Strain WS-68302
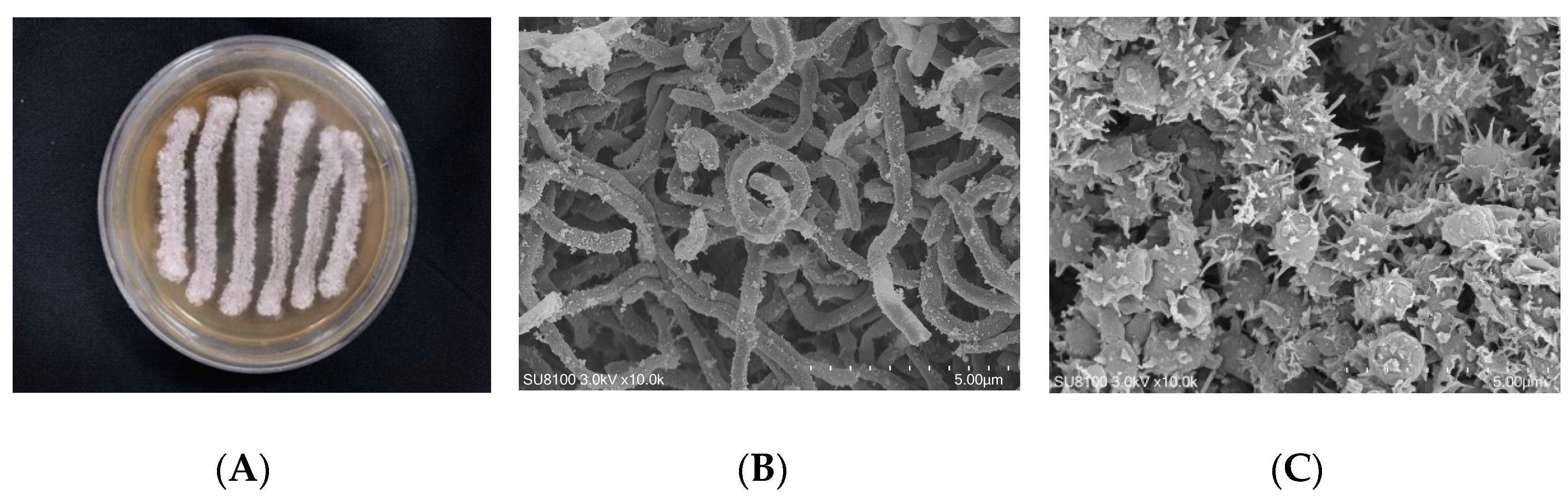
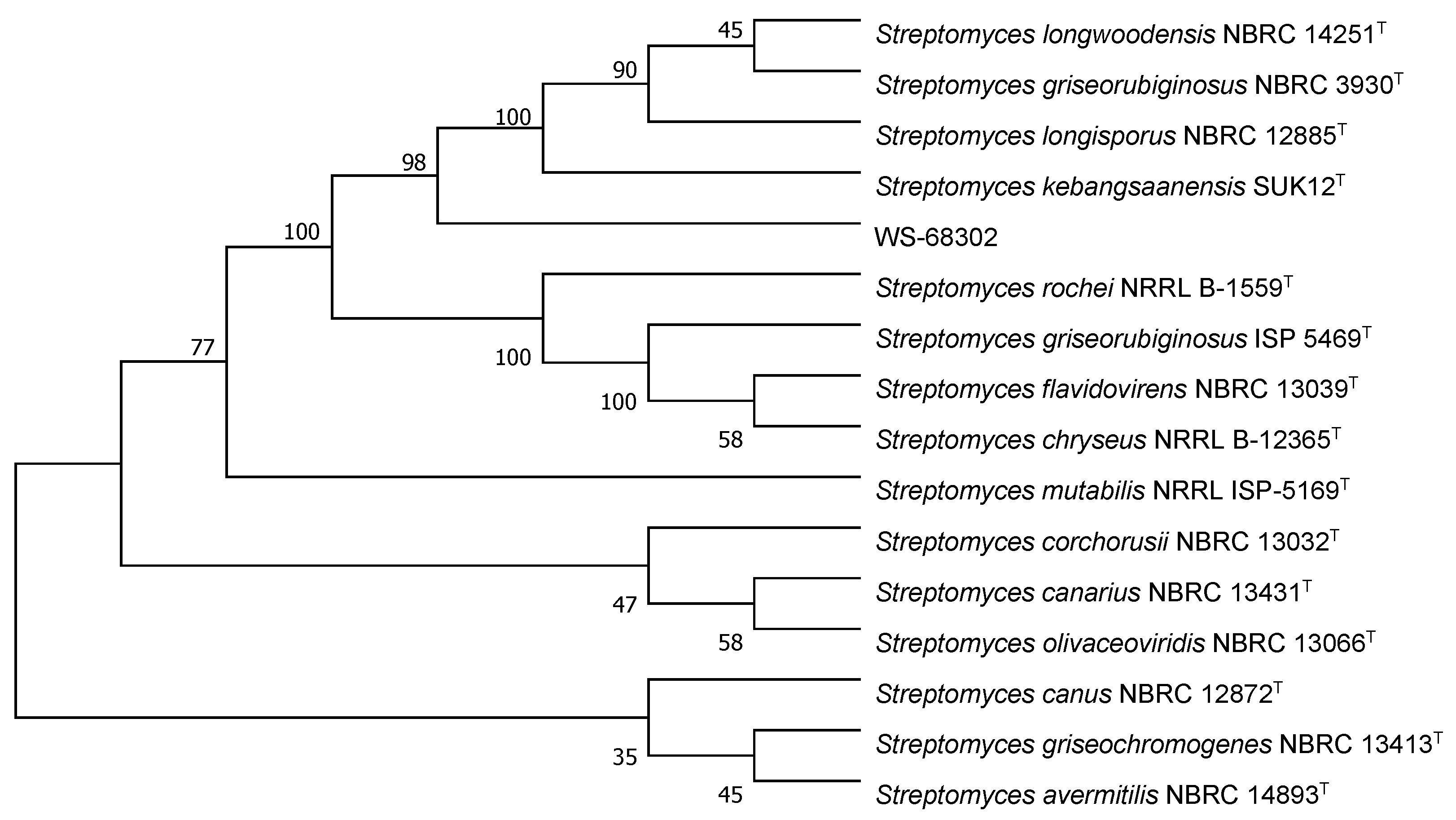
The colonies of strain WS-68302 on ISP-2 agar grew abundant grayish white mycelium (Figure 2A). The aerial mycelia were straight to flexuous and spiral on terminals. (Figure 2B). The spores were mostly ovoid and 0.8–1.2 × 1.2–2.0 μm in size, and the surfaces of the spores were covered with different-length spines (Figure 2C). These morphological characteristics of strain WS-68302 were in total agreement with the strain Streptomyces kebangsaanensis SUK12, which was identified as a novel species of the genus Streptomyces in 2013, isolated from Malaysian ethnomedicinal plant Portulaca olerace [27]. The 16S rRNA sequence of the strain WS-68302 was deposited in the NCBI GenBank database (accession number OP787989), and the BLAST result of the sequence also verified the closest similarity (98.99%) to that of S. kebangsaanensis SUK12. The phylogenetic tree generated using the neighbor-joining method clearly depicted the evolutionary relationship of the strain WS-68302 with Streptomyces (Figure 3). Accordingly, the strain WS-68302 was identified as S. kebangsaanensis and designated S. kebangsaanensis WS-68302.
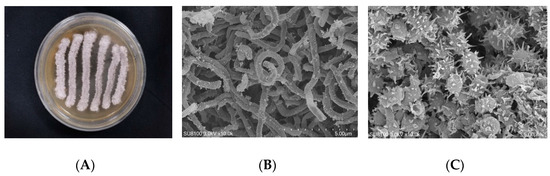
Figure 2.
Identification of the strain WS-68302. (A) The colonies cultured in ISP-2 for 14 days at 28 ℃; (B) scanning electron micrograph of the aerial mycelium (bar, 5.0 μm); (C) scanning electron micrograph of the spores (bar, 5.0 μm).
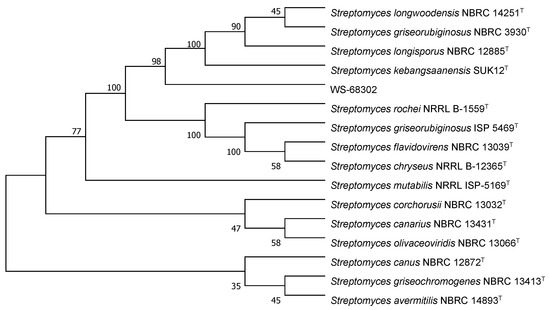
Figure 3.
Phylogenetic tree based on 16S rRNA sequences of the S. kebangsaanensis WS-68302.
2.2. Structural Elucidation
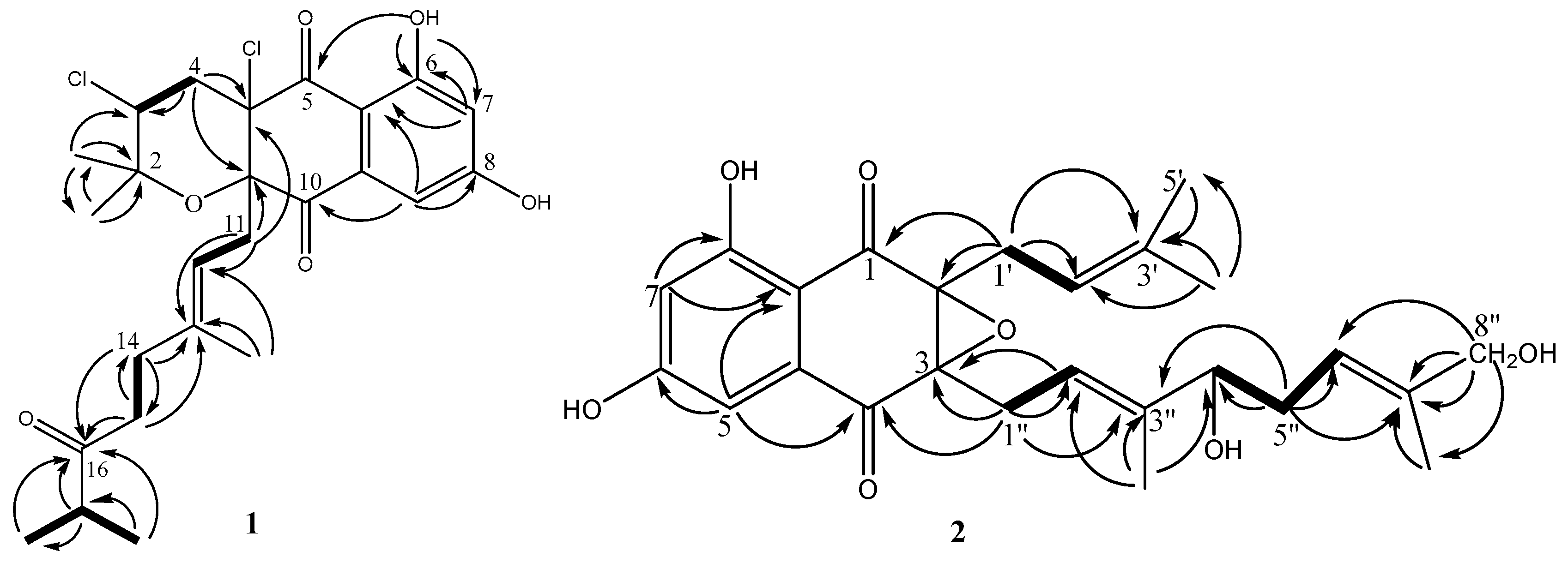
Compound 1 was obtained as pale yellow oil. In the electrospray ionization mass spectrometry spectrum (ESIMS), compound 1 showed a positive ion cluster at m/z 497.4/499.2/501.4 in the ratio of 100:66:10, indicating the presence of two chlorine atoms. HRESIMS analysis gave an ion consistent with the molecular formula C25H30Cl2O6Na (calculated for C25H31Cl2O6Na 519.1312 [M + Na]+). The IR spectrum of 1 displayed bands at 3360 cm−1 for hydroxyl groups, 1697 cm−1 for conjugated carbonyl groups. The 1H NMR spectrum (Table 1) of 1 revealed an exchangeable proton singlet at δH 11.85, two broad singlet aromatic protons at δH 7.17 and 6.72, a triplet of methine doublet proton at δH 4.70 (d, J = 8.5 Hz), a double coupled methine proton [δH 4.42 (dd, J = 12.0, 4.0 Hz)], a methine multiple proton at δH 2.53 (m), four sets of methylene protons [δH 2.68 (brd, J = 8.1 Hz), 2.48 (dd, J = 14.3, 4.0 Hz) and 2.43 (d, J = 12.0 Hz), 2.15 (m) and 2.10 (m), and 1.93 (m) and 1.88 (m)], and five methyl protons [δH 1.50 (s), 1.32 (s), 1.18 (s), 1.06 (t, J = 6.4 Hz), and 1.06 (t, J = 6.4 Hz)]. The 13C NMR spectrum (Table 1) of 1 showed resonances for three carbonyl carbons (δC 215.0, 195.5 and 194.0), two oxygenated aromatic carbons (δC 164.8 and 164.4), three unsaturated methine carbons (δC 115.7, 109.5, and 108.2), six quaternary carbons (δC 141.3, 135.4, 109.9, 83.7, 79.2, and 78.9), two aliphatic methine carbons (δC 58.9 and 41.1), four methylene carbons (δC 42.8, 41.2, 38.0, and 33.3), and five methyl carbons (δC 29.0, 22.4, 18.4, 18.3, and 16.7). Combined with the 2D-NMR spectrum of 1 (Figures S8–S12), these NMR data resembled those of napyradiomycin A1 (3) [16], except for the presence of a keto carbonyl carbon at C-16 (δC 215.0) and an aliphatic methine group at C-17 [(δC 41.1, δH 2.53 (1H, m)] in 1, and the absence of the signal of an olefinic proton at δH 4.89 (brs, H-16) and the terminal double bond at C-16 (δC 123.9) and C-17 (δC 131.9) in 3. The orthotropic 1H-1H COSY correlations (Figure 4) from 17-CH3 to H-17 and HMBC correlations (Figure 4) from H-17, 17-CH3, H-14 and H-15 to C-16, as well as H-17 to 17-CH3, support the above conclusion. Thus, the planar structure of 1 could be designated as napyradiomycin A4 (Figure 4). The coupling constant of H-3 (dd, J = 12.0, 4.0 Hz) indicated that orientation of H-3 was axial. The correlations between H-11 and 2-CH3 ax (δ 1.18), H-11 and H-4 ax (δ 2.43) (Figure S12) suggested that the relative configuration of C-3 and C-10a was syn. Compound 1 showed that characteristic Cotton effects (CEs) (Figure S13) fit well with those of napyradiomycin A1 (3) (Figure S14), indicating that the absolute configurations of benzoquinone of 1 were the same as those of napyradiomycin A1. Therefore, the absolute configurations of 1 were determined as 3R, 4aR, and 10aS.

Table 1.
1H (700 MHz) and 13C (175 MHz) NMR data of compound 1 in CDCl3.
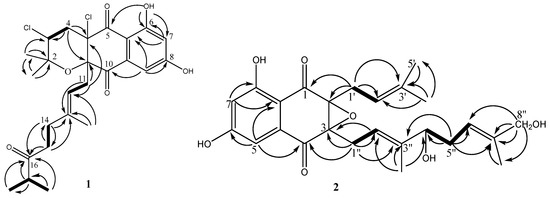
Figure 4.
1H–1H COSY (bold lines) and HMBC (arrows) correlations of compounds 1 and 2.
Compound 2 was obtained as yellow oil. Its molecular formula was determined to be C25H30O7 on the basis of 13C NMR and HRESIMS (m/z 443.2067 [M + H]+, calcd for C25H31O7 443.2064 [M + H]+). Comparison of the NMR spectroscopic data (Table 2) of 2 with those of A80915G (5) revealed that a methene group in A90915G (5) is replaced by the hydroxymethine group [δC 77.0; δH 4.12 (1H, t, J = 6.7 Hz)] in 2, and a methyl group in A90915G (5) is replaced by the hydroxymethyl group [δC 68.9; (δH 3.97 (2H, m)] in 2. The 1H-1H COSY correlations (Figure 4) of H-4″/H-5″/H-6″, and the HMBC correlations (Figure 4) from H-4″ to C-6″, H-5″ to C-4″ and C-6″, as well as H-10″ to C-3″ and C-4″, confirmed the hydroxy-bearing methine at C-4″. The hydroxymethyl group was assigned to C-8″ according to the HMBC correlations from H2-8″ to C-6″, C-7″, and C-9″. Thus, the structure of compound 2 is depicted in Figure 4, and named A80915H.

Table 2.
1H (600 MHz) and 13C (175 MHz) NMR data of compound 2 in CDCl3.
The absolute configurations at C-2 and C-3 of 2 were determined to be 2S and 3S by comparing its CD spectrum (Figure S25) with that of A80915G (Figure S26), whose structure was confirmed by spectroscopic data and chemical synthesis [19,28]. However, the absolute configuration of C-4″ was not determined due to scarcity of 2 for modified Mosher’s analysis [29].
Compounds 3–7 were identified as napyradiomycin A1 (3) [16], napyradiomycin B1 (4) [16], A80915G (5), A80915G-8″-acid (6) [30], and naphthomevalin (7) [31,32] (Figure 1), respectively, by comparing their MS, 1H and 13C ΜMR spectroscopic data with those previously reported.
2.3. Biological Activities
2.3.1. Antiviral Activities of 1–7 against PRV In Vitro
The in vitro antiviral activities of 1–7 against PRV in Marc-145 cells were evaluated by MTT assay according to the protocol. Compounds 1 and 3–6 exhibited significant antiviral activity against PRV, with half maximal inhibitory concentration (IC50) values ranging from 2.056 to 26.47 μM, whereas 2 and 7 did not display any apparent anti-PRV activity (Table 3). It is notable that compounds 1 and 3 exhibited excellent antiviral activity against PRV, with IC50 values of 2.056 and 2.208 μM, respectively—much stronger than that of the positive control ribavirin (IC50 = 58.032 μM). However, the new compound 1 showed a higher therapeutic ratio (TI = 14.98) than 3 (TI = 2.62). Although their structures are closely similar, there is a carbonyl group at C-16 for compound 1 and a double bond at C-16 and C-17 for compound 2, which led to a difference in toxicity between the two compounds and indicated that the substituent of the 16 position is important for toxicity. Despite compound 5 demonstrating a strong activity against PRV with IC50 value of 8.26 μM, its new derivative 2 was inactive against PRV, which suggested the hydroxyl substitution at C-4″ and C-8″ of 5 had an effect on the anti-PRV activity. Compounds 4 and 6 displayed a lower therapeutic index (TI < 2.0). As a result, compound 1 might have potential for the development of an antiviral agent.

Table 3.
In vitro antiviral activities of compounds 1–7 against PRV in the Marc-145 cells.
2.3.2. Indirect Immunofluorescence Assay
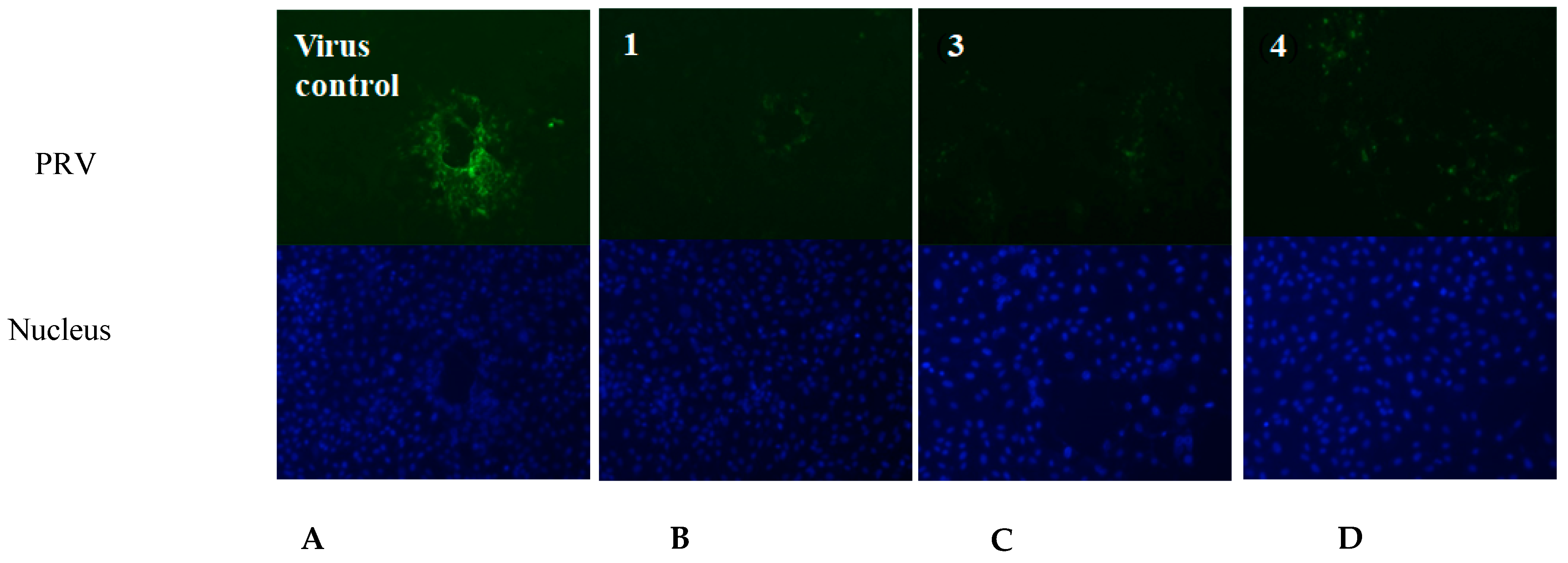
Based on the above antiviral activity, we selected the active compounds 1, 3 and 4 to test the immunofluorescence effect at concentrations of 2, 5 and 10 μg/mL, respectively, by indirect immunofluorescence assay. The three compounds were co-incubated with PRV in Marc-145 cells, respectively. After 24 h, the nucleocapsid (DAPI, blue) and PRV (FITC, green) responses to the indirect immunofluorescence effect are shown in Figure 5. The green fluorescence intensity in the compound-treated cells (Figure 5B–D) was obvious lower than in the virus control group (Figure 5A), indicating that the viral protein synthesis was significantly suppressed by the three active compounds, 1, 3 and 4. Furthermore, the new compound 1 showed lower immunofluorescence effect against PRV and lower cytotoxicity than the known compounds 3 and 4 in the Marc-145 cells.
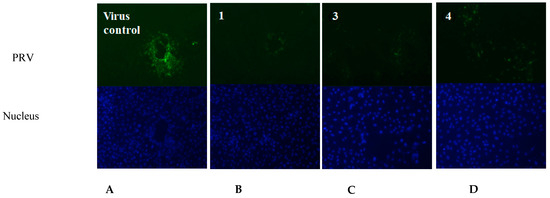
Figure 5.
The fluorescent images of the virus control group and the compound-treated cells groups. (A) Virus control (PRV); (B) compound 1; (C) compound 3; (D) compound 4. (The nucleus was stained with DAPI (blue), and the green foci indicate the presence of PRV protein).
2.3.3. Antibacterial Activities
Compounds 1–7 were evaluated using a microdilution broth method for antibacterial activity against S. aureus and the animal pathogens S. suis, E. rhusiopathiae, and E. coli. Penicillin G sodium salt and cefpirome sulfate were used as positive controls. As shown in Table 4, compounds 3, 4 and 7 displayed potent activities comparable to the positive control cefpirome sulfate (MIC < 0.78 μg/mL) against bacteria S. aureus ATCC 25923, while the new compounds 1 and 6 showed moderate activity against the bacteria, with MIC values of 25 and 50 μg/mL, respectively. Compounds 3 and 4 also showed higher activities against the swine pathogenic S. suis than the positive control penicillin G sodium salt, with MIC values of 3.125 and 6.25 μg/mL, respectively, whereas the new compound 1 exhibited weak activity against the bacteria. Compounds 1 and 3–6 exhibited moderate antibacterial activity against the swine pathogenic E. rhusiopathiae, with MIC values ranging from 25 to 50 μg/mL. There are slight differences on a side chain in the structures of compounds 2, 5 and 6, but compound 2 did not show any activity against the three tested Gram-positive bacteria. Compound 5, previously isolated from Streptomyces aculeolatus A80915, had good antibacterial activity against aerobic Gram-positive Staphylococcus, Streptococcus and Enterococcus bacterial strains (MIC 2–16 μg/mL) [19]. While compound 6, an oxidized compound of 5, only had weak antibacterial activity against Bacillus subtilis ATCC6633 (MIC 64 μg/mL) [30]. Therefore, it was suggested that hydroxyl substitution at C-4″ and C-8″of the side chains on the semi-naphthoquinone nucleus had a great influence on the activities of compound 2. As reported in the literature [15,17,19], these tested compounds were inactive to the Gram-negative bacteria E. coli ATCC 25922.

Table 4.
Antibacterial activities of compounds 1–7 (MIC μg/mL).
3. Conclusions
In conclusion, seven napyradiomycins, including two new ones, napyradiomycin A4 (1) and A80915H (2), were obtained from the fermentation culture of Streptomyces kebangsaanensis WS-68302. To the best of our knowledge, this is the first time that the potent antiviral activities of napyradiomycins against PRV in vitro have been observed. Impressively, the new compound, napyradiomycin A4 (1), and napyradiomycin A1 (3) both showed much better inhibitory activity against PRV than ribavirin. In addition, napyradiomycin A1 (3) and B1 (4) and naphthomevalin (7) displayed potent activities comparable to the positive control cefpirome sulfate against the bacteria S. aureus. Compounds 3 and 4 also showed higher activities against swine pathogen S. suis than the positive control penicillin G sodium salt. This study suggested that the napyradiomycin A4 could serve as a promising antiviral agent against PRV.
4. Experimental Section
4.1. General Experimental Procedures
Optical rotations were measured in MeOH on an MPC 500 (Waltham) polarimeter (PerkinElmer Ltd., Waltham, MA, USA). UV spectrum was recorded on a Shimadzu UV-2600 PC spectrometer (Shimadzu Corporation, Kyoto, Japan). IR spectra were obtained on a NICOLET IS50 FT-IR (Thermo Fisher Scientific, Waltham, MA, USA). NMR spectra, including HSQC, HMBC and COSY, were recorded on a Bruker AC 700 MHz spectrometer using tetramethylsilane as standard (Bruker BioSpin group, Rheinstetten, German). ESIMS data was detected on a waters Xevo TQD UPLC-MS (Waters Corporation, Milford, MA, USA), with a Waters ACQUITY®BEH C18 column (2.1 × 100 mm, 1.7 μm, Ireland). HR-ESI-MS spectra: Thermo Q-T of Micromass spectrometer (Thermo Electron Corporation, Waltham, MA, USA). Preparative HPLC was carried on a Waters 2525 pump and a Waters 2998 DAD detector coupled with Waters 2767 Autopurifcation System (Waters Corporation, Milford, MA, USA) using Sunfire Prep C18 OBD column (19 × 250 mm, 5 μm, Waters Corporation, Milford, MA, USA).
4.2. Isolation of Strain and Morphological Identification
The strain WS-68302 was isolated from a soil sample collected from a hillside in SuiXian County, Hubei Province, China, in April 2015. For the taxonomic studies of the actinomycetes, ISP-2 agar (glucose 0.4%, malt extract 1%, yeast extract 0.4%, agar 1.8%, and pH 7.2) was used to investigate the morphological and physiological characteristics. The strain was incubated at 28 ℃ for 2–3 weeks. Morphological properties were observed under a scanning electron microscope (Model Hitachi SU8100). Genomic DNA extraction, PCR amplification and sequencing of 16S rRNA were developed with the universal methods [33]. The strain was identified based on sequence analysis of 16S rRNA Sequence under the BLAST search.
4.3. Fermentation
A stock culture of the strain S. kebangsaanensis WS-68302 was inoculated into 500 mL Erlenmeyer flasks containing 100 mL of the seed medium ISP-2. The flasks were incubated at 28 ℃ on a rotary shaker at 150 rpm, for 96 h. 10% seed culture was transferred to 500 mL Erlenmeyer flasks containing 100 mL of the production medium ISP-2 under sterile conditions. The flasks were cultivated at 28 ℃ on a rotary shaker at 150 rpm for 120 h.
4.4. Extraction and Isolation
The freeze-dried culture broth (30 L) S. kebangsaanensis WS-68302 was extracted with an equal volume of ethyl acetate 2 times. The ethyl acetate phase was combined, filtrated and evaporated under vacuum to give the crude extract (27 g). The crude extract was subjected to silica gel CC column using gradient elution with a petroleum ether and EtOAc mixture from 100/10 to 0/100 (v/v) to give ten fractions (Fr.1-Fr.10). Fr.4 (2.48 g) was purified by preparative HPLC with an OBD C18 column (Sunfire, 250 × 19 mm, 5 μm) to obtain 1 (3.16 mg), using a gradient solvent system (0–2 min, 5% CH3CN; 2–27 min, 5–100% CH3CN; 27–32 min, 100% CH3CN; 32–37 min, 100–5% CH3CN; 37–40 min, 5% CH3CN). Fr.1 (4.0 g) was further separated on silica gel CC eluting with a gradient petroleum ether–EtOAc solvent system from 20/1 to 6/1 (v/v) to afford eight fractions (Frs.1-1–Fr.1-8). Next, Frs.1-2 and Frs.1-3 were further purified by preparative HPLC to yield 3 (15.6 mg), 4 (10.87 mg), 5 (3.59 mg) and 7 (5.26 mg), under the HPLC conditions (0–2 min, 20% CH3CN; 2–22 min, 20–100% CH3CN; 22–26 min, 100% CH3CN; 26–30 min, 100–20% CH3CN; 30–33 min, 20% CH3CN). Fr.6 (4.4 g) was isolated and purified by preparative HPLC to obtain 6 (4.14 mg). Fr.7 (4.68 g) was subjected to silica gel CC eluting with a gradient dichloromethane and acetone solvent system from 10/1 to 1/1 (v/v) to get eleven fractions (Frs.7-1–Fr.7-11). Fr.7-7 and Fr.7-8 were also purified by preparative HPLC with an OBD C18 column (Sunfire, 250 × 19 mm, 5 μm) to obtain 2 (4.97 mg), with the HPC method (0–2 min, 35% CH3CN; 2–22 min, 35–100% CH3CN; 22–26 min, 100% CH3CN; 26–29 min, 100–35% CH3CN; 29–32 min, 35% CH3CN).
Napyradiomycin A4 (1). Yellow brownish oil; [α]25 D −4.2 (c 0.1 MeOH); IR (KBr) υmax 3360, 2926, 2855, 2359, 1698, 1636, 1614, 1456, 1385, 1371, 1258, 1186, 1134, 1074, 1015, 862, 725 cm−1; UV (MeOH) λmax (log ε): 249 (4.14), 270 (2.99), 359 (1.59) μM; ESIMS m/z 497.4, 499.2, 501.4 [M + H]+ (Figure S3); HRESIMS m/z 519.1316 [M + Na]+ (calcd. for C25H30Cl2O6Na, 519.1312) (Figure S1); 1H and 13C NMR data see Table 1. CD (c 0.3 MeOH): λmax (Δε): 220 nm (−0.69), 250 nm (+0.31), 273 nm (−0.27), 300 nm (+0.23), 330 nm (−0.04), 360 nm (+0.15).
A80915H (2). Yellow oil; [α]25 D +59.35 (c 0.2 MeOH); UV (MeOH) λmax (log ε): 251 (2.15), 270 (1.57), 363 (0.86) nm; ESIMS m/z 441.6 [M − H]− (Figure S15); HRESIMS m/z 443.2067 [M + H]+ (calcd. for C25H31O7, 443.2064) (Figure S16); 1H and 13C NMR data see Table 2. CD (c 0.2 MeOH): λmax (Δε): 220 nm (−0.29), 252 nm (+0.21), 270 nm (−0.03), 295 nm (+0.09), 335 nm (−0.07), 362 nm (+0.24).
4.5. Antiviral and Antibacterial Assays
4.5.1. Antiviral Assays
Cells, Virus and Reagents
Marc-145 cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 100 μg/mL streptomycin, and 100 IU/mL penicillin, at 37 °C in 5% CO2 atmosphere. The PRV was obtained from Professor Tan Chen of Huazhong Agricultural University. The virus was propagated in Marc-145 cells and stored at −80 ℃ until used. DMEM containing high glucose levels and Sodium Pyruvate was purchased from Hyclone. FBS was purchased from Gemini. Ribavirin, 0.25% tyrisin and MTT were purchased from Solarbio.
Chemicals, Antibodies and Reagents
The anti-Pseudorabies Virus antibody (ab3534), goat anti-rabbit IgG H&L (Alexa Fluor 488) (ab150077), and DAPI staining solution (ab228549) were purchased from Abcam.
Cell Proliferation
The supernatant was discarded upon Marc proliferation; cells were washed with PBS, and trypsin was added until the cells were digested into singles at 37 ℃. Cells were dispersed in DMEM with 10% FBS and cultured at 37 ℃ for 2–3 days.
Cell Bioability Assay
Cell viability was assessed using the MTT according to the protocol. Briefly, cells in 96-well plates were treated with compounds in different concentration for 48 h. Assays were performed in triplicate. Then, MTT was added to the cells and they were incubated in the dark for 4 h at 37 ℃. The supernatant was discarded and DMSO was added. After 10 min incubation, the OD 450 of each well was read using a spectrophotometer (Thermo). The cell viability (%) was calculated as (compound average OD450μM value/cell control average OD450μM value) × 100%. Compounds were added to monolayer in different concentration. After 48h incubation, the cell viability (%) was calculated by MTT assay, and the cytotoxic effect (CC50) was analyzed by SPSS 16.0 software. Compounds in different concentrations (40, 20, 10, 5, 2.5, 1.25, 0.625, 0.3125 μg/mL) and PRV (100 TCID50) were added to monolayer at 37℃. After 72h incubation, the cell viability (%) was calculated by MTT assay, and the half maximal inhibitory concentration (IC50) was analyzed by SPSS 16.0 software. Ribavirin was used as a positive control.
Therapeutic index (TI) was calculated as CC50/IC50.
Indirect Immunofluorescence Assay
The monolayers of Marc-145 cells cultured in 24-well plates were treated with PRV and drug mixture. After 24 h incubation, the cells were washed with PBS twice and fixed with cold absolute ethanol at 4 °C for 15 min, then permeabilized with 0.1% Triton X-100 in PBS for 10 min, and blocked with 1% BSA in PBS for 1 h. The cells were then incubated with 1:200 anti-Pseudorabies Virus antibody in PBS containing 1% BSA at 4 °C overnight. After washing three times with PBST, the cells were stained with 1:2000 goat anti-rabbit IgG H&L. To show the nucleus of the cells, DAPI (40, 6-diamidino-2-phenylindole) was used to stain the cellular DNA. The fluorescent images were observed using an inverted fluorescence microscope (Olympus IX73 (Olympus Corporation, Tokyo, Japan)).
4.5.2. Antibacterial Assays
The minimum inhibitory concentrations of 1–7 were determined against the four bacterial strains (Staphylococcus aureus ATCC 25923, Erysipelothrix rhusiopathiae WH13013, Streptococcus suis SC19, Escherichia coli ATCC 25922) following the Clinical and Laboratory Standards Institute (CLSI) guidelines [34]. The microdilution broth method was performed in 96-well plates (Corning Costar® 3599 Corning, Corning, NY, USA) using MHB (Hopebio, Qingdao, China). The final concentration of the culture was 5 × 105 colony-forming units (CFU)/mL, and measurements were repeated at least in triplicate. Penicillin G sodium salt and cefpirome sulfate were purchased from Solarbio.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules28020640/s1, Figures S1–S13: HR-ESI-MS, ESI-MS, UV, IR, 1H-NMR, 13C-NMR, DEPT, HSQC, HMBC, COSY, NOESY, and CD spectrum of compound 1; Figure S14: CD spectrum of compound 3; Figures S15–S25: HR-ESI-MS, ESI-MS, UV, 1H-NMR, 13C-NMR, DEPT, HSQC, HMBC, COSY, and CD spectrum of compound 2; Figure S26: CD spectrum of compound 5.
Author Contributions
Conceptualization, W.F. and K.W.; methodology, M.L. and W.F.; software, Z.W. (Zhaoyuan Wu); validation, Z.W. (Zhongyi Wan) and F.L.; formal analysis, Z.Z.; investigation, L.S.; resources, Z.W. (Zhongyi Wan); data curation, F.L.; writing—original draft preparation, W.F.; writing—review and editing, Y.Z.; visualization, W.F.; supervision, Z.W. (Zhaoyuan Wu); project administration, L.S.; funding acquisition, M.L and K.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Key Research and Development Program of China (2021YFD1800402), the Science and Technology Major Program of Hubei Province (2021ABA005), and by the Hubei Agricultural Science and Technology Innovation Center (2021-620-000-001-27).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented are available in the manuscript and supplementary materials.
Acknowledgments
The authors thank Yueying Wang (Hubei Biopesticide Engineering Research Centre) for the strain identification.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are not available from the authors.
References
- Ma, W.J.; Lager, K.M.; Richt, J.A.; Stoffregen, W.C.; Zhou, F.H.; Yoon, K.J. Development of real-time polymerase chain reaction assays for rapid detection and differentiation of wild-type pseudorabies and gene-deleted vaccine viruses. J. Vet. Diagn. Investig. 2008, 20, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.H.; Tong, W.Z.; Song, X.; Jia, R.Y.; Li, L.X.; Zou, Y.F.; He, C.L.; Liang, X.X.; Lv, C.; Jing, B. Antiviral effect of resveratrol in piglets infected with virulent pseudorabies virus. Viruses 2018, 10, 457. [Google Scholar] [CrossRef] [PubMed]
- Brittle, E.E.; Reynolds, A.E.; Enquist, L.W. Two modes of pseudorabies virus neuroinvasion and lethality in mice. J. Virol. 2004, 78, 12951–12963. [Google Scholar] [CrossRef] [PubMed]
- Pomeranz, L.E.; Reynolds, A.E.; Hengartner, C.J. Molecular biology of pseudorabies virus: Impact on neurovirology and veterinary medicine. Microbiol. Mol. Biol. Rev. 2005, 69, 462–500. [Google Scholar] [CrossRef]
- An, T.Q.; Peng, J.M.; Tian, Z.J.; Zhao, H.Y.; Li, N.; Liu, Y.M.; Chen, J.Z.; Leng, C.L.; Sun, Y.; Chang, D.; et al. Pseudorabies virus variant in Bartha-K61-vaccinated pigs, China, 2012. Emerg. Infect. Dis. 2013, 19, 1749–1755. [Google Scholar] [CrossRef] [PubMed]
- Freuling, C.M.; Muller, T.F.; Mettenleiter, T.C. Vaccines against pseudorabies virus (PrV). Vet. Microbiol. 2017, 206, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.F.; Zhang, Z.D.; Lv, L.; Xiao, Y.H.; Qu, Y.J.; Ma, H.Y.; Niu, Y.J.; Wang, G.W.; Liu, S.D. Outbreak of variant pseudorabies virus in Bartha-K61-vaccinated piglets in central Shandong Province, China. J. Vet. Diagn. Investig. 2015, 27, 600–605. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.M.; Zhou, Q.; Song, W.B.; Sun, E.C.; Zhang, M.M.; He, Q.G.; Chen, H.C.; Wu, B.; Liu, Z.F. Novel pseudorabies virus variant with defects in TK, gE and gI protects growing pigs against lethal challenge. Vaccine 2015, 33, 5733–5740. [Google Scholar] [CrossRef]
- Liu, X.; Lv, L.; Jiang, C.L.; Bai, J.; Gao, Y.N.; Ma, Z.H.; Jian, P. A natural product, (S)-10-Hydroxycamptothecin inhibits pseudorabies virus proliferation through DNA damage dependent antiviral innate immunity. Vet. Microbiol. 2022, 265, 109313–109323. [Google Scholar] [CrossRef]
- Gottschalk, M.; Segura, M.; Xu, J.G. Streptococcus suis infections in humans: The Chinese experience and the situation in North America. Anim. Health Res. Rev. 2007, 8, 29–45. [Google Scholar] [CrossRef]
- Lun, Z.R.; Wang, Q.P.; Chen, X.G.; Li, A.X.; Zhu, X.Q. Streptococcus suis: An emerging zoonotic pathogen. Lancet. Infect. Dis. 2007, 7, 201–209. [Google Scholar] [CrossRef]
- Shimoji, Y. Pathogenicity of Erysipelothrix husiopathiae: Virulence factors and protective immunity. Microbes Infect. 2000, 2, 965–972. [Google Scholar] [CrossRef] [PubMed]
- Josephine Brooke, C.; Riley, T.V. Erysipelothrix rhusiopathiae: Bacteriology, epidemiology and clinical manifestations of an occupational pathogen. J. Med. Microbiol. 1999, 48, 789–799. [Google Scholar] [CrossRef]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L. Discovery, research, and development of new antibiotics: The WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef]
- Shiomi, K.; Iinuma, H.; Hamada, M.; Naganawa, H.; Manabe, M.; Matsuki, C.; Takeuchi, T.; Umezawa, H. Novel antibiotics napyradiomycins. Production, isolation, physicochemical properties and biological activity. J. Antibiot. 1986, 39, 487–493. [Google Scholar] [CrossRef]
- Shiomi, K.; Nakamura, H.; Iinuma, H.; Naganawa, H.; Isshiki, K.; Takeuchi, T.; Umezawa, H. Structures of new antibiotics napyradiomycins. J. Antibiot. 1986, 39, 494–501. [Google Scholar] [CrossRef]
- Shomura, A.; Gomi, S.; Ito, M.; Yoshida, J.; Tanaka, E.; Amano, S.; Watabe, H.; Ohuchi, S.; Itoh, J.; Sezaki, M.; et al. Studies on new antibiotics SF2415. I. Taxonomy, fermentation, isolation, physico-chemical properties and biological activities. J. Antibiot. 1987, 40, 732–739. [Google Scholar] [CrossRef] [PubMed]
- Gomi, S.; Ohuchi, S.; Sasaki, T.; Itoh, J.; Sezaki, M. Studies on new antibiotics SF2415. 2. The structural elucidation. J. Antibiot. 1987, 40, 740–749. [Google Scholar] [CrossRef]
- Fukuda, D.S.; Mynderse, J.S.; Baker, P.J.; Berry, D.M.; Boeck, L.D.; Yao, R.C.; Mertz, F.P.; Nakatsukasa, W.M.; Mabe, J.; Ott, J.; et al. A80915, a new antibiotic complex produced by Streptomyces aculeolatus. Discovery, taxonomy, fermentation, isolation, characterization, and antibacterial evaluation. J. Antibiot. 1990, 43, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Dantzig, A.H.; Minor, P.L.; Garrigus, J.L.; Fukuda, D.S.; Mynderse, J.S. Studies on the mechanism of action of A80915A, a semi-naphthoquinone natural product, as an inhibitor of gastric (H+-K+)-ATPase. Biochem. Pharmacol. 1991, 42, 2019–2026. [Google Scholar] [CrossRef]
- Hori, Y.; Abe, Y.; Shigematsu, N.; Goto, T.; Okuhara, M.; Kohsaka, M. Napyradiomycin A and B1: Non-steroidal estrogen-receptor antagonists produced by a Streptomyces. J. Antibiot. 1993, 46, 1890–1893. [Google Scholar] [CrossRef] [PubMed]
- Farnaes, L.L.L. Novel Analogs and a Protein Target for the Napyradiomycins. Ph.D. Thesis, University of California, San Diego, CA, USA, 2009. [Google Scholar]
- Cheng, Y.B.; Jensen, P.R.; Fenical, W. Cytotoxic and antimicrobial napyradiomycins from two marine-derived Streptomyces Strains. Eur. J. Organ. Chem. 2013, 2013, 3751–3757. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.C.; Li, S.M.; Li, J.; Chen, Y.C.; Saurav, K.; Zhang, Q.B.; Zhang, H.B.; Zhang, W.J.; Zhang, W.M.; Zhang, S.; et al. Antibacterial and cytotoxic new napyradiomycins from the marine-derived Streptomyces sp. SCSIO 10428. Mar. Drugs 2013, 11, 2113–2125. [Google Scholar] [CrossRef]
- Pereira, F.; Almeida, J.R.; Paulino, M.; Grilo, I.R.; Macedo, H.; Cunha, I.; Sobral, R.G.; Vasconcelos, V.; Gaudêncio, S.P. Antifouling napyradiomycins from marine-derived actinomycetes Streptomyces aculeolatus. Mar. Drugs 2020, 18, 63. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.W.; Kim, G.J.; Kim, H.J.; Nam, J.W.; Kim, J.; Chin, J.W.; Park, J.H.; Choi, H.; Park, K.D. Identification and evaluation of a napyradiomycin as a potent Nrf2 activator: Anti-oxidative and anti-inflammatory activities. Bioorganic Chem. 2020, 105, 104434. [Google Scholar] [CrossRef] [PubMed]
- Sarmin, N.I.M.; Tan, G.Y.A.; Franco, C.M.M.; Edrada-Ebel, R.; Latip, J.; Zin, N.M. Streptomyces kebangsaanensis sp. nov., an endophytic actinomycete isolated from an ethnomedicinal plant, which produces phenazine-1-carboxylic acid. Int. J. Syst. Evol. Micr. 2013, 63, 3733–3738. [Google Scholar] [CrossRef]
- Ohtani, I.; Kusumi, T.; Ishitsuka, M.O.; Kakisawa, H. Absolute configurations of marine diterpenes possessing a xenicane skeleton an application of an advanced Mosher’s method. Tetrahedron Lett. 1989, 30, 3147–3150. [Google Scholar] [CrossRef]
- Takemura, S.; Hirayama, A.; Tokunaga, J.; Kawamura, F.; Inagaki, K.; Hashimoto, K.; Nakata, M. A concise total synthesis of (+)-A80915G, a member of the napyradiomycin family of antibiotics. Tetrahedron Lett. 1999, 40, 7501–7505. [Google Scholar] [CrossRef]
- Motohashi, K.; Irie, K.; Toda, T.; Matsuo, Y.; Kasai, H.; Sue, M.; Furihata, K.; Seto, H. Studies on terpenoids produced by actinomycetes. 5-Dimethylallylindole-3-carboxylic acid and A80915G-8″-acid produced by marine-derived Streptomyces sp. MS239. J. Antibiot. 2008, 61, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Henkel, T.; Zeeck, A. Secondary metabolites by chemical screening. 15. Structure and absolute configuration of naphthomevalin, a new dihydro-naphthoquinone antibiotic from Streptomyces sp. J. Antibiot. 1991, 44, 665–669. [Google Scholar] [CrossRef]
- Li, S.F.; Hu, X.X.; Li, L.L.; Hu, X.M.; Wang, J.; Hu, X.W.; Liu, H.Y.; Yu, L.Y.; You, X.F.; Jiang, B.Y.; et al. 1-hydroxy-7-oxolavanducyanin and Δ7″,8″-6″-hydroxynaphthomevalin from Streptomyces sp. CPCC 203577. J. Antibiot. 2020, 73, 324–328. [Google Scholar] [CrossRef] [PubMed]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [PubMed]
- Lu, H.; Lu, W.J.; Zhu, Y.W.; Wang, C.C.; Shi, L.M.; Li, X.D.; Wu, Z.Y.; Wang, G.Y.; Dong, W.; Tan, C.; et al. Auranofin has advantages over first-Line drugs in the treatment of severe Streptococcus suis infections. Antibiotics 2021, 10, 26. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).