Abstract
Pt-based catalysts exhibit unique catalytic properties in many chemical reactions. In particular, metal–support interactions (MSI) greatly improve catalytic activity. However, the current MSI mechanism between platinum (Pt) and the support is not clear enough. In this paper, the interaction of 1 wt% Pt nanoparticles (NPs) on β-MnO2 in carbon monoxide (CO) oxidation was studied. The Pt on β-MnO2 inhibited CO oxidation below 210 °C but promoted it above 210 °C. A Pt/β-MnO2 catalyst contains more Pt4+ and less Pt2+. The results of operando DRIFTS-MS show that surface-terminal-type oxygen (M=O) plays an important role in CO oxidation. When the temperature was below 210 °C, Mn=O consumption on Pt/β-MnO2 was less than β-MnO2 due to Pt4+ inhibition on CO oxidation. When the temperature was above 210 °C, Pt4+ was reduced to Pt2+, and Mn=O consumption due to CO oxidation was greater than β-MnO2. The interaction of Pt and β-MnO2 is proposed.
1. Introduction
CO is a colorless, odorless, but toxic, gas [1]. CO is mainly emitted from vehicle combustion and industrial processes [2,3]. It is not only hazardous to human health, but also indirectly contributes to global warming [4]. Catalytic oxidation technology is a considerably effective method for reducing CO emission because it has high economic feasibility, lower cost, and lower secondary pollution generation [5]. Transition metal oxide catalysts have shown promising applications due to their low cost and good poisoning and sintering resistance [6]. MnO2 is widely used in catalytic oxidation reactions due to its variable valence states and good oxygen-storage and oxygen-release capacities [7,8,9]. MnO2 can form a variety of crystal structures, such as α-, β-, γ-, and δ-MnO2, according to [MnO6] as a backbone arrangement [7]. Among them, β-MnO2 has a thermodynamically stable phase and high crystallinity, which has become one of the current research hotspots [10]. It is still a challenge for MnO2 to perform CO oxidation at low temperatures [11,12].
Loading a noble metal onto a support is an effective way to improve catalytic activity. Noble metal–supported catalysts have been widely used in CO oxidation due to the MSI [2]. Among them, platinum (Pt)-based catalysts have attracted wide attention for their excellent catalytic activity [13]. Zhu et al. used a Pt/Al2O3 catalyst to convert formaldehyde at room temperature [14]. Huang et al. prepared a Pt/CeO2-TiO2 catalyst and found that it has high catalytic activity and selectivity for CO + NO reactions [15]. Cui et al. prepared an octahedral Fe3O4 to support Pt to remove formaldehyde and found that the catalyst has a high activity and stability [16]. Ru et al. synthesized Pt/(α-, γ-, and δ-) MnO2 catalysts for methanol oxidation, and showed that the temperatures for methanol oxidation on Pt/(α-, γ-, and δ-) MnO2 catalysts can be reduced in comparison with MnO2 [17].
MnO2 has two typical oxygen species: terminal-type oxygen (Mn=O) and bridge-type oxygen (Mn-O-Mn) [18]. Mn=O plays an important role in the CO oxidation process [19]. MSI can inhibit the growth of small noble metal nanoparticles and optimize the bonding structure and electron transfer, and, thus, improve catalytic performance [13]. Dispersion of Pt NPs onto MnO2 is an efficient method to weaken Mn=O and Mn-O-Mn bonds and generate oxygen vacancies through MSI between interfaces of Pt NPs and MnO2 [20]. However, some studies have shown that the strong interactions may limit catalytic reactions and weaken catalytic activity [13]. Miao et al. found that excessively strong MSI does not promote O2 adsorption [13,21]. At present, the interaction between Pt and the support is not clear enough. This study aims to gain insight into the interaction of Pt and MnO2 in CO oxidation.
Operando diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS) can monitor surface species and oxygen vacancies under a real reaction condition and is often used to study catalytic reaction mechanisms [10]. Xu et al. used H2 as a probe molecule to study the role of oxygen species on the surface oxygen species of β-MnO2 of a low amount of M=O using operando DRIFTS coupled with mass spectrometry (MS) [10]. Li et al. studied the effects of acid gases (NO, HCl, and SO2) on mercury oxidation over CeO2-WO3/TiO2 catalysts through in situ DRIFTS and showed that NO2 and nitrate are active centers of mercury oxidation [22]. Using CO as a probe molecule and in situ DRIFTS, Jang, et al. quantified a part of the catalytic active sites on PdOx, further elucidating the surface reconstruction of Pt-Pd bimetallic particles during the reaction [23]. Zhang et al. confirmed that Rh atoms are dispersed on Al2O3 and TiO2 using Fourier transform infrared spectroscopy (FTIR) [24].
In this study, 1 wt% Pt NPs loaded on β-MnO2 prepared with deposition–precipitation is used to explore the metal–support interaction of Pt and β-MnO2 during CO oxidation. The physicochemical properties of β-MnO2 and Pt/MnO2 catalysts are characterized using an X-ray diffractometer (XRD), high-resolution transmission microscopy (HRTEM), X-ray photoelectron spectroscopy (XPS), and paramagnetic resonance spectroscopy (EPR). The role of oxygen species on the surfaces of β-MnO2 and Pt/β-MnO2 catalysts in CO oxidation is revealed using operando DRIFTS-MS. The interaction of Pt and β-MnO2 in CO oxidation is proposed.
2. Results and Discussion
2.1. Physical Phase Characterization
Figure 1 shows the XRD patterns of two catalysts. The diffraction peaks of β-MnO2 correspond to JCPDS 24-0735, and 2θ = 28.7°, 37.4°, 42.8°, 56.7°, and 59.5° correspond to (110), (101), (111), (211), and (220) crystal planes, respectively. β-MnO2 exhibits sharp and narrow diffraction peaks, suggesting that β-MnO2 has a high degree of crystallinity [25]. No diffraction peaks of Pt have been observed from XRD patterns, which may be due to small particle size, low Pt loading, or high dispersion of the Pt NPs [13,26,27,28,29]. The positions of the diffraction peaks of Pt/β-MnO2 are consistent with that of β-MnO2, indicating that the loading Pt did not change the crystal structure of β-MnO2. However, after loading Pt, the intensities of the diffraction peaks decreased, indicating that the crystallinity of β-MnO2 can be affected by Pt loading [30].
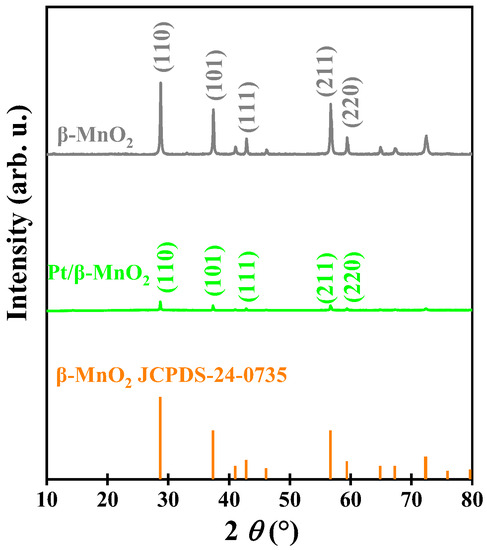
Figure 1.
XRD patterns.
Figure 2 shows the HRTEM images of the two catalysts. The lattice spacing of β-MnO2 is 0.311 nm (Figure 2a), which is consistent with the (110) crystal face of β-MnO2. After loading Pt on β-MnO2, it was found that there are some crystal-plane blurs between the normal lattice stripes (inside the red circles in Figure 2b). This fact implied that the loading of Pt NPs caused defects in the catalyst [31]. Although no crystal planes of Pt species were observed in HRTEM, the presence of Pt NPs could be confirmed from HRTEM images (Figure 2c,d). The sizes of the Pt NPs were between 1.0 and 2.0 nm.
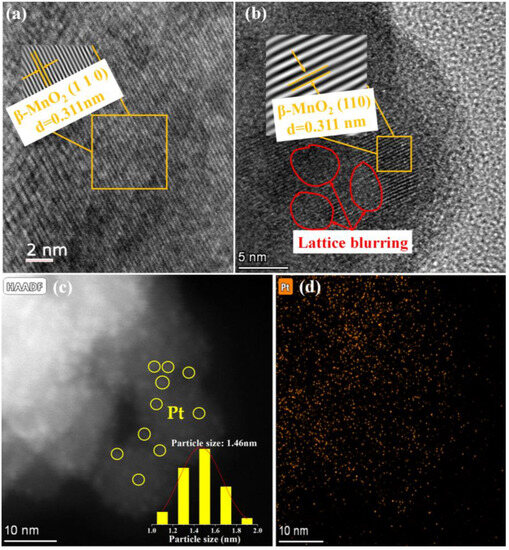
Figure 2.
HRTEM images. (a) β-MnO2; (b) and (c) Pt/β-MnO2; (d) mapping of Pt on Pt/β-MnO2.
The N2 adsorption isotherm and pore-size distribution curves of the two catalysts are shown in Figure 3. The physical and chemical properties of the two catalysts are summarized in Table 1. According to the IUPAC classification, the N2 adsorption isotherms of both catalysts are type IV isotherms. The specific surfaces before and after Pt loading are the same. However, the BJH pore-size distribution showed that β-MnO2 mainly has a microporous structure (<2 nm), while Pt/β-MnO2 is mainly of a mesoporous structure (2–50 nm). After loading Pt NPs, the pore volume of β-MnO2 decreased and the pore diameter increased, indicating that the small pores in β-MnO2 were blocked by Pt NPs.
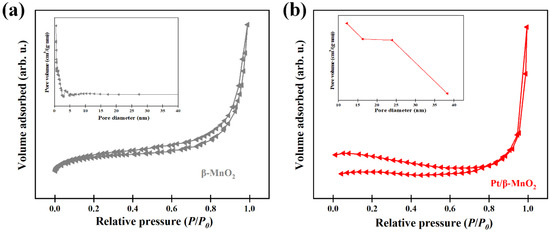
Figure 3.
N2 adsorption/desorption isotherms and pore size distributions. (a) β-MnO2 and (b) Pt/β-MnO2.

Table 1.
Specific surface areas, pore volumes, and pore diameters.
2.2. Catalytic Activity
The catalytic activities of the two catalysts are shown in Table 1 and Figure 4. Pt/β-MnO2 has T50 and T90 values, lower than β-MnO2. This means that loading Pt can enhance the activity of β-MnO2. The TOF value of Pt/β-MnO2 at 222 °C (T90) was 0.06 s−1; this TOF value is around the order of Pt NPs on SiO2 [32].
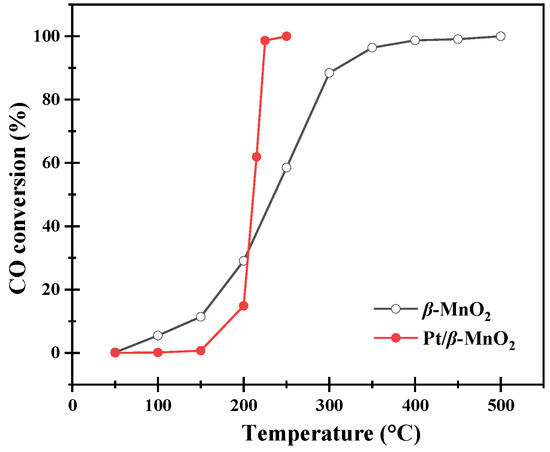
Figure 4.
CO conversion at various temperatures.
It should be noted that CO oxidation on Pt/β-MnO2 is lower than that of β-MnO2 at a temperature below 210 °C. This result suggested that Pt NPs inhibited CO oxidation below 210 °C by Pt NPs alone or in combination with β-MnO2. The inhibition mechanism is discussed later in Section 3.
2.3. Surface Chemical Structures
The surface chemical structures of the two catalysts were characterized using XPS and Raman. Figure 5 shows the XPS spectra of the two catalysts. Each element is qualitatively identified and semiquantitatively calculated using the peak areas of each element [28,29]. The results are listed in Table 2. The peaks of 641.6, 642.5, and 643.6 eV (Figure 5a) correspond to Mn2+, Mn3+, and Mn4+, respectively [10]. The peak-area ratios of Mn cations with different valence states are shown in Table 2. Before and after Pt loading, the (Mn2+ + Mn3+)/Mn4+ ratio increased from 3.55 to 4.00, proving that Pt NPs on β-MnO2 can promote the formation of Mn2+ and Mn3+; those contribute to the formation of oxygen vacancies [33]. Moreover, the increase in the Mn2+ and Mn3+ ratio is also related to the formation of Pt-O-Mn [20].
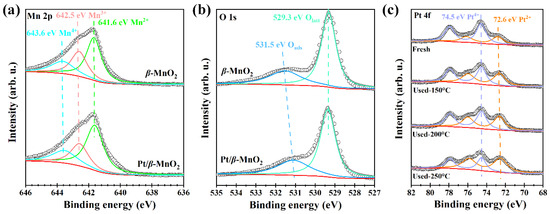
Figure 5.
XPS spectra. (a) Mn 2p; (b) O 1s; and (c) Pt 4f.

Table 2.
XPS and Raman analysis results.
The XPS spectra of O1s are shown in Figure 5b. The peaks at 529.3 and 531.5 eV correspond to lattice oxygen (Olatt) and surface-adsorbed oxygen (Oads), respectively [10]. The peak-area ratios of the surface oxygen species on the two catalysts are listed in Table 2. The Oads/(Oads + Olatt) ratios of β-MnO2 and Pt/β-MnO2 are 0.30 and 0.33, respectively. Pt/β-MnO2 has more adsorbed oxygen than β-MnO2, which supports the fact that a higher (Mn2+ + Mn3+)/Mn4+ ratio can generate more oxygen vacancies on Pt/β-MnO2. The more surface-adsorbed oxygen is essential for catalytic oxidation [13]. Our finding also confirmed that catalysts containing more surface-adsorbed oxygen have better catalytic activity (Figure 4a). The XPS spectra of Pt 4f have broad peaks in the Pt 4f region (Figure 5c), indicating that Pt has different valence states. The peaks of 72.6 and 74.5 eV correspond to Pt2+ and Pt4+, respectively [13,20]. The peak-area ratios of different valence states before and after CO oxidation are given in Table 2. The Pt/β-MnO2 catalyst before CO oxidation mainly has Pt+4 and Pt+2, among which the Pt4+ ratio is as high as 0.68, showing that Pt4+ is the mainstay. The emergence of Pt2+ can be explained by the generation of Pt-O-Mn bonds, meaning that there is an interaction between Pt and β-MnO2 [33]. After the reaction at 150, 200, and 250 °C, the ratio of Pt2+/Pttotal gradually increased to 0.64. At the same time, the ratio of Pt4+/Pttotal decreased to 0.36. This finding clearly explores that Pt4+ was continuously reduced to Pt2+ during CO oxidation. Since Pt2+ is associated with the formation of Pt-O-Mn, it is speculated that Pt-O-Mn can promote CO oxidation. Combined with the low activity of Pt/β-MnO2 below 210 °C, it is concluded that Pt/β-MnO2 containing more Pt4+ and less Pt-O-Mn is not in favor for CO oxidation.
Figure 6 shows the Raman profiles of the two catalysts. The peak located in 500–700 cm−1 is attributed to the stretching of the [MnO6] octahedron [34]. The peak around 637 cm−1 is the symmetrical Mn=O tensile vibration perpendicular to the [MnO6] octahedral double strand. The low-intensity peak with a low wavenumber is attributed to the deformation mode of the metal–oxygen chain (Mn-O-Mn) in the MnO2 octahedral lattice [35]. Compared with β-MnO2, the vibration intensities of Mn=O and Mn-O-Mn after Pt loading have been weakened. This indicates that the mobility of lattice oxygen (Mn-O-Mn) increases after Pt loading [20]. The peak areas of Mn=O (AMn=O) and Mn-O-Mn (AMn-O-Mn) were calculated using LabSpec software. Pt/β-MnO2 has a higher AMn=O/(AMn-O-Mn + AMn-O) ratio than β-MnO2 (Table 2), implying that Pt/β-MnO2 has more adsorbed oxygen (Mn=O) than β-MnO2.
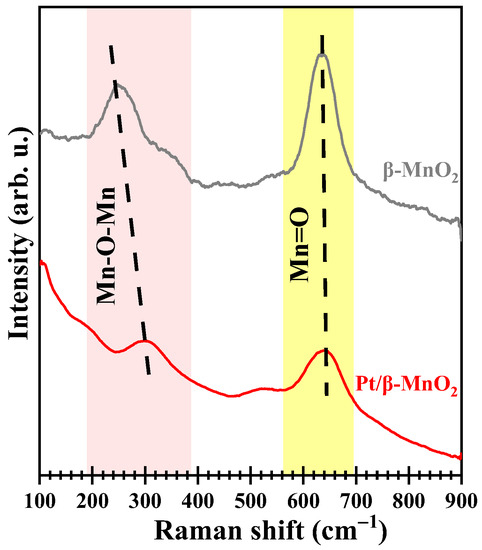
Figure 6.
Raman profiles.
2.4. Reduction, Oxygen-Vacancy, and Electron-Transfer Analyses
To further understand the interaction of Pt and β-MnO2, H2-TPR and EPR were performed. Figure 7a shows the H2-TPR profiles for two catalysts. The reduction temperature can be divided into two regions, R0 (50–200 °C) and RI (200–600 °C). Two reduction peaks (319 and 455 °C) were observed on β-MnO2, which were attributed to the reduction of Mn4+ to Mn3+ and then to Mn2+ [13,20]. After loading Pt, the R0 region has the reduction peak of PtOx at 88 °C and surface-adsorbed oxygen (Oads) at 143 °C [36]. Since the reduction peak in the R0 region is attributed to H2 spillover effects, which are caused by the formation of Pt-O-Mn bonds between β-MnO2 and adjacent Pt NPs [17,37], the Pt/β-MnO2 of peaks in the R0 region interacts between Pt and β-MnO2. Figure 7b shows the EPR spectra of the two catalysts. β-MnO2 has obvious electron paramagnetic resonance signal peaks at g = 2.005 [36], and the peak intensity can also reflect the oxygen-vacancy concentration. After loading Pt on β-MnO2, the signal at g = 2.005 is offset. This proves that there is electron transfer between Pt NPs and β-MnO2, which is consistent with typical MSI phenomena [38]. In addition, it is clear that the oxygen-vacancy concentration order is Pt/β-MnO2 > β-MnO2. Higher oxygen-vacancy concentrations correspond to better oxygen-mobility capacity, which is consistent with the characterization of H2-TPR. The oxygen vacancies of Pt/β-MnO2 increased significantly, mainly due to the fact that MSI affects the Mn=O bonds of the vector and forms more oxygen vacancies [36].
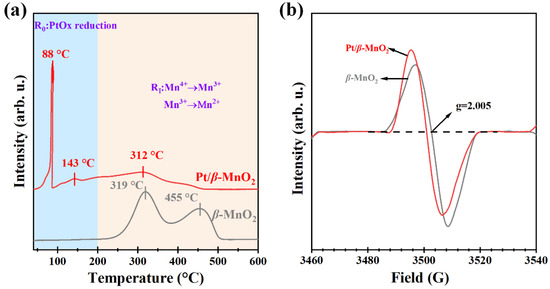
Figure 7.
(a) H2-TPR profiles and (b) EPR spectra.
2.5. Surface Reactions Using Operando DRIFTS-MS
2.5.1. Surface Reactions in CO Atmosphere
Figure 8 shows the operando DRIFTS spectra during CO oxidation on the two catalysts in a 1% CO/Ar atmosphere without O2. The peaks on β-MnO2 at 2173 and 2114 cm−1 belong to the gas-phase CO. On Pt/β-MnO2, the peak at 2173 cm−1 belongs to the gas-phase CO [19], the peak at 2121 cm−1 belongs to CO-Ptδ+ [39,40], and the peak at 2068 cm−1 is assigned to CO-Pt0 [39,40]. Peaks at 1300, 1112, 947, and 770 cm−1 are attributed to Mn=O, Mn+-O2−, Mn+-O22−, and Mn+-O2−-Mn+, respectively [10,19]. The peak in 1605–1688 cm−1 is attributed to bidentate carbonate (v(OCO)), and the peak in 1463–1560 cm−1 belongs to monodentate carbonate (v(CO32−)) [19].
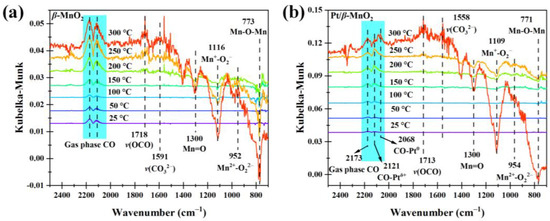
Figure 8.
Operando DRIFTS spectra of two catalysts in 1% CO/Ar atmosphere. (a) β-MnO2 and (b) Pt/β-MnO2.
Figure 9 shows the normalized peak heights of the surface species and MS signals during CO oxidation on β-MnO2 and Pt/β-MnO2 in a CO atmosphere. As the temperature increased, the normalized intensities of Mn=O and Mn-O-Mn on β-MnO2 and Pt/β-MnO2 decreased (Figure 9a,b), indicating that oxygen species are continuously consumed due to their reactions with CO following Equations (1)–(4). The initial consumption temperature of Mn=O on β-MnO2 is 50 °C, obviously lower than that (150 °C) on Pt/β-MnO2, suggesting that Mn=O on β-MnO2 can react more easily with CO than that on Pt/β-MnO2. This fact also implied that Pt inhibited CO oxidation by the O in Mn=O. The initial consumption temperatures of Mn-O-Mn on β-MnO2 and Pt/β-MnO2 are higher than 100 °C, but the normalized peak height of Mn-O-Mn on β-MnO2 is less than that on Pt/β-MnO2 (Figure 9b), also suggesting that Mn-O-Mn on β-MnO2 can react easier with CO than that on Pt/β-MnO2; Pt inhibited CO oxidation by the O in Mn-O-Mn. The reactions of Mn=O and Mn-O-Mn with CO result in the formation of Mn=□ and Mn2+-□-Mn, where □ denotes an oxygen vacancy. As the initial adsorbed O2 in (O2−)Mn2+-O-Mn is reduced to (O22−)Mn2+-□-Mn by CO (Equations (5) and (6)), the decrease in the normalized peak height of (O2−)Mn2+-O-Mn and increase in Mn2+-□-Mn above 50 °C (Figure 9c) proved the reactions in Equations (5) and (6). The CO oxidation products include surface carbonate and gaseous CO2. Figure 9d shows the normalized peak heights of monodentate carbonate (v(CO32−)) on β-MnO2 and Pt/β-MnO2. The remarkable differences between β-MnO2 and Pt/β-MnO2 can be found within the temperature range of 100 and 300 °C, in which the normalized peak heights of monodentate carbonate on β-MnO2 are higher than that on Pt/β-MnO2, indicating that Pt inhibited CO oxidation to monodentate carbonate (Equation (7)). Figure 9e illustrates no obvious difference in the normalized peak heights of bidentate carbonate on β-MnO2 and Pt/β-MnO2; thus, Pt has no influence on bidentate carbonate formation following Equation (9) [41].
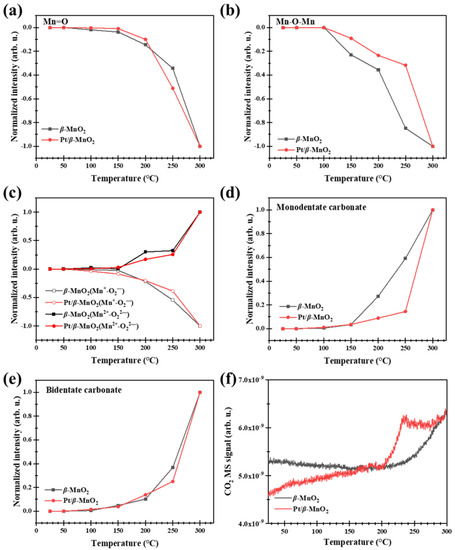
Figure 9.
Normalized intensities of surface species on the surfaces of β-MnO2 and Pt/β-MnO2 catalysts (a–e) and MS signals (f) in a 1%CO/Ar atmosphere.
The CO2 MS signal from β-MnO2 is higher than that from Pt/β-MnO2 in the temperature range of 50–210 °C (Figure 9f), as Pt inhibited CO oxidation with O atoms in Mn=O and Mn-O-Mn.
Mn=O + CO → Mn=□ + CO2
Mn=O + CO-Ptδ+ → Mn=□ + CO2
Mn-O-Mn + CO → Mn-□-Mn + CO2
Mn-O-Mn + CO-Ptδ+ → Mn-□-Mn+CO2
(O2−)Mn-O-Mn + CO+ → (O22−)Mn2+-□-Mn + CO2
(O2−)Mn-O-Mn + CO-Ptδ+ → (O22−)Mn2+-□-Mn + Ptδ+ + CO2
Mn-O-Mn=O + CO → Mn-□-Mn-O-CO2
Mn=O + CO2 → Mn-O-CO2
Mn2+-O22−+CO2 → Mn=O2-CO
2.5.2. Surface Reactions in CO/O2 Atmosphere
Figure 10 shows the DRIFTS-MS spectra of two catalysts during CO oxidation in a 1%CO/20%O2/Ar atmosphere. The normalized intensities of Mn=O and Mn-O-Mn obviously decreased above 150 and 100 °C, respectively (Figure 11a,b), which are similar to those (Figure 9a,b) during CO oxidation in the absence of O2. The normalized intensities of (O2−)Mn-O-Mn and Mn2+-□-Mn on Pt/β-MnO2 changed at 50 °C, lower than that (100 °C) on β-MnO2. Since the changes of (O2−)Mn-O-Mn and Mn2+-□-Mn are related to the formation of oxygen vacancy, Pt can promote the formation of oxygen vacancy in the presence of O2. The normalized intensities of monodentate carbonate β-MnO2 and Pt/β-MnO2 (Figure 11e) are similar to those (Figure 9e), explaining that O2 and Pt cannot influence the formation of monodentate carbonate.
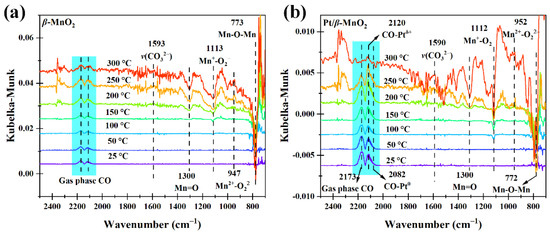
Figure 10.
Operando DRIFTS spectra of two catalysts in a 1%CO/20% O2/Ar atmosphere. (a) β-MnO2 and (b) Pt/β-MnO2.
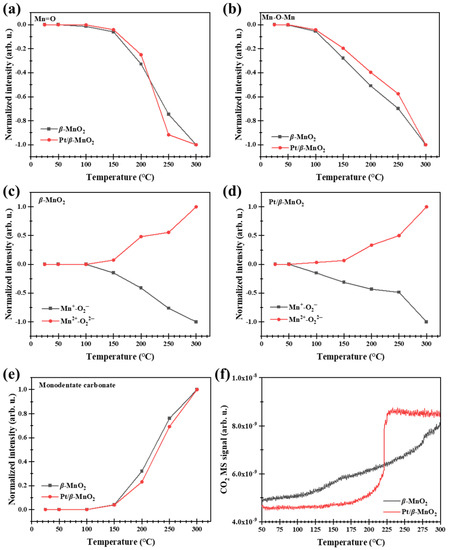
Figure 11.
Normalized intensities of surface species on the surfaces of β-MnO2 and Pt/β-MnO2 catalysts (a–e) and MS signals (f) in a CO/O2/Ar atmosphere.
2.5.3. Surface Reactions in O2 Atmosphere
The normalized intensities of Mn=O and Mn-O-Mn after CO oxidation with or without O2 are all negative (Figure 9 and Figure 11), indicating that the regeneration rates of oxygen vacancies (Mn=□ and Mn-□-Mn) are lower than their consumption rates. To determine whether the oxygen vacancies can be regenerated, the catalysts with oxygen vacancies (generated via CO oxidation in a 1%CO/Ar atmosphere for 30 min at 300 °C) were heated from 25 °C to 300 °C with a ramp of 10 °C min−1 in a 20%O2/Ar atmosphere. The regeneration results are shown in Figure 12. Figure 12a,b show the DRIFTS spectra on β-MnO2 and Pt/β-MnO2 during regeneration. Figure 12c,d demonstrate that the normalized intensities of Mn=O and Mn-O-Mn increased to 0 at 300 °C, indicating that O2 decomposition at Mn=□ and Mn-□-Mn happened, resulting in the complete regeneration of Mn=O and Mn-O-Mn, where the O2 decomposition obeys Epling–Xu mechanism (Equation (10)) [42].
(O22−)Mn-□-Mn → O=Mn-O-Mn
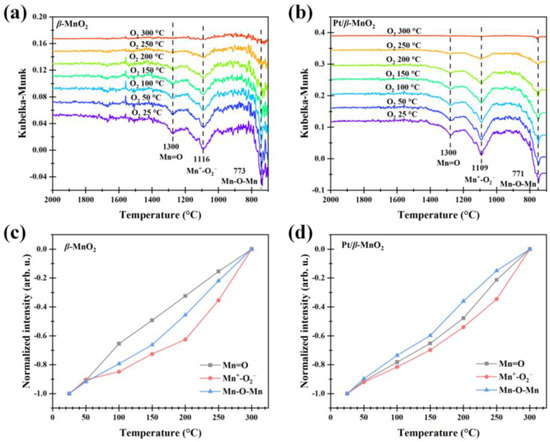
Figure 12.
Operando DRIFT spectra of β-MnO2 (a) and Pt/β-MnO2 (b) in an O2 atmosphere. Normalized intensities of β-MnO2 (c) and Pt/β-MnO2 (d).
3. Interaction of Pt and β-MnO2 in CO Oxidation
Figure 13 shows the interaction of Pt NPs and β-MnO2 in CO oxidation. Pt NPs are anchored on MnO2 by Pt-O-Mn bonding, resulting in Pt+4 and Pt+2 valence states. CO is mainly adsorbed at Mn=O sites as the Pt loading is as low as 1 wt%. CO oxidation required additional O, which is from Mn=O or Pt-O-Mn and Mn-O-Mn. Step (a) indicates that O from Pt+4-O-Mn is difficult, causing the inhibition of CO oxidation. Step (b) is the easy process of O supplement from Pt+2-O-Mn to CO for its oxidation to CO2. At a temperature below 210 °C, the O in Pt+4-O-Mn cannot function as an O source, and Pt loading reduces the active sites of MnO2 for CO oxidation, as Pt is anchored at places having defects. Those are active sites for CO oxidation, and this results in the inhibition of Pt on CO oxidation. When the temperature is above 210 °C, the ratio of Pt+4 decreases, and the Pt+2 ratio increases, resulting in an increase in CO oxidation activity.
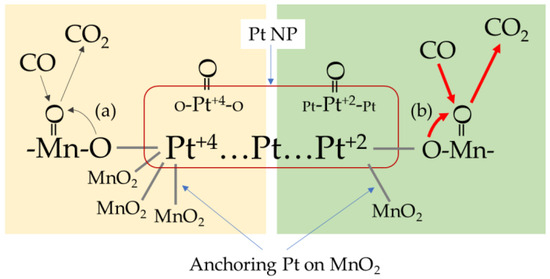
Figure 13.
The interaction of Pt and β-MnO2 in CO oxidation.
4. Materials and Methods
4.1. Catalyst Preparation
β-MnO2 was purchased from Aladdin, Shanghai, China. Chloroauric acid (H2Cl6Pt·xH2O) was from the Shanghai McLean Company (Shanghai, China).
The 1 wt% Pt/β-MnO2 was prepared using the deposition–precipitation method according to the method reported by Koo et al. [43]. Add H2Cl6Pt·xH2O (0.1 g mL−1), β-MnO2 (4 g), and distilled water (100 mL) to a beaker and mix thoroughly. Place the beaker in a 60 °C water bath and stir for 1 h; NH3·H2O was used to adjust the pH of the mixture to around 9 and then the mixture was stirred at 60 °C for 8 h. After stirring, the mixture was filtered and washed with hot distilled water and then dried at 60 °C for 12 h. After drying, the catalyst was put into a muffle furnace and calcined in an air atmosphere at 300 °C for 2 h.
4.2. Evaluation of Catalytic Activity
The CO oxidation activity of two catalysts (200 mg, 40–60 mesh) was measured in a fixed-bed flow reactor fed with 1% CO/20% O2 balanced with N2 at 100 mL min−1, with a gas-space velocity of 60,000 mL g−1 h−1 [27]. CO and CO2 concentrations were analyzed online using a gas chromatograph (GC9790II, Fuli, Taizhou, China) equipped with a flame ion detector with a catalyst convert to reduce CO and CO2 to CH4. Each catalyst was pretreated in 20% O2 with an N2 balance (80 mL min−1) atmosphere at 300 °C for 30 min. The CO conversion (x) and turnover frequency (TOF, s−1) were calculated using Equation (11) and Equation (12), respectively.
where [CO]in and [CO]out are the CO concentrations in gas streams from the inlet and outlet of the reactor, respectively.
where F is the total gas flow rate in mol s−1, mcat is the amount of catalyst in g, mPt is the weight ratio of Pt in the catalyst, and MPt is the molar atomic weight of Pt (195.08).
4.3. Catalyst Characterization
The operando DRIFTS-MS was used to monitor surface species and gaseous products during CO oxidation using the method reported elsewhere [10]. The catalyst characterization was performed as per [10].
5. Conclusions
In this study, 1 wt% Pt NPs loaded on β-MnO2 using deposition–precipitation was used to explore the effect of the metal–support interaction of Pt and β-MnO2 in CO oxidation. The main conclusions are as follows:
- (1)
- After loading Pt on β-MnO2, it was found that Pt NPs (1–2 nm) were anchored on β-MnO2 and formed Pt-O-Mn interfaces. CO oxidation was inhibited at a temperature below 210 °C and promoted above 210 °C;
- (2)
- The ratio of Pt+2 dominates CO oxidation. The ratios of Pt+2 before the reaction and after the reaction at 150, 200, and 250 °C were Pt/β-MnO2-fresh (0.32) < Pt/β-MnO2-used-150 °C (0.48) < Pt/β-MnO2-used-200 °C (0.51) < Pt/β-MnO2-used-250 °C (0.64). Pt4+ can convert to Pt+2 by heating;
- (3)
- The Operando DRIFTS-MS results show that Mn=O plays an important role in CO oxidation. Below 210 °C, more Mn=O was consumed on β-MnO2 than on Pt/β-MnO2. When the temperature is higher than 210 °C, more Mn=O was consumed on Pt/β-MnO2 than on β-MnO2. It is speculated that the inhibition of activity below 200 °C after Pt loading is due to Pt+4, which causes less Mn=O consumption for CO oxidation;
- (4)
- The mechanism of Pt and β-MnO2 interaction is proposed, where the Pt+4 and Pt+2 functions are clearly illustrated.
Author Contributions
T.Z. Conceptualization, Investigation, Formal analysis, Methodology, Writing—original draft; J.X.: Data curation; Y.S. Data curation; S.F. Data curation. Z.W. Data curation; E.G. Validation; J.Z. Methodology; W.W. Validation; S.Y. Conceptualization, Validation; Revision, Supervision, Funding, Project administration; J.L.: Validation; Revision. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Natural Science Foundation of China (Grant No. 12075037).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data are available from the corresponding author upon reasonable request.
Acknowledgments
All authors are grateful for the financial support of this work provided by the National Natural Science Foundation of China.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are not available from the authors.
References
- Dey, S.; Mehta, N.S. Selection of Manganese oxide catalysts for catalytic oxidation of Carbon monoxide at ambient conditions. Resour. Environ. Sustain. 2020, 1, 100003. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, L.; Li, J.; Zhang, W.; Shi, X.; Huang, Y.; Hojamberdiev, M.; Zhu, G. Metal support interaction of defective-rich CuO and Au with enhanced CO low-temperature catalytic oxidation and moisture resistance. Adv. Powder Mater. 2023, 2, 100119. [Google Scholar] [CrossRef]
- Wu, X.; Lang, J.; Sun, Z.; Jin, F.; Hu, Y.H. Photocatalytic conversion of carbon monoxide: From pollutant removal to fuel production. Appl. Catal. B Environ. 2021, 295, 120312. [Google Scholar] [CrossRef]
- Dey, S.; Dhal, G.C. Highly active palladium nanocatalysts for low-temperature carbon monoxide oxidation. Polytechnica 2020, 3, 1–25. [Google Scholar] [CrossRef]
- Guo, Y.; Wen, M.; Li, G.; An, T. Recent advances in VOC elimination by catalytic oxidation technology onto various nanoparticles catalysts: A critical review. Appl. Catal. B Environ. 2021, 281, 119447. [Google Scholar] [CrossRef]
- Guo, N.; Zhang, J.; Jiang, L.; Wang, D.; Wang, Z. Highly efficient and selective Ru and Ce modified ZSM-5 catalysts for catalytic oxidation of toluene. Colloids Surf. A Physicochem. Eng. Asp. 2022, 651, 129709. [Google Scholar] [CrossRef]
- Chen, L.; Liu, Y.; Fang, X.; Cheng, Y. Simple strategy for the construction of oxygen vacancies on α-MnO2 catalyst to improve toluene catalytic oxidation. J. Hazard. Mater. 2021, 409, 125020. [Google Scholar] [CrossRef]
- Shi, J.; Qi, T.; Sun, B.-C.; Chu, G.-W.; Chen, J.-F. Catalytic oxidation of benzyl alcohol over MnO2: Structure-activity description and reaction mechanism. Chem. Eng. J. 2022, 440, 135802. [Google Scholar] [CrossRef]
- Gu, W.; Li, C.; Qiu, J.; Yao, J. Facile fabrication of flower-like MnO2 hollow microspheres as high-performance catalysts for toluene oxidation. J. Hazard. Mater. 2021, 408, 124458. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, T.; Fang, S.; Li, J.; Wu, Z.; Wang, W.; Zhu, J.; Gao, E.; Yao, S. Exploring the roles of oxygen species in H2 oxidation at β-MnO2 surfaces using operando DRIFTS-MS. Commun. Chem. 2022, 5, 97. [Google Scholar] [CrossRef]
- Liu, D.; Hu, F.; Yan, Y.; Ye, R.; Chen, X.; Han, B.; Lu, Z.-H.; Zhou, L.; Feng, G.; Zhang, R. Promotion of oxygen vacancies and metal-support interaction over 3DOM Au/CeO2 catalyst for CO oxidation. Appl. Surf. Sci. 2023, 629, 157438. [Google Scholar] [CrossRef]
- Zou, G.; Zhang, Q.; Peng, S.; She, J.; Teng, D.; Jin, C.; Che, Y. Influence of geological factors on coal permeability in the Sihe coal mine. Int. J. Coal Sci. Technol. 2022, 9, 6. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, Z.; Tan, W.; Ji, J.; Cai, Y.; Tong, Q.; Xiong, Y.; Wan, H.; Dong, L. Thermal-Driven Optimization of the Strong Metal–Support Interaction of a Platinum–Manganese Oxide Octahedral Molecular Sieve to Promote Toluene Oxidation: Effect of the Interface Pt2+–Ov–Mnδ+. ACS Appl. Mater. Interfaces 2022, 14, 56790–56800. [Google Scholar] [CrossRef]
- Zhu, X.; Yu, J.; Jiang, C.; Cheng, B. Catalytic decomposition and mechanism of formaldehyde over Pt–Al2O3 molecular sieves at room temperature. Phys. Chem. Chem. Phys. 2017, 19, 6957–6963. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Lin, L.; Yang, K.; Dai, W.; Chen, X.; Fu, X. Promotion effect of ultraviolet light on NO+ CO reaction over Pt/TiO2 and Pt/CeO2–TiO2 catalysts. Appl. Catal. B Environ. 2015, 179, 395–406. [Google Scholar] [CrossRef]
- Cui, W.; Xue, D.; Tan, N.; Zheng, B.; Jia, M.; Zhang, W. Pt supported on octahedral Fe3O4 microcrystals as a catalyst for removal of formaldehyde under ambient conditions. Chin. J. Catal. 2018, 39, 1534–1542. [Google Scholar] [CrossRef]
- Ru, X.; Li, W.; Wang, X.; Shi, Z.; Wen, X.; Mo, S.; Zhang, Q.; Mo, D. Regulating the surface local environment of MnO2 materials via metal-support interaction in Pt/MnO2 hetero-catalysts for boosting methanol oxidation. Chem. Eng. Sci. 2023, 281, 119079. [Google Scholar] [CrossRef]
- Williams, O.C.; Sievers, C. Active oxygen species in heterogeneously catalyzed oxidation reactions. Appl. Catal. A Gen. 2021, 614, 118057. [Google Scholar] [CrossRef]
- Xu, J.; Wu, Z.; Gao, E.; Zhu, J.; Yao, S.; Li, J. Revealing the role of oxygen vacancies on α-MnO2 of different morphologies in CO oxidation using operando DRIFTS-MS. Appl. Surf. Sci. 2023, 618, 156643. [Google Scholar] [CrossRef]
- Xie, J.; Wang, S.; Zhao, K.; Wu, M.; Wang, F. Regulating the Pt–MnO2 Interaction and Interface for Room Temperature Formaldehyde Oxidation. Inorg. Chem. 2023, 62, 904–915. [Google Scholar] [CrossRef]
- Miao, S.; Deng, Y. Study on total cooxidation of H2 and CO over Au(Pt)/Co3O4 catalysts under oxygen-excessive condition. Chin. J. Catal. 2001, 22, 461–464. [Google Scholar]
- Li, G.; Wang, S.; Wu, Q.; Li, J.; You, X.; Shao, S.; Liu, K. Exploration of reaction mechanism between acid gases and elemental mercury on the CeO2–WO3/TiO2 catalyst via in situ DRIFTS. Fuel 2019, 239, 162–172. [Google Scholar] [CrossRef]
- Jang, E.J.; Lee, J.; Oh, D.G.; Kwak, J.H. CH4 oxidation activity in pd and Pt–Pd bimetallic catalysts: Correlation with surface PdO x quantified from the DRIFTS study. ACS Catal. 2021, 11, 5894–5905. [Google Scholar] [CrossRef]
- Zhang, J.; Asokan, C.; Zakem, G.; Christopher, P.; Medlin, J.W. Enhancing sintering resistance of atomically dispersed catalysts in reducing environments with organic monolayers. Green Energy Environ. 2022, 7, 1263–1269. [Google Scholar] [CrossRef]
- Gao, F.; Tang, X.; Yi, H.; Chu, C.; Li, N.; Li, J.; Zhao, S. In-situ DRIFTS for the mechanistic studies of NO oxidation over α-MnO2, β-MnO2 and γ-MnO2 catalysts. Chem. Eng. J. 2017, 322, 525–537. [Google Scholar] [CrossRef]
- Sun, H.; Yu, X.; Guo, Y.; Deng, J.; Ge, M. Achieving efficient toluene oxidation over metal–organic framework-derived Pt/CeO2-Co3O4 catalyst. Appl. Surf. Sci. 2022, 591, 153225. [Google Scholar] [CrossRef]
- Zhang, Q.; Mo, S.; Li, J.; Sun, Y.; Zhang, M.; Chen, P.; Fu, M.; Wu, J.; Chen, L.; Ye, D. In situ DRIFT spectroscopy insights into the reaction mechanism of CO and toluene co-oxidation over Pt-based catalysts. Catal. Sci. Technol. 2019, 9, 4538–4551. [Google Scholar] [CrossRef]
- Shi, Y.; Wan, J.; Kong, F.; Wang, Y.; Zhou, R. Influence of Pt dispersibility and chemical states on catalytic performance of Pt/CeO2-TiO2 catalysts for VOCs low-temperature removal. Colloids Surf. A Physicochem. Eng. Asp. 2022, 652, 129932. [Google Scholar] [CrossRef]
- Fan, J.; Sun, Y.; Fu, M.; Li, J.; Ye, D. Modulate the metal support interactions to optimize the surface-interface features of Pt/CeO2 catalysts for enhancing the toluene oxidation. J. Hazard. Mater. 2022, 424, 127505. [Google Scholar] [CrossRef]
- Gao, E.; Jin, Q.; Zhang, T.; Han, L.; Li, N.; Xu, J.; Yao, S.; Wu, Z.; Li, J.; Zhu, J.; et al. Unraveling the promotional effects of K-doping on the mobility of surface oxygen species of CoCr2O4 for improved formaldehyde catalytic oxidation: The weakened metal-oxygen bond strength. Chem. Eng. J. 2023, 474, 145618. [Google Scholar] [CrossRef]
- Yang, W.; Su, Z.A.; Xu, Z.; Yang, W.; Peng, Y.; Li, J. Comparative study of α-, β-, γ- and δ-MnO2 on toluene oxidation: Oxygen vacancies and reaction intermediates. Appl. Catal. B Environ. 2020, 260, 118150. [Google Scholar] [CrossRef]
- Lin, J.; Wang, X.; Zhang, T. Recent progress in CO oxidation over Pt-group-metal catalysts at low temperatures. Chin. J. Catal. 2016, 37, 1805–1813. [Google Scholar] [CrossRef]
- Ye, J.; Zhou, M.; Le, Y.; Cheng, B.; Yu, J. Three-dimensional carbon foam supported MnO2/Pt for rapid capture and catalytic oxidation of formaldehyde at room temperature. Appl. Catal. B Environ. 2020, 267, 118689. [Google Scholar] [CrossRef]
- Liang, S.; Teng, F.; Bulgan, G.; Zong, R.; Zhu, Y. Effect of Phase Structure of MnO2 Nanorod Catalyst on the Activity for CO Oxidation. J. Phys. Chem. C 2008, 112, 5307–5315. [Google Scholar] [CrossRef]
- Ede, S.R.; Ramadoss, A.; Anantharaj, S.; Nithiyanantham, U.; Kundu, S. Enhanced catalytic and supercapacitor activities of DNA encapsulated β-MnO2 nanomaterials. Phys. Chem. Chem. Phys. 2014, 16, 21846–21859. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Mo, S.; Li, Y.; Zhang, Y.; Wu, J.; Fu, M.; Niu, X.; Hu, Y.; Ye, D. Pt/MnOx for toluene mineralization via ozonation catalysis at low temperature: SMSI optimization of surface oxygen species. Chemosphere 2022, 286, 131754. [Google Scholar] [CrossRef] [PubMed]
- Mo, S.; Li, J.; Liao, R.; Peng, P.; Li, J.; Wu, J.; Fu, M.; Liao, L.; Shen, T.; Xie, Q.; et al. Unraveling the decisive role of surface CeO2 nanoparticles in the Pt-CeO2/MnO2 hetero-catalysts for boosting toluene oxidation: Synergistic effect of surface decorated and intrinsic O-vacancies. Chem. Eng. J. 2021, 418, 129399. [Google Scholar] [CrossRef]
- Liu, W.; Sheng, H.; Zhu, L.; Zhang, Y.; Liu, W.; Zhao, Y.; Li, Q.; Peng, Y.; Wang, Z. The preparation of ultrastable Al3+ doped CeO2 supported Au catalysts: Strong metal-support interaction for superior catalytic activity towards CO oxidation. J. Colloid Interface Sci. 2022, 627, 53–63. [Google Scholar] [CrossRef]
- He, K. In situ DRIFTS and TPD studies on surface properties affecting SO2-resistance of Pt/TiO2 catalyst in low-temperature CO oxidation. Surf. Sci. 2023, 734, 122315. [Google Scholar] [CrossRef]
- Xu, J.; Xu, X.-C.; Ouyang, L.; Yang, X.-J.; Mao, W.; Su, J.; Han, Y.-F. Mechanistic study of preferential CO oxidation on a Pt/NaY zeolite catalyst. J. Catal. 2012, 287, 114–123. [Google Scholar] [CrossRef]
- Meunier, F.C.; Tibiletti, D.; Goguet, A.; Reid, D.; Burch, R. On the reactivity of carbonate species on a Pt/CeO2 catalyst under various reaction atmospheres: Application of the isotopic exchange technique. Appl. Catal. A Gen. 2005, 289, 104–112. [Google Scholar] [CrossRef]
- Zhang, T.; Xu, J.; Sun, Y.; Fang, S.; Wu, Z.; Gao, E.; Zhu, J.; Wang, W.; Dai, L.; Liu, W.; et al. Exploring the key components of Au catalyst during CO oxidation using TG-MS and operando DRIFTS-MS. Mol. Catal. 2023, 547, 113361. [Google Scholar] [CrossRef]
- Koo, K.Y.; Jung, U.H.; Yoon, W.L. A highly dispersed Pt/γ-Al2O3 catalyst prepared via deposition–precipitation method for preferential CO oxidation. Int. J. Hydrogen Energy 2014, 39, 5696–5703. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).