Abstract
In order to develop a new type of antioxidants with high efficiency, a series of β-ionone thiazolylhydrazone derivatives were designed and synthesized from β-ionone, and their structures were characterized by 1H-NMR, 13C-NMR, FT-IR, and HR-MS. The antioxidant test in vitro indicated that most of the target compounds had high biological activity. Among them, compound 1k exhibited very strong DPPH (1,1-diphenyl-2-picrylhydrazyl radical)-scavenging activity with a half-maximal effective concentration (IC50) of 86.525 μM. Furthermore, in the ABTS (2,2-azinobis (3-ethylbenzothiazoline-6-sulfonate) diammonium salt)-scavenging experiment, compound 1m exhibited excellent activity with an IC50 of 65.408 μM. Their biological activities were significantly better than those of the positive control Trolox. These two compounds, which have good free-radical-scavenging activity in vitro, were used as representative compounds in the anti-browning experiment of fresh-cut potatoes. The results showed that 1k and 1m had the same anti-browning ability as kojic acid, and they were effective browning inhibitors. In addition, it is well known that microbial infection is one of the reasons for food oxidation. Therefore, we investigated the antifungal activity of 25 target compounds against eight plant fungi at a concentration of 125 mg/L. The results indicated that these compounds all have some antifungal activity and may become new potential fungicides. Notably, compound 1u showed the best inhibitory effect against Poria vaporaria, with an inhibition rate as high as 77.71%; it is expected to become the dominant structure for the development of new antifungal agents.
1. Introduction
Microbial contamination and nutrient oxidation are considered to be the two most important causes of food deterioration in the process of food processing, storage, transportation, and marketing, which seriously affect human health and economic benefits [1]. It is particularly important to take some measures to prevent the oxidation of fruits and vegetables with short shelf-life and easy oxidation. The browning reaction is the most common form of fruit and vegetable oxidation, which is mainly divided into enzymatic browning and non-enzymatic browning [2]. The most intuitive effect of browning involves adverse changes in the sensory characteristics of fruits and vegetables, and some cases of browning will even produce toxic substances, which will directly affect the acceptance of consumers, resulting in serious economic losses. Therefore, preventing the oxidation of fruits and vegetables during storage or processing has become the primary concern of the food industry, and the development and application of antioxidants have naturally become a current research focus [3].
Ionone, a monoterpene found in natural products [4], is an important raw material widely used in the fields of medicine and perfume [5]. Experimental results have shown that the β-isomer of ionone has good biological activity [6,7]. In the field of science, the thiazole hydrazone skeleton is a key pharmacophore as it is widely present in compounds with various biological properties [8,9,10,11,12]. Petrou [13] reviewed 124 relevant studies in the past 5 years and found that many natural and synthetic thiazoles and their derivatives show remarkable biological activities, such as antioxidant, analgesic, antifungal, anticancer, antiallergic, antihypertensive, anti-inflammatory, antimalarial, antifungal, and antipsychotic [14,15,16]. Due to the significant biological activities of thiazolidines, scientists are actively involved in the design of new thiazolidine derivatives.
In recent years, there have been an increasing number of studies on antioxidants, and Kauthale [17] and Djafarou [18] have conducted systematic studies on the antioxidant properties of thiazolhydrazone compounds. What is different is that the scavenging activity of the compounds against ABTS free radicals was also studied in this paper, and the effect of introducing more types of functional groups on the antioxidant activity of the compounds was studied. In the study of natural compounds, the biological activity of antioxidants is often characterized by directly eliminating free radicals or providing reducing power to counteract the oxidative stress caused by free radicals. Lee [19] evaluated the antioxidant activity of about 700 plant extracts using the DPPH free-radical-scavenging assay. The antioxidant activity of thiazole hydrazone compounds can be demonstrated by their ability to scavenge DPPH and ABTS radicals [20,21], which is expected to provide assistance in the development of antioxidant drugs [22].
Previous research in our laboratory demonstrated that the six membered cy-clic monoterpenoid thiazole derivatives have good biological activity [23]. In this paper, 25 compounds containing the structure of thiazole hydrazone were designed and synthesized using β-ionone as the lead compound. The newly synthesized thiazole hydrazone derivatives with different substituent groups were characterized by 1H-NMR, 13C-NMR, FT-IR, and HRMS. All compounds were tested for free-radical scavenging and antifungal activity in vitro, and the potential of these compounds for food preservation in vivo was evaluated using fresh-cut potato as an example. The synthesis and bioactivity testing of these compounds can provide some reference for research on food antioxidants and antifungal drugs.
2. Results and Discussion
2.1. Synthesis and Characterization
Compounds 1a~1y were synthesized according to the multi-step synthetic route described in Scheme 1. Specifically, β-ionone (2) was condensed with thiosemicarbazide to form intermediate 3. Then, intermediate 3 and 2-bromoacetophenone (4a~4y) were cyclized to synthesize thiazole hydrazone derivatives (1a~1y).

Scheme 1.
Synthesis of compounds 3 and 1a~1y.
All compounds were characterized by infrared spectroscopy in the range of 400–4000 cm−1. In compounds 1a~1y, the peak near 3200 cm−1 was caused by N–H stretching vibrations. The absorption peak of the C=N bond appeared around 1600 cm−1. The absorption peak of the C–S bond appeared around 1100 cm−1. The stretching vibration of the aromatic ring (C–H) was observed at around 3000 cm−1. In addition, the aromatic ring could be further confirmed by the continuous peak packets appearing in the shape of fingers at 1400–1600 cm−1. In the 1H-NMR spectrum of compounds, the chemical shift of the proton on the imino group appeared at about 12.49 ppm, presenting as a single peak. The protons of the aromatic rings (Ar–H) appeared in the range of about 7.85–7.45 ppm. In the spectrum simulated by MestReNova 14 program, these aromatic protons also showed similar chemical shifts and shapes. In addition, the chemical shift of the proton on the thiazole ring appeared at about 6.80 ppm. Lastly, chemical shifts of protons on saturated carbon appeared in the range of 2.36–1.02 ppm. In the 13C-NMR spectrum of compounds, it was obvious that the carbon on the benzene ring appeared in the range of 120–160 ppm. The three carbons in the thiazole ring appeared at about 169 ppm, 140 ppm, and 125 ppm, and the carbon atoms in the methyl group appeared at about 20–30 ppm. The remaining carbon peaks appeared around 14–40 ppm. From the above characterization data, the correctness of the compound structure could be basically established. In addition, the measured values of HRMS (ESI) of all compounds were generally consistent with the predicted values.
2.2. Antioxidant Activity
Free-radical-scavenging tests such as DPPH and ABTS are widely used to evaluate the antioxidant capacity of compounds, and they have become simple, rapid, and low-cost methods. Through regression analysis, it was found that there was a linear relationship between the concentration of compounds and the radical-scavenging rate, and the IC50 value was calculated using the obtained linear regression equation, enabling a comparison of the strength of the free-radical-scavenging ability of each compound. Trolox is a vitamin E antioxidant with strong scavenging capacity against DPPH and ABTS radicals with IC50 values of 108.334 and 91.897 μM, respectively. The experimental results exhibited that compounds 1a~1y had a good scavenging effect on the above two free radicals, and the correlation coefficients of the linear regression equations were all greater than 0.9, which indicated that their scavenging effect on free radicals would be enhanced with the increase in compound concentration.
As shown in Table 1 and Figure 1, eight compounds (1a, 1d, 1e, 1j, 1k, 1p, 1q, 1t) showed significantly better DPPH radical-scavenging activity than the positive control Trolox. In contrast to compound 1a, which contains no substituents, it was found that the introduction of functional groups such as hydroxyl, methyl, and halogen bromide favored DPPH scavenging. Among them, compound 1k showed the greatest potential scavenging activity on DPPH free radicals with an IC50 value of 86.525 μM. The introduction of different halogen atoms also significantly changed the scavenging activity of target compounds on DPPH free radicals. It is worth noting that the introduction of bromine atoms enhanced the DPPH-scavenging ability of the compounds, while the introduction of fluorine atoms and chlorine atoms weakened the DPPH-scavenging ability, decreasing more obviously with the increase in fluorine atoms and chlorine atoms. This result may be due to the fact that fluorine atoms and chlorine atoms absorb more electricity than bromine atoms. The nitro group is a strong electron-withdrawing group, and its introduction weakened the scavenging ability of target compounds to DPPH, further proving that the introduction of electron-withdrawing groups weakened the scavenging ability of target compounds to DPPH free radicals. In addition, phenolic hydroxyl groups can react with DPPH free radicals [24]; therefore, compounds 1k, 1e, and 1q containing phenolic hydroxyl groups had excellent DPPH free-radical-scavenging ability. However, when the hydroxyl group was replaced by methoxy group, the ability of the compound to scavenge DPPH radicals was greatly reduced [25].

Table 1.
Scavenging activity of DPPH radicals.
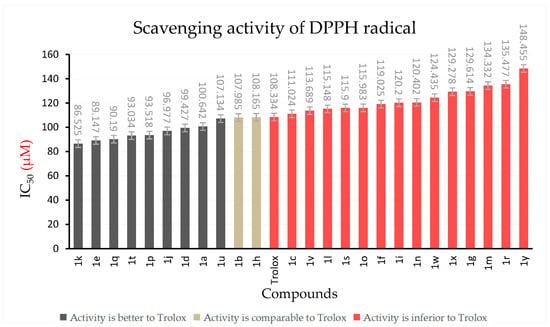
Figure 1.
Scavenging activity of compounds 1a~1y and Trolox against DPPH radicals.
As shown in Table 2 and Figure 2, 22 compounds (except 1a, 1p, and 1x) showed significantly better ABTS radical-scavenging activity than the positive control Trolox. Compared with compound 1a, it was found that the introduction of substituents could significantly enhance the ability of the compounds to scavenge ABTS free radicals. Among them, compound 1m had the strongest ABTS free-radical-scavenging activity, and its IC50 value was 65.408 μM. Compounds containing electron-donating groups (hydroxyl group, methyl group, and methoxy group) had a better ABTS radical-scavenging effect than other compounds, especially with regard to the methoxy group. In general, the effect of introducing halogen atoms on the ABTS radical-scavenging activity was slightly worse than that of other functional groups; however, when halogen atoms were introduced at the same position of the compound, compounds containing fluorine atoms were often the most active. In addition, it was found that, when the same functional group was introduced in the compound, introduction at the 3-position produced better ABTS free-radical-scavenging activity than at other positions.

Table 2.
Scavenging activity of ABTS radical.

Figure 2.
Scavenging activity of compounds 1a~1y and Trolox against ABTS radical.
The radical-scavenging activity of thiazole hydrazones may be related to the existence of N–H active groups in the hydrazone moiety, which has the ability to provide hydrogen atoms to eliminate free radicals [26]. The scavenging effect of these compounds on ABTS free radicals is better than that on DPPH free radicals, which may be due to the different scavenging mechanisms of the two radicals. The ABTS free-radical-scavenging mechanism of thiazole hydrazone compounds is based on hydrogen atom transfer (HAT), and the DPPH free radical-scavenging mechanism is based on hydrogen atom transfer (HAT) and single-electron transfer (SET) [24].
2.3. Anti-Browning Effect on Fresh-Cut Potatoes
In the process of food processing, the tissue damage of fresh fruits and vegetables leads to oxidative browning, and the degree of browning gradually deepens within a certain period. In order to verify whether these compounds have an anti-browning effect, compounds 1k and 1m, which showed good results in the free-radical-scavenging test in vitro, were selected for anti-browning experiments using fresh-cut potato slices. The experimental results showed that this effect was equivalent to that of the positive control kojic acid. It is evident from Figure 3 that the color of the potatoes gradually darkened with prolonged storage, indicating that browning occurred. In this study, L* and ΔE values were selected as key parameters to evaluate the anti-browning activity of compounds 1k and 1m on fresh-cut potato slices. As shown in Figure 4, the initial L* values of the experimental and control groups were statistically close or equal, both showing a downward trend with longer storage time. Compared with potato chips soaked in distilled water, kojic acid and other compounds added to the soaking solution had a significant effect on slowing the browning of potatoes. According to the degree of decrease in L* values, compounds 1k and 1m showed comparable browning resistance to kojic acid, with compound 1k showing slightly stronger browning resistance than kojic acid. According to the ΔE value calculated by the formula, a similar conclusion was presented. Combined with the experimental data and visual sensory evaluation, it can be concluded that these two compounds had a certain anti-browning effect on fresh-cut fruits and vegetables. Compounds containing similar structures are of great interest in the development of fruit and vegetable preservatives.
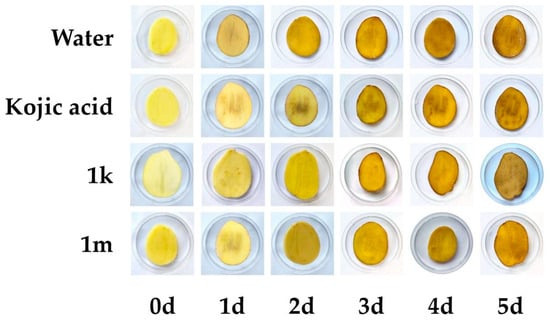
Figure 3.
Visual observation of browning at 20 °C for 5 days.

Figure 4.
L* value changes (A); ΔE value changes (B).
2.4. Antifungal Activity
The antifungal activities of compounds 1a~1y against eight fungi (Sphaeropsis sapinea, Colletotrichum acutatum, Rhizoctonia solani AG1, Coriolus versicolor, Poria vaporaria, Phytophthora parasitica var. nicotianae, Fusarium oxysporum f. sp. niveum, and Fusarium verticillioides) were evaluated at the concentration of 125 mg/L. These eight kinds of fungi are the most common plant pathogenic fungi affecting growth processes. The diameter of each colony was measured, and the inhibition rate was calculated. The broad-spectrum fungicide azoxystrobin was used as a positive control, and the results are shown in Table 3. All compounds had a certain antifungal activity.

Table 3.
Antifungal activity of compounds 1a~1y.
Importantly, the results obtained in our study suggested that compounds with fluorine-containing substituents (1b, 1h, 1n, 1u, 1w) possessed potent antifungal activities against most of the fungi, especially against Poria vaporaria fungi, all of which showed inhibition rates of more than 45%. It has been reported that fluorine atoms can effectively enhance the stability and biological activity of compounds in many aspects owing to their small atomic radius and strong electronegativity, thus improving the hydrophobicity and liposolubility of compounds [27,28,29,30]. Compounds containing nitro and methoxy groups (1f, 1g, 1l, 1m, 1r, 1s, 1y) usually had less effective antifungal effects on the eight kinds of fungi. In addition, the inhibition rate of target compounds 1n and 1u on Poria vaporaria was over 75%, which indicated that it had the potential to control tree rot. Therefore, compounds 1n and 1u could be used as a lead compound to control tree rot, which deserves further study. Our research also found that these compounds have relatively weak inhibitory effects on three fungi: Coriolus versicolor, Fusarium oxysporum f. sp. niveum, and Fusarium verticillioides.
3. Experimental
3.1. Materials and Methods
All chemicals were reagent-grade and without purification purchased from Xi-Long Co. (Shantou, China) and Aladdin. All plant fungi were provided by the Plant Pathology Laboratory of Agricultural College of Jiangxi Agricultural University. FT-IR spectra of the samples were performed between 400 and 4000 cm−1 from KBr pellets on. 1H-NMR and 13C-NMR spectra were carried out by AV 400 MHz spectrometer (CDCl3 as solvents). High-resolution mass spectrometry (HRMS) spectra were recorded on a triple time-of-flight TOF 5600+ (AB Sciex) mass spectrometer (Concord, ON, Canada). Absorbance was measured using a microplate reader (Multikan-FC). Compound melting points were determined using a melting point apparatus (44X-6T). The color change of potato samples was recorded using a colorimetric spectrophotometer (LS171, Linshang, Shenzhen, China).
3.2. Synthesis
3.2.1. (E)-2-((E)-4-(2,6,6-Trimethylcyclohex-1-en-1-yl)but-3-en-2-ylidene)hydrazine-1-carbothioamide (3)
β-Ionone (2) (1 eq, 20 mmol, 3.85 g) and thiosemicarbazide (1.2 eq, 24 mmol, 2.19 g) were introduced in a flask and dissolved in ethanol; then, diluted hydrochloric acid (2.87 mol, 3 mL) was added and refluxed at 80 °C for 1 h. The reaction was monitored by TLC. When the reaction was completed, the obtained yellow solid was filtered [31]. It was recrystallized from 70% ethanol. After filtration and drying, compound 3 was obtained: m.p. 130–133 °C decomposition; yield: 86.84% (4.62 g); FT-IR v (cm−1): 3208 (v N–H), 3027 (v C=C-H), 2814 (v C–H), 1603 (v C–N), 1088 (v C=S) cm−1; 1H-NMR (400 MHz, CDCl3) δ 8.68 (s, 1H, NH), 6.61 (d, J = 16.55 Hz, 1H, NH), 6.44–6.32 (m, 1H, 4-CH), 6.16–6.06 (m, 1H, 3-CH), 2.03 (d, J = 2.15 Hz, 5H, 1-CH3, 3′-CH2), 1.71 (s, 3H, 7′-CH3), 1.61 (d, J = 6.19 Hz, 2H, 4′-CH2), 1.47 (dd, J = 2.34, 6.13 Hz, 2H, 5′-CH2), 1.03 (s, 6H, 8′-CH3, 9′-CH3). 13C-NMR (101 MHz, CDCl3) δ 178.94 (C1″), 149.11 (C2), 136.69 (C4), 134.76 (C1′), 131.96 (C2′), 120.63 (C3), 39.59 (C5′), 34.19 (C6′), 33.16 (C3′), 28.88 (C8′, C9′), 21.67 (C7′), 19.07 (C4′), 11.13 (C1). HRMS calculated for C14H23N3S [M + H]+ 266.1613, found 266.1673.
3.2.2. β-Ionone Thiazole Hydrazone Derivatives (1a~1y)
The synthesis methods were modified from Chen [31] and Babu [32]. Briefly, the above condensation product 3 (1 eq, 20 mmol, 5.32 g) was introduced in a flask and dissolved in ethanol. To this solution, 2-bromoacetophenone (1.2 eq, 24 mmol) was added, and the mixture was stirred at 50 °C. The reaction was monitored by TLC. When completion of the reaction was reached, it was recrystallized from 70% ethanol. After filtration and drying, compound β-ionone thiazole hydrazone derivatives (1a~1y) were obtained. All the obtained results indicated that compounds 1a~1y were successfully synthesized in this study (see Supplementary Materials).
3.3. Antioxidant Activity
3.3.1. DPPH Assay
The antioxidant activities of the compounds were measured by measuring the change in DPPH radical absorbance, referring to the methods in [33,34]. DPPH was weighed accurately, and 100 mL of an ethanol solution of DPPH (200 μM) was shaken and stored at room temperature in the dark. Then quantities of the compounds were weighed to prepare test solutions with different concentrations (200, 160, 120, 80, 40 μM). Then, 200 μL of DPPH–ethanol solution taken accurately was mixed with 50 μL of different concentrations of test solutions. After incubation in the dark for 30 min, the absorbance of the mixed solution was recorded at 517 nm wavelength as A2. In addition, 200 μL of DPPH solution and 50 μL of ethanol were mixed as blank, and the absorbance A0 was measured. Lastly, 200 μL of ethanol and 50 μL of the test solutions was mixed as a control, and the absorbance A1 was measured. The calculation formula used for the determination of the DPPH radical-scavenging rate was as follows:
where A0 is the measured absorbance of ethanol and DPPH, A1 is the measured absorbance of test solution and ethanol, and A2 is the measured absorbance of test solution and DPPH.
3.3.2. ABTS Assay
The scavenging effect of the compounds on ABTS free radicals was determined, referring to the methods in [35,36,37]. ABTS solution (7 mmol) and K2S2O8 solution (2.45 mmol) were prepared with ultrapure water, mixed in equal volume, and placed at 4 °C in the dark for 24 h. The stock solution was valid for 2 days. The ABTS solution was diluted with ethanol to an absorbance of 0.7 ± 0.02 Abs at 734 nm. Then, quantities of the compounds were weighed to prepare test solutions with different concentrations (200, 160, 120, 80, 40 μM). A volume of 200 μL of ABTS solution was mixed with 50 μL of different concentrations of test solutions. After incubation in the dark for 10 min, the absorbance of the mixed solution was recorded at 734 nm. In addition, 200 μL of ABTS solution and 50 μL of ethanol were mixed as the blank, and the absorbance A0 was measured. Then, 200 μL of ethanol and 50 μL of the test solutions were mixed as a control, and the absorbance A1 was measured. The calculation formula used for determination of the ABTS radical\-scavenging rate was as follows:
where A0 is the measured absorbance of ethanol and ABTS, A1 is the measured absorbance of test solution and ethanol, and A2 is the measured absorbance of test solution and ABTS.
3.4. Anti-Browning Effect on Fresh-Cut Potatoes
Potatoes were purchased from local supermarkets and were of similar size, without damage or disease. The washed potatoes were cut into thin slices about 5 mm thick and completely immersed in the prepared solution (500 μM) for 5 min. They were drained and placed in sterile Petri dishes and stored at 20 °C. Distilled water and kojic acid were selected as control groups. The L* (lightness), a* (red-green), and b* (yellow-blue) values of potatoes at different times were measured using a colorimetric spectrophotometer [18,38]. To increase the confidence of the experimental results, all experiments were repeated several times. The total color difference (ΔE) was calculated as follows:
ΔE = [(Lt* − Linitial*)2 + (at* − ainitial*)2 + (bt* − binitial*)2]0.5
3.5. Antifungal Activity
In this research, eight plant fungi, namely, Sphaeropsis sapinea, Colletotrichum acutatum, Rhizoctonia solani AG1, Coriolus versicolor, Poria vaporaria, Phytophthora parasitica var. nicotianae, Fusarium oxysporum f. sp. Niveum and Fusarium verticillioides were used to determine the antifungal activity of the compounds according to the mycelial growth rate method. The compounds were dissolved in dimethyl sulfoxide to generate stock solutions (1.0 × 104 mg/L), and then were further diluted to 125 mg/L on PDA plates. After PDA solidification, an inoculation loop was used to take 5 mm diameter fungus cakes from the edge of the 7-day-cultured pathogenic fungi colony. The fungus cake was moved to the center of the petri dishes, with the hyphae facing down; after the treatment, the petri dishes were placed in a constant-temperature biochemical incubator at 28 °C for cultivation, and the growth diameter of the hyphae was measured after 3–10 days. As a positive control, azoxystrobin (antifungal agent) was used, while the solution without the test compound was used as a blank control. All experiments were independently repeated three times [39,40].
4. Conclusions
In this paper, 25 new unreported thiazole hydrazone compounds were successfully synthesized using β-ionone as the main raw material and verified by a series of characterization data. In the in vitro antioxidant experiments, all target compounds showed a good ability to scavenge free radicals. Compared with the positive control Trolox, it was found that eight compounds were more effective in scavenging DPPH free radicals, and 22 compounds were more effective in scavenging ABTS free radicals. This result may be closely related to the structure of hydrazone in the target compounds. In the anti-browning experiment of freshly cut potatoes, compounds 1k and 1m showed similar anti-browning effects to kojic acid, and compound 1k was even slightly more effective than kojic acid. This result further confirmed that the target compounds had excellent antioxidant activity. Although most compounds showed weaker effects than the positive control in antifungal experiments, there are few antifungal agents with this structure on the market. It is of great significance for the development of antifungal agents to further optimize this structure and improve the efficacy. To sum up, the excellent antioxidant properties of these target products are of great significance for developing more efficient antioxidants.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules28186713/s1.
Author Contributions
C.H., methodology, validation, investigation, data curation, writing—original draft, and writing—review and editing; Y.Z., data analysis; R.Z., investigation; J.W., conceptualization and methodology; Q.F., data analysis; S.X., data analysis; J.Z., writing—review and editing; Z.W., conceptualization and methodology; D.P., conceptualization, methodology, and project administration; S.C., writing—review and editing, visualization, supervision, project administration, and funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation (No. 32160660, No. 31960295 and No. 21562022), the Jiangxi Province Academic and Technical Leaders Training Program Leading Talents Project (20204BCJ22022), Special Funding for Major Scientific and Technological Research and Development in Jiangxi Province (20203ABC28W016), the Special Research Project on Camphor Tree (KRPCT) of Jiangxi Forestry Department (grant no. 2020CXZX07), the Research Foundation of Jiangxi Provincial Drug Administration [2021-03 and 2022-03], the Earmarked Fund for the Youthful Innovation Research Team of Jiangxi Agricultural University-05.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Not applicable.
References
- Sun, X.; Wang, J.; Dong, M.; Zhang, H.; Li, L.; Wang, L. Food spoilage, bioactive food fresh-keeping films and functional edible coatings: Research status, existing problems and development trend. Trends Food Sci. Technol. 2022, 119, 122–132. [Google Scholar] [CrossRef]
- Harasym, J.; Oledzki, R. Effect of fruit and vegetable antioxidants on total antioxidant capacity of blood plasma. Nutrition 2014, 30, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Suri, K.; Shevkani, K.; Kaur, A.; Kaur, A.; Singh, N. Enzymatic browning of fruit and vegetables: A review. Enzym. Food Technol. Improv. Innov. 2018, 63–78. [Google Scholar] [CrossRef]
- Yang, J.; Mu, W.-W.; Cao, Y.-X.; Liu, G.-Y. Synthesis and biological evaluation of β-ionone oriented proapoptosis agents by enhancing the ROS generation. Bioorganic Chem. 2020, 104, 104273. [Google Scholar] [CrossRef]
- Hu, L.; Du, M.; Zhang, J.; Wang, Y. Chemistry of the main component of essential oil of Litsea cubeba and its derivatives. Open J. For. 2014, 4, 457. [Google Scholar]
- Paparella, A.; Shaltiel-Harpaza, L.; Ibdah, M. β-Ionone: Its occurrence and biological function and metabolic engineering. Plants 2021, 10, 754. [Google Scholar] [CrossRef]
- Grabarczyk, M.; Wińska, K.; Mączka, W.; Żarowska, B.; Maciejewska, G.; Dancewicz, K.; Gabryś, B.; Anioł, M. Synthesis, biotransformation and biological activity of halolactones obtained from β-ionone. Tetrahedron 2016, 72, 637–644. [Google Scholar] [CrossRef]
- Adole, V.A.; More, R.A.; Jagdale, B.S.; Pawar, T.B.; Chobe, S.S. Efficient Synthesis, Antibacterial, Antifungal, Antioxidant and Cytotoxicity Study of 2-(2-Hydrazineyl)thiazole Derivatives. ChemistrySelect 2020, 5, 2778–2786. [Google Scholar] [CrossRef]
- Ayati, A.; Emami, S.; Asadipour, A.; Shafiee, A.; Foroumadi, A. Recent applications of 1, 3-thiazole core structure in the identification of new lead compounds and drug discovery. Eur. J. Med. Chem. 2015, 97, 699–718. [Google Scholar] [CrossRef]
- Al-Omair, M.A.; Sayed, A.R.; Youssef, M.M. Synthesis and Biological Evaluation of Bisthiazoles and Polythiazoles. Molecules 2018, 23, 1133. [Google Scholar] [CrossRef]
- Mohammad, H.; Reddy, P.N.; Monteleone, D.; Mayhoub, A.S.; Cushman, M.; Hammac, G.K.; Seleem, M.N. Antibacterial characterization of novel synthetic thiazole compounds against methicillin-resistant Staphylococcus pseudintermedius. PLoS ONE 2015, 10, e0130385. [Google Scholar] [CrossRef]
- Kumar, S.; Saini, V.; Maurya, I.K.; Sindhu, J.; Kumari, M.; Kataria, R.; Kumar, V. Design, synthesis, DFT, docking studies and ADME prediction of some new coumarinyl linked pyrazolylthiazoles: Potential standalone or adjuvant antimicrobial agents. PLoS ONE 2018, 13, e0196016. [Google Scholar] [CrossRef] [PubMed]
- Petrou, A.; Fesatidou, M.; Geronikaki, A. Thiazole Ring-A Biologically Active Scaffold. Molecules 2021, 26, 3166. [Google Scholar] [CrossRef] [PubMed]
- Makam, P.; Thakur, P.K.; Kannan, T. In vitro and in silico antimalarial activity of 2-(2-hydrazinyl) thiazole derivatives. Eur. J. Pharm. Sci. 2014, 52, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Pattnaik, S.; Pathak, K.; Kumar, S.; Pathak, D.; Jain, S.; Vaidya, A. Anticancer potential of thiazole derivatives: A retrospective review. Mini Rev. Med. Chem. 2018, 18, 640–655. [Google Scholar] [CrossRef] [PubMed]
- Łączkowski, K.Z.; Konklewska, N.; Biernasiuk, A.; Malm, A.; Sałat, K.; Furgała, A.; Dzitko, K.; Bekier, A.; Baranowska-Łączkowska, A.; Paneth, A. Thiazoles with cyclopropyl fragment as antifungal, anticonvulsant, and anti-Toxoplasma gondii agents: Synthesis, toxicity evaluation, and molecular docking study. Med. Chem. Res. 2018, 27, 2125–2140. [Google Scholar] [CrossRef]
- Kauthale, S.; Tekale, S.; Damale, M.; Sangshetti, J.; Pawar, R. Synthesis, antioxidant, antifungal, molecular docking and ADMET studies of some thiazolyl hydrazones. Bioorganic Med. Chem. Lett. 2017, 27, 3891–3896. [Google Scholar] [CrossRef]
- Djafarou, S.; Mermer, A.; Barut, B.; Yılmaz, G.T.; Amine Khodja, I.; Boulebd, H. Synthesis and evaluation of the antioxidant and anti-tyrosinase activities of thiazolyl hydrazone derivatives and their application in the anti-browning of fresh-cut potato. Food Chem. 2023, 414, 135745. [Google Scholar] [CrossRef]
- Lee, S.K.; Mbwambo, Z.; Chung, H.; Luyengi, L.; Gamez, E.; Mehta, R.; Kinghorn, A.; Pezzuto, J. Evaluation of the antioxidant potential of natural products. Comb. Chem. High Throughput Screen. 1998, 1, 35–46. [Google Scholar] [CrossRef]
- Li, X.; Huang, J.; Wang, Z.; Jiang, X.; Yu, W.; Zheng, Y.; Li, Q.; He, N. Alkaline extraction and acid precipitation of phenolic compounds from longan (Dimocarpus longan L.) seeds. Sep. Purif. Technol. 2014, 124, 201–206. [Google Scholar] [CrossRef]
- Adelakun, O.E.; Kudanga, T.; Parker, A.; Green, I.R.; le Roes-Hill, M.; Burton, S.G. Laccase-catalyzed dimerization of ferulic acid amplifies antioxidant activity. J. Mol. Catal. B Enzym. 2012, 74, 29–35. [Google Scholar] [CrossRef]
- Borcea, A.M.; Ionut, I.; Crisan, O.; Oniga, O. An Overview of the Synthesis and Antimicrobial, Antiprotozoal, and Antitumor Activity of Thiazole and Bisthiazole Derivatives. Molecules 2021, 26, 624. [Google Scholar] [CrossRef]
- Duan, X.; Zhang, L.; Si, H.; Song, J.; Wang, P.; Chen, S.; Luo, H.; Rao, X.; Wang, Z.; Liao, S. Synthesis, Antifungal Activity, Cytotoxicity and QSAR Study of Camphor Derivatives. J. Fungi 2022, 8, 762. [Google Scholar] [CrossRef] [PubMed]
- Giri, L.; Belwal, T.; Bahukhandi, A.; Suyal, R.; Bhatt, I.D.; Rawal, R.S.; Nandi, S.K. Oxidative DNA damage protective activity and antioxidant potential of Ashtvarga species growing in the Indian Himalayan Region. Ind. Crops Prod. 2017, 102, 173–179. [Google Scholar] [CrossRef]
- Jianqing, Z.; Bo, L.; Qi, J.; Yiming, L.; Weiliang, Z.; Kaixian, C. Advances in the structure-activity relationship study of natural flavonoids and its derivatives. Acta Pharm. Sin. 2011, 46, 622–630. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, Y.; Zhao, Y.; Ma, C.; Xu, X.; Gu, W.; Yang, Y.; Wang, S. Design, Synthesis and Antioxidant Application of Camphorsulfonic Acid Thiazolylhydrazone Derivatives. Chin. J. Org. Chem. 2019, 39, 2616–2624. [Google Scholar] [CrossRef]
- Wang, Q.Q.; Zhang, S.G.; Jiao, J.; Dai, P.; Zhang, W.H. Novel Fluorinated 7-Hydroxycoumarin Derivatives Containing an Oxime Ether Moiety: Design, Synthesis, Crystal Structure and Biological Evaluation. Molecules 2021, 26, 372. [Google Scholar] [CrossRef]
- Jayprakash, R.C.; Vinit, R.; Jin-Hyung, L.; Jintae, L. Antifungal activities of fluoroindoles against the postharvest pathogen Botrytis cinerea: In vitro and in silico approaches. Int. J. Food Microbiol. 2022, 362, 109492. [Google Scholar]
- Chen, A.P.-C.; Chen, Y.-H.; Liu, H.-P.; Li, Y.-C.; Chen, C.-T.; Liang, P.-H. Synthesis and application of a fluorescent substrate analogue to study ligand interactions for undecaprenyl pyrophosphate synthase. J. Am. Chem. Soc. 2002, 124, 15217–15224. [Google Scholar] [CrossRef]
- Ferreira, M.L.; Pastoriza-Gallego, M.J.; Araujo, J.M.; Canongia Lopes, J.N.; Rebelo, L.P.N.; M. Piñeiro, M.; Shimizu, K.; Pereiro, A.B. Influence of nanosegregation on the phase behavior of fluorinated ionic liquids. J. Phys. Chem. C 2017, 121, 5415–5427. [Google Scholar] [CrossRef]
- Chen, Z.; Duan, W.; Lin, G.; Zhang, R.; Luo, M.; Yang, Z. Synthesis and antifungal activity of novel myrtenal-based thiazole-hydrazone compounds. Sci. Silvae Sin. 2017, 53, 93–101. [Google Scholar]
- Babu, B.H.; Vijay, K.; Murali Krishna, K.B.; Sharmila, N.; Ramana, M.B. An efficient PEG-400 mediated catalyst free green synthesis of 2-amino-thiazoles from α-diazoketones and thiourea. J. Chem. Sci. 2016, 128, 1475–1478. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.-E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Nagarajan, S.; Nagarajan, R.; Kumar, J.; Salemme, A.; Togna, A.R.; Saso, L.; Bruno, F. Antioxidant Activity of Synthetic Polymers of Phenolic Compounds. Polymers 2020, 12, 1646. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhang, X.; Liu, Q.; Lin, Y.; Zhang, Z.; Li, S. Study on Extraction and Antioxidant Activity of Flavonoids from Hemerocallis fulva (Daylily) Leaves. Molecules 2022, 27, 2916. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Wang, W.; Wu, M.; Fang, Y.; Wang, S.; Yang, Y.; Ye, C.; Xiang, F. Antioxidant and antibacterial activities of essential oil from Atractylodes lancea rhizomes. Ind. Crops Prod. 2020, 153, 112552. [Google Scholar] [CrossRef]
- Osipova, V.; Polovinkina, M.; Gracheva, Y.; Shpakovsky, D.; Osipova, A.; Berberova, N. Antioxidant activity of some organosulfur compounds in vitro. Arab. J. Chem. 2021, 14, 103068. [Google Scholar] [CrossRef]
- Peng, Z.; Li, Y.; Tan, L.; Chen, L.; Shi, Q.; Zeng, Q.-H.; Liu, H.; Wang, J.J.; Zhao, Y. Anti-tyrosinase, antioxidant and antibacterial activities of gallic acid-benzylidenehydrazine hybrids and their application in preservation of fresh-cut apples and shrimps. Food Chem. 2022, 378, 132127. [Google Scholar] [CrossRef]
- Sabt, A.; Abdelrahman, M.T.; Abdelraof, M.; Rashdan, H.R. Investigation of Novel Mucorales Fungal Inhibitors: Synthesis, In-Silico Study and Anti-Fungal Potency of Novel Class of Coumarin-6-Sulfonamides-Thiazole and Thiadiazole Hybrids. ChemistrySelect 2022, 7, e202200691. [Google Scholar] [CrossRef]
- Sirsat, D.M.; Bhale, P.S.; Chavan, H.V.; Karape, S.M.; Bachute, M.T. Synthesis and Anti-Microbial, Anti-Oxidant and Anti-Inflammatory Activities of Thiazole-Pyrazole Based Pyrimidine Derivatives. Rasayan J. Chem. 2020, 13, 1589–1597. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).