Influence of Different Types, Utilization Times, and Volumes of Aging Barrels on the Metabolite Profile of Red Wine Revealed by 1H-NMR Metabolomics Approach
Abstract
1. Introduction
2. Results and Discussion
2.1. Acidity and Major Chemical Composition of Wine Samples
2.2. 1H-NMR Data Acquisition and Metabolite Identification
2.3. Overall Comparative Metabolite Profiling of Wine Samples
2.4. Influence of Aging Container Type on the Metabolite Profiles of Wine Samples
2.5. Influence of Barrel Age on the Metabolite Profiles of Wine Samples
2.6. Influence of Aging Barrel Volume on the Metabolite Profiles of Wine Samples
3. Materials and Methods
3.1. Wine Samples
3.2. Determination of pH and Titratable Acidity
3.3. Determination of Alcoholic Strength by Volume
3.4. Determination of Residual Sugar Content
3.5. Determination of Total Phenolic Content
3.6. Sample Preparation and 1H-NMR Analysis
3.7. 1H-NMR Spectra Processing and Data Acquisition
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Jackson, R.S. Wine Science: Principles and Applications, 5th ed.; Elsevier Science: Amsterdam, The Netherlands, 2020; pp. 1–20, 573–723. [Google Scholar]
- Amarando, M.; Assenov, I.; Visuthismajarn, P. Sustainable wine tourism and vineyards’ environmental consciousness in Thailand. Afr. J. Hosp. Tour. Leis. 2019, 8, 1–13. [Google Scholar]
- Chong, K.L. Thailand wine tourism: A dream or a reality? Asia Pac. J. Tour. Res. 2017, 22, 604–614. [Google Scholar] [CrossRef][Green Version]
- Alañón, M.E.; Pérez-Coello, M.S.; Marina, M.L. Wine science in the metabolomics era. TrAC Trends Anal. Chem. 2015, 74, 1–20. [Google Scholar] [CrossRef]
- Sáenz-Navajas, M.P.; Jeffery, D.W. Perspectives on wines of provenance: Sensory typicality, quality, and authenticity. ACS Food Sci. Technol. 2021, 1, 986–992. [Google Scholar] [CrossRef]
- Popîrdă, A.; Luchian, C.E.; Cotea, V.V.; Colibaba, L.C.; Scutarașu, E.C.; Toader, A.M. A review of representative methods used in wine authentication. Agriculture 2021, 11, 225. [Google Scholar] [CrossRef]
- Mao, I.L.; Da Costa, G.; Richard, T. 1H-NMR metabolomics for wine screening and analysis. OENO One 2023, 57, 15–31. [Google Scholar] [CrossRef]
- Wishart, D.S. Metabolomics: Applications to food science and nutrition research. Trends Food Sci. Technol. 2008, 19, 482–493. [Google Scholar] [CrossRef]
- Pinu, F.R. Grape and wine metabolomics to develop new insights using untargeted and targeted approaches. Fermentation 2018, 4, 92. [Google Scholar] [CrossRef]
- Valls Fonayet, J.; Loupit, G.; Richard, T. Chapter 10 MS- and NMR-metabolomic tools for the discrimination of wines: Applications for authenticity. In Advances in Botanical Research; Pétriacq, P., Bouchereau, A., Eds.; Academic Press: Cambridge, MA, USA, 2021; Volume 98, pp. 297–357. [Google Scholar]
- Bordet, F.; Roullier-Gall, C.; Ballester, J.; Vichi, S.; Quintanilla-Casas, B.; Gougeon, R.D.; Julien-Ortiz, A.; Kopplin, P.S.; Alexandre, H. Different wines from different yeasts? “Saccharomyces cerevisiae intraspecies differentiation by metabolomic signature and sensory patterns in wine”. Microorganisms 2021, 9, 2327. [Google Scholar] [CrossRef]
- Lee, J.E.; Hong, Y.S.; Lee, C.H. Characterization of fermentative behaviors of lactic acid bacteria in grape wines through 1H-NMR- and GC-based metabolic profiling. J. Agric. Food Chem. 2009, 57, 4810–4817. [Google Scholar] [CrossRef]
- Liu, S.; Lou, Y.; Li, Y.; Zhao, Y.; Laaksonen, O.; Li, P.; Zhang, J.; Battino, M.; Yang, B.; Gu, Q. Aroma characteristics of volatile compounds brought by variations in microbes in winemaking. Food Chem. 2023, 420, 136075. [Google Scholar] [CrossRef] [PubMed]
- Arapitsas, P.; Speri, G.; Angeli, A.; Perenzoni, D.; Mattivi, F. The influence of storage on the “chemical age” of red wines. Metabolomics 2014, 10, 816–832. [Google Scholar] [CrossRef]
- Flamini, R.; Panighel, A.; De Marchi, F. Mass spectrometry in the study of wood compounds released in the barrel-aged wine and spirits. Mass Spectrom. Rev. 2023, 42, 1174–1220. [Google Scholar] [CrossRef]
- Rosso, M.D.; Panighel, A.; Vedova, A.D.; Stella, L.; Flamini, R. Changes in chemical composition of a red wine aged in acacia, cherry, chestnut, mulberry, and oak wood barrels. J. Agric. Food Chem. 2009, 57, 1915–1920. [Google Scholar] [CrossRef] [PubMed]
- Herrero, P.; Sáenz-Navajas, M.P.; Avizcuri, J.M.; Culleré, L.; Balda, P.; Antón, E.C.; Ferreira, V.; Escudero, A. Study of Chardonnay and Sauvignon blanc wines from D.O.Ca Rioja (Spain) aged in different French oak wood barrels: Chemical and aroma quality aspects. Food Res. Int. 2016, 89, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Zamora, F. Chapter 9—Barrel Aging: Types of Wood. In Red Wine Technology, 1st ed.; Morata, A., Ed.; Academic Press: London, UK, 2019; pp. 125–147. [Google Scholar]
- Carpena, M.; Pereira, A.G.; Prieto, M.A.; Simal-Gandara, J. Wine aging technology: Fundamental role of wood barrels. Foods 2020, 9, 1160. [Google Scholar] [CrossRef]
- Wang, C.; Wang, C.; Tang, K.; Rao, Z.; Chen, J. Effects of different aging methods on the phenolic compounds and antioxidant activity of red wine. Fermentation 2022, 8, 592. [Google Scholar] [CrossRef]
- Maioli, F.; Picchi, M.; Guerrini, L.; Parenti, A.; Domizio, P.; Andrenelli, L.; Zanoni, B.; Canuti, V. Monitoring of Sangiovese red wine chemical and sensory parameters along one-year aging in different tank materials and glass bottle. ACS Food Sci. Technol. 2022, 2, 221–239. [Google Scholar] [CrossRef]
- Garde-Cerdán, T.; Ancín-Azpilicueta, C. Review of quality factors on wine ageing in oak barrels. Trends Food Sci. Technol. 2006, 17, 438–447. [Google Scholar] [CrossRef]
- Nunes, P.; Muxagata, S.; Correia, A.C.; Nunes, F.M.; Cosme, F.; Jordão, A.M. Effect of oak wood barrel capacity and utilization time on phenolic and sensorial profile evolution of an Encruzado white wine. J. Sci. Food Agric. 2017, 97, 4847–4856. [Google Scholar] [CrossRef]
- Pérez-Prieto, L.J.; López-Roca, J.M.; Martínez-Cutillas, A.; Pardo Mínguez, F.; Gómez-Plaza, E. Maturing wines in oak barrels. Effects of origin, volume, and age of the barrel on the wine volatile composition. J. Agric. Food Chem. 2002, 50, 3272–3276. [Google Scholar] [CrossRef] [PubMed]
- Gougeon, L.; Da Costa, G.; Le Mao, I.; Ma, W.; Teissedre, P.L.; Guyon, F.; Richard, T. Wine analysis and authenticity using 1H-NMR metabolomics data: Application to Chinese wines. Food Anal. Methods 2018, 11, 3425–3434. [Google Scholar] [CrossRef]
- Mascellani, A.; Hoca, G.; Babisz, M.; Krska, P.; Kloucek, P.; Havlik, J. 1H-NMR chemometric models for classification of Czech wine type and variety. Food Chem. 2021, 339, 127852. [Google Scholar] [CrossRef] [PubMed]
- Gougeon, L.; da Costa, G.; Guyon, F.; Richard, T. 1H-NMR metabolomics applied to Bordeaux red wines. Food Chem. 2019, 301, 125257. [Google Scholar] [CrossRef] [PubMed]
- Le Mao, I.; Da Costa, G.; Bautista, C.; de Revel, G.; Richard, T. Application of 1H-NMR metabolomics to French sparkling wines. Food Control 2023, 145, 109423. [Google Scholar] [CrossRef]
- López-Rituerto, E.; Sørensen, K.M.; Savorani, F.; Engelsen, S.B.; Avenoza, A.; Peregrina, J.M.; Busto, J.H. Monitoring of the Rioja red wine production process by 1H-NMR spectroscopy. J. Sci. Food Agric. 2022, 102, 3808–3816. [Google Scholar] [CrossRef]
- Solovyev, P.A.; Fauhl-Hassek, C.; Riedl, J.; Esslinger, S.; Bontempo, L.; Camin, F. NMR spectroscopy in wine authentication: An official control perspective. Compr. Rev. Food. Sci. Food Saf. 2021, 20, 2040–2062. [Google Scholar] [CrossRef]
- Luangwilai, M.; Duangmal, K.; Chantaprasarn, N.; Settachaimongkon, S. Comparative metabolite profiling of raw milk from subclinical and clinical mastitis cows using 1H-NMR combined with chemometric analysis. Int. J. Food Sci. Technol. 2021, 56, 493–503. [Google Scholar] [CrossRef]
- Phuenpong, T.; Kongboonkird, M.; Duangmal, K.; Lerdvorasap, W.; Suksawwawimon, M.; Mekboonsonglarp, W.; Nuamchit, J.; Chantaprasarn, N.; Settachaimongkon, S. Molecular discrimination between organic and conventional liquid milk products in Thailand using 1H-NMR metabolomics approach. Trop. Anim. Sci. J. 2021, 44, 478–488. [Google Scholar] [CrossRef]
- Lv, Y.; Ma, F.L.; Wang, J.N.; Zhang, Y.; Jiang, Y.; Ge, Q.; Yu, Y.J. A strategy for differentiating oak barrel aged and non-oak barrel aged wines by using UHPLC–HRMS combined with chemometrics. Chemosensors 2023, 11, 165. [Google Scholar] [CrossRef]
- Navarro, M.; Kontoudakis, N.; Gómez-Alonso, S.; García-Romero, E.; Canals, J.M.; Hermosín-Gutíerrez, I.; Zamora, F. Influence of the volatile substances released by oak barrels into a Cabernet Sauvignon red wine and a discolored Macabeo white wine on sensory appreciation by a trained panel. Eur. Food Res. Technol. 2018, 244, 245–258. [Google Scholar] [CrossRef]
- Casassa, L.F.; Ceja, G.M.; Vega-Osorno, A.; du Fresne, F.; Llodrá, D. Detailed chemical composition of Cabernet Sauvignon wines aged in French oak barrels coopered with three different stave bending techniques. Food Chem. 2021, 340, 127573. [Google Scholar] [CrossRef] [PubMed]
- Morata, A.; Bañuelos, M.A.; López, C.; Song, C.; Vejarano, R.; Loira, I.; Palomero, F.; Suarez Lepe, J.A. Use of fumaric acid to control pH and inhibit malolactic fermentation in wines. Food Addit. Contam.—Part A 2020, 37, 228–238. [Google Scholar] [CrossRef]
- Martínez-Gil, A.; Del Alamo-Sanza, M.; Nevares, I. Evolution of red wine in oak barrels with different oxygen transmission rates. Phenolic compounds and colour. LWT 2022, 158, 113133. [Google Scholar] [CrossRef]
- Gutiérrez-Gamboa, G.; Garde-Cerdán, T.; Moreno-Simunovic, Y.; Pérez-Álvarez, E.P. 10—Amino acid composition of grape juice and wine: Principal factors that determine its content and contribution to the human diet. In Nutrients in Beverages, 1st ed.; Grumezescu, A.M., Holban, A.M., Eds.; Academic Press: London, UK, 2019; pp. 369–391. [Google Scholar]
- Waterhouse, A.L.; Miao, Y. Can chemical analysis predict wine aging capacity? Foods 2021, 10, 654. [Google Scholar] [CrossRef] [PubMed]
- Müller, N.; Rauhut, D. Recent developments on the origin and nature of reductive sulfurous off-odours in wine. Fermentation 2018, 4, 62. [Google Scholar] [CrossRef]
- Cordente, A.G.; Nandorfy, D.E.; Solomon, M.; Schulkin, A.; Kolouchova, R.; Francis, I.L.; Schmidt, S.A. Aromatic higher alcohols in wine: Implication on aroma and palate attributes during chardonnay aging. Molecules 2021, 26, 4979. [Google Scholar] [CrossRef]
- Scutarașu, E.C.; Luchian, C.E.; Cioroiu, I.B.; Trincă, L.C.; Cotea, V.V. Increasing amino acids content of white wines with enzymes treatments. Agronomy 2022, 12, 1406. [Google Scholar] [CrossRef]
- Bordiga, M.; Guzzon, R.; Larcher, R.; Travaglia, F.; Arlorio, M.; Coïsson, J.D. Influence of different commercial active dry yeasts on histaminol production during wine alcoholic fermentation. Int. J. Food Sci. Technol. 2017, 52, 1333–1340. [Google Scholar] [CrossRef]
- Zhang, X.-K.; Lan, Y.-B.; Zhu, B.-Q.; Xiang, X.-F.; Duan, C.-Q.; Shi, Y. Changes in monosaccharides, organic acids and amino acids during Cabernet Sauvignon wine ageing based on a simultaneous analysis using gas chromatography–mass spectrometry. J. Sci. Food Agric. 2018, 98, 104–112. [Google Scholar] [CrossRef]
- Pérez-Prieto, L.J.; López-Roca, J.M.; Martínez-Cutillas, A.; Pardo-Mínguez, F.; Gómez-Plaza, E. Extraction and formation dynamic of oak-related volatile compounds from different volume barrels to wine and their behavior during bottle storage. J. Agric. Food Chem. 2003, 51, 5444–5449. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Rodríguez, P.; Bautista-Ortín, A.B.; Gómez-Plaza, E. Increasing wine quality through the use of oak barrels: Factors that will influence aged wine color and aroma. In Wine: Types, Production and Health, 1st ed.; Peeters, A.S., Ed.; Nova Science Publishers, Inc.: New York, NY, USA, 2012; pp. 301–317. [Google Scholar]
- Scutarașu, E.C.; Teliban, I.V.; Zamfir, C.I.; Luchian, C.E.; Colibaba, L.C.; Niculaua, M.; Cotea, V.V. Effect of different winemaking conditions on organic acids compounds of white wines. Foods 2021, 10, 2569. [Google Scholar] [CrossRef] [PubMed]
- Butkhup, L.; Chowtivannakul, S.; Gaensakoo, R.; Prathepha, P.; Samappito, S. Study of the phenolic composition of Shiraz red grape cultivar (Vitis vinifera L.) cultivated in North-eastern Thailand and its antioxidant and antimicrobial activity. S. Afr. J. Enol. Vitic. 2016, 31, 89–98. [Google Scholar] [CrossRef]
- AOAC 962.12-1963; Acidity (Titratable) of Wines: Official Methods of Analysis of AOAC INTERNATIONAL. AOAC International: Gaithersburg, MD, USA, 1962.
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Kupina, S.; Fields, C.; Roman, M.C.; Brunelle, S.L. Determination of total phenolic content using the Folin-C assay: Single-laboratory validation, first action 2017.13. J. AOAC Int. 2019, 101, 1466–1472. [Google Scholar] [CrossRef]
- Settachaimongkon, S.; Homyog, K.; Mekboonsonglarp, W.; Soonoue, P.; Lerdamnuaylarp, T.; Prayoonpeeraput, P.; Theil, P.K.; Nuntapaitoon, M. Dynamics of fatty acid and non-volatile polar metabolite profiles in colostrum and milk depending on the lactation stage and parity number of sows. Sci. Rep. 2023, 13, 1989. [Google Scholar] [CrossRef]
 ), new (
), new ( ), medium-used (
), medium-used ( ) and old (
) and old ( ) oak wood barrels. The dendrogram represents sample clusters based on Pearson’s correlation coefficient with average linkage. Each square in the heatmap expresses normalized metabolite content within the color range. The red color indicates a higher content of the corresponding metabolite compound. For interpretation of the color range used in this figure, the reader is referred to the relative quantification of metabolites in Supplementary Table S2.
) oak wood barrels. The dendrogram represents sample clusters based on Pearson’s correlation coefficient with average linkage. Each square in the heatmap expresses normalized metabolite content within the color range. The red color indicates a higher content of the corresponding metabolite compound. For interpretation of the color range used in this figure, the reader is referred to the relative quantification of metabolites in Supplementary Table S2.
 ), new (
), new ( ), medium-used (
), medium-used ( ) and old (
) and old ( ) oak wood barrels. The dendrogram represents sample clusters based on Pearson’s correlation coefficient with average linkage. Each square in the heatmap expresses normalized metabolite content within the color range. The red color indicates a higher content of the corresponding metabolite compound. For interpretation of the color range used in this figure, the reader is referred to the relative quantification of metabolites in Supplementary Table S2.
) oak wood barrels. The dendrogram represents sample clusters based on Pearson’s correlation coefficient with average linkage. Each square in the heatmap expresses normalized metabolite content within the color range. The red color indicates a higher content of the corresponding metabolite compound. For interpretation of the color range used in this figure, the reader is referred to the relative quantification of metabolites in Supplementary Table S2.
 ), new (
), new ( ), medium-used (
), medium-used ( ) and old (
) and old ( ) oak wood barrels. Squares in the VIP score panel express normalized non-volatile polar metabolite content within the color range. The red color indicates a higher content of the corresponding compound.
) oak wood barrels. Squares in the VIP score panel express normalized non-volatile polar metabolite content within the color range. The red color indicates a higher content of the corresponding compound.
 ), new (
), new ( ), medium-used (
), medium-used ( ) and old (
) and old ( ) oak wood barrels. Squares in the VIP score panel express normalized non-volatile polar metabolite content within the color range. The red color indicates a higher content of the corresponding compound.
) oak wood barrels. Squares in the VIP score panel express normalized non-volatile polar metabolite content within the color range. The red color indicates a higher content of the corresponding compound.
 ), medium-used (
), medium-used ( ) and old used (
) and old used ( ) oak wood barrels (A) and discrimination between wine samples aged in small (
) oak wood barrels (A) and discrimination between wine samples aged in small ( ) and medium (
) and medium ( ) size barrels (B). The lower and upper edges of the box denote the 25th and 75th percentile of observation, respectively; the bold line within the box denotes the median value; the yellow rhombus spot (◊) within the box denotes the average value; whiskers denote the 5th and 95th percentiles.
) size barrels (B). The lower and upper edges of the box denote the 25th and 75th percentile of observation, respectively; the bold line within the box denotes the median value; the yellow rhombus spot (◊) within the box denotes the average value; whiskers denote the 5th and 95th percentiles.
 ), medium-used (
), medium-used ( ) and old used (
) and old used ( ) oak wood barrels (A) and discrimination between wine samples aged in small (
) oak wood barrels (A) and discrimination between wine samples aged in small ( ) and medium (
) and medium ( ) size barrels (B). The lower and upper edges of the box denote the 25th and 75th percentile of observation, respectively; the bold line within the box denotes the median value; the yellow rhombus spot (◊) within the box denotes the average value; whiskers denote the 5th and 95th percentiles.
) size barrels (B). The lower and upper edges of the box denote the 25th and 75th percentile of observation, respectively; the bold line within the box denotes the median value; the yellow rhombus spot (◊) within the box denotes the average value; whiskers denote the 5th and 95th percentiles.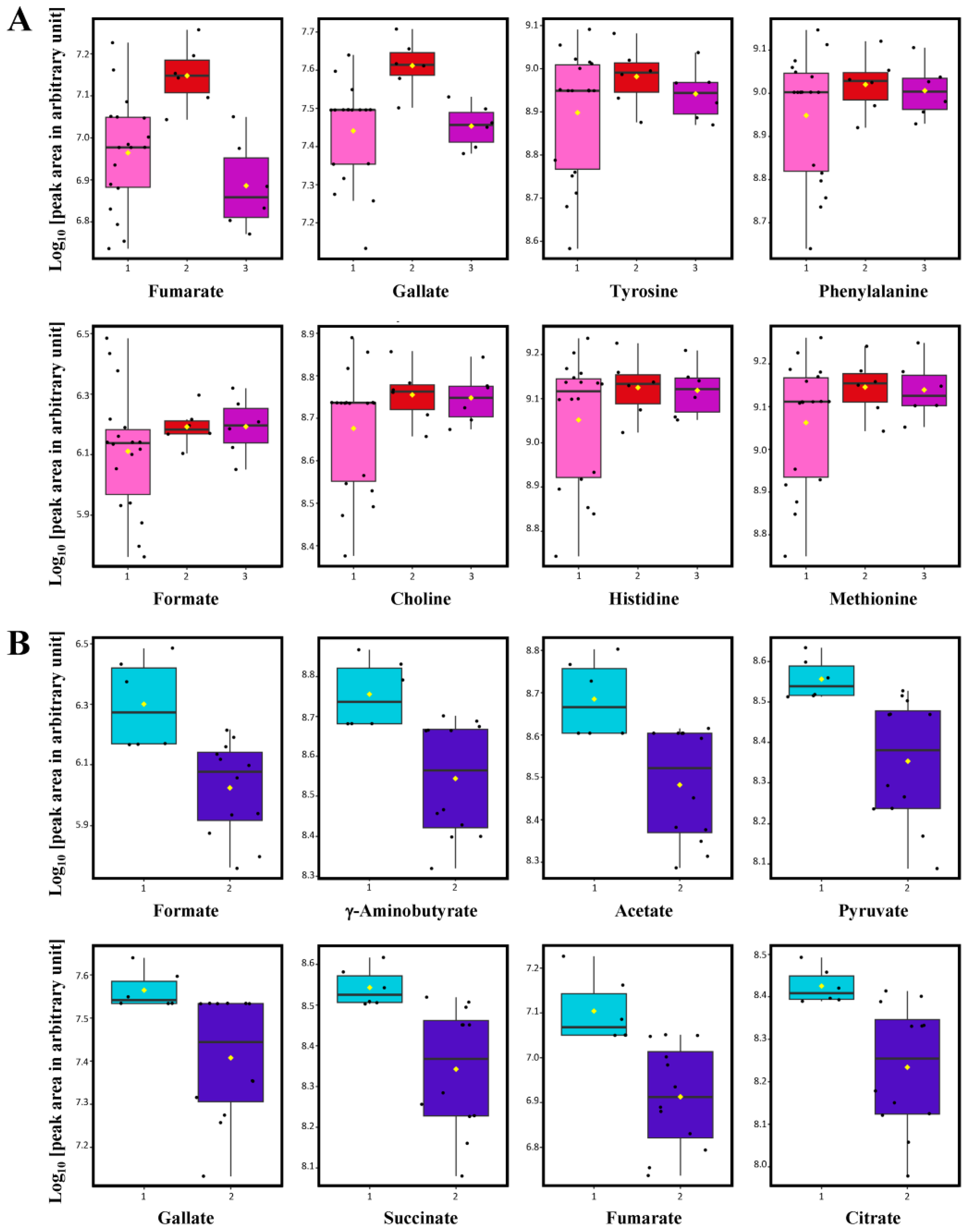
| Type of Aging Container | Sample Code | pH | Titratable Acidity (g Tartaric Acid/L) | Alcohol (%vol) | Residual Sugar (g Glucose/L) | Total Phenolics (mg GAE/L) |
|---|---|---|---|---|---|---|
| New oak | O-RD-G7 (225 L) | 3.68 * ± 0.02 c** | 6.80 ± 0.02 f | 14.08 ± 0.02 d | 7.44 ± 0.76 c | 2579.44 ± 39.38 d |
| O-RD-G5 (225 L) | 3.62 ± 0.01 b | 6.60 ± 0.02 e | 15.54 ± 0.01 g | 6.82 ± 0.45 bc | 2646.11 ± 41.11 d | |
| O-RD-A18 (500 L) | 3.65 ± 0.02 b | 6.53 ± 0.02 d | 13.90 ± 0.01 cd | 6.12 ± 0.32 ab | 2287.78 ± 25.46 bc | |
| O-SM-A3 (500 L) | 3.61 ± 0.02 ab | 6.15 ± 0.02 a | 14.24 ± 0.02 e | 6.97 ± 0.30 bc | 2585.00 ± 80.36 d | |
| O-SV-A8 (500 L) | 3.60 ± 0.01 ab | 6.21 ± 0.01 b | 14.28 ± 0.01 e | 6.86 ± 0.33 bc | 2626.67 ± 72.65 d | |
| O-TS-A12 (500 L) | 3.69 ± 0.02 c | 6.26 ± 0.02 b | 13.84 ± 0.01 c | 7.00 ± 0.35 bc | 2673.89 ± 119.70 d | |
| Medium-used oak | O-RD-E6 (2000 L) | 3.67 ± 0.01 c | 6.64 ± 0.02 e | 13.29 ± 0.02 a | 6.35 ± 0.38 abc | 2537.78 ± 62.55 d |
| Old oak | O-FF-FF1 (2000 L) | 3.55 ± 0.02 a | 6.43 ± 0.02 c | 13.32 ± 0.01 a | 5.35 ± 0.12 a | 1582.22 ± 67.55 a |
| O-AB-ASS1 (2000 L) | 3.66 ± 0.02 bc | 6.25 ± 0.01 b | 14.42 ± 0.01 f | 6.25 ± 0.62 abc | 2326.67 ± 25.00 c | |
| Stainless steel | SS (2000 L) | 3.59 ± 0.02 a | 6.18 ± 0.02 ab | 13.42 ± 0.01 b | 6.13 ± 0.17 ab | 2112.78 ± 62.55 b |
| Group | Sample Code | Replicate | Type of Aging Container | Brand of Barrels * | Age of Barrels ** | Container Size *** |
|---|---|---|---|---|---|---|
| 1 | SS | 6 | Stainless steel | NA | NA | Large |
| 2 | O-RD-G7 | 3 | Oak | A | New | Small |
| 3 | O-RD-G5 | 3 | Oak | A | New | Small |
| 4 | O-RD-A18 | 3 | Oak | A | New | Medium |
| 5 | O-RD-E6 | 6 | Oak | A | Medium | Large |
| 6 | O-SM-A3 | 3 | Oak | B | New | Medium |
| 7 | O-SV-A8 | 3 | Oak | C | New | Medium |
| 8 | O-TS-A12 | 3 | Oak | D | New | Medium |
| 9 | O-FF-FF1 | 3 | Oak | E | Old | Large |
| 10 | O-AB-ASS1 | 3 | Oak | F | Old | Large |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Denchai, S.; Sasomsin, S.; Prakitchaiwattana, C.; Phuenpong, T.; Homyog, K.; Mekboonsonglarp, W.; Settachaimongkon, S. Influence of Different Types, Utilization Times, and Volumes of Aging Barrels on the Metabolite Profile of Red Wine Revealed by 1H-NMR Metabolomics Approach. Molecules 2023, 28, 6716. https://doi.org/10.3390/molecules28186716
Denchai S, Sasomsin S, Prakitchaiwattana C, Phuenpong T, Homyog K, Mekboonsonglarp W, Settachaimongkon S. Influence of Different Types, Utilization Times, and Volumes of Aging Barrels on the Metabolite Profile of Red Wine Revealed by 1H-NMR Metabolomics Approach. Molecules. 2023; 28(18):6716. https://doi.org/10.3390/molecules28186716
Chicago/Turabian StyleDenchai, Suwanan, Suppached Sasomsin, Cheunjit Prakitchaiwattana, Thanitaporn Phuenpong, Kunaporn Homyog, Wanwimon Mekboonsonglarp, and Sarn Settachaimongkon. 2023. "Influence of Different Types, Utilization Times, and Volumes of Aging Barrels on the Metabolite Profile of Red Wine Revealed by 1H-NMR Metabolomics Approach" Molecules 28, no. 18: 6716. https://doi.org/10.3390/molecules28186716
APA StyleDenchai, S., Sasomsin, S., Prakitchaiwattana, C., Phuenpong, T., Homyog, K., Mekboonsonglarp, W., & Settachaimongkon, S. (2023). Influence of Different Types, Utilization Times, and Volumes of Aging Barrels on the Metabolite Profile of Red Wine Revealed by 1H-NMR Metabolomics Approach. Molecules, 28(18), 6716. https://doi.org/10.3390/molecules28186716






