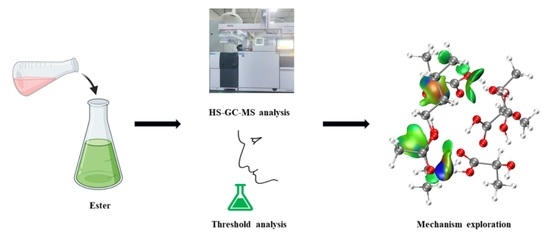
Effects of Organic Acids on the Release of Fruity Esters in Water: An Insight at the Molecular Level
Abstract
:1. Introduction
2. Results and Discussion
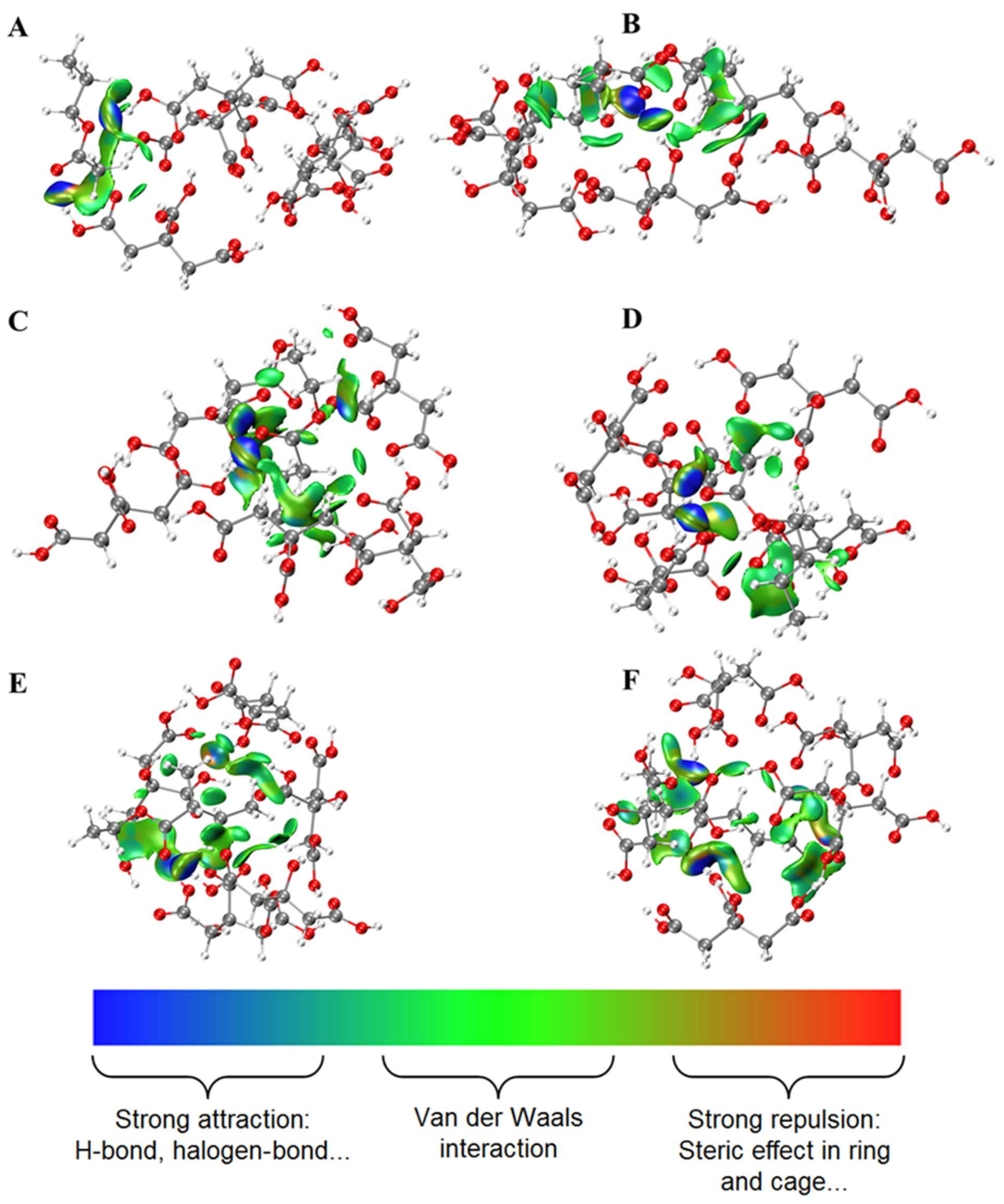
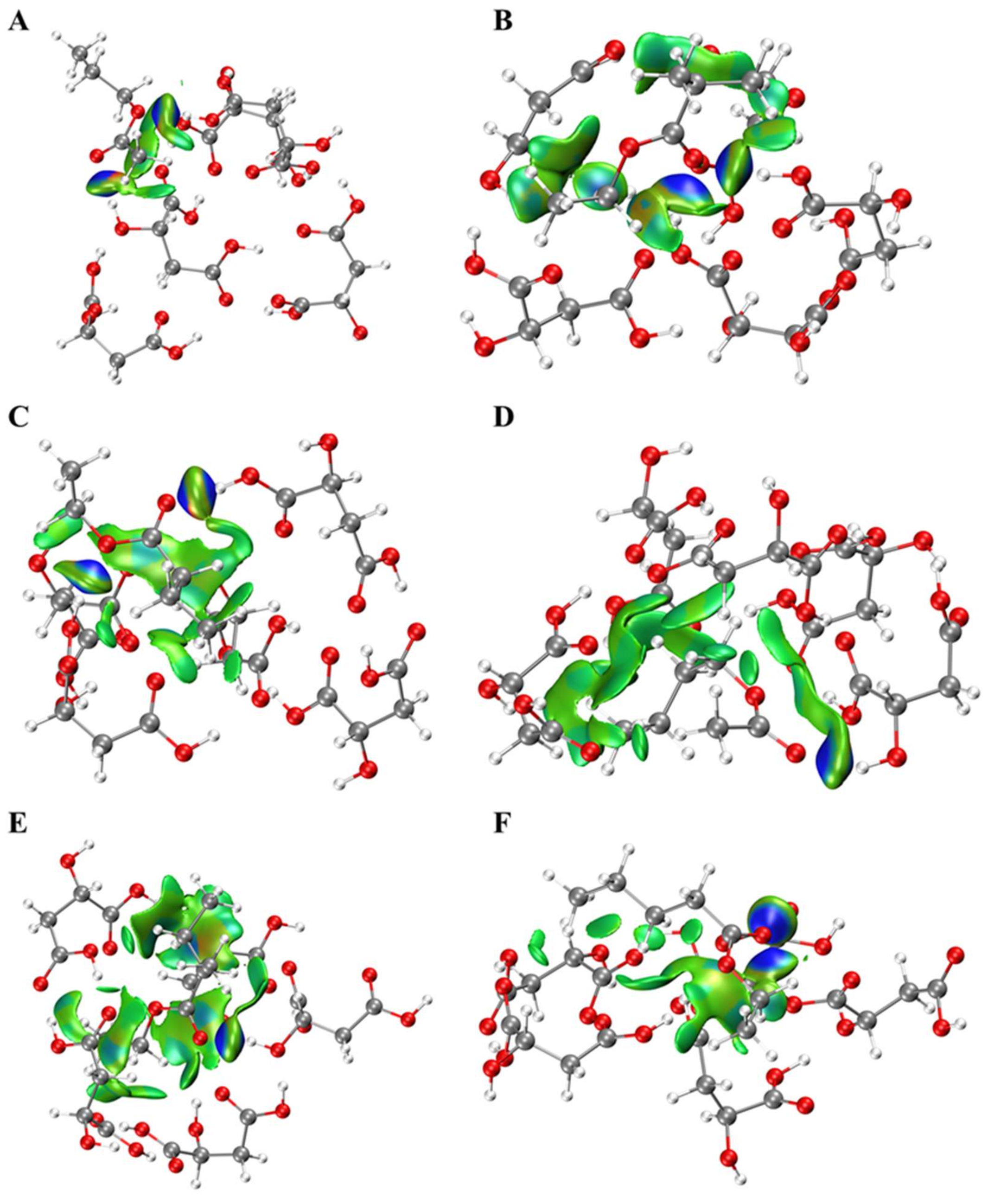
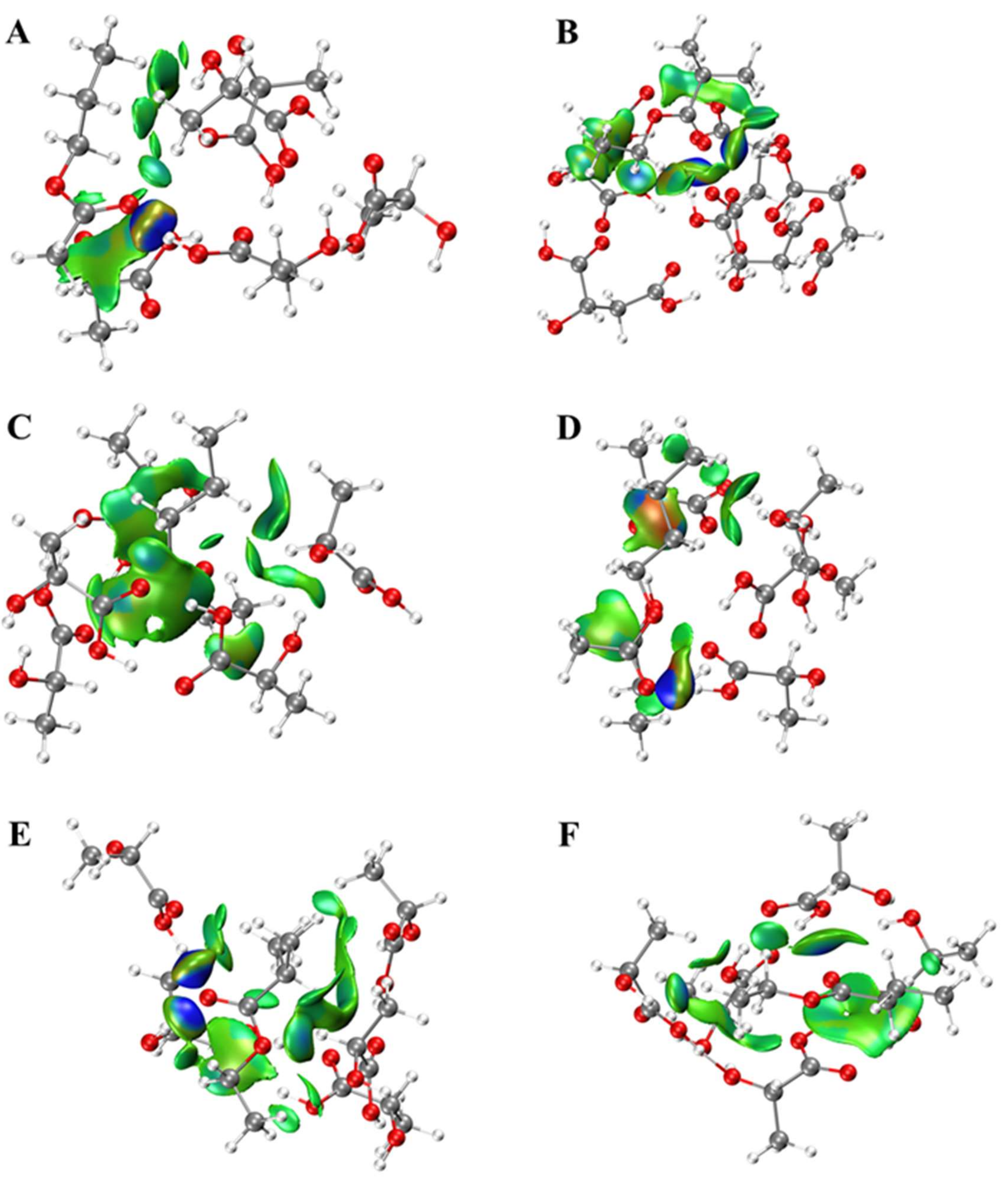
2.1. Interactions between Esters and OAs
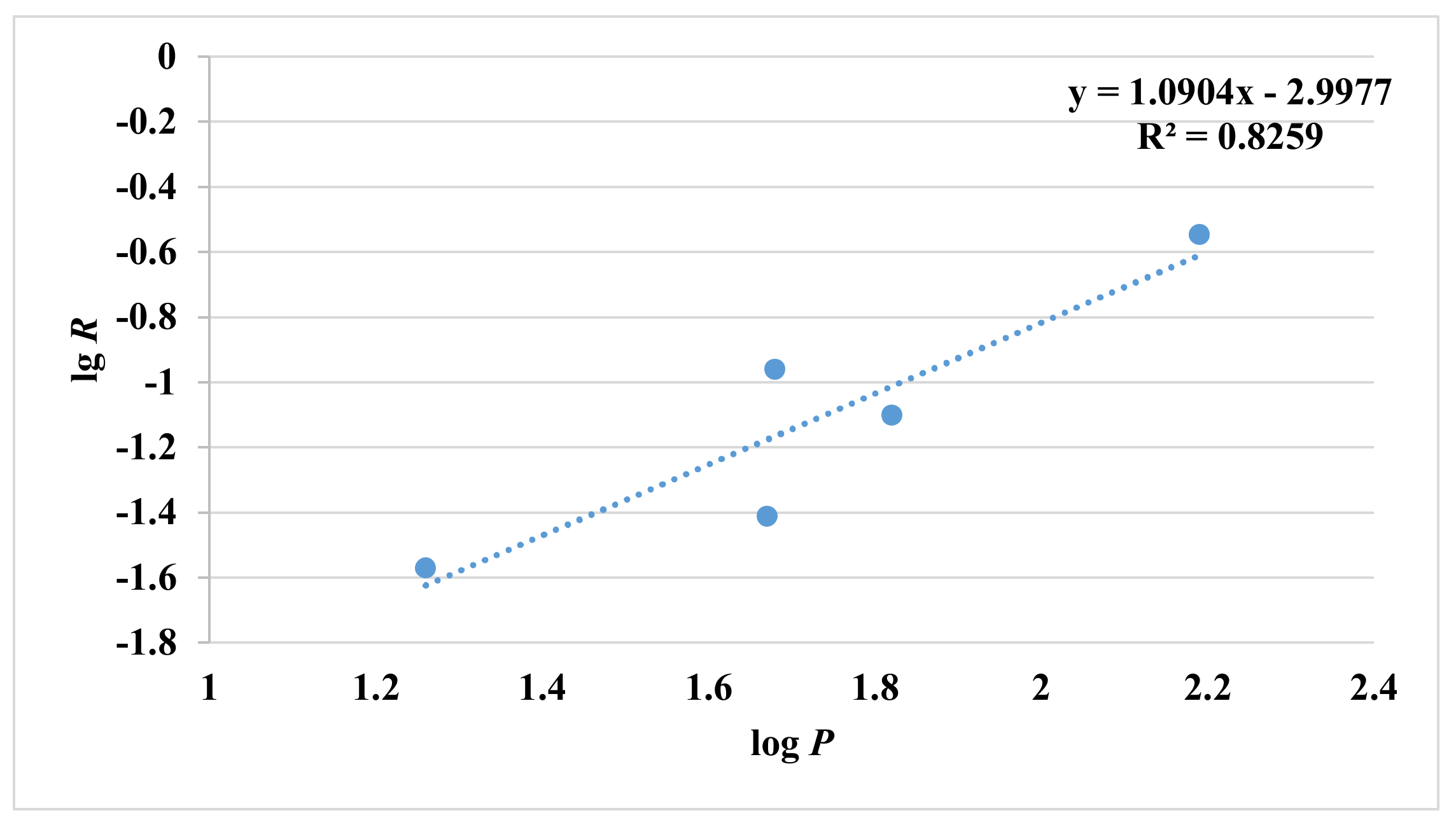
2.2. Release of Esters in Aqueous Solutions
2.3. Release of Esters in OAs Solution
2.3.1. Effect of Citric Acid Concentration
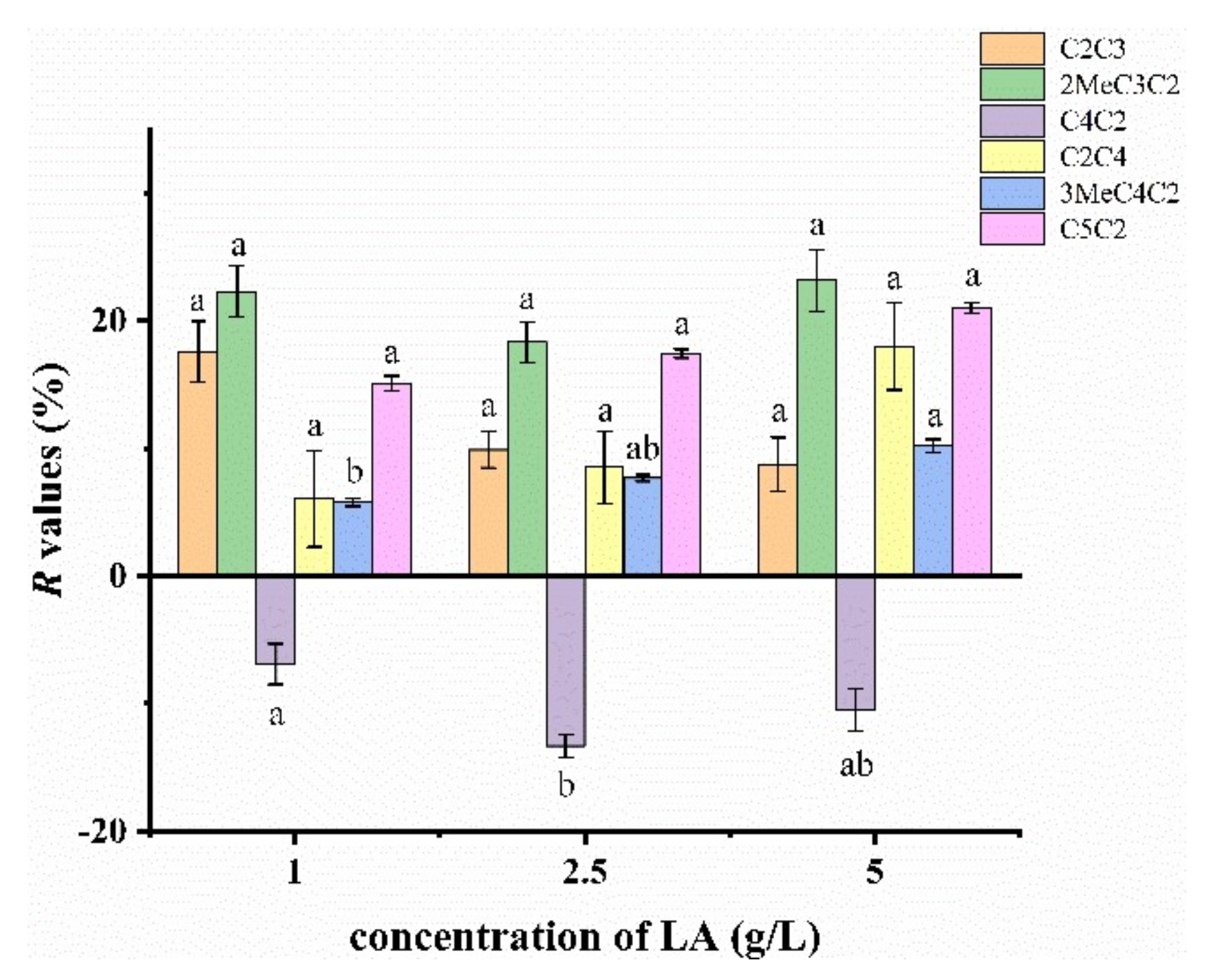
2.3.2. Effect of Lactic Acid Concentration
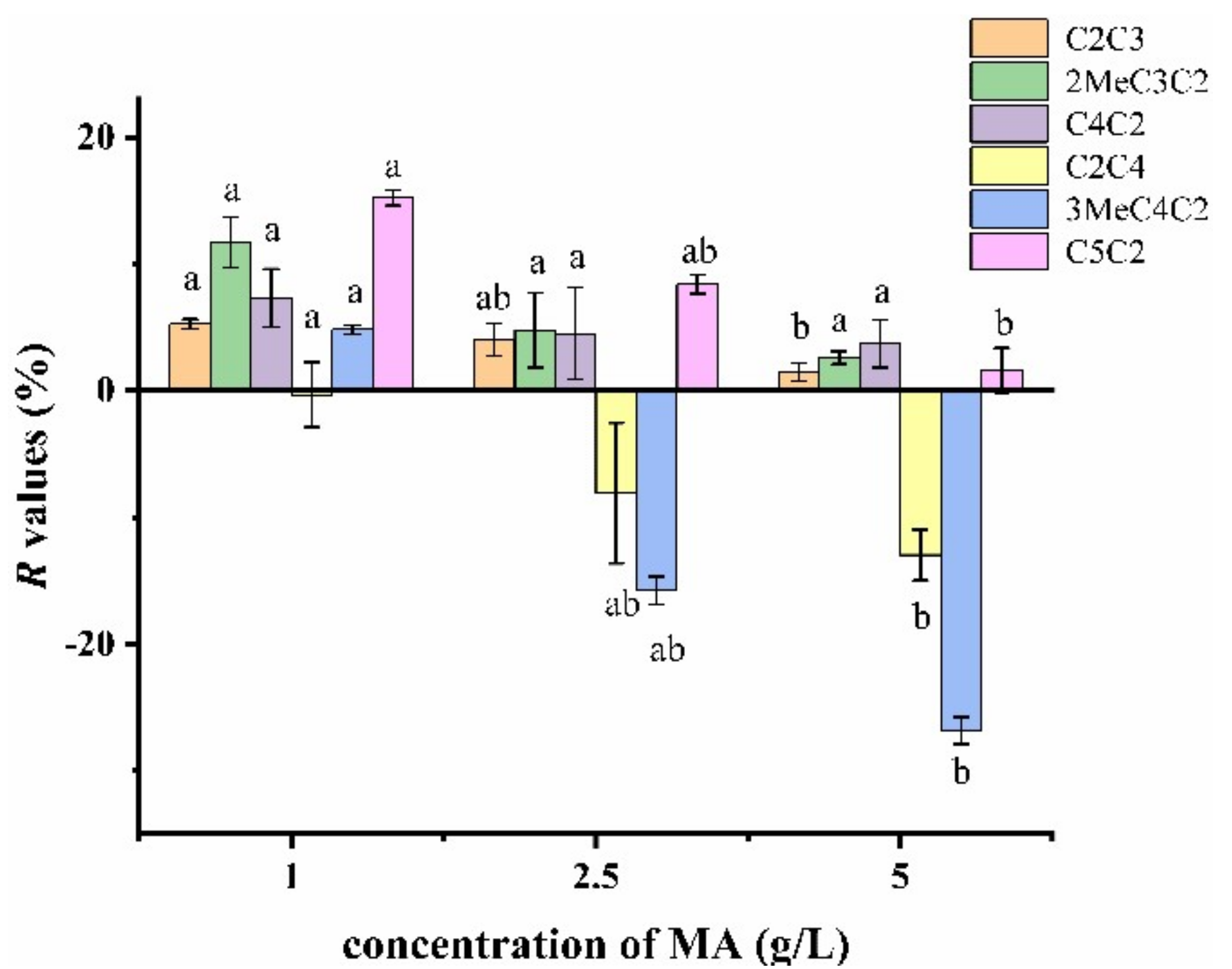
2.3.3. Effect of Malic Acid Concentration
2.4. Odour Detection Thresholds of Esters and Esters Added with Organic Acids
3. Materials and Methods
3.1. Chemicals
3.2. Experimental Methods
3.2.1. Chromatographic Conditions
3.2.2. Calculation of Partition Coefficients
3.2.3. Determination of Odour Detection Thresholds
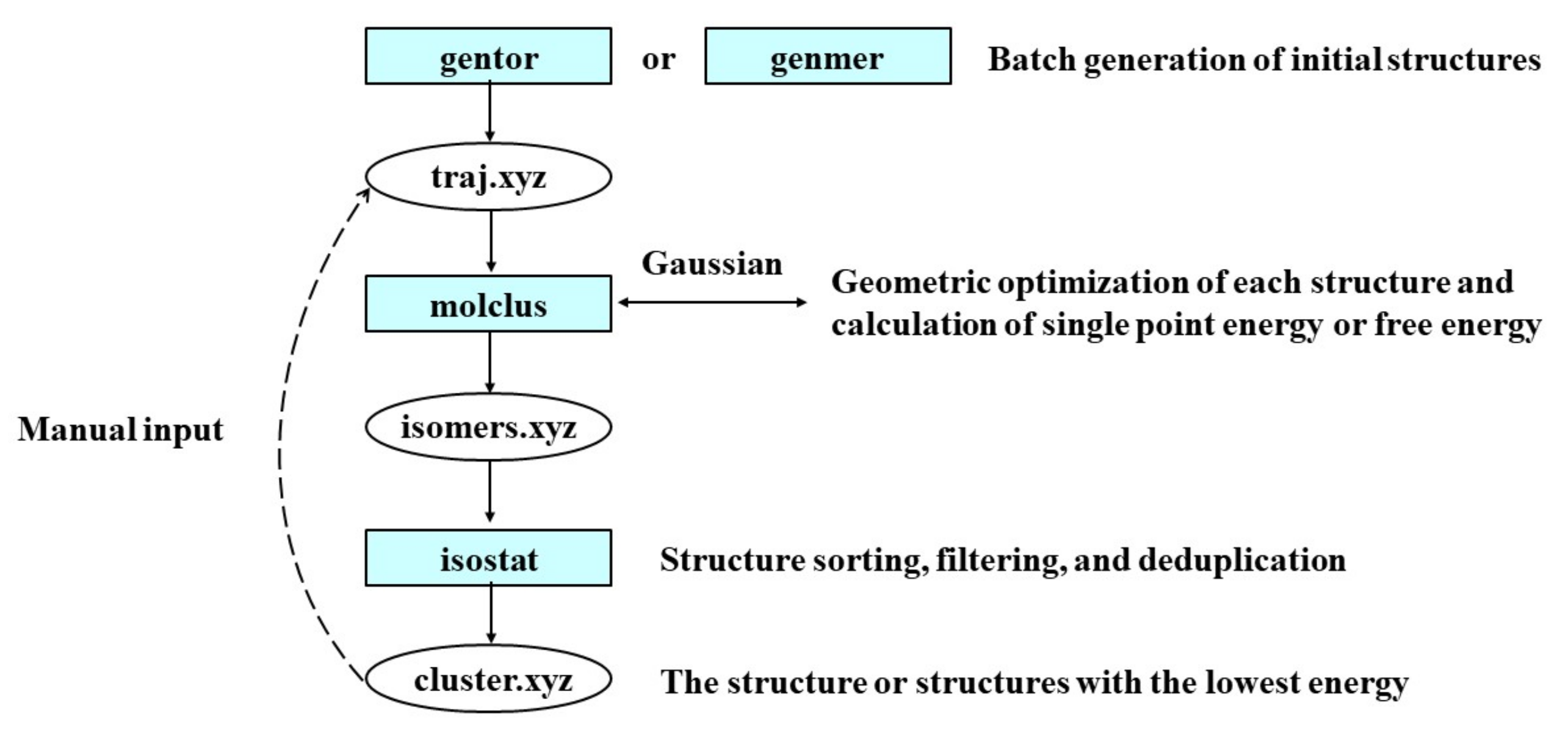
3.2.4. Theoretical Methods
3.3. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Druaux, C.; Voilley, A. Effect of food composition and microstructure on volatile flavour release. Trends Food Sci. Technol. 1997, 8, 364–368. [Google Scholar] [CrossRef]
- Salvador, D.; Bakker, J.; Langley, K.R.; Potjewijd, R.; Martin, A.; Elmore, J.S. Flavour release of diacetyl from water, sunflower oil and emulsions in model systems. Food Qual. Prefer. 1994, 5, 103–107. [Google Scholar] [CrossRef]
- Xiao, Z.; Luo, J.; Niu, Y.; Wang, P.; Wang, R.; Sun, X. Olfactory impact of esters on rose essential oil floral alcohol aroma expression in model solution. Food Res. Int. 2019, 116, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Zhang, Y.; Zeng, S.; Luo, L.; Xi, H.; Li, P.; Wang, D.; Liao, T.; Chen, J.; Sun, S.; et al. The effect of long-chain alkanes on flavour release and olfactory characteristics of rose essential oil. Flavour Fragr. J. 2021, 37, 72–80. [Google Scholar] [CrossRef]
- Ployon, S.; Morzel, M.; Canon, F. The role of saliva in aroma release and perception. Food Chem. 2017, 226, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Piornos, J.A.; Delgado, A.; de La Burgade, R.C.; Methven, L.; Balagiannis, D.P.; Koussissi, E.; Brouwer, E.; Parker, J.K. Orthonasal and retronasal detection thresholds of 26 aroma compounds in a model alcohol-free beer: Effect of threshold calculation method. Food Res. Int. 2019, 123, 317–326. [Google Scholar] [CrossRef]
- Hansson, A.; Andersson, J.; Leufven, A.; Pehrson, K. Effect of changes in pH on the release of flavour compounds from a soft drink-related model system. Food Chem. 2001, 74, 429–435. [Google Scholar] [CrossRef]
- Baldwin, E.A.; Goodner, K.; Plotto, A. Interaction of Volatiles, Sugars, and Acids on Perception of Tomato Aroma and Flavor Descriptors. J. Food Sci. 2008, 73, S294–S307. [Google Scholar] [CrossRef]
- Ribeiro, J.C.B.; Granato, D.; Masson, M.L.; Andriot, I.; Mosca, A.C.; Salles, C.; Guichard, E. Effect of lactobionic acid on the acidification, rheological properties and aroma release of dairy gels. Food Chem. 2016, 207, 101–106. [Google Scholar] [CrossRef]
- Arvisenet, G.; Ballester, J.; Ayed, C.; Sémon, E.; Andriot, I.; Le Quere, J.L.; Guichard, E. Effect of sugar and acid composition, aroma release and assessment conditions on aroma enhancement by taste in model wines. Food Qual. Prefer. 2018, 71, 172–180. [Google Scholar] [CrossRef] [Green Version]
- Hewson, L.; Hollowood, T.; Chandra, S.; Hort, J. Taste–aroma interactions in a citrus flavoured model beverage system: Similarities and differences between acid and sugar type. Food Qual. Prefer. 2008, 19, 323–334. [Google Scholar] [CrossRef]
- Niimi, J.; Eddy, A.I.; Overington, A.R.; Heenan, S.P.; Silcock, P.; Bremer, P.J.; Delahunty, C.M. Aroma–taste interactions between a model cheese aroma and five basic tastes in solution. Food Qual. Prefer. 2014, 31, 1–9. [Google Scholar] [CrossRef]
- Roussis, I.G.; Sergianitis, S. Protection of some aroma volatiles in a model wine medium by sulphur dioxide and mixtures of glutathione with caffeic acid or gallic acid. Flavour Fragr. J. 2008, 23, 35–39. [Google Scholar] [CrossRef]
- Covarrubias-Cervantes, M.; Champion, D.; Bongard, S.; Voilley, A. Effects of the nature and concentration of substrates in aqueous solutions on the solubility of aroma compounds. Flavour Fragr. J. 2004, 20, 265–273. [Google Scholar] [CrossRef]
- Pozo-Bayón, M.Á.; Reineccius, G. Interactions Between Wine Matrix Macro-Components and Aroma Compounds; Springer: New York, NY, USA, 2009. [Google Scholar]
- Margaux, C.; Georgia, L.; Jean-Christophe, B. Static headspace analysis using low-pressure gas chromatography and mass spectrometry, application to determining multiple partition coefficients: A practical tool for understanding red wine fruity volatile perception and the sensory impact of higher alcohols. Anal. Chem. 2018, 90, 10812–10818. [Google Scholar]
- Guth, H.; Rusu, M. Food matrices–determination of odorant partition coefficients and application of models for their prediction. Food Chem. 2008, 108, 1208–1216. [Google Scholar] [CrossRef]
- Guyot, C.; Bonnafont, C.; Lesschaeve, I.; Issanchou, S.; Voilley, A.; Spinnler, H.E. Effect of Fat Content on Odor Intensity of Three Aroma Compounds in Model Emulsions: δ-Decalactone, Diacetyl, and Butyric Acid. J. Agric. Food Chem. 1996, 44, 2341–2348. [Google Scholar] [CrossRef]
- Ettre, L.S.; Kolb, B. Headspace-gas chromatography: The influence of sample volume on analytical results. Chromatographia 1991, 32, 5–12. [Google Scholar] [CrossRef]
- Ettre, L.S.; Welter, C.; Kolb, B. Determination of gas-liquid partition coefficients by automatic equilibrium headspace-gas chromatography utilizing the phase ratio variation method. Chromatographia 1993, 35, 73–84. [Google Scholar] [CrossRef]
- Heilig, A.; Sonne, A.; Schieberle, P.; Hinrichs, J. Determination of aroma compound partition coefficients in aqueous, polysaccharide and dairy matrices using the Phase Ratio Variation (PRV) method: A review and modeling approach. J. Agric. Food Chem. 2016, 64, 4450–4470. [Google Scholar] [CrossRef]
- Lloyd, N.W.; Dungan, S.R.; Ebeler, S.E. Measuring gas-liquid partition coefficients of aroma compounds by solid phase mi-croextraction, sampling either headspace or liquid. Analyst 2011, 136, 3375–3383. [Google Scholar] [CrossRef] [PubMed]
- Helburn, R.; Albritton, J.; Howe, G.; Michael, L.; Franke, D. Henry’s Law constants for fragrance and organic solvent compounds in aqueous in-dustrial surfactants. Chem. Eng. Data 2008, 53, 1071–1079. [Google Scholar] [CrossRef]
- Welke, B.; Ettlinger, K.; Riederer, M. Sorption of volatile organic chemicals in plant surfaces. Environ. Sci. Technol. 1998, 32, 1099–1104. [Google Scholar] [CrossRef]
- Dewulf, J.; Langenhove, H.V.; Everaert, P. Determination of Henry’s law coefficients by combination of the equilibrium parti-tioning in closed systems and solid-phase microextraction techniques. J. Chromatogr. A 1999, 830, 353–363. [Google Scholar] [CrossRef]
- Robbins, G.A.; Wang, S.; Stuart, J.D. Using the static headspace method to determine Henry’s law constants. Anal. Chem. 1993, 65, 3113–3118. [Google Scholar] [CrossRef]
- Xu, J.; He, Z.; Zeng, M.; Li, B.; Qin, F.; Wang, L.; Wu, S.; Chen, J. Effect of xanthan gum on the release of strawberry flavor in formulated soy beverage. Food Chem. 2017, 228, 595–601. [Google Scholar] [CrossRef]
- Van Ruth, S.M.; King, C.; Giannouli, P. Influence of Lipid Fraction, Emulsifier Fraction, and Mean Particle Diameter of Oil-in-Water Emulsions on the Release of 20 Aroma Compounds. J. Agric. Food Chem. 2002, 50, 2365–2371. [Google Scholar] [CrossRef]
- Zhang, Y.; Barringer, S. Effect of hydrocolloids, sugar, and citric acid on strawberry volatiles in a gummy candy. J. Food Process. Preserv. 2018, 42, e13327. [Google Scholar] [CrossRef]
- Apelblat, A.; Korin, E.; Manzurola, E. Thermodynamic properties of aqueous solutions with citrate ions. Compressibility studies in aqueous solutions of citric acid. J. Chem. Thermodyn. 2013, 64, 14–21. [Google Scholar] [CrossRef]
- Mahiuddin, S.; Minofar, B.; Borah, J.M.; Das, M.R.; Jungwirth, P. Propensities of oxalic, citric, succinic, and maleic acids for the aqueous solu-tion/vapour interface: Surface tension measurements and molecular dynamics simulations. Chem. Phys. Lett. 2008, 462, 217–221. [Google Scholar] [CrossRef]
- Żarska, M.; Dzida, M.; Apelblat, A. Surface tensions and densities of concentrated aqueous solutions of citric acid. J. Mol. Liq. 2017, 228, 91–95. [Google Scholar] [CrossRef]
- Tsachaki, M.; Linforth, R.S.T.; Taylor, A.J. Aroma Release from Wines under Dynamic Conditions. J. Agric. Food Chem. 2009, 57, 6976–6981. [Google Scholar] [CrossRef] [PubMed]
- Villamor, R.R.; Evans, M.A.; Mattinson, D.S.; Ross, C.F. Effects of ethanol, tannin and fructose on the headspace concentration and potential sensory significance of odorants in a model wine. Food Res. Int. 2013, 50, 38–45. [Google Scholar] [CrossRef]
- Losada, M.; Tran, H.; Xu, Y. Lactic acid in solution: Investigations of lactic acid self-aggregation and hydrogen bonding in-teractions with water and methanol using vibrational absorption and vibrational circular dichroism spectroscopies. J. Chem. Phys. 2008, 128, 232. [Google Scholar] [CrossRef]
- Rodríguez-Bencomo, J.J.; Muñoz-González, C.; Andújar-Ortiz, I.; Martín-Álvarez, P.J.; Moreno-Arribas, M.V.; Pozo-Bayón, M.Á. Assessment of the effect of the non-volatile wine matrix on the volatility of typical wine aroma compounds by headspace solid phase microextraction/gas chromatography analysis. J. Sci. Food Agr. 2011, 91, 2484–2494. [Google Scholar] [CrossRef] [Green Version]
- Lorrain, B.; Tempere, S.; Iturmendi, N.; Moine, V.; de Revel, G.; Teissedre, P.L. Influence of phenolic compounds on the sensorial perception and volatility of red wine esters in model solution: An insight at the molecular level. Food Chem. 2013, 140, 76–82. [Google Scholar] [CrossRef]
- Pineau, B.; Barbe, J.C.; Van Leeuwen, C.; Dubourdieu, D. Which impact for β-damascenone on red wines aroma? J. Agric. Food Chem. 2007, 55, 4103–4108. [Google Scholar] [CrossRef]
- Maragò, E.; Michelozzi, M.; Calamai, L.; Camangi, F.; Sebastiani, L. Antioxidant properties, sensory characteristics and volatile compounds profile of apple juices from ancient Tuscany (Italy) apple varieties. Eur. J. Hortic. Sci. 2016, 81, 255–263. [Google Scholar] [CrossRef]
- Perestrelo, R.; Silva, C.; Silva, P.; Medina, S.; Câmara, J.S. Differentiation of Fresh and Processed Fruit Juices Using Volatile Composition. Molecules 2019, 24, 974. [Google Scholar] [CrossRef] [Green Version]
- Pino, J.A. Odour-active compounds in mango (Mangifera indica L. cv. Corazón). Int. J. Food Sci. Technol. 2012, 47, 1944–1950. [Google Scholar] [CrossRef]
- ISO-13001; Sensory Analysis–Methodology General Guidance for Measuring Odour, Flavour and Test Detection Thresholds by a Three-Alternative Forced-Choice (3-AFC) Procedure. International Organization for Standardization (ISO): Geneva, Switzerland, 2018.
- Lu, T. Molclus Program, Version 1.9.5; Beijing Kein Research Center for Natural Science: Beijing, China, 2016. Available online: http://www.keinsci.com/research/molclus.html(accessed on 6 November 2021).
- Frisch, M.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.J.R.A.; et al. Gaussian 16 Rev. C.01; Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Yan, Z.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, non-covalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 2008, 119, 525. [Google Scholar]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104–154119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lefebvre, C.; Rubez, G.; Khartabil, H.; Boisson, J.-C.; Contreras-García, J.; Hénon, E. Accurately extracting the signature of intermolecular interactions present in the NCI plot of the reduced density gradient versus electron density. Phys. Chem. Chem. Phys. 2017, 19, 17928–17936. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Feiwu, C. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]

| Odour Detection Threshold (μg/L) in Solution | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Water | 1 g/L CA | 2.5 g/L CA | 5 g/L CA | 1 g/L LA | 2.5 g/L LA | 5 g/L LA | 1 g/L MA | 2.5 g/L MA | 5 g/L MA | |
| Propyl acetate | 2.27 | 4.19 | 2.74 | 2.49 | 6.12 | 3.98 | 3.46 | 2.74 | 2.84 | 2.41 |
| Butyl acetate | 68.0 | 80.4 | 93.1 | 57.0 | 136 | 111 | 136 | 64.0 | 33.9 | 37.5 |
| Ethyl butyrate | 0.691 | 1.76 | 0.614 | 0.42 | 0.442 | 0.345 | 0.425 | 0.817 | 0.781 | 0.666 |
| Ethyl isobutyrate | 0.262 | 0.249 | 0.249 | 0.131 | 0.621 | 0.552 | 0.584 | 0.552 | 0.395 | 0.276 |
| Ethyl valerate | 18.2 | 63.5 | 55.0 | 34.4 | 46.7 | 58.9 | 47.6 | 46.7 | 34.4 | 28.1 |
| Ethyl isovalerate | 0.201 | 0.184 | 0.0884 | 0.0582 | 0.276 | 0.260 | 0.295 | 0.276 | 0.0650 | 0.0552 |
| Compound | Aromatic Descriptor 1 | Abbreviation | Water Solubility 2 (g/L) | Log P 3 | Vapor Pressure 2 (mm Hg) | Concentration (μg/L) |
|---|---|---|---|---|---|---|
| Propyl acetate [39,40] | Celery, floral, pear | C2C3 | 10.670 | +1.26 | 35.1 | 500 |
| Ethyl isobutyrate [39,41] | Kiwi, strawberry, solvent | 2MeC3C2 | 3.172 | +1.67 | 24.2 | 400 |
| Ethyl butyrate [39,40] | Apple, butter, cheese | C4C2 | 2.745 | +1.68 | 14.6 | 400 |
| Butyl acetate [39] | Apple, banana | C2C4 | 3.128 | +1.82 | 11.9 | 400 |
| Ethyl isovalerate [41] | Apple, fruit, pineapple | 3MeC4C2 | 1.070 | +1.9 | 7.98 | 500 |
| Ethyl valerate [39] | Apple, dry fish, herb | C5C2 | 0.926 | +2.19 | 4.8 | 500 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Xi, H.; Fu, Y.; Li, P.; Sun, S.; Zong, Y. Effects of Organic Acids on the Release of Fruity Esters in Water: An Insight at the Molecular Level. Molecules 2022, 27, 2942. https://doi.org/10.3390/molecules27092942
Liu Y, Xi H, Fu Y, Li P, Sun S, Zong Y. Effects of Organic Acids on the Release of Fruity Esters in Water: An Insight at the Molecular Level. Molecules. 2022; 27(9):2942. https://doi.org/10.3390/molecules27092942
Chicago/Turabian StyleLiu, Yu, Hui Xi, Yingjie Fu, Peng Li, Shihao Sun, and Yongli Zong. 2022. "Effects of Organic Acids on the Release of Fruity Esters in Water: An Insight at the Molecular Level" Molecules 27, no. 9: 2942. https://doi.org/10.3390/molecules27092942
APA StyleLiu, Y., Xi, H., Fu, Y., Li, P., Sun, S., & Zong, Y. (2022). Effects of Organic Acids on the Release of Fruity Esters in Water: An Insight at the Molecular Level. Molecules, 27(9), 2942. https://doi.org/10.3390/molecules27092942