Abstract
Melatonin, N-acetyl-5-hydroxytryptamine, is a hormone that synchronizes the internal environment with the photoperiod. It is synthesized in the pineal gland and greatly depends on the endogenous circadian clock located in the suprachiasmatic nucleus and the retina’s exposure to different light intensities. Among its most studied functions are the regulation of the waking-sleep rhythm and body temperature. Furthermore, melatonin has pleiotropic actions, which affect, for instance, the modulation of the immune and the cardiovascular systems, as well as the neuroprotection achieved by scavenging free radicals. Recent research has supported that melatonin contributes to neuronal survival, proliferation, and differentiation, such as dendritogenesis and axogenesis, and its processes are similar to those caused by Nerve Growth Factor, Brain-Derived Neurotrophic Factor, Neurotrophin-3, and Neurotrophin-4/5. Furthermore, this indolamine has apoptotic and anti-inflammatory actions in specific brain regions akin to those exerted by neurotrophic factors. This review presents evidence suggesting melatonin’s role as a neurotrophic factor, describes the signaling pathways involved in these processes, and, lastly, highlights the therapeutic implications involved.
1. Introduction
Melatonin (MEL) N-acetyl-5-methoxytryptamine is a hormone synthesized by the pineal gland. Discovered and isolated from bovine pineal glands by Aaron Lerner, MEL was injected in Lerner’s groundbreaking experiment into dermatosis patients who, contrary to his expectations, did not experience depigmentation but drowsiness. With these initial findings, he began to investigate MEL [1]. Later, MEL was described in plants as phytomelatonin [2,3,4], and, nowadays, it is known that MEL is present in practically all organisms along the phylogenetic scale [5].
The pineal gland produces MEL in the dark phase of the photoperiod. MEL is secreted into the general circulation and in the cerebrospinal fluid and, in this manner, circulates throughout the body and the brain [6]. Its synthesis is regulated by a clock located in the suprachiasmatic nuclei (SCN) at the hypothalamus. This clock determines the circadian rhythm of MEL secretion. In the brain, extrapineal MEL behaves similarly to Neurotrophic Factors (NTFs) [7,8] and is capable of modulating cell survival, proliferation, and differentiation, by signaling pathways that can be triggered in response to stimulation of membrane and intracellular receptors.
In this regard, NTFs play a crucial role in brain neuroplasticity and neurodevelopment, promoting its growth and survival [9,10]. These peptide molecules function as signals that trigger biological processes allowing adaptation to the environment and survival of neurons. In the early stages of development, they modulate both differentiation and maturation of neuronal precursors. Some factors are only present at the early stages of development and others throughout life [10,11,12]. Besides neurogenesis, NTFs are also needed for the maintenance of neuronal function and the neuron’s structural integrity [11,13]. NTFs are also expressed in non-neuronal tissues like lung components including nasal and bronchial epithelium, smooth muscle, nerves, immune cells, kidney, spermatozoa, and ovarium [9,14,15]. These peptides are secreted into the cellular medium and may act in a paracrine manner signaling neighboring cells. If required, cells can also synthesize and secrete these factors in response to autocrine stimulation [16].
MEL can also be synthesized in other organs such as the intestine, retina, placenta, specific brain regions, and skin [17,18] (Figure 1). MEL is released at micromolar concentrations from extrapineal sites of synthesis and has paracrine, autocrine, and antioxidant actions [19]. The pleiotropic effects of indolamine have been widely described. For this reason, in this review, we will compare NTFs’ features with MEL synthesis, focusing on their role in neurogenesis in the adult brain, as well as on the mechanisms of action involved in survival, proliferation, and neurodevelopment.
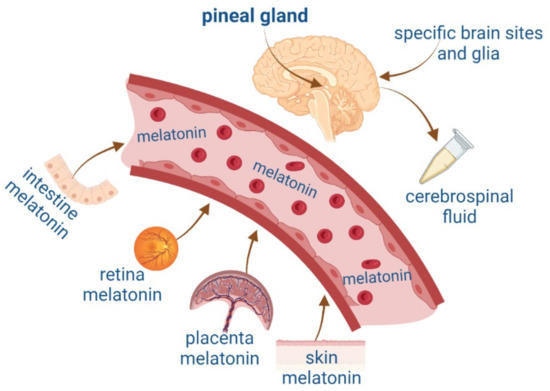
Figure 1.
Melatonin synthesis in the pineal gland and extrapineal sites such as the intestine, retina, placenta, and skin and their blood circulation. Figure created by Biorender.com.
2. Melatonin and Neurotrophic Factors Synthesis
MEL is an indolamine with a 3-amido group and a 5-methoxy group, which confers its amphiphilic properties to MEL (N-[2-(5-methoxy-1H-indol-3-yl)ethyl] ethanamide) [20]. MEL biosynthesis starts from the amino acid tryptophan which is transformed into serotonin. In the pineal gland, serotonin will undergo two enzymatic reactions: First, N-acetylation by N-acetytransferase (NAT) will produce N-acetyl-serotonin. Then, a methyl group is transferred from S-adenosylmethionine to the 5-hydroxy group of N-acetylserotonin by the action of hydroxyindol-O-methyltransferase (HIOMT) [21]. NAT is the limiting step for MEL synthesis, and it has a high amplitude rhythm with a nocturnal activity that is 50 to 100 times greater than diurnal activity. HIOMT, on the other hand, catalyzes the last step of the synthesis and has a very low rhythmic amplitude (Figure 2) [21].
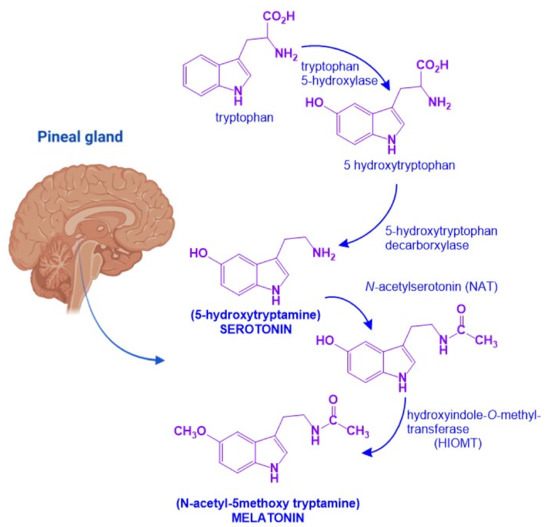
Figure 2.
Melatonin biosynthetic pathway. Melatonin biosynthesis in the pineal gland starts from tryptophan and involves four sequential enzymatic steps to render 5-hydroxytryptophan, N-acetylserotonin, 5-hydroxytryptamine (serotonin), and N-acetyltryptamine (melatonin). Figure created with Biorender.com software.
MEL in circulation is primarily catabolized in the liver and the brain. In the hepatocytes, MEL undergoes 80% of hydroxylation by the cytochrome p450 1A2 (CYP1A2); afterward, in the kidney, it is sulpho-conjugated (70 to 80% of total catabolism) or conjugated with glucuronic acid (5% of total catabolism) [22]. CYP1A2 ensures hepatic catabolism [23]. Even at the hepatic level, there are also deacetylation and demethylation pathways. After deacetylation, MEL is transformed into 5-methoxytryptamine and after oxidation to 5-methoxyindole acetic acid [24]. Cleavage of the pyrrole nucleus of MEL results in N-acetyl-5-methoxykynurenamine through the N-formyl intermediate [24]. 70% of this metabolite is eliminated by urine and 20% in the feces in 24 h. The main route of elimination is, therefore, urinary and will lead to the formation of various metabolites present in the urine: 6-hydroxymelatonin in the form of sulfate (70–80%) and glucuronide (5%) and kynuramines (15%), among them. In summary, MEL urinary elimination comprises MEL (1%), 5-methoxyindoleacetic acid (0.5%), and 6-sulfatoxymelatonin, which is the major urinary metabolite [25].
On the other hand, NTFs have significant differences in their biosynthesis and catabolism. MEL is an indolamine, while NTFs are peptides synthesized from protein precursors. Despite the great number of molecules considered as NTFs that can regulate neuronal function, we will focus on just four of them with similar structure and functions: Nerve Growth Factor (NGF), Brain-Derived Neurotrophic Factor (BDNF), Neurotrophin-3 (NT3), and Neurotrophin-4/5 (NT4/5).
NTFs are synthesized by neuronal cells as inactive precursors (pro-NTFs, 27 kDa). Synthesis of Pro-NTFs occurs in the endoplasmic reticulum and then a cleavage is caused by furin enzymes in the intracellular compartment to produce active molecules. Also, cleavage is produced by plasmin metalloproteinases in the extracellular space. Pro-NTFs are further processed to generate mature NTFs nearly 13–15 kDa polypeptides with extensive homology. The mature forms of BDNF, NT4, and NT3 have approximately 50% amino acid identity to NGF [9,26]. Mature NTFs form homodimers by noncovalent binding and contain a conserved terminal fold and a cysteine “knot”, which consists of three disulfide bonds in the polypeptide chain [16,27].
3. The Neurodevelopment in the Adult Brain
The organisms could survive and adapt to their environment through neuroplastic changes produced in the brain. Neuronal activity can modify the number and strength of synaptic connections [28]. The activity-dependent modulation of synapses is a critical factor for brain development alongside many cognitive functions and behavior. It has been postulated that from the embryonic stages and during adulthood, various cytotypes, tissues, and organs release NTFs. These factors are essential for new neuron formation, their survival as well as differentiation, and the correct organization of the nervous system [29].
NTFs expression is regulated by neuronal activity and these molecules modulate the efficiency of synaptic transmission, dendrite, and axon growth, as well as the elements necessary for synaptogenesis [30]. These processes are adaptative changes in which the number of neurons correlates with needs and the number of neurons in the innervated target neurons. The “neurotrophic factor hypothesis” was conceptualized by Victor Hamburger and Rita Levi Montalcini, who identified the first of these factors in NGF [31]. In the adult mammalian brain, there are several neurogenic niches: the hippocampal dentate gyrus, the subventricular zone of the lateral cerebral ventricle, the substantia nigra and the cerebellum, the amygdala, the spinal cord, the hypothalamus, and the cerebral neocortex [32,33].
Neurogenesis and neuronal differentiation take place at three stages: (1) Nervous cell generation through cellular proliferation, (2) Migration of neuronal precursor to the area where they will be established, (3) Morphofunctional polarization of neurons in the somatodendritic and axonal domains [34]. Communication between distal neurons occurs in the third stage of neurogenesis, in which axons and dendrites are formed, synapses are formed according to electric activity patterns, and nonfunctional connections are eliminated [34]. This process begins with the neuronal secretion of neurotrophic factors. The nerve terminals of neighbor neurons capture these factors, internalize them, and transport them in a retrograde direction to the soma. Growth factors are constantly secreted to establish functional connections, as shown in Figure 3. Finally, the neurons that are not exposed to the secreted NTFs enter apoptosis and die (Figure 3).
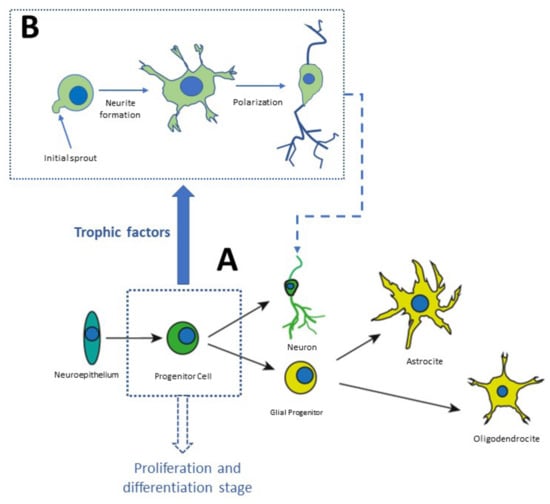
Figure 3.
Stages of neurodevelopment in vitro. Stem cells derived from the neuronal niches located at specific regions in the brain or in the olfactory neuroepithelium can be plated in Petri dishes. In culture, they proliferate and amplify the population of progenitor cells which differentiate into neurons or glial cells, as shown in panel (A). Panel (B) shows distinct phases of differentiation [35,36].
In a similar fashion as NTFs, pineal MEL and extrapineal MEL, which are released at specific brain regions, promote and improve neurogenesis, synaptogenesis, and growth of axons as well as dendrites. This claim will be addressed in the next paragraphs by describing the evidence about MEL effects on these neurodevelopmental processes and by answering our guiding question: Does MEL act on neuronal plasticity and the formation of new synapsis as the NTFs, even though it is a low mass indole molecule?
4. Evidence That Supports Melatonin Acts as a Neurotrophic Factor
Like the neurotrophic factors, MEL also participates in brain neuroplasticity and neurodevelopment [37,38]. One important feature of neurogenesis is the proliferation and survival of neuronal precursors. MEL in vitro at 10−6 M stimulates both processes [39]. Moreover, with MEL at 10−7 M, the formation of new neurons increased by approximately 70% in comparison to the control. On the other hand, the indolamine administered for 14 days to mice increases the number of “newborn” neurons in the dentate gyrus of the hippocampus [40]. However, there was no increase in the proliferation rate. Instead, an increase in the survival of neuronal precursors was observed during the proliferation process. By contrast, other authors observed increased proliferation of new neurons (158%) in the subventricular zone of the adult mouse brain and rat embryos, with concentrations ranging between 10−7 M and 10−6 M [39,41]. These effects were dose-dependent because higher MEL concentrations increased cell survival [39,41]. Environmental factors in combination with MEL also promote neuroplastic changes, for instance, physical activity plus MEL administration enhances neurogenesis in mice [42].
Recently, we found increased neurogenesis in human olfactory neuronal precursors incubated with MEL, ketamine, and a combination of these compounds. This effect was similar to what has been observed in relation to neurotrophic factors BDNF, Epithelial Grow Factor (EGF), and Fibroblast Growth Factor (FGF) in the dentate gyrus of the hippocampus. The increased neurogenesis was associated with the antidepressant-like behavior produced by these NTFs and the combination MEL/KET [43,44].
MEL also stimulates dendritogenesis, dendrite’s spine formation, dendritic arborization, and synaptogenesis [37,45]. The indolamine maintains the neuronal somatodendritic domain [46] through structural morphofunctional polarization thanks to cytoskeletal rearrangements. The three cytoskeletal components (microtubules, microfilaments, and intermediate filaments) are reorganized in the presence of this indolamine [46]. Hence, an important function of MEL for neurodevelopment is the modulation of the cytoskeletal organization. Cytoskeletal rearrangements play a crucial role in the formation and enlargement of axons and dendrites, as well as in the synaptic assembly (for review see [46,47]).
Regarding dendritogenesis, there is a vast literature about the effects of MEL in dendrite formation in organotypic cultures of hippocampus and animal models. Besides the survival-promoting effect, MEL also increases new neuron maturation in adult brains [48], by augmenting the ramifications of the dendritic trees in the hilus of the hippocampus. In this sense, the systemic administration of MEL to mice for 14 days produced an increase in the number of dendritic arborizations. Of utmost importance is that the loss of dendrites in epilepsy and Alzheimer’s (AD) diseases also occurs in this brain region [45,49] and that enhanced dendritic complexity, which is measured in arborizations in the dorsal-ventral regions of the dentate gyrus in male Balb/C mice, is associated with an antidepressant-like behavior [50]. MEL has also a neuroprotective effect on dendrite formation, for example, administration of MEL to rats, which were previously submitted to global ischemia, prevents the impairment of place learning and memory, both of which are integrated into the hippocampus. Just as importantly, MEL partly preserves the density of spines, mushroom spines, and dendrites pyramidal neurons, which are necessary for an adequate synapsis formation [51]. The indolamine not only counteracted and protected the neurons against apoptosis; it also prevented and reversed the dendritic arborization retraction [52], and fostered synapse formation in hippocampal organotypic cultures at 100 nM. This was evidenced by staining with an anti-synapsin antibody which labels synapsin, a protein localized in the presynapsis [45].
The evidence that supports the stimulation of axogenesis by MEL was obtained in cultured neurons and brain tissue. In cultured human olfactory neuronal precursors, MEL at 10−7 and 10−5 M increased by 15% the axonal formation [37]. Moreover, neurite formation—the primary neurodevelopment step that antecedes axogenesis—is stimulated by MEL in N1E-115 cells [47]. In addition, experiments in rodents support that MEL stimulates axogenesis. Daily administration of MEL at 8 mg/kg for one or six months to male Balb/C mice increased the granular cell layer in the dentate gyrus by 11–33% and the volume of suprapyramidal and infrapyramidal mossy fiber projection of granule neurons in the dentate gyrus of the hippocampus. Also, an increase in the volume of the CA3 region was observed in this work [53]. Recently, we observed spine formation in a clone of human olfactory neuronal precursors stimulated by MEL (preliminary data). The evidence described in this section suggests that MEL, analogous to NTFs, promotes the distinct stages of the neurodevelopmental process in the adult brain. Therefore, it is possible to consider that MEL acts as a neurotrophic factor. Although, in order to highlight its similarities to NTFs, it is relevant to present the pathways underlying neurogenesis, proliferation, and neuronal survival, pathways that reveal the role of MEL in these stages [44].
Mechanism of Action Involved in Neurogenesis and Neural Differentiation: Neurotrophic Factors and Melatonin
The biological activity of NTFs and MEL occurs thanks to their interaction with transmembrane receptor proteins. In the case of NTFs receptors, they have an extracellular domain, where the NTFs bind [12,26], and a cytosolic domain with catalytic and regulatory activity. In contrast, MEL receptors have seven transmembrane domains with an extracellular N-terminal and an intracellular C-terminal [54]. Given that the transactivation of Trk receptors is well documented [55,56], we will briefly describe the pathways involved in neurogenesis, proliferation, and survival. MEL participates in all these stages (Figure 4).
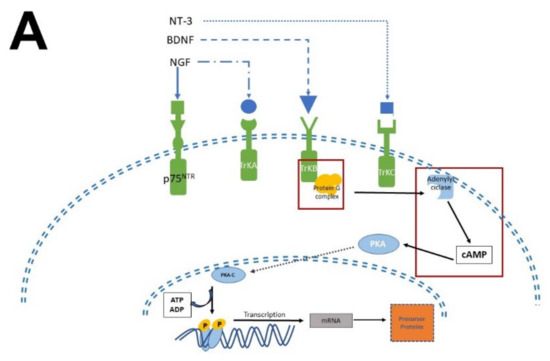
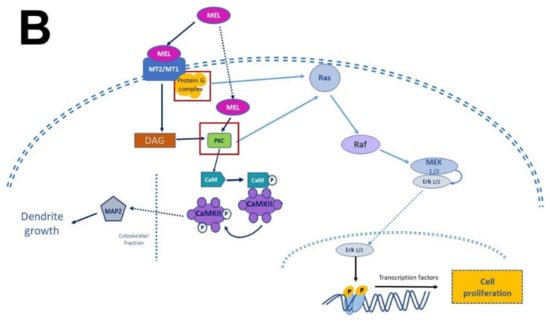
Figure 4.
Simplified scheme of neurotrophic factors and melatonin signaling pathways. Panel (A) shows the interaction of neurotrophins (NT-3, BDNF, NGF) with their receptors (p75NTR, TrkA, TrkB, TrkC), the activation of G proteins, which in turn activate protein kinase A (PKA) and protein kinase C (PKC) to generate transcription factors and precursor proteins that promote neuronal survival. Panel (B) display melatonin (MEL) signaling pathways that promote cell proliferation through binding to melatonin receptors (MT1, MT2), which activate the protein G complex and fibrosarcoma kinases (Raf), mitogen-activated protein kinase (MEK) and extracellular signal-regulated kinase (Erk). MEL can also cross plasmatic membranes and bind to calmodulin (CaM); the complex CaM/MEL binds to the enzyme calmodulin kinase II and enhance its activity to stimulate dendritic development and growth. Red squares in both panels indicate the common features in these pathways.
There are two types of receptors for NTFs: a low NTFs affinity pan-NTF receptor p75NTR that belongs to Tumoral Necrosis Factor (TNF) family, and the high-affinity tropomyosin-related kinase (Trk) receptors; both activate multiple signaling pathways [26,29,56,57]. NGF preferentially activates TrkA, BDNF, and NT-4 activates TrkB, while NT-3 activates TrkC [58]. Once NTFs are bound, the receptor dimerizes and phosphorylates itself on the cytoplasmic domain. When the receptor is phosphorylated, it forms the core of the binding site, adapting proteins and enzymes that mediate the rapid (seconds to minutes) activation of downstream signaling cascades. The main signaling pathways activated by Trk receptors are Ras, phosphoinositide-3 kinase (PI3K), and phospholipase (PLC-γ1); which in turn activate their downstream effectors [30], including stimulation of the mitogenic protein kinase (MAP) cascade and protein kinase B (Akt). Afterward, diacylglycerol (DAG) and inositol triphosphate (IP3) are produced by IP3K activity and PLCγ1, leading to calcium (Ca2+) mobilization. These pathways activate transcription factors involved in cell differentiation, survival, growth, and apoptosis, all of which are processes that occur in hours or days. In addition, NTFs modulate plasma membrane receptors, such as NMDA which are ionic channels permeable to Ca2+ and Na+; both Ca2+ and Na+ are crucial for neuronal function and differentiation [59,60].
Melatonin receptor 1 (MT1) and melatonin receptor 2 (MT2) share amino acid sequences. However, other sequences distinguish them as “fingerprints”. MT1 and MT2 receptors are coupled to Galpha proteins which are stimulatory (Gs) or inhibitory (Gi). MT1 and MT2 can be coupled to Gi which in turn inhibits the cAMP production or to a Gq which activates beta-type PLC-β leading to the production of PIP2, IP3, and DAG [54,61]. Similarly, to NTFs that activate TrKs receptors, MEL activates the same signaling pathways but through its binding with its specific receptors. In addition, MEL can cross the plasmatic membranes thanks to its amphiphilic features and binds to intracellular proteins such as calmodulin (CaM), calreticulin, and PKC, which transduce the biological responses triggered by Ca2+. Furthermore, MEL binds to the quinone reductase-2 (the MT3 receptor) in the cytosol and the orphan retinoid receptors in the nucleus [61,62].
The participation of MEL receptors in all stages of neurodevelopment has been evidenced by the administration of luzindole, a non-selective antagonist of these receptors [40,49,63,64], and also by using the pertussis toxin that uncouples adenylate cyclase, which inhibits the downstream signaling of MT1 and MT2 receptors [62]. The investigations of Sotthibundhu [41] and Tocharus [65] support these findings.
In addition, in the first stage of neurodevelopment, that is, in proliferation and cell survival, MEL receptors signaling downstream activate fibrosarcoma kinases, mitogen-activated protein kinase, and extracellular signal-regulated kinase 1 and 2 (Raf/MEK/ERK1/2). These kinases stimulate transcription factors that lead to gene expression [65]. Remarkably, similarly to MEL, this downstream pathway is activated by BDNF [29,56].
During neurodevelopment, there is a higher energy demand and an increased oxygen consumption with a higher ATP/ADP turnover rate. MEL enhances mitochondrial metabolism, increasing the expression of mitochondrial DNA. Therefore, the indolamine participates in the formation of mitochondrial mass and the development of oxidative phosphorylation complexes which, ultimately, confer energy to cells [66]. Furthermore, MEL as a free radical scavenger protects immature neurons by eliminating reactive oxygen species generated during this process [67]. The voltage-dependent L-type membrane channels are further MEL signaling pathways involved in neural differentiation. This molecule activates protein kinases A/B, which causes a transitory increase in the intracellular Ca2+ concentration and thus activates CaM [68]. In this regard, dendrite formation and arborization (which is a crucial step for the establishment of neuronal connections in neurodevelopment) are stimulated by MEL through autophosphorylation of calcium calmodulin kinase II (CAMKII), activation of PKC, and the phosphorylation of ERK 1/2. The involvement of these pathways is evident because dendrite formation stimulated by MEL is inhibited by KN-26, an antagonist of CaMKII, and by bisindolylmaleimide, an inhibitor of PKC [49]. Thus, data suggest that PKC acts upstream of CAMKII and downstream via MT1 and MT2 receptors [49,68].
Studies with cellular lines, such as human olfactory neuronal precursors, verified MEL effects in pluripotential cells [36,63,69,70]. These results concur with the effects observed in PC12 cells, mouse fibroblasts, and cells isolated from amniotic fluid [69]. Not only do NTFs elicit similar responses as MEL; MEL concentration determines the differentiation of pluripotential cells at a specific lineage. The indolamine drives differentiation to the neuronal lineage at 10−7 M (physiological concentrations), or to oligodendrocytes at 5 × 10−6 M. Hence, MEL concentration is critical to governing cell differentiation at specific lineages [71]. The mechanisms involved in MEL’s recruitment of cell linage are the activation of the tyrosine kinase signaling cascade, the ERK-mediated cell proliferation pathway, and the activation of intracellular Ca2+ and CAMKII [49]. In concordance with this evidence, we recently found that MEL, by binding to CaM in presence of Ca2+ and in an aqueous microenvironment, makes this protein adopt a specific structural conformation able to increase the activity of CAMKII, as shown in Figure 4 [72].
Finally, it is worth mentioning that the Jun-kinase pathway is one of the most important pathways activated by NTFs. The signaling of NFTs involves protein 53 (p53) activation and apoptosis. Among the various targets of p53 is the proapoptotic gene Bax [73]. Activation of one of the NTFs receptor p75NTR can also control the activity of Rho GTPase proteins, resulting in the inhibition of axonal growth and thus leading to selective pruning in neurodevelopment. In contrast, MEL modulates the apoptotic process via COX-2, p300, and the nuclear factor kappa β (NF-κβ) signaling, and, in addition, it suppresses p300 histone acetyltransferase (HAT) activity and p300-mediated NF-κβ acetylation [74]. The information recapitulated shows that the effects of MEL are comparable to other NTFs’ functions, such as the proliferation of neuronal precursors, their survival, and their consequent differentiation between neurons or glial cells. Both Mel and NTFs share IP3 and Ca2+-CaM signaling pathways.
5. Therapeutic Implications
As we have described, the regulation of neurodevelopment through mechanisms elicited by NTFs allows to claiming that the use of these molecules could be beneficial in the treatment of neuropsychiatric diseases. Moreover, neurogenesis studies have been a valuable tool for the development of new therapeutical drugs for neurodegenerative and affective disorders. In this context, it is important to mention that most antidepressants cause the release of NTFs that in turn stimulate neurogenesis in the hippocampus [75], synaptogenesis in the prefrontal cortex [76], and the production of neurogenic transcription factors [26,27,29,58,77,78,79]. One limitation in the treatment of affective disorders with NTFs is that they have poor pharmacokinetics and bioavailability, in addition to their inability to cross the blood-brain barrier [27]. Hence, several efforts have been directed to develop new molecules with other physicochemical features. In this regard, MEL as an amphiphilic molecule can cross the blood-brain barrier and its endogenous effects can be potentialized by exogen administration.
Evidence accumulated in the last decade shows that MEL antidepressant-like effects in rodents are associated with increased neurogenesis in the dentate gyrus of the hippocampus [11,80,81,82]. Administration of MEL reduced the immobility time in the forced swimming test (FST) and in the tail suspension test (TST) paradigms, providing evidence for the antidepressant effects of MEL [83,84]. In this respect, one must add that the effect of MEL was potentiated depending on the time of administration [82].
The neurogenesis stimulation by MEL is documented in rodents that were submitted to behavioral paradigms. For example, a study with BalbC mice reports that intraperitoneal administration of MEL for 14 days potentiates the effect of citalopram, an SSRIs antidepressant, and stimulates neurogenesis in the hippocampal dentate gyrus [85]. Moreover, we recently showed that triple administration of non-effective doses of MEL combined with low doses of ketamine elicits antidepressant-like effects in Swiss Webster mice and also increases neurogenesis in the dentate gyrus hippocampus [43]. Noteworthy is that this combination increased neurogenesis in a clone derived from human olfactory neural precursors similar to FGF, EGF, and BDNF neurotrophic factors [44]. In addition to being effective as an antidepressant in mouse models, MEL reduces the levels of proinflammatory interleukins and TNF-α release, likewise, MEL reduces oxidative stress and increases BDNF expression [83]. MEL is also useful in the treatment of other neuropathic or neuropsychiatric diseases, such as fibromyalgia, a chronic muscle-skeletal disorder characterized by generalized muscular pain and chronic fatigue, sleep disruption, depression, and anxiety. In a clinical study, MEL was found to decrease anxiety, pain, stiffness, and depressive symptoms similarly to fluoxetine in a concentration-depended manner [86]. The anti-inflammatory and antidepressant effects of MEL are exerted by the scavenger properties of MEL [87,88] and through its receptors [89,90]. For this reason, MEL could prove useful for treating inflammatory and pain-related diseases [90].
In neurodegenerative diseases, such as AD, Huntington’s, or Parkinson’s, MEL can increase cell survival in specific brain regions, such as the cerebral cortex [91,92]. To support this claim, it is important to mention that AD, which is a complex neurodegenerative disorder, is characterized by oxidative stress and developed by constant overproduction of free radicals coming from different pathways in areas where β-amyloid forms aggregate and trigger an imbalance of state homeostatic redox. Thus, MEL as a scavenger of free radicals modulates the neuroinflammatory response, diminishes the oxidative stress, and directly interacts with β-amyloid, preventing its aggregation [93]. Additionally, therapeutic MEL effects in AD are also possible thanks to the activation of the MT1 receptor and downstream pathways. Together they promote transcription proteins; for instance, the expression of the cAMP-response element binding protein gene (Creb1) and Bdnf lead to an increase in neurotrophic factors in the hippocampus and reduce memory impairment [80]. Learning and memory impairment in a model of AD in mice can be improved by MEL. Administered as a prophylactic, Mel can up-regulate CREB/BDNF signaling and cholinergic transmission in the prefrontal cortex [81].
Furthermore, MEL reduces age-related cognitive impairment [94]. Factors such as weight gain [95], constant exposure to stress [96], or even the use of medications for the treatment of chronic diseases [97,98] are risk factors in the development and progression of cognitive loss. For example, in a model of induced obesity in C57BL6 mice, cognitive functions were, on the one hand, evaluated with behavioral tests, and, on the other, the inflammatory cytokines associated with cognitive impairment and the BDNF levels as neurogenesis markers were also determined. In comparison to the control group, mice treated with MEL for eight weeks showed a reduction in inflammatory cytokines as well as an increase in BDNF [95]. Moreover, MEL can potentiate its effects when accompanied by physical activity [99]. The chronic administration of MEL also diminished proinflammatory cytokines levels in aged mice, demonstrating that its use may prevent memory impairment in aging [94]. In addition, MEL found in different food sources including walnuts, may be part of the mechanism involved in lowering inflammation and further preventing some age-related diseases, thus should be useful in the intake of these seeds [100].
Additionally, treatment with increasing doses of MEL is effective for improving the failed cognition induced by chronic stress exposure in rats, enhancing mood state, neurogenesis, and synaptogenesis [96]. The prolonged administration of MEL as a prophylactic treatment has been proven to enhance neurogenesis. For instance, in a chronic stress model of sleep deprivation, mice treated with the indolamine showed that in comparison to the non-treated groups, MEL promotes antiapoptotic and antioxidant molecules [101]. Hence, this molecule could alleviate neuroinflammation, cognitive, and mood impairments.
Indeed, the characteristics of MEL related to its structure as an antioxidant, the nature of its receptors, and its signaling pathways confer neuroprotective effects, for example, MEL increased the effect of antioxidant enzymes, maintained the balance of free radical production, and increased the production of BDNF in rodent models subjected to a neurotoxic administration scheme with methotrexate, which is used for the treatment of cancer [97], or valproate which is used in the treatment of epilepsy [98].
Furthermore, in neurodegenerative diseases like Multiple Sclerosis, MEL treatment increases neurogenesis and transcriptional markers that lead to neuron survival in the mouse hippocampus [102]. In a neurodegenerative animal model, the transplant of neurons cultured with MEL developed and increased proliferation and differentiation of neurons at the transplanted brain region and shows a clear contrast with the transplant without MEL treatment [66]. Despite the proposed therapeutic uses for MEL (Table 1), it is crucial to further explore its mechanisms and whether combining MEL with other substances could potentiate its effects. For example, the spinal or supraspinal MEL system might be involved in the modulation of neuropathic pain because MEL intrathecal and oral treatment has been shown to relieve the pain and deceased tactile allodynia induced by spinal denervation in rats; the antiallodynic MEL effect was here mediated by the MT2 extrapineal receptor and opioid receptors, possibly by increasing the β-endorphin release [103]. However, specific research is required in this field to indicate whether this type of therapy does not affect other brain regions and whether neural connections develop efficiently in the long term and without affecting the behavior of individuals.

Table 1.
MEL effects that support its neurotrophic actions.
6. Final Considerations: Is Melatonin a Neurotrophic Factor?
In this review, we collected evidence indicating that MEL is a factor that modulates neuronal survival, proliferation, differentiation, apoptosis, and the structural polarization of neurons (Table 1). Remarkably, NTFs and MEL act similarly although they are chemically distinct. The former are peptides in nature while the latter is an indolamine with a low molecular mass. In addition, MEL is endogenously produced like NTFs and its actions can be mediated by specific receptors that activate signaling pathways promoting these processes. In addition, and similarly to NTFs, MEL stimulates neurogenesis in organisms at the early stages of their development and in the adult brain, promoting dendritogenesis and the extension of dendritic trees, particularly in the hippocampus.
Despite considerable efforts made in this field by specialists, MEL’s mechanisms of action have not been thoroughly understood and more knowledge gaps arise when it is used in combination with other substances. For this reason, it is necessary to conduct more research at the molecular level to determine, firstly, whether these types of interactions exist and, secondly, to understand these mechanisms in depth together with the signaling pathways that are activated to conduct their functions.
7. Method
This review was conducted through a broad search of information using books and the PubMed and ScienceDirect databases. To detect original and review articles, the following search terms were used: MEL AND neurotrophic; MEL AND central nervous system; MEL AND trophic factor; MEL AND neurogenesis; MEL AND neuronal precursor cultures (NSCs); MEL AND neural differentiation. Titles and abstracts were read and inclusion and exclusion criteria were applied.
The inclusion criteria considered the following (1) articles from original and review journals, (2) books or book chapters with ISSN registration, (3) documents in English, (4) articles published in the last ten years, except for seminal or high-impact articles in the area, for which the year of publication was not considered. The following exclusion criteria were applied: (1) articles that did not present the search terms in the title and abstract; (2) repeated articles, (3) journal articles without an impact factor. One author (AMR) performed data extraction which was confirmed by others (GBK, RER, and GO). Search results are displayed in the description and in tables and figures.
Author Contributions
Conceptualization, investigation, methodology, discussion, and writing, G.B.-K.; design of the paper, investigation, figure preparation, and manuscript writing, A.M.-R.; conception proposed, investigation, and conceptualization, G.G.O.; investigation, R.E.-R. investigation, contributed to the research design, writing, figure preparation and reviewing of the paper, E.D.T.-S.; searching method design, S.C.-G. All authors have read and agreed to the published version of the manuscript.
Funding
Funding of this study was provided by “Consejo Nacional de Ciencia y Tecnología” (CONACyT) Grant No 290526 to G.B.-K. CONACyT had no further role in study design, nor in the writing of the article or and in the decision to submit the paper for publication.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Instituto Nacional de Psiquiatría Ramón de la Fuente Muñiz, protocol code: CEI/071/2017 on 9 October 2017.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lerner, A.B.; Case, J.D.; Takahashi, Y.; Lee, T.H.; Mori, W. Isolation of Melatonin, Pineal Factor That Lightens Melanocytes. J. Am. Chem. Soc. 1958, 80, 2587. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tan, D.X.; Zhou, Z.; Cruz, M.H.C.; Fuentes-Broto, L.; Galano, A. Phytomelatonin: Assisting Plants to Survive and Thrive. Molecules 2015, 20, 7396–7437. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.K.; Atigadda, V.R.; Brzeminski, P.; Fabisiak, A.; Tang, E.K.Y.; Tuckey, R.C.; Reiter, R.J.; Slominski, A.T. Detection of Serotonin, Melatonin, and Their Metabolites in Honey. ACS Food Sci. Technol. 2021, 1, 1228–1235. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.K.; Fabisiak, A.; Brzeminski, P.; Reiter, R.J.; Slominski, A.T. Serotonin, Melatonin and Their Precursors and Metabolites and Vitamin D3 Derivatives in Honey. Melatonin Res. 2022, 5, 374–380. [Google Scholar] [CrossRef]
- Zhao, D.; Yu, Y.; Shen, Y.; Liu, Q.; Zhao, Z.; Sharma, R.; Reiter, R.J. Melatonin Synthesis and Function: Evolutionary History in Animals and Plants. Front. Endocrinol. 2019, 10, 249. [Google Scholar] [CrossRef]
- Tricoire, H.; Møller, M.; Chemineau, P.; Malpaux, B. Origin of Cerebrospinal Fluid Melatonin and Possible Function in the Integration of Photoperiod. Reprod. Suppl. 2003, 61, 311–321. [Google Scholar] [CrossRef]
- Hardeland, R.; Pandi-Perumal, S.R.; Cardinali, D.P. Melatonin. Int. J. Biochem. Cell Biol. 2006, 38, 313–316. [Google Scholar] [CrossRef]
- Reiter, R.J. Melatonin: The Chemical Expression of Darkness. Mol. Cell. Endocrinol. 1991, 79, C153–C158. [Google Scholar] [CrossRef]
- Prakash, Y.; Thompson, M.A.; Meuchel, L.; Pabelick, C.M.; Mantilla, C.B.; Zaidi, S.; Martin, R.J. Neurotrophins in Lung Health and Disease. Expert Rev. Respir. Med. 2010, 4, 395–411. [Google Scholar] [CrossRef]
- Thoenen, H. Neurotrophins and Neuronal Plasticity. Science 1995, 270, 593–598. [Google Scholar] [CrossRef]
- Duman, R.S.; Nakagawa, S.; Malberg, J. Regulation of Adult Neurogenesis by Antidepressant Treatment. Neuropsychopharmacology 2001, 25, 836–844. [Google Scholar] [CrossRef]
- Landreth, G.E. Growth Factors. In Basic Neurochemistry: Molecular, Cellular and Medical Aspects; Siegel, G.J., Albers, R.W., Brady, S.T., Price, D.L., Eds.; Elsevier: Oxford, UK, 2006; pp. 471–484. ISBN 0-12-088397-X. [Google Scholar]
- Duman, R.S.; Monteggia, L.M. A Neurotrophic Model for Stress-Related Mood Disorders. Biol. Psychiatry 2006, 59, 1116–1127. [Google Scholar] [CrossRef] [PubMed]
- Sariola, H. The Neurotrophic Factors in Non-Neuronal Tissues. Cell. Mol. Life Sci. 2001, 58, 1061–1066. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.M.; Wu, H.C.; Sun, Z.G.; Lian, F.; Leung, P.C.K. Neurotrophins and Glial Cell Linederived Neurotrophic Factor in the Ovary: Physiological and Pathophysiological Implications. Hum. Reprod. Update 2019, 25, 224–242. [Google Scholar] [CrossRef]
- Skaper, S.D. Nerve Growth Factor: A Neuroimmune Crosstalk Mediator for All Seasons. Immunology 2017, 151, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Acuña-Castroviejo, D.; Escames, G.; Venegas, C.; Díaz-Casado, M.E.; Lima-Cabello, E.; López, L.C.; Rosales-Corral, S.; Tan, D.-X.X.; Reiter, R.J. Extrapineal Melatonin: Sources, Regulation, and Potential Functions. Cell. Mol. Life Sci. 2014, 71, 2997–3025. [Google Scholar] [CrossRef]
- Slominski, A.T.; Zmijewski, M.A.; Semak, I.; Kim, T.K.; Janjetovic, Z.; Slominski, R.M.; Zmijewski, J.W. Melatonin, Mitochondria, and the Skin. Cell. Mol. Life Sci. 2017, 74, 3913–3925. [Google Scholar] [CrossRef]
- Tan, D.X.; Manchester, L.C.; Hardeland, R.; Lopez-Burillo, S.; Mayo, J.C.; Sainz, R.M.; Reiter, R.J. Melatonin: A Hormone, a Tissue Factor, an Autocoid, a Paracoid, and an Antioxidant Vitamin. J. Pineal Res. 2003, 34, 75–78. [Google Scholar] [CrossRef]
- Mannino, G.; Pernici, C.; Serio, G.; Gentile, C.; Bertea, C.M. Melatonin and Phytomelatonin: Chemistry, Biosynthesis, Metabolism, Distribution and Bioactivity in Plants and Animals-An Overview. Int. J. Mol. Sci. 2021, 22, 9996. [Google Scholar] [CrossRef]
- Schomerus, C.; Korf, H.W. Mechanisms Regulating Melatonin Synthesis in the Mammalian Pineal Organ. Ann. N. Y. Acad. Sci. 2005, 1057, 372–383. [Google Scholar] [CrossRef]
- Leone, A.M.; Francis, P.L.; McKenzie-Gray, B. Rapid and Simple Synthesis for the Sulphate Esters of 6-Hydroxy-Melatonin and N-Acetyl-Serotonin. J. Pineal Res. 1988, 5, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Skene, D.J.; Papagiannidou, E.; Hashemi, E.; Snelling, J.; Lewis, D.F.V.; Fernandez, M.; Ioannides, C. Contribution of CYP1A2 in the Hepatic Metabolism of Melatonin: Studies with Isolated Microsomal Preparations and Liver Slices. J. Pineal Res. 2001, 31, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Hardeland, R. Melatonin Metabolism in the Central Nervous System. Curr. Neuropharmacol. 2010, 8, 168. [Google Scholar] [CrossRef] [PubMed]
- Francis, P.L.; Leone, A.M.; Young, I.M.; Stovell, P.; Silman, R.E. Gas Chromatographic-Mass Spectrometric Assay for 6-Hydroxymelatonin Sulfate and 6-Hydroxymelatonin Glucuronide in Urine. Clin. Chem. 1987, 33, 453–457. [Google Scholar] [CrossRef]
- Keefe, K.; Sheikh, I.; Smith, G. Targeting Neurotrophins to Specific Populations of Neurons: NGF, BDNF, and NT-3 and Their Relevance for Treatment of Spinal Cord Injury. Int. J. Mol. Sci. 2017, 18, 548. [Google Scholar] [CrossRef]
- Skaper, S.D. Neurotrophic Factors: An Overview. In Methods in Molecular Biology; Humana Press Inc.: Totowa, NJ, USA, 2018; Volume 1727, pp. 1–17. [Google Scholar]
- Jackman, S.L.; Regehr, W.G. The Mechanisms and Functions of Synaptic Facilitation. Neuron 2017, 94, 447–464. [Google Scholar] [CrossRef]
- Song, M.; Martinowich, K.; Lee, F.S. BDNF at the Synapse: Why Location Matters. Mol. Psychiatry 2017, 22, 1370–1375. [Google Scholar] [CrossRef]
- Huang, E.J.; Reichardt, L.F. Neurotrophins: Roles in Neuronal Development and Function. Annu. Rev. Neurosci. 2001, 24, 677–736. [Google Scholar] [CrossRef]
- Levi-Montalcini, R. The Nerve Growth Factor 35 Years Later. Science 1987, 237, 1154–1162. [Google Scholar] [CrossRef]
- Alvarez-Buylla, A.; García-Verdugo, J.M.; Tramontin, A.D. A Unified Hypothesis on the Lineage of Neural Stem Cells. Nat. Rev. Neurosci. 2001, 2, 287–293. [Google Scholar] [CrossRef]
- Nualart, F. Unconventional Neurogenic Niches and Neurogenesis Modulation by Vitamins. J. Stem Cell Res. Ther. 2014, 4, 3. [Google Scholar] [CrossRef] [PubMed]
- Alberts, B.; Bray, D.; Lewis, J.; Raff, M.; Roberts, K.; Watson, D.J. Biología Molecular de La Célula, 3rd ed.; Durfort, M., Llobera, M., Eds.; Ediciones Omega: Barcelona, Spain, 2002; ISBN 84-282-1011-X. [Google Scholar]
- Da Silva, J.S.; Dotti, C.G. Breaking the Neuronal Sphere: Regulation of the Actin Cytoskeleton in Neuritogenesis. Nat. Rev. Neurosci. 2002, 3, 694–704. [Google Scholar] [CrossRef] [PubMed]
- Shu, T.; Wu, T.; Pang, M.; Liu, C.; Wang, X.; Wang, J.; Liu, B.; Rong, L. Effects and Mechanisms of Melatonin on Neural Differentiation of Induced Pluripotent Stem Cells. Biochem. Biophys. Res. Commun. 2016, 474, 566–571. [Google Scholar] [CrossRef] [PubMed]
- Galván-Arrieta, T.; Trueta, C.; Cercós, M.G.; Valdés-Tovar, M.; Alarcón, S.; Oikawa, J.; Zamudio-Meza, H.; Benítez-King, G. The Role of Melatonin in the Neurodevelopmental Etiology of Schizophrenia: A Study in Human Olfactory Neuronal Precursors. J. Pineal Res. 2017, 63, e12421. [Google Scholar] [CrossRef]
- Valdés-Tovar, M.; Estrada-Reyes, R.; Solís-Chagoyán, H.; Argueta, J.; Dorantes-Barrón, A.M.; Quero-Chávez, D.; Cruz-Garduño, R.; Cercós, M.G.; Trueta, C.; Oikawa-Sala, J.; et al. Circadian Modulation of Neuroplasticity by Melatonin: A Target in the Treatment of Depression. Br. J. Pharmacol. 2018, 175, 3200–3208. [Google Scholar] [CrossRef] [PubMed]
- Moriya, T.; Horie, N.; Mitome, M.; Shinohara, K. Melatonin Influences the Proliferative and Differentiative Activity of Neural Stem Cells. J. Pineal Res. 2007, 42, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Rodríguez, G.; Klempin, F.; Babu, H.; Benítez-King, G.; Kempermann, G. Melatonin Modulates Cell Survival of New Neurons in the Hippocampus of Adult Mice. Neuropsychopharmacology 2009, 34, 2180–2191. [Google Scholar] [CrossRef]
- Sotthibundhu, A.; Phansuwan-Pujito, P.; Govitrapong, P. Melatonin Increases Proliferation of Cultured Neural Stem Cells Obtained from Adult Mouse Subventricular Zone. J. Pineal Res. 2010, 49, 291–300. [Google Scholar] [CrossRef]
- Liu, J.; Somera-Molina, K.C.; Hudson, R.L.; Dubocovich, M.L. Melatonin Potentiates Running Wheel-Induced Neurogenesis in the Dentate Gyrus of Adult C3H/HeN Mice Hippocampus. J. Pineal Res. 2013, 54, 222–231. [Google Scholar] [CrossRef]
- Estrada-Reyes, R.; Quero-Chávez, D.B.; Trueta, C.; Miranda, A.; Valdés-Tovar, M.; Alarcón-Elizalde, S.; Oikawa-Sala, J.; Argueta, J.; Constantino-Jonapa, L.A.; Muñoz-Estrada, J.; et al. Low Doses of Ketamine and Melatonin in Combination Produce Additive Antidepressant-like Effects in Mice. Int. J. Mol. Sci. 2021, 22, 9225. [Google Scholar] [CrossRef]
- Estrada-Reyes, R.; Quero-Chávez, D.B.; Alarcón-Elizalde, S.; Cercós, M.G.; Trueta, C.; Constantino-Jonapa, L.A.; Oikawa-Sala, J.; Argueta, J.; Cruz-Garduño, R.; Dubocovich, M.L.; et al. Antidepressant Low Doses of Ketamine and Melatonin in Combination Produce Additive Neurogenesis in Human Olfactory Neuronal Precursors. Molecules 2022, 27, 5650. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Alonso, A.; Ramírez-Rodríguez, G.; Benítez-King, G. Melatonin Increases Dendritogenesis in the Hilus of Hippocampal Organotypic Cultures. J. Pineal Res. 2012, 52, 427–436. [Google Scholar] [CrossRef]
- Benítez-King, G. Melatonin as a Cytoskeletal Modulator: Implications for Cell Physiology and Disease. J. Pineal Res. 2006, 40, 1–9. [Google Scholar] [CrossRef]
- Bellon, A.; Ortíz-López, L.; Ramírez-Rodríguez, G.; Antón-Tay, F.; Benítez-King, G. Melatonin Induces Neuritogenesis at Early Stages in N1E-115 Cells through Actin Rearrangements via Activation of Protein Kinase C and Rho-Associated Kinase. J. Pineal Res. 2007, 42, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Rodriguez, G.; Ortíz-López, L.; Domínguez-Alonso, A.; Benítez-King, G.A.; Kempermann, G. Chronic Treatment with Melatonin Stimulates Dendrite Maturation and Complexity in Adult Hippocampal Neurogenesis of Mice. J. Pineal Res. 2011, 50, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Alonso, A.; Valdés-Tovar, M.; Solís-Chagoyán, H.; Benítez-King, G. Melatonin Stimulates Dendrite Formation and Complexity in the Hilar Zone of the Rat Hippocampus: Participation of the Ca++/Calmodulin Complex. Int. J. Mol. Sci. 2015, 16, 1907–1927. [Google Scholar] [CrossRef]
- Ramírez-Rodríguez, G.B.; Palacios-Cabriales, D.M.; Ortiz-López, L.; Estrada-Camarena, E.M.; Vega-Rivera, N.M. Melatonin Modulates Dendrite Maturation and Complexity in the Dorsal-and Ventral-Dentate Gyrus Concomitantly with Its Antidepressant-like Effect in Male Balb/c Mice. Int. J. Mol. Sci. 2020, 21, 1724. [Google Scholar] [CrossRef]
- González-Burgos, I.; Letechipía-Vallejo, G.; López-Loeza, E.; Moralí, G.; Cervantes, M. Long-Term Study of Dendritic Spines from Hippocampal CA1 Pyramidal Cells, after Neuroprotective Melatonin Treatment Following Global Cerebral Ischemia in Rats. Neurosci. Lett. 2007, 423, 162–166. [Google Scholar] [CrossRef]
- Solís-Chagoyán, H.; Domínguez-Alonso, A.; Valdés-Tovar, M.; Argueta, J.; Sánchez-Florentino, Z.A.; Calixto, E.; Benítez-King, G. Melatonin Rescues the Dendrite Collapse Induced by the Pro-Oxidant Toxin Okadaic Acid in Organotypic Cultures of Rat Hilar Hippocampus. Molecules 2020, 25, 5508. [Google Scholar] [CrossRef]
- Ramírez-Rodríguez, G.B.; Olvera-Hernández, S.; Vega-Rivera, N.M.; Ortiz-López, L. Melatonin Influences Structural Plasticity in the Axons of Granule Cells in the Dentate Gyrus of Balb/C Mice. Int. J. Mol. Sci. 2018, 20, 73. [Google Scholar] [CrossRef]
- Jockers, R.; Delagrange, P.; Dubocovich, M.L.; Markus, R.P.; Renault, N.; Tosini, G.; Cecon, E.; Zlotos, D.P. Update on Melatonin Receptors: IUPHAR Review 20. Br. J. Pharmacol. 2016, 173, 2702–2725. [Google Scholar] [CrossRef] [PubMed]
- Delcourt, N.; Bockaert, J.; Marin, P. GPCR-Jacking: From a New Route in RTK Signalling to a New Concept in GPCR Activation. Trends Pharmacol. Sci. 2007, 28, 602–607. [Google Scholar] [CrossRef] [PubMed]
- Lemmon, M.A.; Schlessinger, J. Cell Signaling by Receptor Tyrosine Kinases. Cell 2010, 141, 1117–1134. [Google Scholar] [CrossRef] [PubMed]
- Deinhardt, K.; Kim, T.; Spellman, D.S.; Mains, R.E.; Eipper, B.A.; Neubert, T.A.; Chao, M.V.; Hempstead, B.L. Neuronal Growth Cone Retraction Relies on Proneurotrophin Receptor Signaling through Rac. Sci. Signal. 2011, 4, ra82. [Google Scholar] [CrossRef]
- Barford, K.; Deppmann, C.; Winckler, B. The Neurotrophin Receptor Signaling Endosome: Where Trafficking Meets Signaling. Dev. Neurobiol. 2017, 77, 405–418. [Google Scholar] [CrossRef] [PubMed]
- Caldeira, M.V.; Melo, C.V.; Pereira, D.B.; Carvalho, R.F.; Carvalho, A.L.; Duarte, C.B. BDNF Regulates the Expression and Traffic of NMDA Receptors in Cultured Hippocampal Neurons. Mol. Cell. Neurosci. 2007, 35, 208–219. [Google Scholar] [CrossRef]
- Schinder, A.F.; Berninger, B.; Poo, M. ming Postsynaptic Target Specificity of Neurotrophin-Induced Presynaptic Potentiation. Neuron 2000, 25, 151–163. [Google Scholar] [CrossRef]
- Dubocovich, M.L.; Delagrange, P.; Krause, D.N.; Sugden, D.; Cardinali, D.P.; Olcese, J. International Union of Basic and Clinical Pharmacology. LXXV. Nomenclature, Classification, and Pharmacology of G Protein-Coupled Melatonin Receptors. Pharmacol. Rev. 2010, 63, 343–380. [Google Scholar] [CrossRef]
- Hardeland, R. Melatonin: Signaling Mechanisms of a Pleiotropic Agent. Biofactors 2009, 35, 183–192. [Google Scholar] [CrossRef]
- Liu, D.; Wei, N.; Man, H.Y.; Lu, Y.; Zhu, L.Q.; Wang, J.Z. The MT2 Receptor Stimulates Axonogenesis and Enhances Synaptic Transmission by Activating Akt Signaling. Cell Death Differ. 2015, 22, 583–596. [Google Scholar] [CrossRef]
- Liu, J.; Clough, S.J.; Dubocovich, M.L. Role of the MT1 and MT2 Melatonin Receptors in Mediating Depressive- and Anxiety-like Behaviors in C3H/HeN Mice. Genes Brain Behav. 2017, 16, 546–553. [Google Scholar] [CrossRef] [PubMed]
- Tocharus, C.; Puriboriboon, Y.; Junmanee, T.; Tocharus, J.; Ekthuwapranee, K.; Govitrapong, P. Melatonin Enhances Adult Rat Hippocampal Progenitor Cell Proliferation via ERK Signaling Pathway through Melatonin Receptor. Neuroscience 2014, 275, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Mendivil-Perez, M.; Soto-Mercado, V.; Guerra-Librero, A.; Fernandez-Gil, B.I.; Florido, J.; Shen, Y.Q.; Tejada, M.A.; Capilla-Gonzalez, V.; Rusanova, I.; Garcia-Verdugo, J.M.; et al. Melatonin Enhances Neural Stem Cell Differentiation and Engraftment by Increasing Mitochondrial Function. J. Pineal Res. 2017, 63, e12415. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.X.; Manchester, L.C.; Esteban-Zubero, E.; Zhou, Z.; Reiter, R.J. Melatonin as a Potent and Inducible Endogenous Antioxidant: Synthesis and Metabolism. Molecules 2015, 20, 18886–18906. [Google Scholar] [CrossRef]
- De Faria Poloni, J.; Feltes, B.C.; Bonatto, D. Melatonin as a Central Molecule Connecting Neural Development and Calcium Signaling. Funct. Integr. Genom. 2011, 11, 383–388. [Google Scholar] [CrossRef]
- Phonchai, R.; Phermthai, T.; Kitiyanant, N.; Suwanjang, W.; Kotchabhakdi, N.; Chetsawang, B. Potential Effects and Molecular Mechanisms of Melatonin on the Dopaminergic Neuronal Differentiation of Human Amniotic Fluid Mesenchymal Stem Cells. Neurochem. Int. 2019, 124, 82–93. [Google Scholar] [CrossRef]
- Ortiz-López, L.; González-Olvera, J.J.; Vega-Rivera, N.M.; García-Anaya, M.; Carapia-Hernández, A.K.; Velázquez-Escobar, J.C.; Ramírez-Rodríguez, G.B. Human Neural Stem/Progenitor Cells Derived from the Olfactory Epithelium Express the TrkB Receptor and Migrate in Response to BDNF. Neuroscience 2017, 355, 84–100. [Google Scholar] [CrossRef]
- Ghareghani, M.; Sadeghi, H.; Zibara, K.; Danaei, N.; Azari, H.; Ghanbari, A. Melatonin Increases Oligodendrocyte Differentiation in Cultured Neural Stem Cells. Cell. Mol. Neurobiol. 2017, 37, 1319–1324. [Google Scholar] [CrossRef]
- Argueta, J.; Solís-Chagoyán, H.; Estrada-Reyes, R.; Constantino-Jonapa, L.A.; Oikawa-Sala, J.; Velázquez-Moctezuma, J.; Benítez-King, G. Further Evidence of the Melatonin Calmodulin Interaction: Effect on CaMKII Activity. Int. J. Mol. Sci. 2022, 23, 2479. [Google Scholar] [CrossRef]
- Assimakopoulou, M.; Kondyli, M.; Gatzounis, G.; Maraziotis, T.; Varakis, J. Neurotrophin Receptors Expression and JNK Pathway Activation in Human Astrocytomas. BMC Cancer 2007, 7, 202. [Google Scholar] [CrossRef]
- Wang, J.; Xiao, X.; Zhang, Y.; Shi, D.; Chen, W.; Fu, L.; Liu, L.; Xie, F.; Kang, T.; Huang, W.; et al. Simultaneous Modulation of COX-2, P300, Akt, and Apaf-1 Signaling by Melatonin to Inhibit Proliferation and Induce Apoptosis in Breast Cancer Cells. J. Pineal Res. 2012, 53, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Santarelli, L.; Saxe, M.; Gross, C.; Surget, A.; Battaglia, F.; Dulawa, S.; Weisstaub, N.; Lee, J.; Duman, R.; Arancio, O.; et al. Requirement of Hippocampal Neurogenesis for the Behavioral Effects of Antidepressants. Science 2003, 301, 805–809. [Google Scholar] [CrossRef] [PubMed]
- Hare, B.D.; Duman, R.S. Prefrontal Cortex Circuits in Depression and Anxiety: Contribution of Discrete Neuronal Populations and Target Regions. Mol. Psychiatry 2020, 25, 2742–2758. [Google Scholar] [CrossRef] [PubMed]
- Phillips, C.; Baktir, M.A.; Srivatsan, M.; Salehi, A. Neuroprotective Effects of Physical Activity on the Brain: A Closer Look at Trophic Factor Signaling. Front. Cell. Neurosci. 2014, 8, 170. [Google Scholar] [CrossRef]
- Kowiański, P.; Lietzau, G.; Czuba, E.; Waśkow, M.; Steliga, A.; Moryś, J. BDNF: A Key Factor with Multipotent Impact on Brain Signaling and Synaptic Plasticity. Cell. Mol. Neurobiol. 2018, 38, 579–593. [Google Scholar] [CrossRef]
- Numakawa, T.; Odaka, H.; Adachi, N. Actions of Brain-Derived Neurotrophin Factor in the Neurogenesis and Neuronal Function, and Its Involvement in the Pathophysiology of Brain Diseases. Int. J. Mol. Sci. 2018, 19, 3650. [Google Scholar] [CrossRef]
- Labban, S.; Alshehri, F.S.; Kurdi, M.; Alatawi, Y.; Alghamdi, B.S. Melatonin Improves Short-Term Spatial Memory in a Mouse Model of Alzheimer’s Disease. Degener. Neurol. Neuromuscul. Dis. 2021, 11, 15–27. [Google Scholar] [CrossRef]
- Labban, S.; Alghamdi, B.S.; Alshehri, F.S.; Kurdi, M. Effects of Melatonin and Resveratrol on Recognition Memory and Passive Avoidance Performance in a Mouse Model of Alzheimer’s Disease. Behav. Brain Res. 2021, 402, 113100. [Google Scholar] [CrossRef]
- Guaiana, G.; Gupta, S.; Chiodo, D.; Davies, S.J.; Haederle, K.; Koesters, M. Agomelatine versus Other Antidepressive Agents for Major Depression. Cochrane Database Syst. Rev. 2013, 2013, CD008851. [Google Scholar] [CrossRef]
- Taniguti, E.H.; Ferreira, Y.S.; Stupp, I.J.V.; Fraga-Junior, E.B.; Mendonça, C.B.; Rossi, F.L.; Ynoue, H.N.; Doneda, D.L.; Lopes, L.; Lima, E.; et al. Neuroprotective Effect of Melatonin against Lipopolysaccharide-Induced Depressive-like Behavior in Mice. Physiol. Behav. 2018, 188, 270–275. [Google Scholar] [CrossRef]
- Estrada-Reyes, R.; Valdés-Tovar, M.; Arrieta-Baez, D.; Dorantes-Barrón, A.M.; Quero-Chávez, D.; Solís-Chagoyán, H.; Argueta, J.; Dubocovich, M.L.; Benítez-King, G. The Timing of Melatonin Administration Is Crucial for Its Antidepressant-like Effect in Mice. Int. J. Mol. Sci. 2018, 19, 2278. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Rodríguez, G.; Vega-Rivera, N.M.; Oikawa-Sala, J.; Gómez-Sánchez, A.; Ortiz-López, L.; Estrada-Camarena, E. Melatonin Synergizes with Citalopram to Induce Antidepressant-like Behavior and to Promote Hippocampal Neurogenesis in Adult Mice. J. Pineal Res. 2014, 56, 450–461. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.A.R.; Al-Khalifa, I.I.; Jasim, N.A.; Gorial, F.I. Adjuvant Use of Melatonin for Treatment of Fibromyalgia. J. Pineal Res. 2011, 50, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Cuzzocrea, S.; Zingarelli, B.; Gilad, E.; Hake, P.; Salzman, A.L.; Szabó, C. Protective Effect of Melatonin in Carrageenan-Induced Models of Local Inflammation: Relationship to Its Inhibitory Effect on Nitric Oxide Production and Its Peroxynitrite Scavenging Activity. J. Pineal Res. 1997, 23, 106–116. [Google Scholar] [CrossRef]
- Bilici, D.; Akpinar, E.; Kiziltunç, A. Protective effect of melatonin in carrageenan-induced acute local inflammation. Pharmacol. Res. 2002, 46, 133–139. [Google Scholar] [CrossRef]
- Posa, L.; De Gregorio, D.; Gobbi, G.; Comai, S. Targeting Melatonin MT2 Receptors: A Novel Pharmacological Avenue for Inflammatory and Neuropathic Pain. Curr. Med. Chem. 2018, 25, 3866–3882. [Google Scholar] [CrossRef]
- Posa, L.; Lopez-Canul, M.; Rullo, L.; De Gregorio, D.; Dominguez-Lopez, S.; Kaba Aboud, M.; Caputi, F.F.; Candeletti, S.; Romualdi, P.; Gobbi, G. Nociceptive Responses in Melatonin MT2 Receptor Knockout Mice Compared to MT1 and Double MT1/MT2 Receptor Knockout Mice. J. Pineal Res. 2020, 69, e12671. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, T.; Lee, T.H. Cellular Mechanisms of Melatonin: Insight from Neurodegenerative Diseases. Biomolecules 2020, 10, 1158. [Google Scholar] [CrossRef]
- Wander, C.M.; Song, J. The Neurogenic Niche in Alzheimer’s Disease. Neurosci. Lett. 2021, 762, 136109. [Google Scholar] [CrossRef]
- Rosales-Corral, S.; Acuna-Castroviejo, D.; Tan, D.X.; López-Armas, G.; Cruz-Ramos, J.; Munoz, R.; Melnikov, V.G.; Manchester, L.C.; Reiter, R.J. Accumulation of Exogenous Amyloid- Beta Peptide in Hippocampal Mitochondria Causes Their Dysfunction: A Protective Role for Melatonin. Oxidative Med. Cell. Longev. 2012, 2012, 843649. [Google Scholar] [CrossRef]
- Permpoonputtana, K.; Tangweerasing, P.; Mukda, S.; Boontem, P.; Nopparat, C.; Govitrapong, P. Long-Term Administration of Melatonin Attenuates Neuroinflammation in the Aged Mouse Brain. EXCLI J. 2018, 17, 634–646. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, S.; Salari, A.-A.; Abedi, A.; Mohammadi, P.; Amani, M. Melatonin Treatment Improves Cognitive Deficits by Altering Inflammatory and Neurotrophic Factors in the Hippocampus of Obese Mice. Physiol. Behav. 2022, 254, 113919. [Google Scholar] [CrossRef] [PubMed]
- Madhu, L.N.; Kodali, M.; Attaluri, S.; Shuai, B.; Melissari, L.; Rao, X.; Shetty, A.K. Melatonin Improves Brain Function in a Model of Chronic Gulf War Illness with Modulation of Oxidative Stress, NLRP3 Inflammasomes, and BDNF-ERK-CREB Pathway in the Hippocampus. Redox Biol. 2021, 43, 101973. [Google Scholar] [CrossRef] [PubMed]
- Suwannakot, K.; Sritawan, N.; Naewla, S.; Aranarochana, A.; Sirichoat, A.; Pannangrong, W.; Wigmore, P.; Welbat, J.U. Melatonin Attenuates Methotrexate-Induced Reduction of Antioxidant Activity Related to Decreases of Neurogenesis in Adult Rat Hippocampus and Prefrontal Cortex. Oxidative Med. Cell. Longev. 2022, 2022, 1596362. [Google Scholar] [CrossRef] [PubMed]
- Aranarochana, A.; Sirichoat, A.; Pannangrong, W.; Wigmore, P.; Welbat, J.U. Melatonin Ameliorates Valproic Acid-Induced Neurogenesis Impairment: The Role of Oxidative Stress in Adult Rats. Oxidative Med. Cell. Longev. 2021, 2021, 9997582. [Google Scholar] [CrossRef]
- Sugiyama, A.; Kato, H.; Takakura, H.; Osawa, S.; Maeda, Y.; Izawa, T. Effects of Physical Activity and Melatonin on Brain-derived Neurotrophic Factor and Cytokine Expression in the Cerebellum of High-fat Diet-fed Rats. Neuropsychopharmacol. Rep. 2020, 40, 291–296. [Google Scholar] [CrossRef]
- Mateș, L.; Popa, D.-S.; Rusu, M.E.; Fizeșan, I.; Leucuța, D. Walnut Intake Interventions Targeting Biomarkers of Metabolic Syndrome and Inflammation in Middle-Aged and Older Adults: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Antioxidants 2022, 11, 1412. [Google Scholar] [CrossRef]
- López-Armas, G.; Flores-Soto, M.E.; Chaparro-Huerta, V.; Jave-Suarez, L.F.; Soto-Rodríguez, S.; Rusanova, I.; Acuña-Castroviejo, D.; González-Perez, O.; González-Castañeda, R.E. Prophylactic Role of Oral Melatonin Administration on Neurogenesis in Adult Balb/C Mice during REM Sleep Deprivation. Oxidative Med. Cell. Longev. 2016, 2016, 2136902. [Google Scholar] [CrossRef]
- Kim, W.; Hahn, K.R.; Jung, H.Y.; Kwon, H.J.; Nam, S.M.; Kim, J.W.; Park, J.H.; Yoo, D.Y.; Kim, D.W.; Won, M.H.; et al. Melatonin Ameliorates Cuprizone-Induced Reduction of Hippocampal Neurogenesis, Brain-Derived Neurotrophic Factor, and Phosphorylation of Cyclic AMP Response Element-Binding Protein in the Mouse Dentate Gyrus. Brain Behav. 2019, 9, e01388. [Google Scholar] [CrossRef]
- Ambriz-Tututi, M.; Granados-Soto, V. Oral and Spinal Melatonin Reduces Tactile Allodynia in Rats via Activation of MT2 and Opioid Receptors. Pain 2007, 132, 273–280. [Google Scholar] [CrossRef]
- Ortiz-López, L.; Pérez-Beltran, C.; Ramírez-Rodríguez, G. Chronic Administration of a Melatonin Membrane Receptor Antagonist, Luzindole, Affects Hippocampal Neurogenesis without Changes in Hopelessness-like Behavior in Adult Mice. Neuropharmacology 2016, 103, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Sotthibundhu, A.; Ekthuwapranee, K.; Govitrapong, P. Comparison of Melatonin with Growth Factors in Promoting Precursor Cells Proliferation in Adult Mouse Subventricular Zone. EXCLI J. 2016, 15, 829–841. [Google Scholar] [CrossRef] [PubMed]
- Sung, J.Y.; Bae, J.H.; Lee, J.H.; Kim, Y.N.; Kim, D.K. The Melatonin Signaling Pathway in a Long-Term Memory In Vitro Study. Molecules 2018, 23, 737. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).