Influence of Fermentation Time on the Phenolic Compounds, Vitamin C, Color and Antioxidant Activity in the Winemaking Process of Blueberry (Vaccinium corymbosum) Wine Obtained by Maceration
Abstract
1. Introduction
2. Results and Discussion
2.1. Bioactive Compounds of Berries
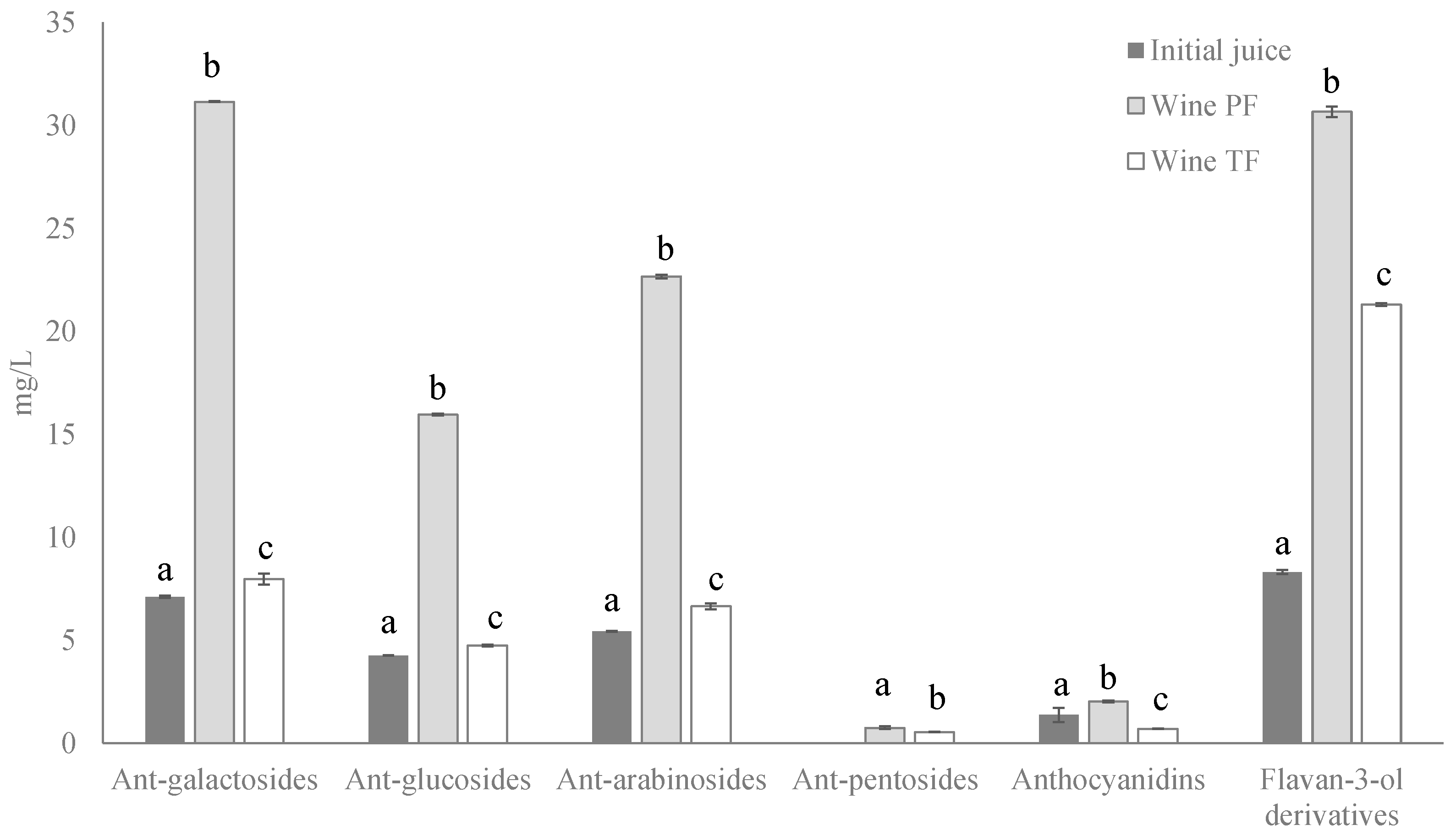
2.2. Bioactive Compounds of Wines
3. Material and Methods
3.1. Material
3.2. Reagents
3.3. Methods of Sample Preparation
3.3.1. Extraction Procedure
3.3.2. Blueberry Winemaking
3.4. Separation and Extraction of Anthocyanins from Juice/Wine
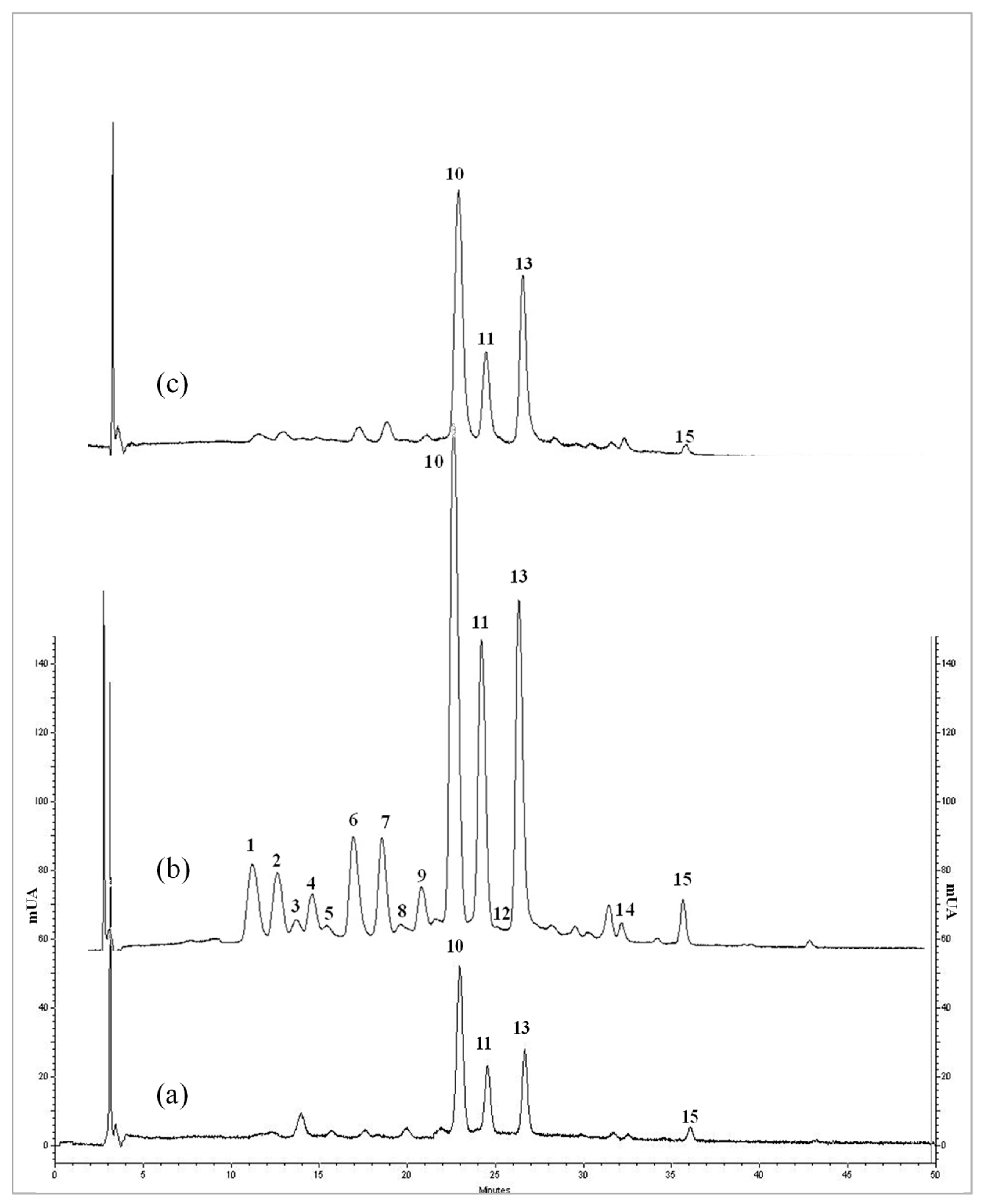
3.5. Identification and Quantification by HPLC-DAD of Anthocyanins
3.6. Identification and Quantification by HPLC-F of Flavan-3-ol Derivatives and Resveratrol
3.7. Vitamin C
3.8. Reducing Sugars
3.9. Spectrophotometric Determinations
3.9.1. Color
3.9.2. Total Tannins
3.9.3. Antioxidant Activity
3.9.4. Ethanol Content of Wines
3.10. Statistical Procedures
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Müller, D.; Schantz, M.; Richling, E. High performance liquid chromatography analysis of anthocyanins in bilberries (Vaccinium myrtillus L.), blueberries (Vaccinium corymbosum L.), and corresponding juices. J. Food Sci. 2014, 77, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Rashidinejad, A. Blueberries. In Nutritional Composition and Antioxidant Properties of Fruits and Vegetables; Jaiswal, A.K., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 695–708. [Google Scholar] [CrossRef]
- Määttä-Riihinen, K.R.; Kähkönen, M.P.; Törrönen, A.R.; Heinonen, I.M. Catechins and procyanidins in berries of Vaccinium species and their antioxidant activity. J. Agric. Food Chem. 2005, 53, 8485–8491. [Google Scholar] [CrossRef]
- Prior, R.L.; Cao, G.; Martin, A.; Sofic, E.; Mcewen, J.; O’brien, C. Antioxidant capacity as influenced by total phenolic and anthocyanin content, maturity, and variety of Vaccinium species. J. Agric. Food Chem. 1998, 46, 2686–2693. [Google Scholar] [CrossRef]
- Wu, X.; Prior, R. Systematic identification and characterization of anthocyanins by HPLC-ESI-MS/MS in common foods in the United States: Fruits and berries. J. Agric. Food Chem. 2005, 53, 2589–2599. [Google Scholar] [CrossRef]
- Pertuzatti, P.B.; Barcia, M.T.; Gómez-Alonso, S.; Godoy, H.T.; Hermosin-Gutierrez, I. Phenolics Profiling by HPLC-DAD-ESI-MSn Aided by Principal Component Analysis to Classify Rabbiteye and Highbush Blueberries. Food Chem. 2021, 340, 127958. [Google Scholar] [CrossRef]
- Prior, R.L.; Lazarus, S.A.; Cao, G.; Muccitelli, H.; Hammerstone, J.F. Identification of procyanidins and anthocyanins in blueberries and cranberries (Vaccinium spp.) using high-performance liquid chromatography/mass spectrometry. J. Agric. Food Chem. 2001, 49, 1270–1276. [Google Scholar] [CrossRef]
- Kalt, W.; Forney, C.F.; Martin, A.; Prior, R. Antioxidant capacity, vitamin C, phenolics, and anthocyanins after fresh storage of Small Fruits. J. Agric. Food Chem. 1999, 47, 4638–4644. [Google Scholar] [CrossRef] [PubMed]
- Pereira-Kechinski, C.; Guimaraes, P.V.R.; Noreña, C.P.Z.; Tessaro, I.C.; Marczak, L.D.F. Degradation kinetics of anthocyanin in blueberry juice during thermal treatment. J. Food Sci. 2010, 75, 173–176. [Google Scholar] [CrossRef]
- López, J.; Uribe, E.; Vega-Gálvez, A.; Miranda, M.; Vergara, J.; Gonzalez, E.; Di Scala, K. Effect of air temperature on drying kinetics, vitamin C, antioxidant activity, total phenolic content, non enzymatic browning and firmness of blueberries variety O’neil. Food Bioprocess. Technol. 2010, 3, 772–777. [Google Scholar] [CrossRef]
- Varo, M.A.; Martín-Gómez, J.; Mérida, J.; Serratosa, M.P. Bioactive Compounds and Antioxidant Activity of Highbush Blueberry (Vaccinium Corymbosum) Grown in Southern Spain. Eur. Food Res. Technol. 2021, 247, 1199–1208. [Google Scholar] [CrossRef]
- Gavrilova, V.; Kajdzanoska, M.; Gjamovski, V.; Stefova, M. Separation, characterization and quantification of phenolic compounds in blueberries and red and black currants by HPLC-DAD-ESI-MSn. J. Agric. Food Chem. 2001, 59, 4009–4018. [Google Scholar] [CrossRef]
- Moyer, R.A.; Hummer, K.E.; Finn, C.E.; Frei, B.; Wrolstad, R.E. Anthocyanins, phenolics, and antioxidant capacity in diverse small fruits: Vaccinium, Rubus, and Ribes. J. Agric. Food Chem. 2002, 50, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Y.; Chen, H.; Camp, M.J.; Ehlenfeldt, M.K. Genotype and growing season influence blueberry antioxidant capacity and other quality attributes. Int. J. Food Sci. 2012, 47, 1540–1549. [Google Scholar] [CrossRef]
- Lyons, M.M.; Yu, C.; Toma, R.B.; Cho, S.Y.; Reiboldt, W.; Lee, J.; Van Breemen, R.B. Resveratrol in raw and baked blueberries and bilberries. J. Agric. Food Chem. 2003, 51, 5867–5870. [Google Scholar] [CrossRef]
- Rimando, A.M.; Kalt, W.; Magee, J.B.; Dewey, J.; Ballington, J.R. Resveratrol, pterostilbene, and piceatannol in Vaccinium berries. J. Agric. Food Chem. 2004, 52, 4713–4719. [Google Scholar] [CrossRef]
- Shanmuganathan, M.; Li, P.C.H. Resveratrol analysis and degradation study in blueberry samples by HPLC with fluorescence detection. J. Liq. Chromatogr. Relat. Technol. 2009, 32, 3038–3048. [Google Scholar] [CrossRef]
- Bravo, L. Polyphenols: Chemistry, dietary sources, metabolism, and nutritional significance. Nutr. Res. Rev. 1980, 56, 317–333. [Google Scholar] [CrossRef] [PubMed]
- Hertog, M.G.; Feskens, E.J.; Kromhout, D. Antioxidant flavonols and coronary heart disease risk. Lancet 1997, 349, 699. [Google Scholar] [CrossRef]
- Keli, S.O.; Hertog, M.G.; Feskens, E.J.; Kromhout, D. Dietary flavonoids, antioxidant vitamins, and incidence of stroke: The Zutphen study. Arch. Intern. Med. 1996, 156, 637–642. [Google Scholar] [CrossRef]
- Knekt, P.; Jarvinen, R.; Seppanen, R.; Hellovaara, M.; Teppo, L.; Pukkala, E.; Aromaa, A. Dietary flavonoids and the risk of lung cancer and other malignant neoplasms. Am. J. Epidemiol. 1997, 146, 223–230. [Google Scholar] [CrossRef]
- Michalska, A.; Łysiak, G. Bioactive Compounds of Blueberries: Post-Harvest Factors Influencing the Nutritional Value of Products. Int. J. Mol. Sci. 2015, 16, 18642–18663. [Google Scholar] [CrossRef]
- Molina, J.M.; Calvo, D.; Medina, J.J.; Barrau, C.; Romero, F. Fruit quality parameters of some southern highbush blueberries (Vaccinium xcorymbosum L.) grown in Andalusia (Spain). Span. J. Agric. Res. 2008, 6, 671–676. [Google Scholar] [CrossRef][Green Version]
- Barrau, C.; De los Santos, B.; Calvo, D.; Medina, J.J.; Molina, J.M.; Romero, F. Low chilling blueberries (Vaccinium spp.) studies in Huelva (Andalusia, SW Spain): Present and future. Acta Hortic. 2004, 649, 305–308. [Google Scholar] [CrossRef]
- Martín-Gómez, J.; García-Martínez, T.; Varo, M.Á.; Mérida, J.; Serratosa, M.P. Phenolic compounds, antioxidant activity and color in the fermentation of mixed blueberry and grape juice with different yeasts. LWT 2021, 146, 111661. [Google Scholar] [CrossRef]
- Amidžić Klarić, D.; Klarić, I.; Mornar, A. Polyphenol, content and antioxidant activity of commercial blackberry wines from Croatia: Application of multivariate analysis for geographic origin differentiation. J. Food Nutr. Res. 2011, 50, 99–209. [Google Scholar]
- Arozarena, I.; Ortiz, J.; Hermosín-Gutierrez, I.; Urretavizcaya, I.; Salvatierra, S.; Cordova, I.; Marín-Arroyo, M.R.; Noriega, M.J.; Navarro, M. Color, ellagitannins, anthocyanins, and antioxidant activity of andean blackberry (Rubus glaucus Benth.) wines. J. Agric. Food Chem. 2012, 60, 7463–7473. [Google Scholar] [CrossRef]
- Johnson, M.H.; Gonzalez de Mejia, E. Comparison of chemical composition and antioxidant capacity of commercially available blueberry and blackberry wines in Illinois. J. Food Sci. 2012, 71, C141–C148. [Google Scholar] [CrossRef]
- Koca, I.; Karadeniz, B. Antioxidant properties of blackberry and blueberry fruits grown in the Black Sea Region of Turkey. Sci. Hortic. 2009, 121, 447–450. [Google Scholar] [CrossRef]
- Skrede, G.; Wrolstad, R.E.; Durst, R.W. Changes in anthocyanins and polyphenolics during juice processing of highbush blueberries (Vaccinium corymbosum L.). J. Food Sci. 2000, 65, 357–363. [Google Scholar] [CrossRef]
- Garcia-Alonso, M.; Pascual-Teresa, S.; Santos-Buelga, C.; Rivas-Gonzalo, J. Evaluation of the antioxidant properties of fruits. Food Chem. 2004, 84, 13–18. [Google Scholar] [CrossRef]
- Proteggente, A.R.; Pannala, A.S.; Paganga, G.; Van Buren, L.; Wagner, E.; Wiseman, S.; Van de Put, F.; Dacombe, C.; Rice-Evans, C.A. The antioxidant activity of regularly consumed fruit and vegetables reflects their phenolic and vitamin C composition. Free Radic. Res. 2002, 36, 217–233. [Google Scholar] [CrossRef] [PubMed]
- Atala, E.; Vásquez, L.; Speisky, H.; Lissi, E.; López-Alarcón, C. Ascorbic acid contribution to ORAC values in berry extracts: An evaluation by the ORAC-pyrogallol red methodology. Food Chem. 2009, 113, 331–335. [Google Scholar] [CrossRef]
- Namiesnik, J.; Vearasilp, K.; Kupska, M.; Ham, K.-S.; Kang, S.-G.; Park, Y.-K.; Barasch, D.; Nemirovski, A.; Gorinstein, A. Antioxidant activities and bioactive components in some berries. Eur. Food Res. Technol. 2013, 237, 819–829. [Google Scholar] [CrossRef]
- Wang, S.Y.; Chen, C.; Sciarappa, W.; Wang, C.Y.; Camp, M.J. Fruit quality, antioxidant capacity, and flavonoid content of organically and conventionally grown blueberries. J. Agric. Food Chem. 2008, 56, 5788–5794. [Google Scholar] [CrossRef]
- Bishayee, A. Cancer Prevention and Treatment with Resveratrol: From Rodent Studies to Clinical Trials. Cancer Prev. Res. 2009, 2, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Folmer, F.; Basavaraju, U.; Jaspars, M.; Hold, G.; El-Omar, E.; Dicato, M.; Diederich, M. Anticancer effects of bioactive berry compounds. Phytochem. Rev. 2014, 13, 295–322. [Google Scholar] [CrossRef]
- Sanchez-Moreno, C.; Cao, G.; Ou, B.; Prior, R.L. Anthocyanin and proanthocyanidin content in selected whiteand red wines. Oxygen radical absorbance capacity comparison with nontraditional wines obtained from highbush blueberry. J. Agric. Food Chem. 2003, 51, 4889–4896. [Google Scholar] [CrossRef] [PubMed]
- Burns, J.; Gardner, P.T.; Matthews, D.; Duthie, G.G.; Lean, M.E.J.; Crozier, A. Extraction of phenolics and changes in antioxidant activity of red wines during vinification. J. Agric. Food Chem. 2001, 49, 5797–5808. [Google Scholar] [CrossRef] [PubMed]
- Canals, R.; Llaudy, M.C.; Vall, J.; Canals, J.M.; Zamora, F. Influence of ethanol concentration on the extraction of color and phenolic compounds from the skin and seeds of Tempranillo grapes at different stages of ripening. J. Agric. Food Chem. 2005, 53, 4019–4025. [Google Scholar] [CrossRef]
- Budic-Leto, I.; Lovrić, T.; Kljusuric, J.G.; Pezo, I.; Vrhovsek, U. Anthocyanin composition of the red wine Babić affected by maceration treatment. Eur. Food Res. Technol. 2006, 222, 397–402. [Google Scholar] [CrossRef]
- Soto-Vázquez, E.; Rio-Segade, S.; Orriols-Fernández, F. Effect of wine-making technique on phenolic composition and chromatic characteristics in young red wines. Eur. Food Res. Technol. 2010, 231, 789–802. [Google Scholar] [CrossRef]
- Boulton, R. The copigmentation of anthocyanins and its role in the color of red wine: A critical review. Am. J. Enol. Vitic. 2001, 52, 67–87. [Google Scholar]
- Fulcrand, H.; Dueñas, M.; Salas, E.; Cheynier, V. Phenolic reactions during winemaking and aging. Am. J. Enol. Vitic. 2006, 57, 289–297. [Google Scholar]
- Chira, K.; Lorrain, B.; Ky, I.; Teissedre, P.L. Tannin Composition of Cabernet-Sauvignon and Merlot Grapes from the Bordeaux Area for Different Vintages (2006 to 2009) and Comparison to Tannin Profile of Five 2009 Vintage Mediterranean Grapes Varieties. Molecules 2011, 16, 1519–1532. [Google Scholar] [CrossRef] [PubMed]
- Marquez, A.; Serratosa, M.P.; Merida, J. Pyranoanthocyanin derived pigments in wine: Structure and formation during winemaking. J. Chem. 2013, 2013, 713028. [Google Scholar] [CrossRef]
- Weber, F.; Greve, K.; Durner, D.; Fisher, U.; Winterhalter, P. Sensory and Chemical Characterization of Phenolic Polymers from Red Wine Obtained by Gel Permeation Chromatography. Am. J. Enol. Vitic. 2013, 64, 15–25. [Google Scholar] [CrossRef]
- Sdiri, S.; Bermejo, A.; Aleza, P.; Navarro, P.; Salvador, A. Phenolic composition, organic acids, sugars, vitamin C and antioxidant activity in the juice of two new triploid late-season mandarins. Food Res. Int. 2012, 49, 462–468. [Google Scholar] [CrossRef]
- C.I.E. Colourimetry. Publication of the International Commission on Illumination, 3rd ed.; Vienna, Austria. 2004. Available online: https://cie.co.at/publications/colorimetry-3rd-edition (accessed on 10 October 2022).
- Ribereau-Gayon, P.; Glories, Y.; Maujean, A.; Dubourdieu, D. Traité d’Œnologie. 2. Chimie du vin. Stabilisation et Traitement; Dunod: Paris, France, 1998. [Google Scholar]
- Alen-Ruiz, F.; García-Falcon, M.S.; Pérez-Lamela, M.C.; Martínez-Carballo, E.; Simal-Gandara, J. Influence of major polyphenols on antioxidant activity in Mencía and Brancellao red wines. Food Chem. 2009, 113, 53–60. [Google Scholar] [CrossRef]
- Crowell, E.A.; Ough, C.S. A modified procedure for alcohol determination by dichromate oxidation. Am. J. Enol. Vitic. 1979, 30, 61–63. [Google Scholar]
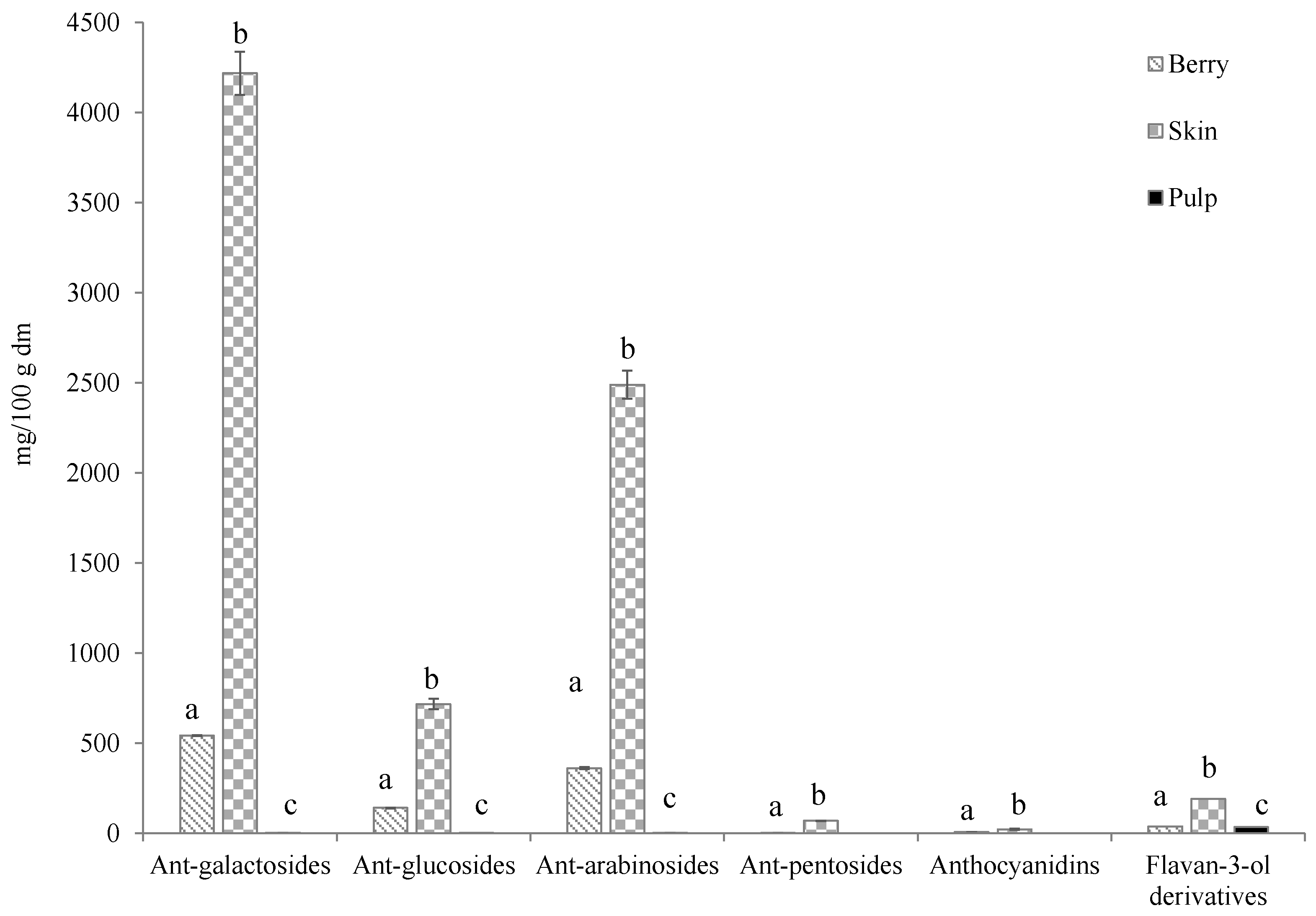


| Berry | Skin | Pulp | |
|---|---|---|---|
| Dp-3-gal | 187 ± 2.90 a | 1059 ± 14.0 b | 0.448 ± 0.148 c |
| Cn-3-gal | 20.0 ± 3.60 a | 162 ± 16.1 b | n.d. |
| Pt-3-gal | 120 ± 0.702 a | 604 ± 24 b | 0.532 ± 0.164 c |
| Pn-3-gal | 7.10 ± 0.430 a | 73.6 ± 4.8 b | n.d. |
| Mv-3-gal | 209 ± 1.50 a | 2321 ± 75.7 b | 2.91 ± 0.012 c |
| Dp-3-glc | 47.9 ± 0.503 a | 178 ± 9.00 b | n.d. |
| Cn-3-glc | 4.3 ± 0.031 a | 22.4 ± 3.82 b | n.d. |
| Pt-3-glc | n.d. | 186 ± 4.20 b | n.d. |
| Mv-3-glc | 88.5 ± 1.70 a | 332 ± 14.0 b | 0.288 ± 0.128 c |
| Dp-3-arb | 124 ± 1.27 a | 728 ± 8.00 b | 0.54 ± 0.101 c |
| Cn-3-arb | 50.5 ± 0.115 a | 188 ± 20.0 b | n.d. |
| Pt-3-arb | 66.8 ± 1.60 a | 460 ± 28.0 b | n.d. |
| Mv-3-arb | 121 ± 2.20 a | 1115 ± 30.0 b | 2.57 ± 0.170 c |
| Pn-3-pentoside | 1.31 ± 0.192 a | 69.5 ± 0.231 b | n.d. |
| Pn | 3.82 ± 0.423 a | n.d. | n.d. |
| Mv | 3.31 ± 0.110 a | 21.8 ± 3.53 b | n.d. |
| Total anthocyanins | 1054 ± 8.80 a | 7519 ± 230 b | 7.30 ± 0.381 c |
| Catechin | 25.4 ± 0.234 a | 98.1 ± 3.64 b | 23.6 ± 0.110 |
| Epicatechin | 4.86 ± 0.038 a | 12.9 ± 0.559 b | 4.11 ± 0.026 |
| Epigallocatechin | n.d. | 13.9 ± 1.09 b | n.d. |
| Epigallocatechin-gallate | 5.84 ± 0.074 a | 57.3 ± 3.02 b | 6.41 ± 0.016 |
| Procyanidin B1 | 1.69 ± 0.006 a | 10.5 ± 0.131 b | 1.04 ± 0.012 |
| Total flavan-3-ol derivatives | 37.8 ± 0.267 a | 193 ± 2.13 b | 35.1 ± 0.061 c |
| Resveratrol | 44.8 ± 0.623 a | 100 ± 0.680 b | n.d. |
| Initial Juice | Wine PF | Wine TF | |
|---|---|---|---|
| Dp-3-gal | 0.642 ± 0.004 a | 4.02 ± 0.047 b | 0.472 ± 0.027 c |
| Cn-3-gal | n.d. | 1.07 ± 0.084 a | 0.422 ± 0.006 b |
| Pt-3-gal | 0.650 ± 0.005 a | 4.83 ± 0.041 b | 0.822 ± 0.006 |
| Pn-3-gal | n.d. | 1.18 ± 0.041 a | 0.465 ± 0.031 b |
| Mv-3-gal | 5.83 ± 0.047 a | 20.0 ± 0.124 b | 5.80 ± 0.261 a |
| Dp-3-glc | 1.31 ± 0.002 a | 3.38 ± 0.011 b | 0.663 ± 0.004 |
| Cn-3-glc | n.d. | 0.938 ± 0.060 a | 0.468 ± 0.034 b |
| Mv-3-glc | 2.95 ± 0.005 a | 11.6 ± 0.035 b | 3.62 ± 0.076 c |
| Dp-3-arb | 0.595 ± 0.002 a | 2.33 ± 0.105 b | 0.498 ± 0.022 a |
| Cn-3-arb | 0.801 ± 0.005 a | 4.34 ± 0.012 b | 1.23 ± 0.016 c |
| Pt-3-arb | 0.825 ± 0.010 a | 2.63 ± 0.073 b | 0.676 ± 0.030 c |
| Mv-3-arb | 3.23 ± 0.016 a | 13.4 ± 0.128 b | 4.25 ± 0.181 c |
| Pn-3-pent | n.d. | 0.759 ± 0.061 a | 0.546 ± 0.009 b |
| Pn | 0.734 ± 0.343 a | 0.549 ± 0.017 a | n.d. |
| Mv | 0.646 ± 0.004 a | 1.48 ± 0.022 b | 0.710 ± 0.013 c |
| Total Anthocyanins | 18.21 ± 0.399 a | 72.5 ± 0.109 b | 21.0 ± 0.437 c |
| Catechin | 2.74 ± 0.036 a | 10.5 ± 0.260 b | 6.40 ± 0.205 c |
| Epicatechin | 0.364 ± 0.019 a | 1.54 ± 0.040 b | 1.40 ± 0.233 b |
| Epigallocatechin | 0.917 ± 0.084 a | 3.04 ± 0.100 b | 3.40 ± 0.196 c |
| Epigallocatechin-gallate | 3.15 ± 0.008 a | 13.4 ± 0.222 b | 8.85 ± 0.026 c |
| Procyanidin B1 | 1.15 ± 0.070 a | 2.15 ± 0.133 b | 1.25 ± 0.185 a |
| Total Flavan-3-ol derivatives | 8.32 ± 0.102 a | 30.7 ± 0.258 b | 21.3 ± 0.067 c |
| Resveratrol | n.d | n.d. | n.d. |
| L* | a* | b* | hab | |
|---|---|---|---|---|
| Initial Juice | 68.5 ± 0.105 a | 68.4 ± 1.01 a | 41.2 ± 0.093 a | 31.1 ± 0.418 a |
| Wine PF | 62.1 ± 0.123 b | 83.4 ± 0.500 b | 38.3 ± 0.094 b | 24.7 ± 0.082 b |
| Wine TF | 67.7 ± 0.008 c | 71.1 ± 0.003 c | 30.9 ± 0.090 c | 23.4 ± 0.209 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varo, M.A.; Serratosa, M.P.; Martín-Gómez, J.; Moyano, L.; Mérida, J. Influence of Fermentation Time on the Phenolic Compounds, Vitamin C, Color and Antioxidant Activity in the Winemaking Process of Blueberry (Vaccinium corymbosum) Wine Obtained by Maceration. Molecules 2022, 27, 7744. https://doi.org/10.3390/molecules27227744
Varo MA, Serratosa MP, Martín-Gómez J, Moyano L, Mérida J. Influence of Fermentation Time on the Phenolic Compounds, Vitamin C, Color and Antioxidant Activity in the Winemaking Process of Blueberry (Vaccinium corymbosum) Wine Obtained by Maceration. Molecules. 2022; 27(22):7744. https://doi.org/10.3390/molecules27227744
Chicago/Turabian StyleVaro, M. Angeles, Maria P. Serratosa, Juan Martín-Gómez, Lourdes Moyano, and Julieta Mérida. 2022. "Influence of Fermentation Time on the Phenolic Compounds, Vitamin C, Color and Antioxidant Activity in the Winemaking Process of Blueberry (Vaccinium corymbosum) Wine Obtained by Maceration" Molecules 27, no. 22: 7744. https://doi.org/10.3390/molecules27227744
APA StyleVaro, M. A., Serratosa, M. P., Martín-Gómez, J., Moyano, L., & Mérida, J. (2022). Influence of Fermentation Time on the Phenolic Compounds, Vitamin C, Color and Antioxidant Activity in the Winemaking Process of Blueberry (Vaccinium corymbosum) Wine Obtained by Maceration. Molecules, 27(22), 7744. https://doi.org/10.3390/molecules27227744






