Simultaneous Determination of Caffeine and Paracetamol in Commercial Formulations Using Greener Normal-Phase and Reversed-Phase HPTLC Methods: A Contrast of Validation Parameters
Abstract
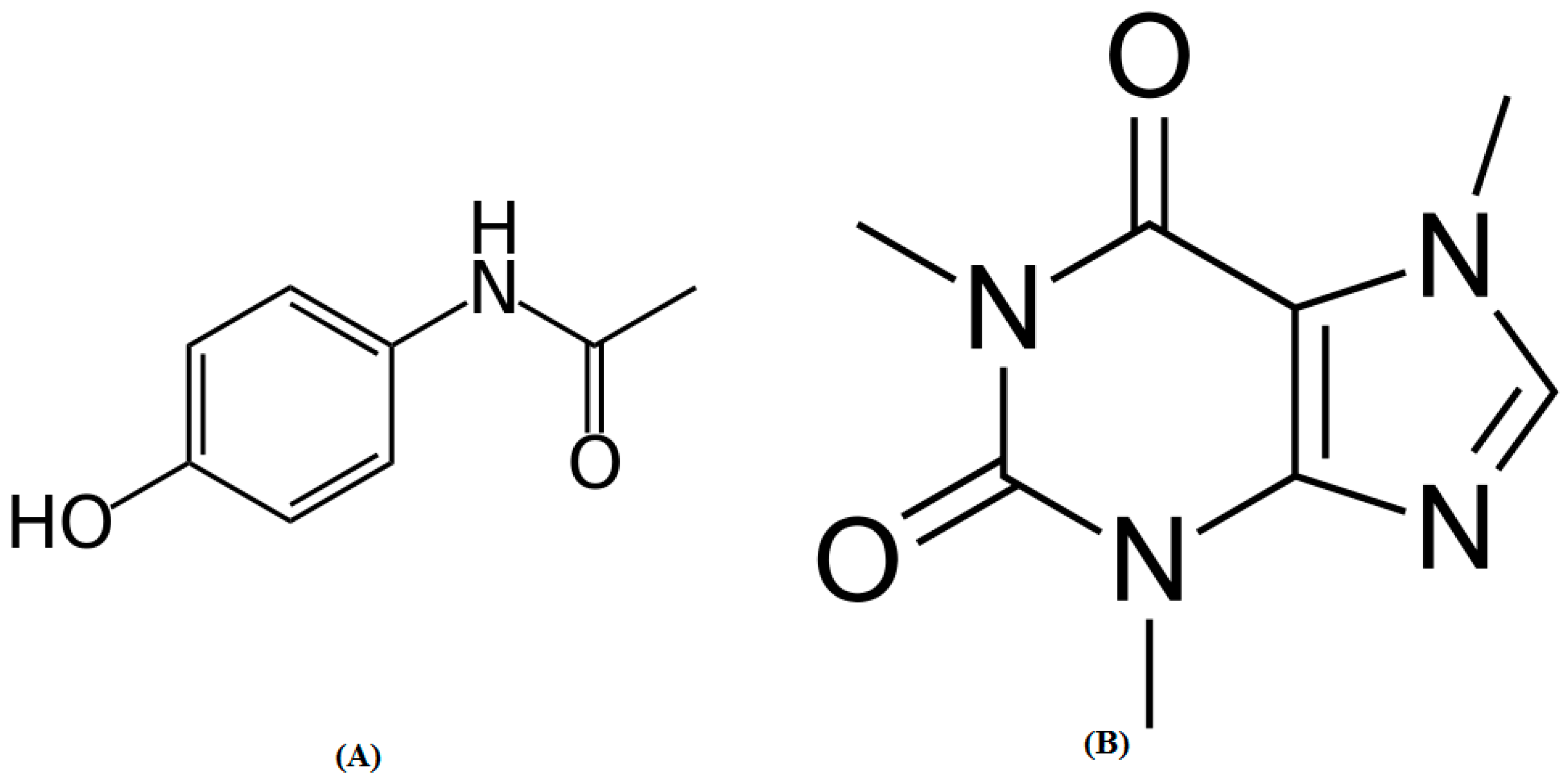
:1. Introduction
2. Results and Discussion
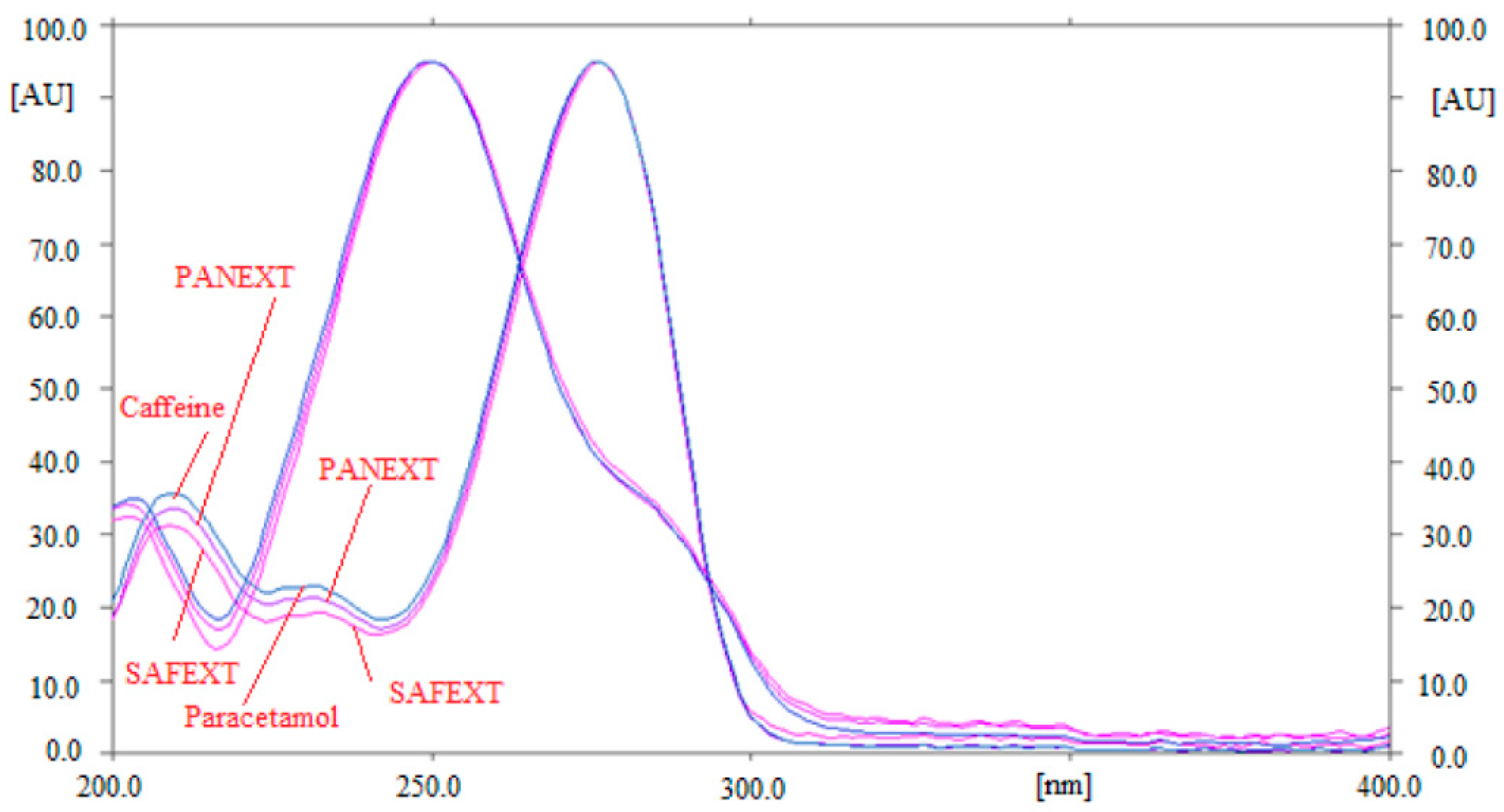
2.1. Method Development
2.2. Method Validation
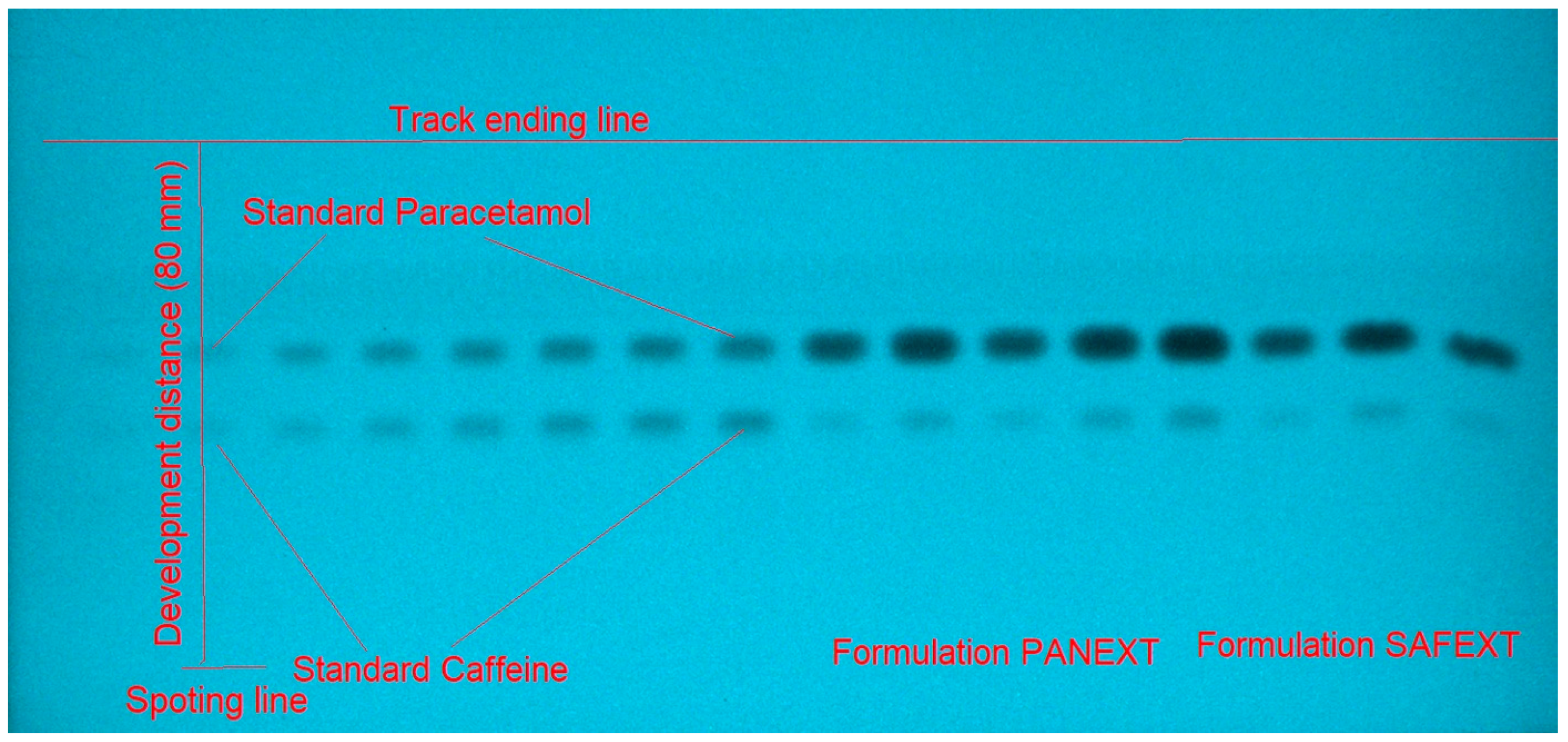
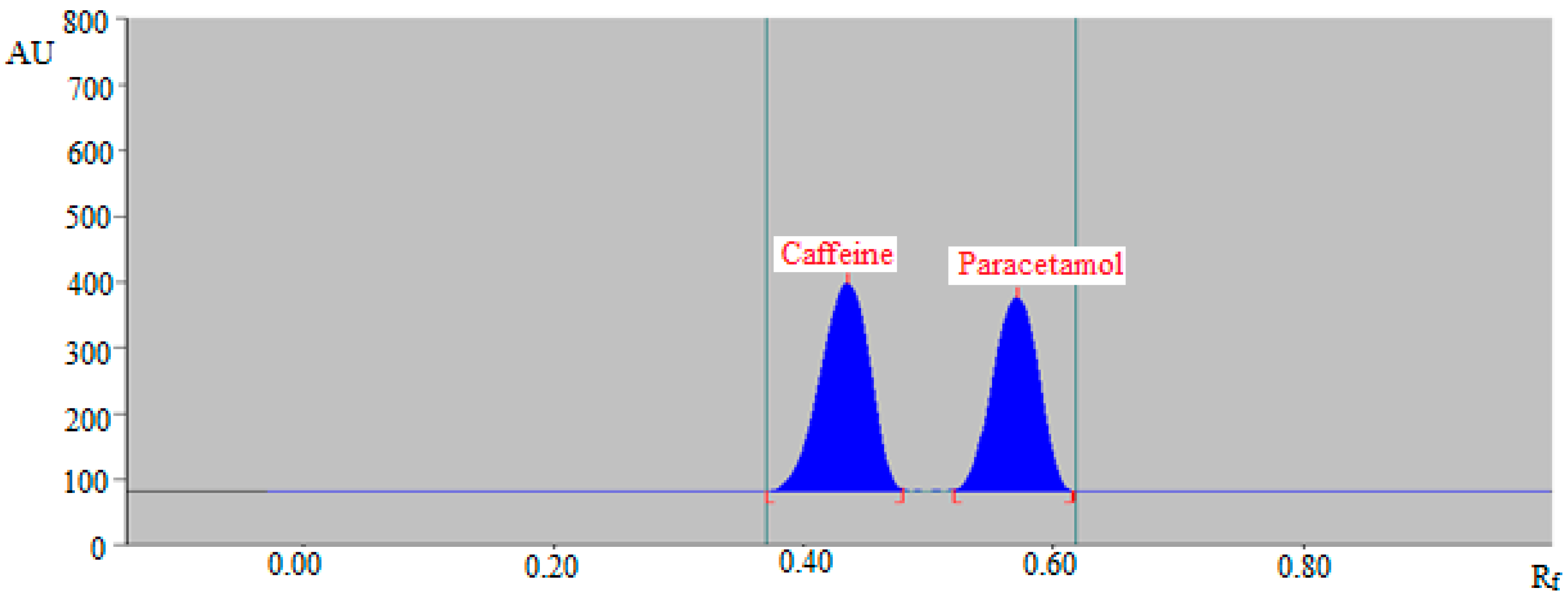
2.3. Application of Greener Normal-Phase and Reversed-Phase HPTLC Aapraches in the SMD of Caffeine and Paracetamol in Commercial Tablets
2.4. Greenness Estimation Using AGREE
3. Materials and Methods
3.1. Materials
3.2. Instrumentation and Analytical Procedures
3.3. Calibration Curves and QC Sample for Caffeine and Paracetamol
3.4. Processing of Samples for the SMD of Caffeine and Paracetamol in Commercial Tablets
3.5. Analytical Method Validation
3.6. Application of Greener Normal-Phase and Reversed-Phase HPTLC Approaches in the SMD of Caffeine and Paracetamol in Commercial Tablets
3.7. Greenness Estimation Using AGREE
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Sample Availability
References and Note
- Jimenez, J.A.; Martinez, F. Thermodynamic study of the solubility of acetaminophen in propylene glycol + water cosolvent mixtures. J. Braz. Chem. Soc. 2006, 17, 125–134. [Google Scholar] [CrossRef] [Green Version]
- Shakeel, F.; Alanazi, F.K.; Alsarra, I.A.; Haq, N. Solubilization behavior of paracetamol in Transcutol-water mixtures at T = (298.15 to 333.15) K. J. Chem. Eng. Data 2012, 58, 3551–3556. [Google Scholar] [CrossRef]
- Shakeel, F.; Ramadan, W. Transdermal delivery of anticancer drug caffeine from water-in-oil nanoemulsions. Colloids Surf. B 2010, 75, 356–362. [Google Scholar] [CrossRef]
- Rahimi, M.; Khorshidi, N.; Heydari, R. Simultaneous determination of paracetamol and caffeine in aqueous samples by ultrasound-assisted emulsification microextraction coupled with high-performance liquid chromatography. Sep. Sci. Plus 2020, 3, 561–570. [Google Scholar] [CrossRef]
- Medina, A.R.; de Cordova, M.L.F.; Molina-Diaz, A. Simultaneous determination of paracetamol, caffeine and acetylsalicylic acid by means of FI ultraviolet pls multioptosensing device. J. Pharm. Biomed. Anal. 1999, 21, 983–992. [Google Scholar] [CrossRef]
- Tavallali, H.; Salami, M. Simultaneous determination of caffeine and paracetamol by zero-crossing second derivative spectrophotometry in pharmaceutical preparations. Asian J. Chem. 2009, 21, 1949–1956. [Google Scholar]
- Tavallali, H.; Sheikhaei, M. Simultaneous kinetic determination of paracetamol and caffeine by H-point standard addition method. Afr. J. Pure Appl. Chem. 2009, 3, 11–19. [Google Scholar]
- Aktas, A.H.; Kitis, F. Spectrophotometric simultaneous determination of caffeine and paracetamol in commercial pharmaceutical by principal component regression, least squares and artificial neural networks chemometric methods. Croat. Chem. Acta 2014, 87, 69–74. [Google Scholar] [CrossRef]
- Uddin, M.N.; Mondol, A.; Karim, M.M.; Jahan, R.A.; Rana, A.A. Chemometrics assisted spectrophotometric method for simultaneous determination of paracetamol and caffeine in pharmaceutical formulations. Bangladesh J. Sci. Ind. Res. 2019, 54, 215–222. [Google Scholar] [CrossRef] [Green Version]
- Sebaiy, M.M.; Mattar, A.A. H-point assay method for simultaneous determination of paracetamol and caffeine in panadol extra dosage forms. Can. J. Biomed. Res. Technol. 2020, 3, 1–6. [Google Scholar]
- Altun, M.L. HPLC method for the analysis of paracetamol, caffeine and dipyrone. Turk. J. Chem. 2002, 26, 521–528. [Google Scholar]
- Issa, I.M.; Hassouna, E.M.; Zayed, A.G. Simultaneous determination of paracetamol, caffeine, domperidone, ergotamine tartrate, propyphenazole, and drotaverine HCl by high performance liquid chromatography. J. Liq. Chromatogr. Rel. Technol. 2012, 35, 2148–2161. [Google Scholar] [CrossRef]
- Tsvetkova, B.; Kostova, B.; Pencheva, I.; Zlatkov, A.; Rachew, D.; Peikov, P. Validated method for simultaneous analysis of paracetamol and caffeine in model tablet formulation. Int. J. Pharm. Pharm. Sci. 2012, 4, 680–684. [Google Scholar]
- Cunha, R.R.; Chaves, S.C.; Ribeiro, M.M.A.C.; Torres, L.M.F.C.; Munoz, R.A.A.; Santos, W.T.P.D.; Richter, E.M. Simultaneous determination of caffeine, paracetamol, and ibuprofen in pharmaceutical formulations by high-performance liquid chromatography with UV detection and by capillary electrophoresis with conductivity detection. J. Sep. Sci. 2015, 38, 1657–1662. [Google Scholar] [CrossRef]
- Acheampong, A.; Gyasi, W.O.; Darko, G.; Apau, J.; Addai-Arhin, S. Validated RP-HPLC method for simultaneous determination and quantification of chlorphenaramine maleate, paracetamol and caffeine in tablet formulation. Springer Plus 2016, 5, E625. [Google Scholar] [CrossRef] [Green Version]
- Narayanan, V.L.; Austin, A. Determination of acetaminophen and caffeine using reverse phase liquid (RP-LC) chromatographic technique. Quest J. Res. Pharm. Sci. 2016, 3, 5–10. [Google Scholar]
- Aminu, N.; Chan, S.-Y.; Khan, N.H.; Farhan, A.B.; Umar, M.N.; Toh, S.-M. A simple stability-indicating HPLC method for simultaneous analysis of paracetamol and caffeine and its application to determinations in fixed-dose combination tablet dosage form. Acta Chromatogr. 2019, 31, 85–91. [Google Scholar] [CrossRef]
- Ali, J.G.; Muhammad, I.; Hamid, S.; Muhammad, A.A.; Shoaib, H.; Tasleem, S. Simultaneous determination and quantification of paracetamol, caffeine and orphenadrine citrate using stability-indicating HPLC method in a fixed dose combination tablet dosage form. Ann. Pharmacol. Pharm. 2020, 5, E118. [Google Scholar]
- Belal, F.; Omar, M.A.; Derayea, S.; Zayed, S.; Hammad, M.A.; Saleh, S.F. Simultaneous determination of paracetamol, caffeine and codeine in tablets and human plasma by micellar liquid chromatography. Eur. J. Chem. 2015, 4, 468–474. [Google Scholar] [CrossRef]
- Wang, A.; Sun, J.; Feng, H.; Gao, S.; He, Z. Simultaneous determination of paracetamol and caffeine in human plasma by LC-ESI-MS. Chromatographia 2008, 67, 281–285. [Google Scholar] [CrossRef]
- Tavallali, H.; Zareiyan, S.F.; Naghian, M. An efficient and simultaneous analysis of caffeine and paracetamol in pharmaceutical formulations using TLC with a fluorescence plate reader. J. AOAC Int. 2011, 94, 1094–1099. [Google Scholar] [CrossRef] [Green Version]
- Chabukswar, A.R.; Thakur, V.G.; Dharam, D.L.; Shah, M.H.; Kuchekar, B.S.; Sharma, S.N. Development and validation of HPTLC method for simultaneous estimation of paracetamol, ibuprofen and caffeine in bulk and pharmaceutical dosage form. Res. J. Pharm. Technol. 2012, 5, 1218–1222. [Google Scholar]
- Halka-Grysinska, A.; Slazak, P.; Zareba, G.; Markowski, W.; Klimek-Turek, A.; Dzido, T.H. Simultaneous determination of acetaminophen, propyphenazone and caffeine in cefalgin preparation by pressurized planar electrochromatography and high-performance thin-layer chromatography. Anal. Methods 2012, 4, 973–982. [Google Scholar] [CrossRef]
- Lau, O.-H.; Luk, S.-F.; Cheung, Y.-M. Simultaneous determination of ascorbic acid, caffeine and paracetamol in drug formulations by differential-pulse voltammetry using a glassy carbon electrode. Analyst 1989, 114, 1047–1051. [Google Scholar] [CrossRef] [PubMed]
- Yigit, A.; Yardrm, Y.; Senturk, Z. Voltametric sensor based on boron-doped diamond electrode for simultaneous determination of paracetamol, caffeine and aspirin in pharmaceutical formulations. IEEE Sens. 2016, 16, 1674–1680. [Google Scholar] [CrossRef]
- Minh, T.T.; Phong, N.H.; Duc, H.V.; Khieu, D.Q. Microwave synthesis and voltametric simultaneous determination of paracetamol and caffeine using an MOF-199-based electrode. J. Mater. Sci. 2018, 53, 2453–2471. [Google Scholar] [CrossRef]
- Hung, N.X.; Quang, D.A.; Toan, T.T.T.; Dung, N.N. The simultaneous determination of ascorbic acid, paracetamol, and caffeine by voltammetry method using cobalt Schiff base complex/SBA-15 modified electrode. ECS J. Solid State Sci. Technol. 2020, 9, E101004. [Google Scholar] [CrossRef]
- Ibrahim, H.; Hamdy, A.M.; Merey, H.A.; Saad, A.S. Simultaneous determination of paracetamol, propyphenazone and caffeine in presence of paracetamol impurities using dual-mode gradient HPLC and TLC densitometry methods. J. Chromatogr. Sci. 2021, 59, 140–147. [Google Scholar] [CrossRef]
- Meter, M.I.V.; Khan, S.M.; Taulbee-Cotton, B.V.; Dimmitt, N.H.; Hubbard, N.D.; Green, A.M.; Webster, G.K.; McVey, P.A. Diagnosis of agglomeration and crystallinity of active pharmaceutical ingredients in over the counter headache medication by electrospray desorption ionization mass spectrometry imaging. Molecules 2021, 26, 610. [Google Scholar] [CrossRef]
- Katseli, V.; Economou, A.; Kokkinos, C. A novel all-3D-printed cell-on-a-chip device as a useful electroanalytical tool: Application to the simultaneous voltametric determination of caffeine and paracetamol. Talanta 2020, 208, 120388. [Google Scholar] [CrossRef]
- Boltia, S.A.; Soudi, A.T.; Elzanfaly, E.S.; Zaazaa, H.E. Effect of genetic algorithm-based wavelength selection as a preprocessing tool on multivariate simultaneous determination of paracetamol, orphenadrine citrate, and caffeine in the presence of p-aminophenol impurity. J. AOAC Int. 2020, 103, 250–256. [Google Scholar] [CrossRef]
- Muntean, D.M.; Alecu, C.; Tomuta, I. Simultaneous quantification of paracetamol and caffeine in powder blends for tableting by NIR-chemometry. J. Spectrosc. 2017, 2017, E7160675. [Google Scholar] [CrossRef] [Green Version]
- Ortega-Barrales, P.; Padilla-Weigand, R.; Molina-Diaz, A. Simultaneous determination of paracetamol and caffeine by flow injection-solid phase spectrometry using C18 silica gel as a sensing support. Anal. Sci. 2002, 18, 1241–1246. [Google Scholar] [CrossRef] [Green Version]
- Kulikov, A.U.; Verushkin, A.G. Simultaneous determination of paracetamol, caffeine, guaifenesin and preservatives in syrups by micellar LC. Chromatographia 2008, 67, 347–355. [Google Scholar] [CrossRef]
- Emre, D.; Ozaltrn, N. simultaneous determination of paracetamol, caffeine and propyphenazone in ternary mixtures by micellar electrokinetic capillary chromatography. J. Chromatogr. B 2007, 847, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Abdelrahman, M.M.; Abdelwahab, N.S.; Hegazy, M.A.; Fares, M.Y.; El-Sayed, G.M. Determination of the abused intravenously administered madness drops (tropicamide) by liquid chromatography in rat plasma; an application to pharmacokinetic study and greenness profile assessment. Microchem. J. 2020, 159, E105582. [Google Scholar] [CrossRef]
- Duan, X.; Liu, X.; Dong, Y.; Yang, J.; Zhang, J.; He, S.; Yang, F.; Wang, Z.; Dong, Y. A green HPLC method for determination of nine sulfonamides in milk and beef, and its greenness assessment with analytical eco-scale and greenness profile. J. AOAC Int. 2020, 103, 1181–1189. [Google Scholar] [CrossRef]
- Pena-Pereira, F.; Wojnowski, W.; Tobiszewski, M. AGREE-Analytical GREEnness metric approach and software. Anal. Chem. 2020, 92, 10076–10082. [Google Scholar] [CrossRef] [PubMed]
- Foudah, A.I.; Shakeel, F.; Alqarni, M.H.; Alam, P. A rapid and sensitive stability-indicating green RP-HPTLC method for the quantitation of flibanserin compared to green NP-HPTLC method: Validation studies and greenness assessment. Microchem J. 2021, 164, E105960. [Google Scholar] [CrossRef]
- Alam, P.; Salem-Bekhit, M.M.; Al-Joufi, F.A.; Alqarni, M.H.; Shakeel, F. Quantitative analysis of cabozantinib in pharmaceutical dosage forms using green RP-HPTLC and green NP-HPTLC methods: A comparative evaluation. Sus. Chem. Pharm. 2021, 21, E100413. [Google Scholar] [CrossRef]
- International Conference on Harmonization (ICH), Q2 (R1): Validation of Analytical Procedures–Text and Methodology, Geneva, Switzerland. 2005.

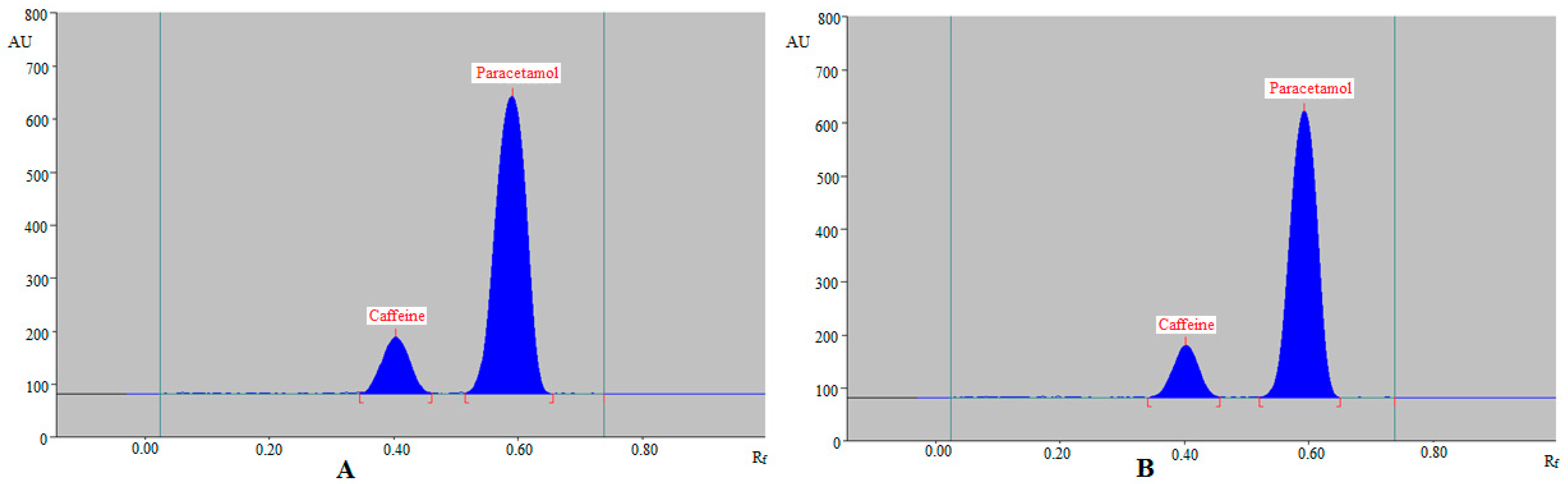
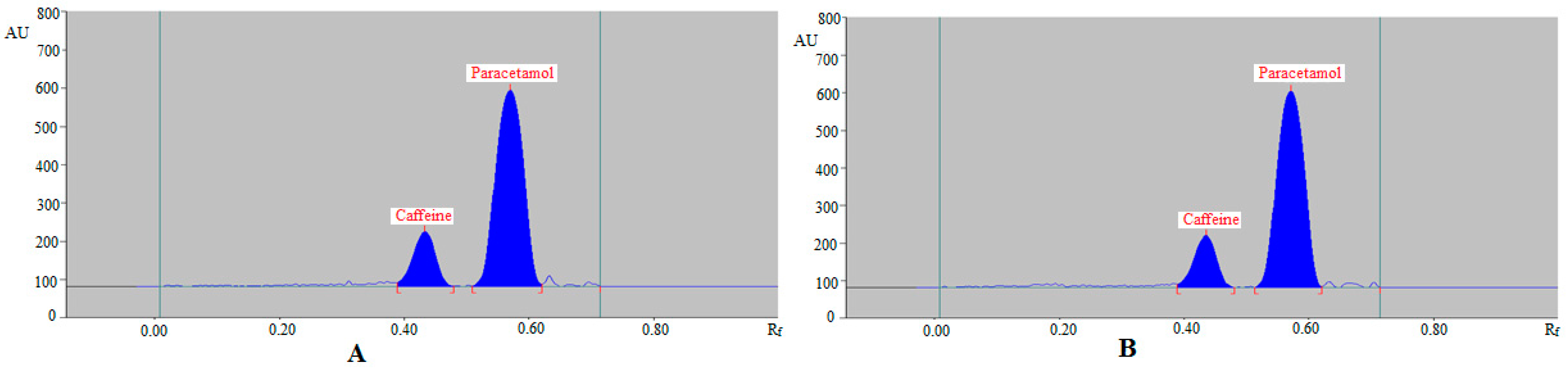

| Parameters | Caffeine | Paracetamol |
|---|---|---|
| linearity range (ng/band) | 50–500 | 50–500 |
| R2 | 0.9928 | 0.9970 |
| R | 0.9963 | 0.9984 |
| slope ± SD | 19.36 ± 0.96 | 18.05 ± 0.54 |
| intercept ± SD | 387.62 ± 4.38 | 95.66 ± 2.14 |
| standard error of slope | 0.39 | 0.22 |
| standard error of intercept | 1.78 | 0.87 |
| 95% confidence interval of slope | 17.68–21.05 | 17.10–19.00 |
| 95% confidence interval of intercept | 379.92–395.31 | 91.90–99.42 |
| LOD ± SD (ng/band) | 16.84 ± 0.27 | 17.05 ± 0.31 |
| LOQ ± SD (ng/band) | 50.52 ± 0.81 | 51.15 ± 0.93 |
| Parameters | Caffeine | Paracetamol |
|---|---|---|
| linearity range (ng/band) | 25–800 | 25–800 |
| R2 | 0.9976 | 0.9966 |
| R | 0.9987 | 0.9982 |
| slope ± SD | 20.54 ± 1.05 | 17.12 ± 0.47 |
| intercept ± SD | 833.46 ± 7.51 | 696.63 ± 6.21 |
| standard error of slope | 0.42 | 0.19 |
| standard error of intercept | 3.06 | 2.53 |
| 95% confidence interval of slope | 18.70–22.38 | 16.29–17.94 |
| 95% confidence interval of intercept | 820.26–846.65 | 685.71–707.54 |
| LOD ± SD (ng/band) | 8.52 ± 0.12 | 8.71 ± 0.13 |
| LOQ ± SD (ng/band) | 25.56 ± 0.36 | 26.13 ± 0.39 |
| Parameters | Caffeine | Paracetamol |
|---|---|---|
| Rf | 0.40 ± 0.01 | 0.59 ± 0.02 |
| As | 1.06 ± 0.02 | 1.08 ± 0.03 |
| N/m | 5245 ± 5.81 | 4978 ± 5.19 |
| Parameters | Caffeine | Paracetamol |
|---|---|---|
| Rf | 0.43 ± 0.01 | 0.57 ± 0.02 |
| As | 1.10 ± 0.03 | 1.09 ± 0.02 |
| N/m | 5182 ± 5.92 | 5367 ± 6.32 |
| Conc. (ng/Band) | Conc. Found (ng/Band) ± SD | Recovery (%) | CV (%) |
|---|---|---|---|
| Caffeine | |||
| 100 | 97.13 ± 2.24 | 97.13 | 2.30 |
| 300 | 314.65 ± 4.87 | 104.88 | 1.54 |
| 500 | 492.31 ± 6.13 | 98.46 | 1.24 |
| Paracetamol | |||
| 100 | 103.23 ± 3.12 | 103.23 | 3.02 |
| 300 | 289.71 ± 7.12 | 96.57 | 2.45 |
| 500 | 514.41 ± 9.12 | 102.88 | 1.77 |
| Conc. (ng/Band) | Conc. Found (ng/Band) ± SD | Recovery (%) | CV (%) |
|---|---|---|---|
| Caffeine | |||
| 50 | 50.31 ± 0.41 | 100.62 | 0.81 |
| 300 | 296.54 ± 1.45 | 98.84 | 0.48 |
| 800 | 795.61 ± 3.45 | 99.45 | 0.43 |
| Paracetamol | |||
| 50 | 49.18 ± 0.41 | 98.36 | 0.83 |
| 300 | 304.51 ± 1.64 | 101.50 | 0.53 |
| 800 | 807.54 ± 4.15 | 100.94 | 0.51 |
| Conc. (ng/Band) | Intraday Precision | Interday Precision | ||||
|---|---|---|---|---|---|---|
| Conc. (ng/Band) ± SD | Standard Error | CV (%) | Conc. (ng/Band) ± SD | Standard Error | CV (%) | |
| Caffeine | ||||||
| 100 | 96.54 ± 2.31 | 0.94 | 2.39 | 103.65 ± 2.65 | 1.08 | 2.55 |
| 300 | 292.97 ± 5.02 | 2.04 | 1.71 | 291.98 ± 5.61 | 2.29 | 1.92 |
| 500 | 512.45 ± 6.68 | 2.72 | 1.30 | 483.27 ± 7.31 | 2.98 | 1.51 |
| Paracetamol | ||||||
| 100 | 96.89 ± 3.32 | 1.35 | 3.42 | 95.61 ± 3.41 | 1.39 | 3.56 |
| 300 | 313.56 ± 7.52 | 3.07 | 2.39 | 286.51 ± 7.59 | 3.09 | 2.64 |
| 500 | 486.67 ± 9.32 | 3.80 | 1.91 | 516.41 ± 9.61 | 3.92 | 1.86 |
| Conc. (ng/Band) | Intraday Precision | Interday Precision | ||||
|---|---|---|---|---|---|---|
| Conc. (ng/Band) ± SD | Standard Error | CV (%) | Conc. (ng/Band) ± SD | Standard Error | CV (%) | |
| Caffeine | ||||||
| 50 | 50.42 ± 0.43 | 0.17 | 0.85 | 49.61 ± 0.39 | 0.15 | 0.78 |
| 300 | 303.21 ± 1.51 | 0.61 | 0.49 | 297.54 ± 1.31 | 0.53 | 0.44 |
| 800 | 806.31 ± 3.61 | 1.47 | 0.40 | 797.61 ± 3.40 | 1.38 | 0.42 |
| Paracetamol | ||||||
| 50 | 49.54 ± 0.48 | 0.19 | 0.96 | 50.21 ± 0.52 | 0.21 | 1.03 |
| 300 | 305.61 ± 1.78 | 0.72 | 0.58 | 296.54 ± 1.81 | 0.73 | 0.61 |
| 800 | 794.65 ± 4.21 | 1.71 | 0.52 | 804.61 ± 4.47 | 1.82 | 0.55 |
| Conc. (ng/Band) | Mobile Phase Composition (Ethyl Acetate/Ethanol) | Results | ||||
|---|---|---|---|---|---|---|
| Original | Used | (ng/Band) ± SD | % CV | Rf | ||
| Caffeine | ||||||
| 87:13 | +2.0 | 294.98 ± 6.43 | 2.17 | 0.39 | ||
| 300 | 85:15 | 85:15 | 0.0 | 302.14 ± 6.87 | 2.27 | 0.40 |
| 83:17 | −2.0 | 305.61 ± 7.13 | 2.33 | 0.41 | ||
| Paracetamol | ||||||
| 87:13 | +2.0 | 287.21 ± 7.14 | 2.48 | 0.58 | ||
| 300 | 85:15 | 85:15 | 0.0 | 291.34 ± 7.72 | 2.64 | 0.59 |
| 83:17 | −2.0 | 304.51 ± 8.02 | 2.63 | 0.60 | ||
| Conc. (ng/Band) | Mobile Phase Composition (Ethanol/Water) | Results | ||||
|---|---|---|---|---|---|---|
| Original | Used | (ng/Band) ± SD | % CV | Rf | ||
| Caffeine | ||||||
| 52:48 | +2.0 | 296.31 ± 2.71 | 0.91 | 0.42 | ||
| 300 | 50:50 | 50:50 | 0.0 | 303.54 ± 2.82 | 0.92 | 0.43 |
| 48:52 | −2.0 | 306.87 ± 2.91 | 0.94 | 0.44 | ||
| Paracetamol | ||||||
| 52:48 | +2.0 | 294.87 ± 2.81 | 0.95 | 0.56 | ||
| 300 | 50:50 | 50:50 | 0.0 | 303.21 ± 2.94 | 0.96 | 0.57 |
| 48:52 | −2.0 | 307.81 ± 3.21 | 1.04 | 0.58 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alam, P.; Shakeel, F.; Ali, A.; Alqarni, M.H.; Foudah, A.I.; Aljarba, T.M.; Alkholifi, F.K.; Alshehri, S.; Ghoneim, M.M.; Ali, A. Simultaneous Determination of Caffeine and Paracetamol in Commercial Formulations Using Greener Normal-Phase and Reversed-Phase HPTLC Methods: A Contrast of Validation Parameters. Molecules 2022, 27, 405. https://doi.org/10.3390/molecules27020405
Alam P, Shakeel F, Ali A, Alqarni MH, Foudah AI, Aljarba TM, Alkholifi FK, Alshehri S, Ghoneim MM, Ali A. Simultaneous Determination of Caffeine and Paracetamol in Commercial Formulations Using Greener Normal-Phase and Reversed-Phase HPTLC Methods: A Contrast of Validation Parameters. Molecules. 2022; 27(2):405. https://doi.org/10.3390/molecules27020405
Chicago/Turabian StyleAlam, Prawez, Faiyaz Shakeel, Abuzer Ali, Mohammed H. Alqarni, Ahmed I. Foudah, Tariq M. Aljarba, Faisal K. Alkholifi, Sultan Alshehri, Mohammed M. Ghoneim, and Amena Ali. 2022. "Simultaneous Determination of Caffeine and Paracetamol in Commercial Formulations Using Greener Normal-Phase and Reversed-Phase HPTLC Methods: A Contrast of Validation Parameters" Molecules 27, no. 2: 405. https://doi.org/10.3390/molecules27020405
APA StyleAlam, P., Shakeel, F., Ali, A., Alqarni, M. H., Foudah, A. I., Aljarba, T. M., Alkholifi, F. K., Alshehri, S., Ghoneim, M. M., & Ali, A. (2022). Simultaneous Determination of Caffeine and Paracetamol in Commercial Formulations Using Greener Normal-Phase and Reversed-Phase HPTLC Methods: A Contrast of Validation Parameters. Molecules, 27(2), 405. https://doi.org/10.3390/molecules27020405








