Growth Properties of Carbon Nanowalls on Nickel and Titanium Interlayers
Abstract
:1. Introduction
2. Experimental Details
2.1. Interlayer Deposition
2.2. Carbon Nanowall Growth
2.3. Analysis and Measurement
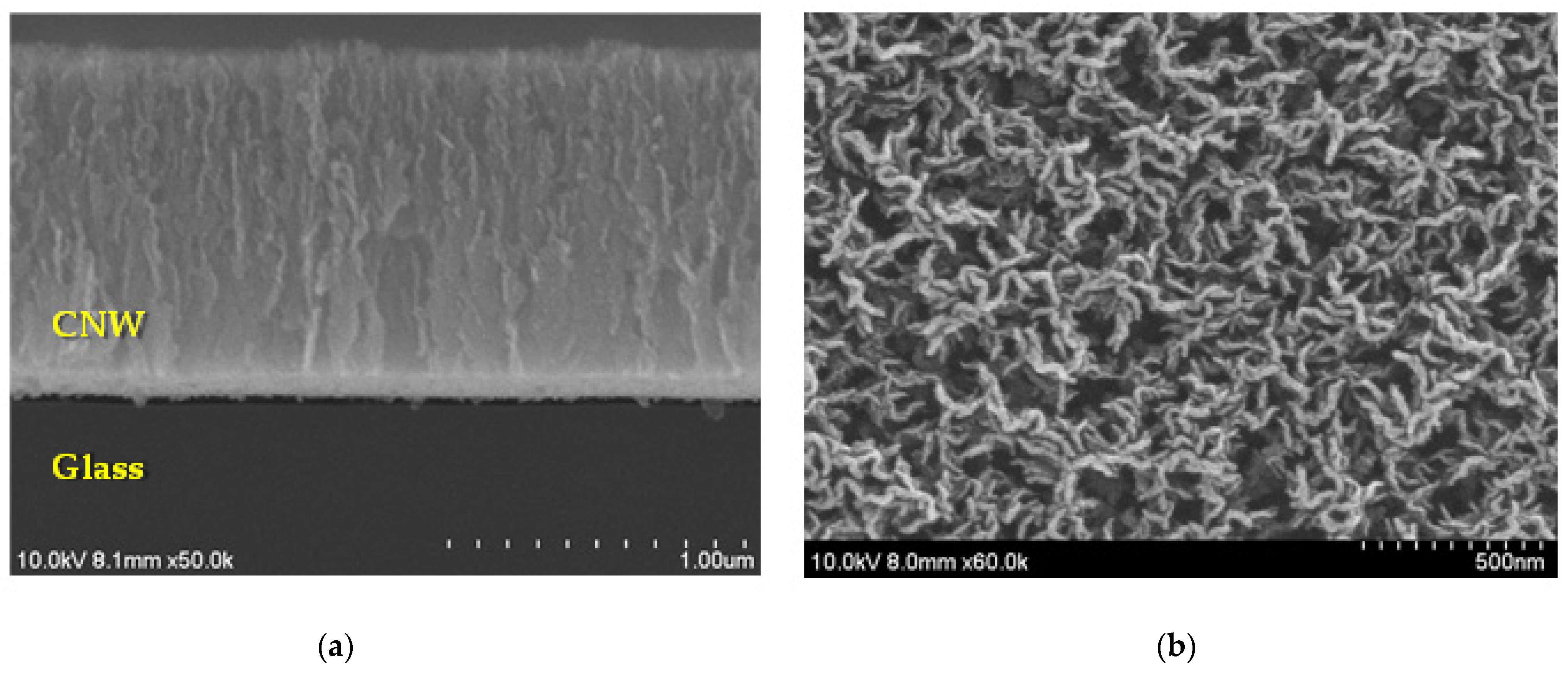
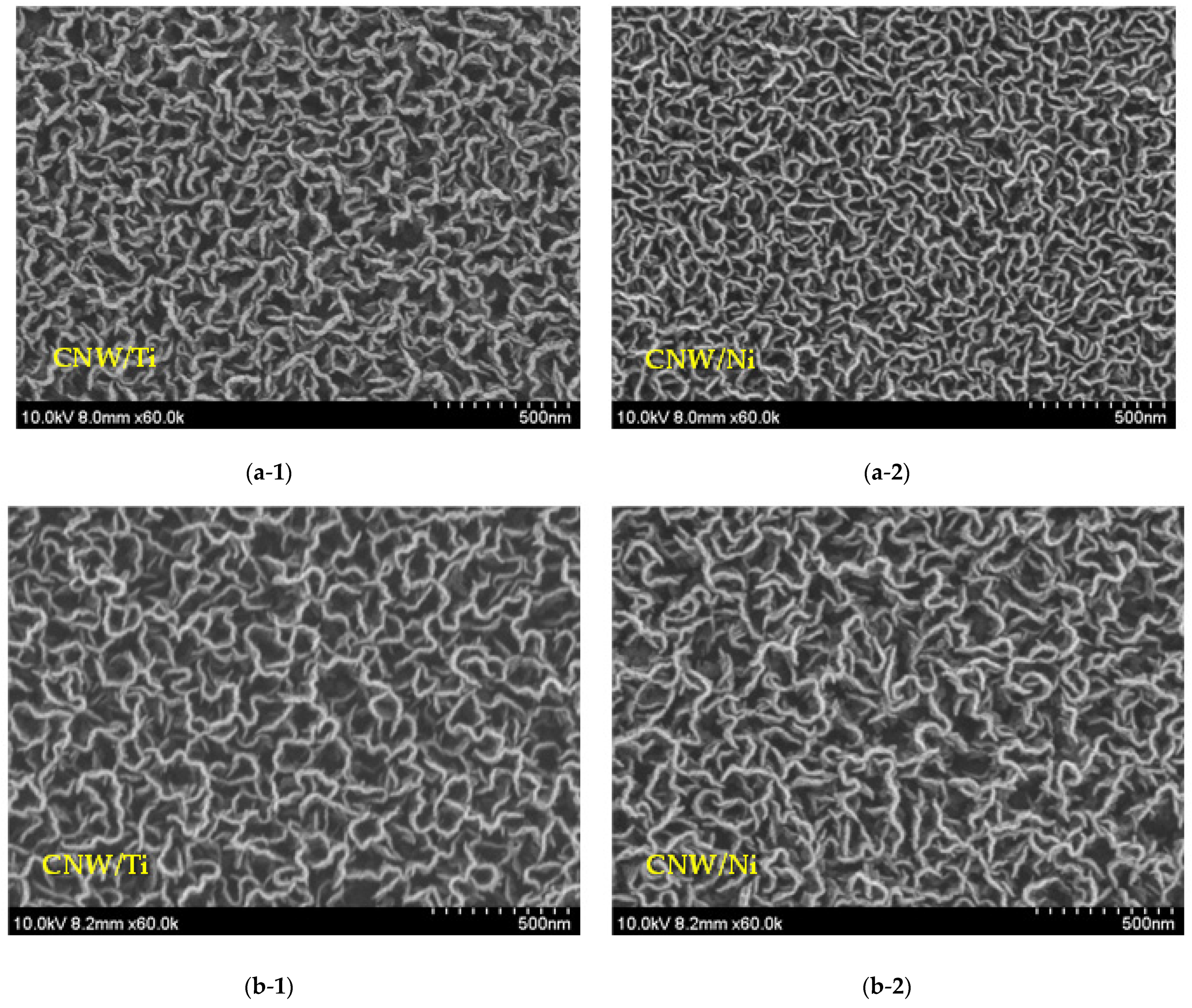
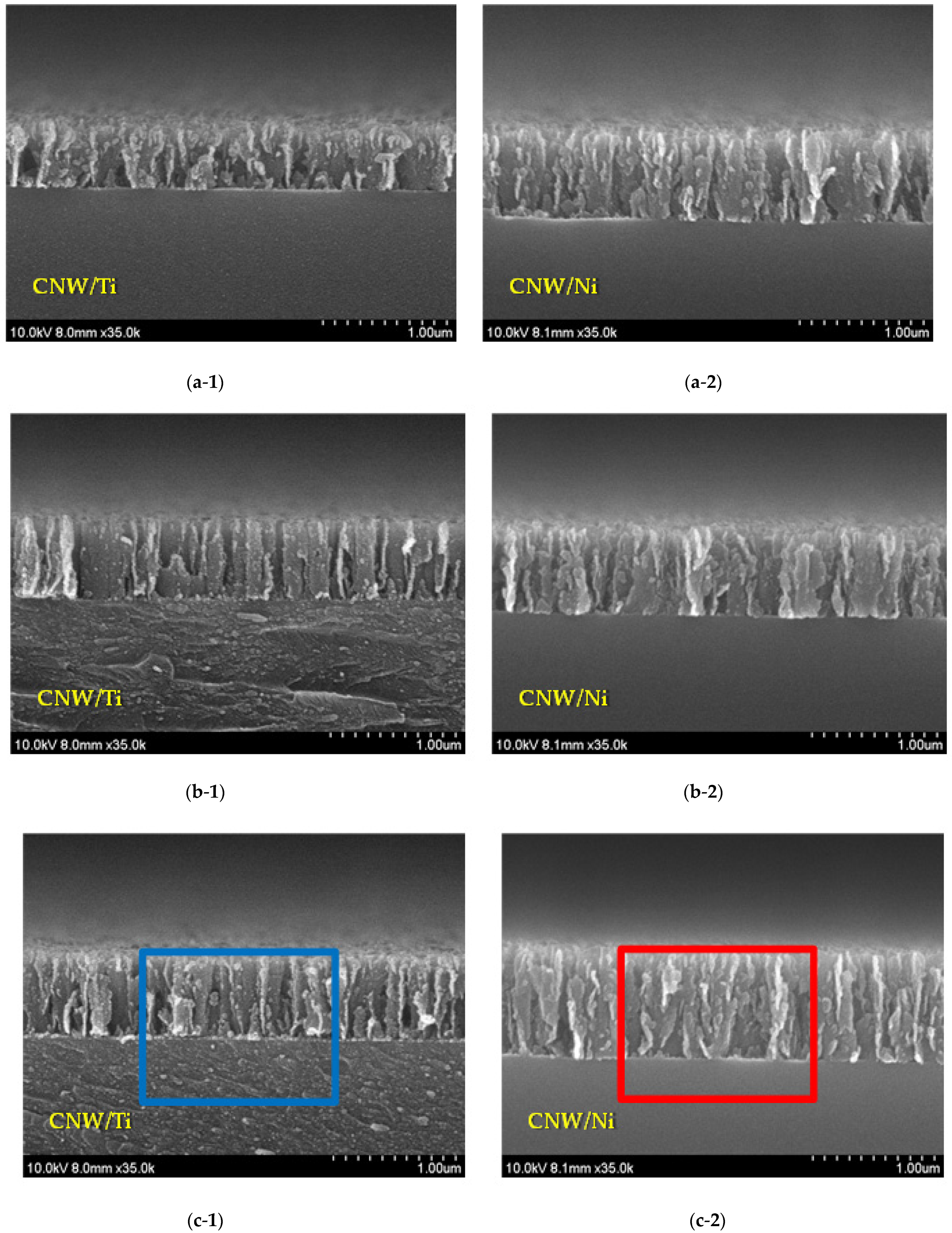
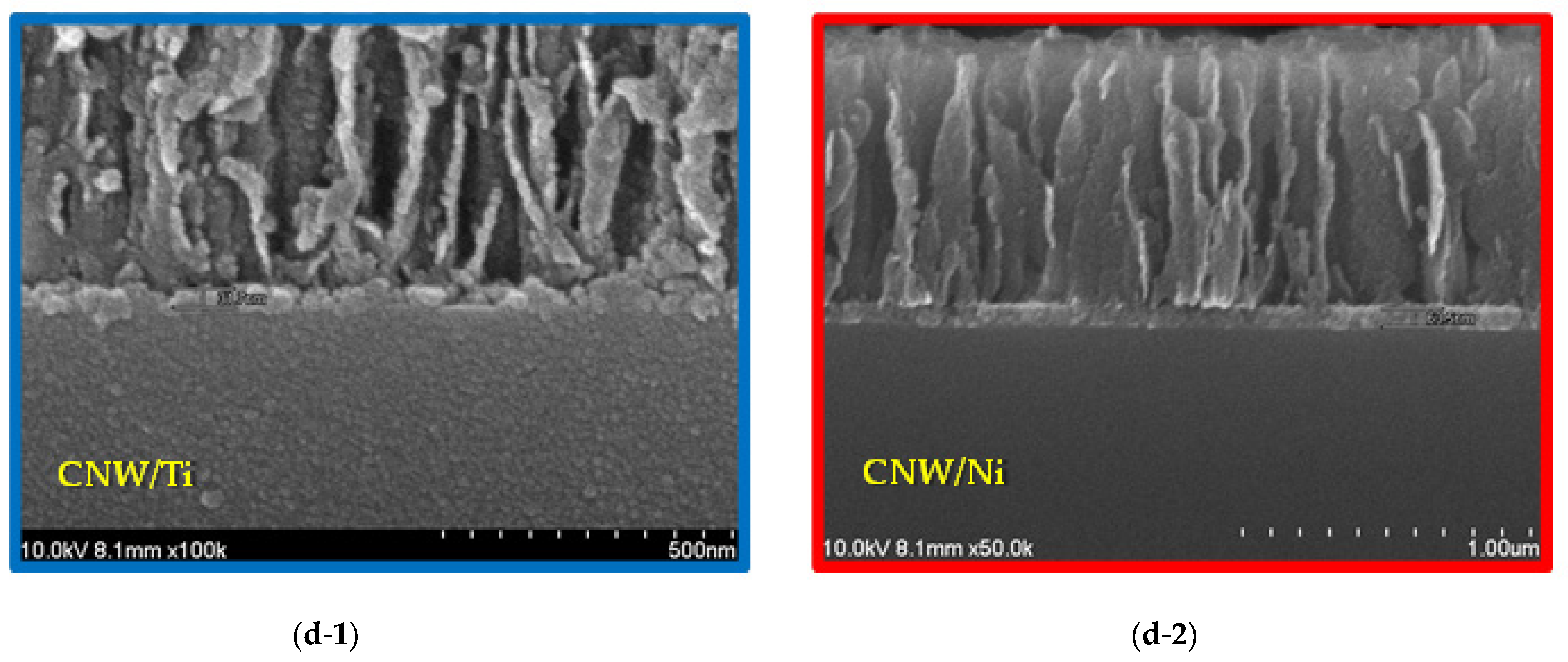
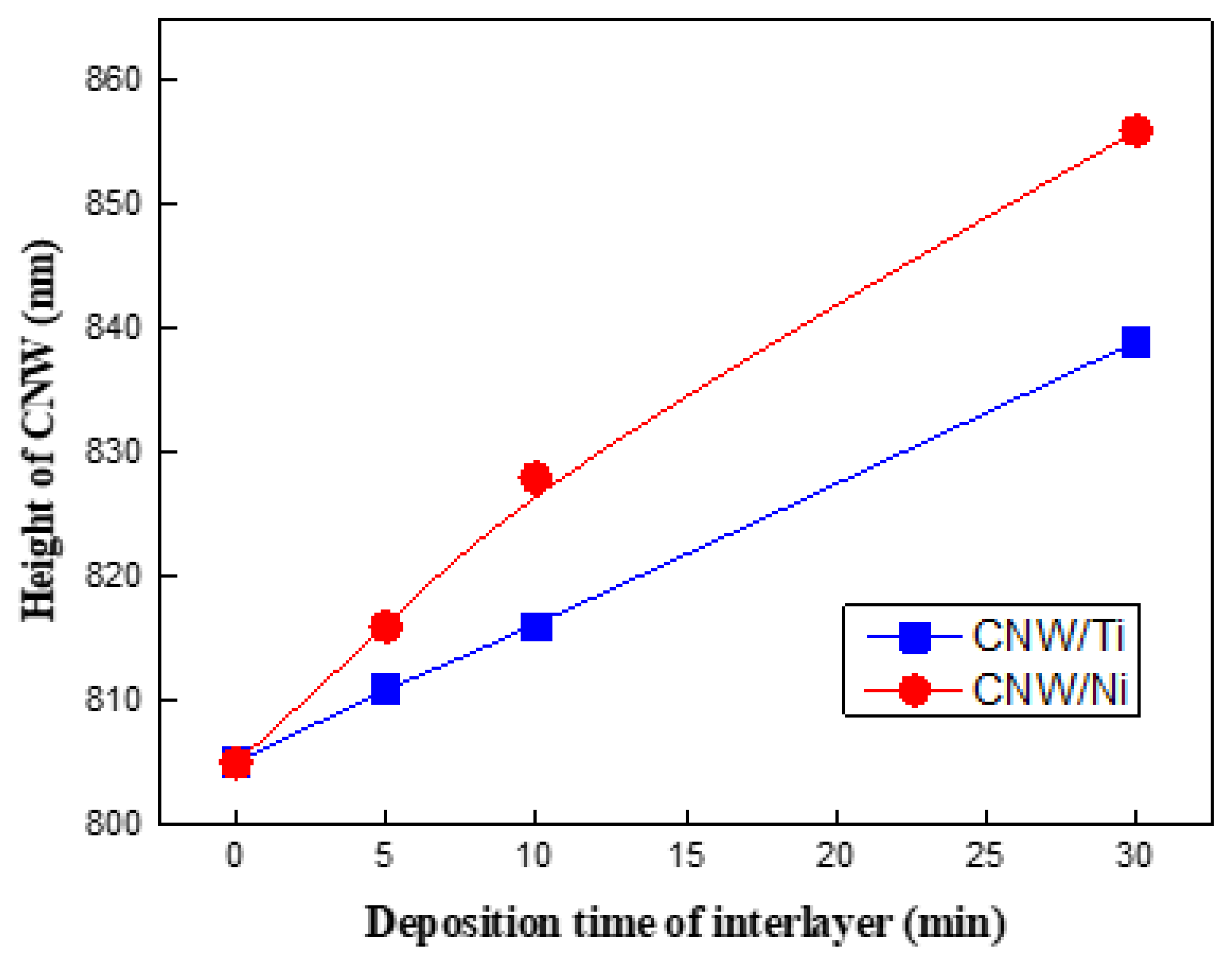
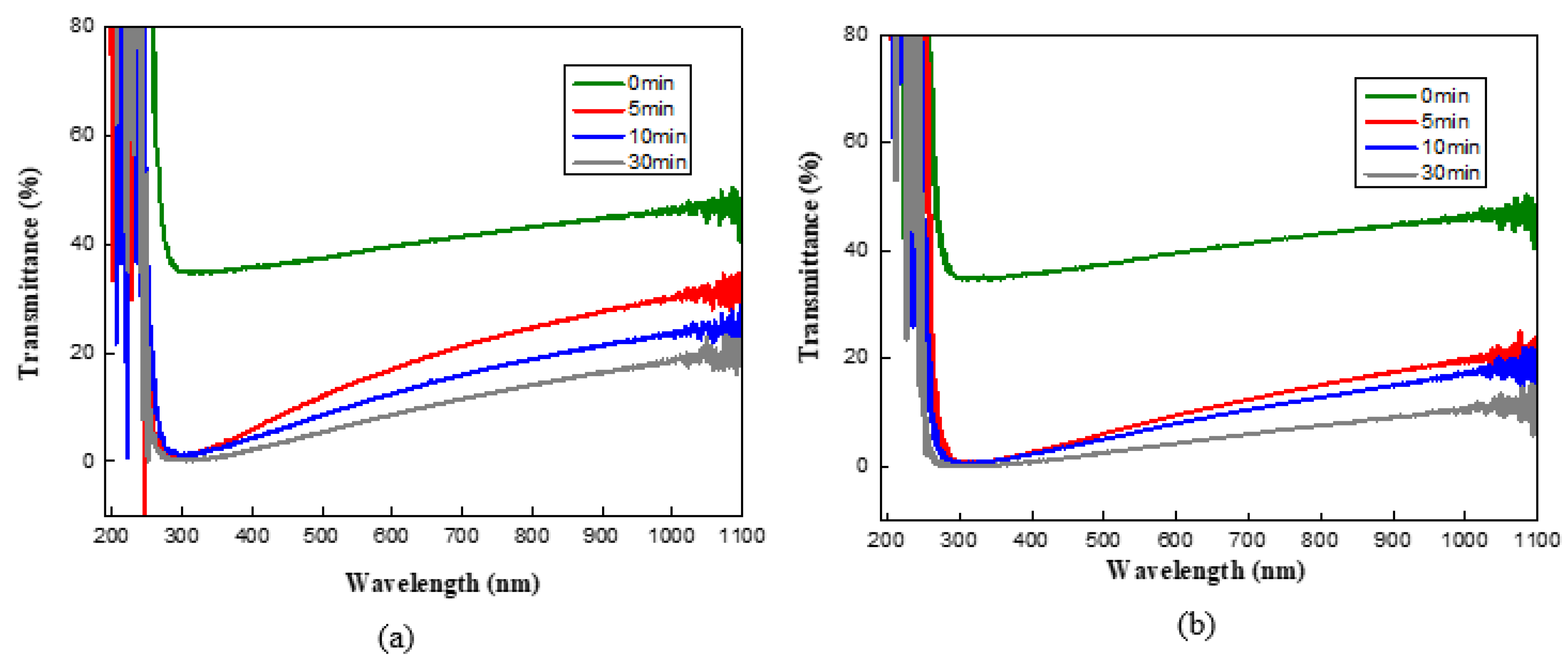
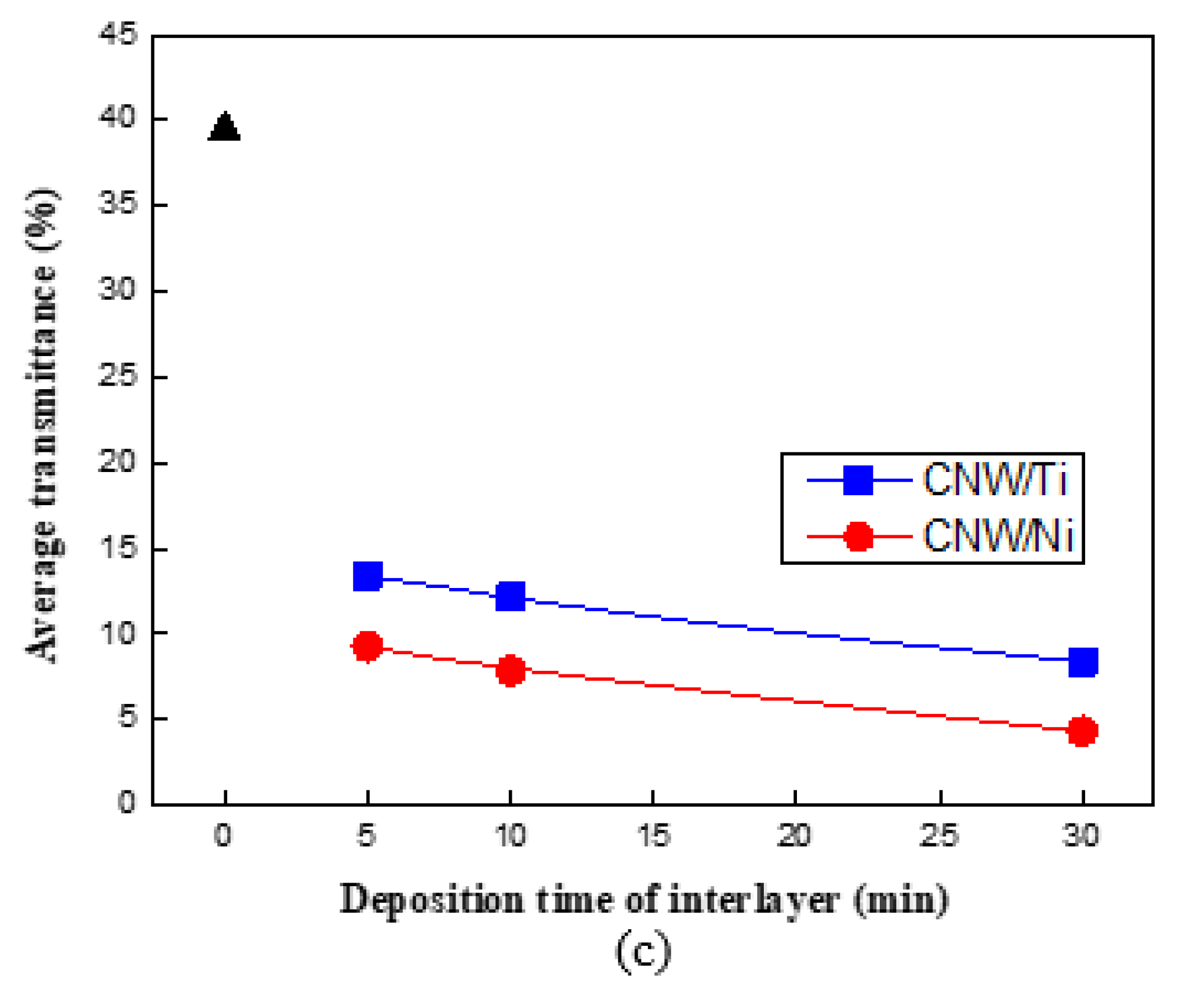
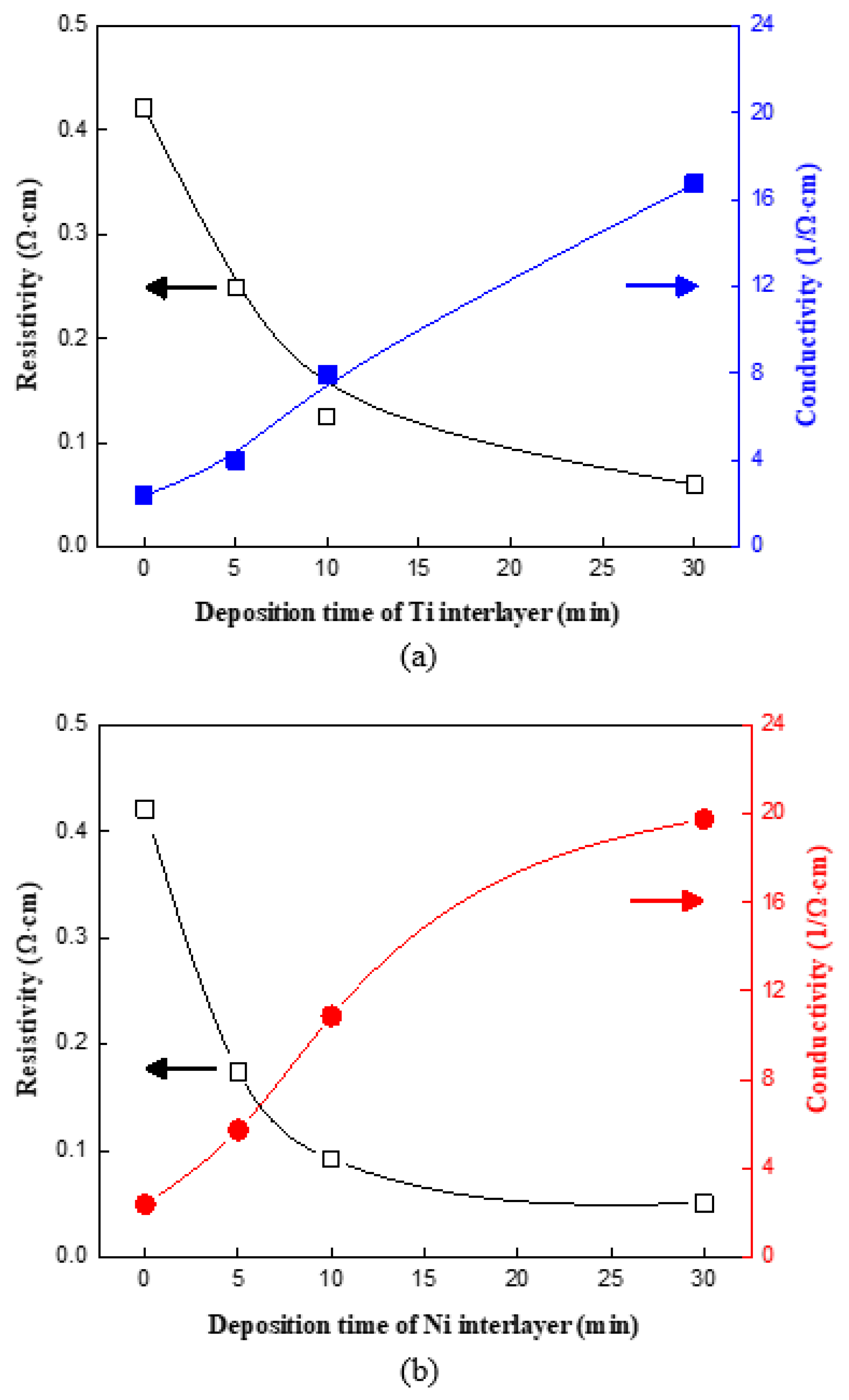
3. Results and Discussion
Surface and Structural Characteristics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, C.; Wei, X.; Kysar, J.W.; Hone, J. Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science 2008, 321, 385. [Google Scholar] [CrossRef]
- Divya, K.C.; Østergaard, J. Battery energy storage technology for power systems-An overview. Electr. Pow. Syst. Res. 2009, 79, 511. [Google Scholar] [CrossRef]
- Huang, Y.; Cai, J.; Zheng, S.; Guo, Y.J. Fabrication of a high-performance Pb-PtCu/CNT catalyst for methanol electro-oxidation. Power Sources 2012, 210, 81. [Google Scholar] [CrossRef]
- Yang, S.; Cheng, Y.; Xiao, X.; Pang, H. Development and application of carbon fiber in batteries. Chem. Eng. J. 2019, 384, 123294. [Google Scholar] [CrossRef]
- Li, Z.; Wang, L.; Yu, L.; Feng, Y. Carbon-based functional nanomaterials: Preparation, properties, and application. Compos. Sci. Technol. 2019, 179, 10–40. [Google Scholar] [CrossRef]
- Fiorani, A.; Merino, J.P.; Zanut, A.; Cariado, A.; Valenti, G.; Prao, M.; Paolucci, F. Advanced carbon nanomaterial for elec-trochemiluminescent biosensor application. Curr. Opin. Electrochem. 2019, 16, 66–74. [Google Scholar] [CrossRef]
- Kuang, Q.; Xie, S.Y.; Jiang, Z.Y.; Zhang, X.-H.; Xie, Z.-X.; Huang, R.-B.; Zheng, L.-S. Low temperature solvothermal synthesis of crumple carbon nanosheets. Carbon 2004, 42, 1737. [Google Scholar] [CrossRef]
- Lee, S.-W.; Muoth, M.; Helbling, T.; Mattmann, M.; Hierold, C. Suppression of resist contamination during photolithography on carbon nanomaterials by a sacrificial layer. Carbon 2014, 66, 295–301. [Google Scholar] [CrossRef]
- Filippidou, M.K.; Chatzichristidi, M.; Chatzandroulis, S. A fabrication process of flexible IDE capacitive, chemical sensors using a two-step lift-off method based on PVA patterning. Sens. Actuators B Chem. 2019, 284, 7–12. [Google Scholar] [CrossRef]
- Aleksandrova, M.; Kurtev, N.; Videkov, V.; Tzanova, S.; Schintke, S. Material alternative to ITO for transparent conductive electrode in flexible display and photovoltaic devices. Microelectron. Eng. 2015, 145, 112–116. [Google Scholar] [CrossRef]
- Barksdale, J. (Ed.) Titanium, Its Occurrence, Chemistry, and Technology; The Ronald Press: New York, NY, USA, 1966; p. 185. [Google Scholar]
- Kong, S.H.; Kim, H.W.; Shin, Y.S.; Kim, M.I. The Effect of Titanium Interlayer on the Adhesion Properties of TiN Coating. J. Korea Soc. Heat Treat. 1992, 5, 1–12. [Google Scholar]
- Wen, F.; Zhao, J.; Yuan, M.; Wang, J. Influence of Ni interlayer on interfacial microstructure and mechanical properties of Ti-6Al-4V/AZ91D bimetals fabricated by a solid-liquid compound casting process. J. Magnes. Alloy. 2021, 9, 1382–1395. [Google Scholar] [CrossRef]
- Kundu, S.; Chatterjee, S. Interfacial microstructure and mechanical properties of diffusion-bonded titanium–stainless steel joints using a nickel interlayer. Mater. Sci. Eng. A 2006, 425, 107–113. [Google Scholar] [CrossRef]
- Choi, H.; Kwon, S.; Kang, H.; Kim, J.H.; Choi, W. Adhesion-Increased Carbon Nanowalls for the Electrodes of Energy Storage Systems. Energies 2019, 12, 4759. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.J.; Choi, W.S. Growth of Carbon Nanowalls on Metal-Coated Substrates via Microwave Plasma Enhanced Chemical Vapor Deposition. J. Nanosci. Nanotechnol. 2014, 14, 9174–9177. [Google Scholar] [CrossRef] [PubMed]
- Helmenstine, A.M. Table of Electrical Resistivity and Conductivity, Science, Tech, Math. 2019. Available online: https://www.thoughtco.com/table-of-electrical-resistivity-conductivity-608499 (accessed on 9 December 2021).

| Parameters | Conditions |
|---|---|
| Substrate lass | Below 1 × 10−6 Torr |
| Working pressure | 3 × 10−3 Torr |
| RF power | 50 W |
| Deposition temperature | Room temperature |
| Target | Ni, Ti |
| Sputtering gas | Ar, 40 sccm |
| Rotation speed | 1700 rph |
| Deposition time | 5 min, 10 min, and 30 min |
| Parameters | Conditions |
|---|---|
| Substrate | Interlayer-coated glass substrates |
| Base pressure | Below 1 × 10−5 Torr |
| Working pressure | 1.1 × 10−2 Torr |
| Microware power | 1200 W |
| Substrate temperature | 500 °C |
| Reaction gas | CH4: 20 sccm |
| Reaction gas | H2: 40 sccm |
| Growth time | 5 min |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tran Thi, M.; Kwon, S.; Kang, H.; Kim, J.-H.; Yoon, Y.-K.; Choi, W. Growth Properties of Carbon Nanowalls on Nickel and Titanium Interlayers. Molecules 2022, 27, 406. https://doi.org/10.3390/molecules27020406
Tran Thi M, Kwon S, Kang H, Kim J-H, Yoon Y-K, Choi W. Growth Properties of Carbon Nanowalls on Nickel and Titanium Interlayers. Molecules. 2022; 27(2):406. https://doi.org/10.3390/molecules27020406
Chicago/Turabian StyleTran Thi, May, Seokhun Kwon, Hyunil Kang, Jung-Hyun Kim, Yong-Kyu Yoon, and Wonseok Choi. 2022. "Growth Properties of Carbon Nanowalls on Nickel and Titanium Interlayers" Molecules 27, no. 2: 406. https://doi.org/10.3390/molecules27020406
APA StyleTran Thi, M., Kwon, S., Kang, H., Kim, J.-H., Yoon, Y.-K., & Choi, W. (2022). Growth Properties of Carbon Nanowalls on Nickel and Titanium Interlayers. Molecules, 27(2), 406. https://doi.org/10.3390/molecules27020406







