Abstract
Cephalanthus tetrandrus (Roxb.) Ridsd. et Badh. F. (CT) belongs to the Rubiaceae family. Its dried leaves are widely used in traditional Chinese medicine to treat enteritis, dysentery, toothache, furuncles, swelling, traumatic injury, fracture, bleeding, and scalding. In order to further clarify the unknown chemical composition of CT, a rapid strategy based on UHPLC-Q-exactive orbitrap was established for this analysis using a Thermo Scientific Hypersil GOLDTM aQ (100 mm × 2.1 mm, 1.9 µm) chromatographic column. The mobile phase was 0.1% formic acid water–acetonitrile, with a flow rate of 0.3 mL/min and injection volume of 2 µL; for mass spectrometry, an ESI ion source in positive and negative ion monitoring modes was adopted. A total of 135 chemicals comprising 67 chlorogenic acid derivatives, 48 flavonoids, and 20 anthocyanin derivatives were identified by comparing the mass spectrum information with standard substances, public databases, and the literature, which were all discovered for the first time in this plant. This result broadly expands the chemical composition of CT, which will contribute to understanding of its effectiveness and enable quality control.
1. Introduction
Cephalanthus tetrandrus (Roxb.) Ridsd. et Badh. F. (CT), known as Ma Yanshu or Water Yangmei in traditional Chinese medicine (TCM) and ‘Bagua Maple’ in Dong medicine, belongs to the Rubiaceae family. The leaves have the effects of clearing away heat and toxic materials, as well as dispelling blood stasis and reducing swelling, and they are used for the treatment of enteritis, dysentery, toothache caused by acute gingivitis and acute pulpitis, furuncles, swelling, traumatic injury, fracture, bleeding, and scalding [1]. This plant is mainly distributed in Hunan, Guangdong, Hainan, and Taiwan provinces. However, no detailed studies on the material basis of its medicinal effects have been reported. To date, most studies on CT have focused on its ornamental value. A previous study reported on its pharmacognosy [2]. Therefore, it is necessary to clarify the unknown chemical composition of CT.
In order to further analyze and discover its chemical composition and pharmacological effects, it is necessary to find a suitable analytical technique to analyze the complex chemical composition in CT. There are many methods for analyzing herbal medicine, including thin-layer chromatography (TLC) [3,4], ultraviolet spectroscopy (UV) [5], infrared spectroscopy (IR) [6], and nuclear magnetic resonance (NMR) [7,8]; however, the components of Chinese herbal medicine are complex and in trace amounts, and these methods cannot accurately characterize them. UV is only applicable to the determination of groups containing unsaturated bonds and aromatic structures, with low quantitative sensitivity and a small application range. NMR also suffers from low sensitivity, with a narrow detection range. TLC and IR easily succumb to interference by many factors, leading to large errors in the analysis results. In recent years, the combination of liquid chromatography and mass spectrometry has resulted in improvements in sensitivity and resolution, and it has been widely used for the analysis of food, environment, drugs, etc., as well as for the qualitative analysis of the components of TCM [9,10,11].
In this study, a strategy based on UHPLC-Q-exactive orbitrap was established for the identification of the unknown chemical components of CT. As a result, a total of 135 chemicals comprising 67 chlorogenic acid derivatives (CGAs), 48 flavonoids, and 20 anthocyanin derivatives, were identified by comparing the mass spectral information with standard substances, public databases, and the literature, all of which are reported for the first time.
2. Results and Discussion
2.1. Scheme for Qualitative Analysis
In this study, a strategy based on UHPLC-Q-exactive orbitrap MS combined with parallel reaction monitoring (PRM), diagnostic fragment ions (DFIs), and neutral loss (NL) was established. First, the total extract was obtained via reflux filtration and rotary evaporation. Secondly, the sample was injected into the UHPLC-Q-exactive orbitrap MS to obtain a high-resolution mass spectrum through a full scan. Thirdly, the mass spectral fragmentation pathway library of each chemical component was established and summarized. Fourthly, the potential chemical was predicted by metabolite workflow in Compound Discoverer 3.0 using the following parameters: the drugs were set as shikimic acid, quinic acid, quercetin, and kaempferol, whereas the groups added were set as a list of the abovementioned substituents. Next, the fragment ions were acquired using UHPLC-Q-exactive orbitrap MS in PRM mode triggered by the list of included ions. Fifthly, a high-resolution extraction ion flow diagram (HREIC) was used to further verify the accuracy of screening. Lastly, the candidate chemicals were identified on the basis of diagnostic fragment ions, neutral loss, and retention time, as well as through comparison with the literature.
2.2. Optimization of Extraction Conditions
Different extraction conditions, including extraction methods (reflux extraction and ultrasonic extraction) and extraction solvents (70% ethanol, 20% methanol, 40% methanol, 60% methanol, 80% methanol, and 100% methanol) were investigated in our study. Eventually, ultrasonic extraction with 70% ethanol at room temperature was chosen as the optimal conditions according to the numbers and intensity of the peak in UHPLC-Q-exactive orbitrap MS.
2.3. UHPLC-ESI-MS2 Qualitative Analysis of CGAs and Flavonoids
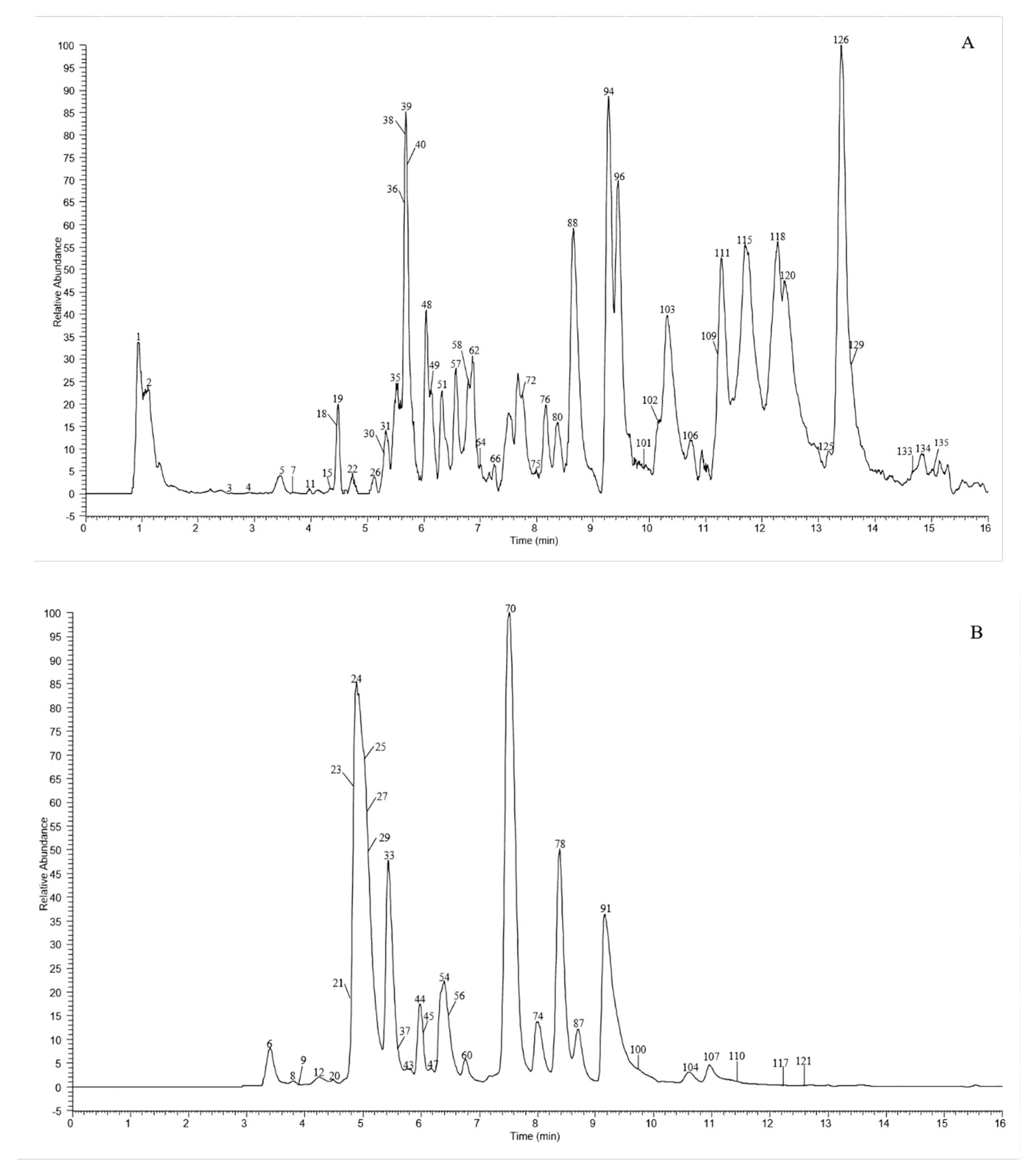
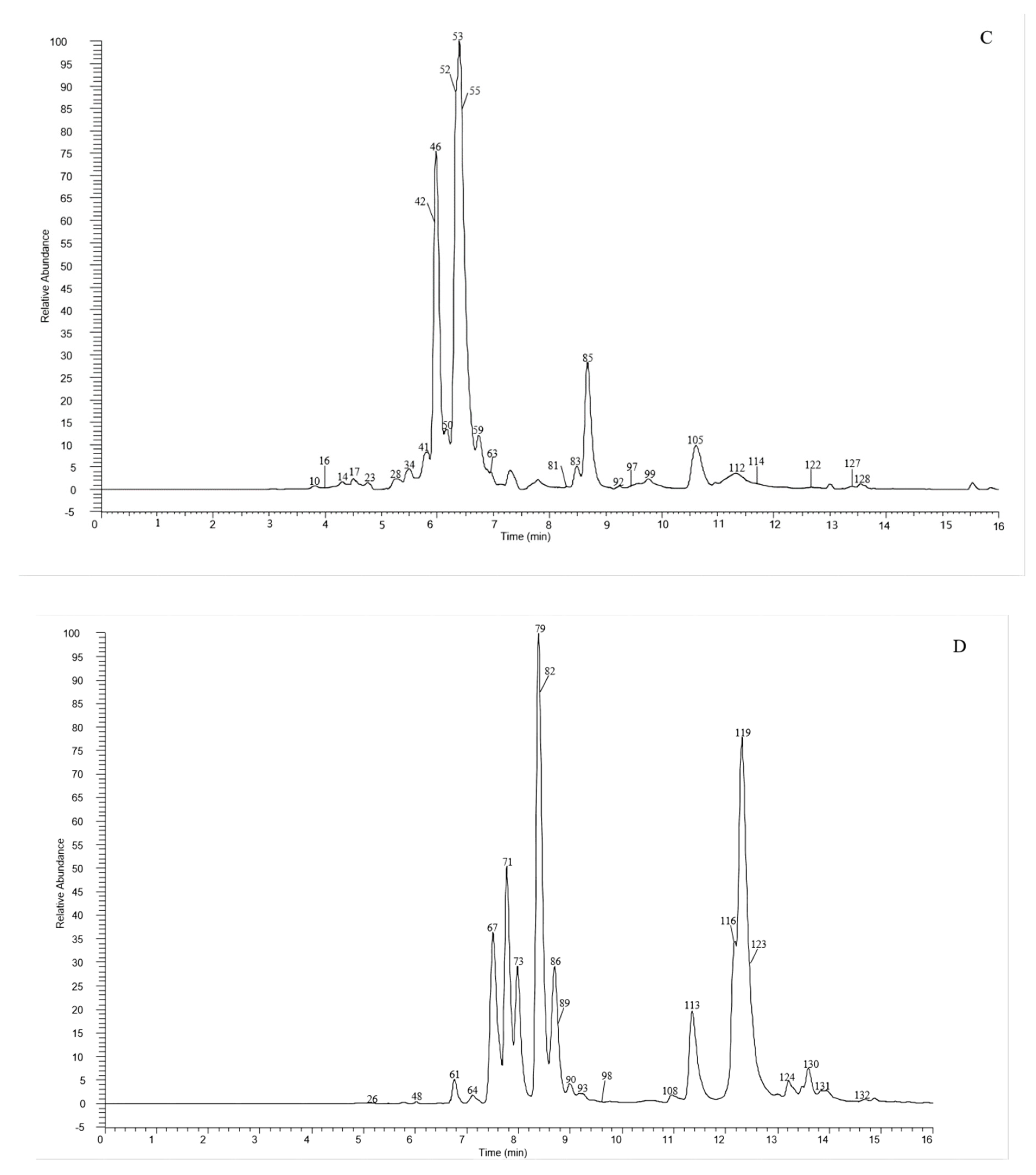
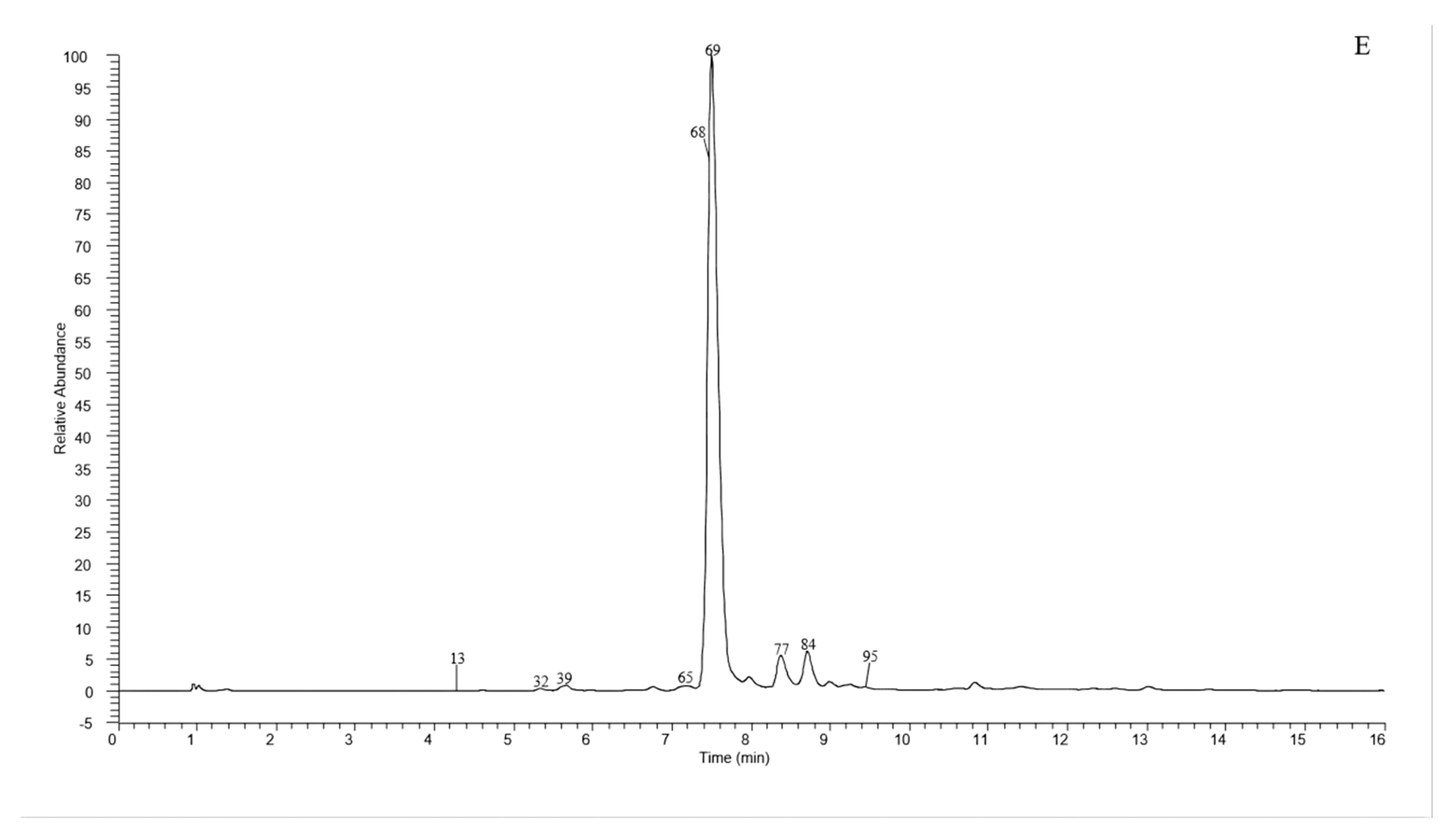
A total of 135 chemical were tentatively identified by UHPLC-Q-exactive orbitrap MS, comprising 67 CGAs, 20 anthocyanins, and 48 flavonoid derivatives. The chromatographic and mass data of those detected constituents are listed in Table 1, and the high-resolution extracted ion chromatograms (HREICs) are shown in Figure 1.

Table 1.
Retention times and mass spectral data of Cephalanthus tetrandrus (Roxb.) Ridsd. et Badh. F.
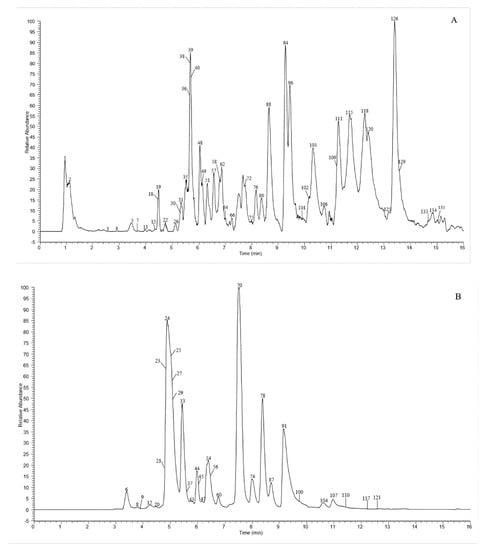
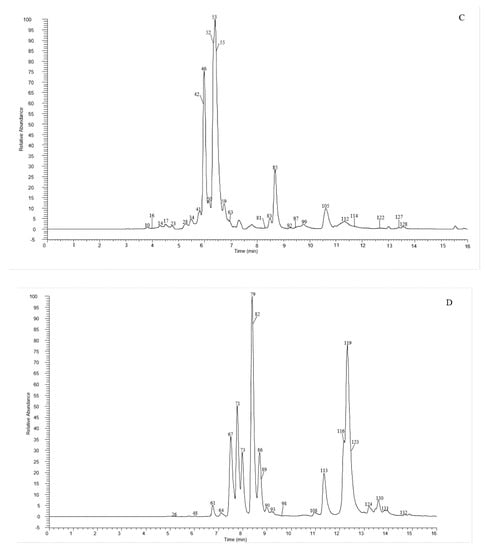
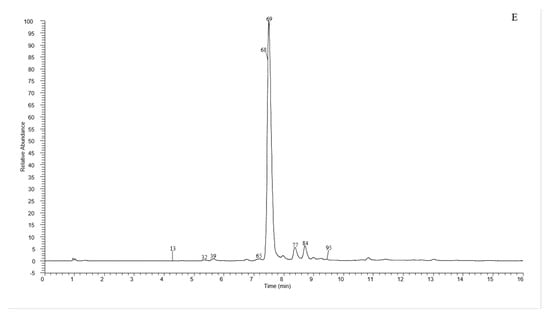
Figure 1.
High-resolution extracted ion chromatogram (HREIC) in 5 ppm for multiple compounds in CT: (A) m/z 269.04554, 271.06119, 283.06119, 285.04046, 287.05611, 289.07176, 301.03537, 303.05102, 353.10893, 431.09837, 433.07763, 465.10384, 497.10893, 497.13006, 529.13514, 579.1497, 625.14102, 677.15119, and 901.24079; (B) m/z 335.07724, 337.09289, 341.08780, 353.08780, 367.10345, 397.11402, 447.09328, 449.10893, 497.13006, 499.12458, 499.14571, 515.11949, 515.14062, 529.15627, 593.15119, 609.1461, 623.16175, 677.19345, 755.20401, 771.19893, and 917.23571; (C) m/z 335.07724, 337.09289, 367.10345, 397.11402, 449.10893, 497.13006, 499.12458, 499.14571, 515.14062, 529.15627, 623.16175, 677.19345, 677.15119, 771.19893, and 917.23571; (D) m/z 757.21856, 597.14501, 741.20252, 465.10275, 595.16574, 625.17631, 479.11840, 609.18139, 579.17083, 741.22365, and 757.19744; (E) m/z 291.08631, 463.12348, 479.11840, 579.1497, 611.16066, and 741.22365. (A–C) EIC in negative mode; (D,E) EIC in positive mode.
2.3.1. Identification Based on Reference Standard
Compounds 6, 24, 25, 75, 78, and 104 were observed at 3.41, 4.89, 5.03, 8.06, 8.34, and 10.60 min, corresponding to trans-3-caffeoylquinic acid (CQA), trans-4-CQA, trans-5-CQA, 3,4-dicaffeoylquinic acid (DiCQA), 3,5-DiCQA, and 4,5-DiCQA, respectively, by comparing the retention time and MS data with reference standards.
Compounds 70, 71, 80, 106, 117, 126, and 135 were identified as rutin, isoquercitrin, kaempferol-3-O-rutinoside, eriodictyol, quercetin, naringenin, and kaempferol, respectively, by comparing the retention time and MS/MS data with reference standards.
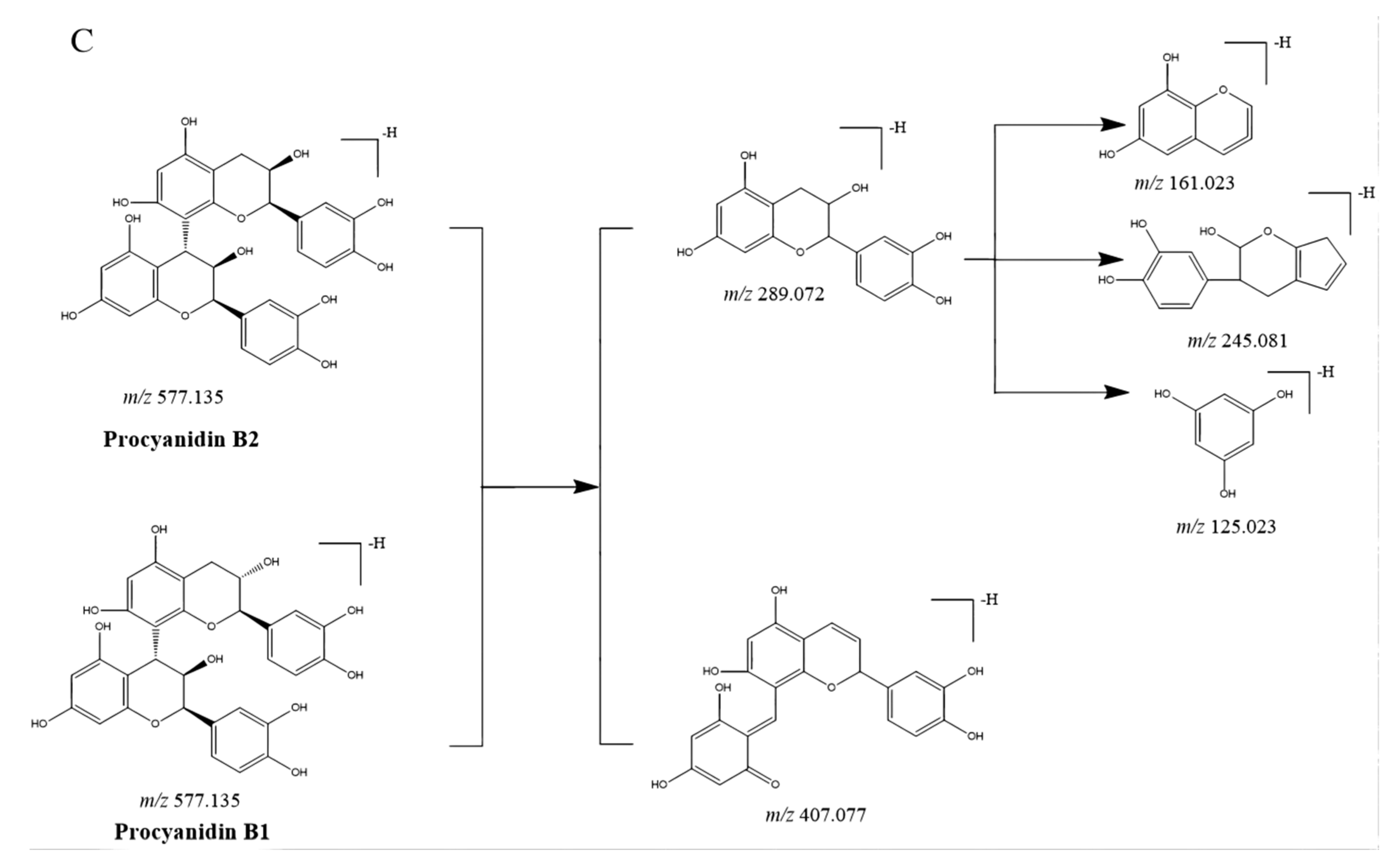
Likewise, compounds 13 and 32 were confirmed as procyanidin B1 and procyanidin B2.
2.3.2. Identification of Speculative Chlorogenic Acid Derivatives
Identification of Monoacyl-Quinic Acids and Monoacyl-Shikimic Acids
Compounds 14, 28, 42, and 52 were observed at 4.29, 5.25, 5.79, and 6.34 min, respectively, with the same molecular ion [M − H]− at m/z 337.0929 (C16H18O8). According to the literature [12,13], they were identified as trans-3-O-p-coumaroylquinic acid (pCoQA), trans-4-pCoQA, cis-4-pCoQA, and cis-5-pCoQA, respectively, according to their retention time and base peak ion in the MS2 spectrum.
Compounds 27, 30, 54, and 57 were observed at 5.21, 5.30, 6.39, and 6.57 min, respectively, with the same molecular ion [M − H]− at m/z 397.1140 (C18H22O10) and fragment ions [M − H]− at m/z 173.0446 and 191.0553; these compounds were identified as sinapoylquinic acids (SQAs). According to their base peak ion and retention time, they were identified as trans-3-SQA, cis-3-SQA, trans-5-SQA, and cis-5-SQA through comparison with the literature data [14].
Compounds 33 and 45 were observed at 5.44 and 5.99 min, respectively, with the quasi-molecular ion [M − H]− at m/z 353.0878 (C16H18O9), exhibiting identical MS1 data and similar MS2 data to trans-3-CQA, trans-4-CQA, and trans-5-CQA. Accordingly, compounds 33 and 45 were inferred as caffeoylquinic acids (CQAs). According to their retention time and MS2 data, as well as the literature [15,16], compounds 33 and 45 were identified as cis-5-CQA and cis-3-CQA, respectively.
Compounds 38, 43, 49, 53, and 56 were observed at 5.67, 5.85, 6.17, 6.37, and 6.49 min, respectively, with the quasi-molecular ion [M − H]− at m/z 335.0772 (C16H16O8), inferring that they may be caffeoylquinic acid lactones (CQLs) or caffeoyl shikimic acids (CSAs). The fragmentation ion at m/z 161.0233 yielded by quinic acid lactones is characteristic of CQLs [17,18,19,20,21]. Hence, compounds 38, 53, and 56 were identified as 3-CQL, 1-COL, and 4-CQL, respectively, on the basis of their retention time and MS2 spectra. Furthermore, compounds 43 and 49 were identified as 5-CSA and 4-CSA, respectively.
Compounds 50, 55, and 59 possessed a deprotonated ion at m/z 367.1035 (C17H20O9), suggesting that they could be feruloyl quinic acids (FQAs). 4-FQA yielded the base peak ion at m/z 173.0445, whereas cis-5-FQA and trans-5-FQA yielded the base peak ion at m/z 191.0554. The configuration of cis or trans was determined from the intensity of these peaks [13,15]. Therefore, compounds 50, 55, and 59 were identified as 4-FQA, trans-5-FQA, and cis-5-FQA.
Compounds 9, 15, 20, 22, 31, and 34 were eluted at 3.81, 4.31, 4.50, 4.74, 5.31, and 5.52 min, respectively, with the quasi-molecular ion [M − H]− at m/z 515.1406 (C22H28O14) and their fragment ions [M − H]− at m/z 191.0554, 161.0235, 173.0447, and 353.1092, respectively, indicating that they possessed a CQA moiety. Considering the neutral loss of 162 Da, they were identified as CQA-hexoside [11]. Likewise, compounds 10 and 23 were identified as 4-pCoQA-hexoside and 5-pCoQA-hexoside, respectively [17,18,19]; compounds 16, 29, and 40 were tentatively characterized as 5-FQA-hexoside; compounds 17 and 44 were identified as CQA-dihexoside; compound 35 was identified as CSA-hexoside; compound 41 was identified as CQL-hexoside; and compound 47 was identified as 4-FQA-hexoside [17,18,19].
Identification of Diacyl-Quinic Acids and Diacyl-Shikimic Acids
Compounds 61, 91, and 107 were obtained at 6.83, 9.15, and 10.96 min, respectively, with the quasi-molecular ion [M − H]− at m/z 515.1195 (C25H24O12), indicating that they were dicaffeoylquinic acids (DiCQAs). Compound 91 yielded the base peak ion [M − H]− at m/z 173.0445 and fragmentation ions at m/z 179.0340 and 191.0552, suggesting that it might be 1,4-DiCQA [22,23]. Compounds 110, 115, and 118 with the quasi-molecular ion [M − H]− at m/z 497.1089 (C25H22O11) were characterized as dicaffeoylquinic acid lactones (DiCQLs) or dicaffeoyl shikimic acids (DiCSAs) on the basis of their fragmentation ions. Hence, compounds 110, 115, and 118 were identified as DiCSA, DiCSA, and DiCQL, respectively.
Compounds 97, 99, 112, and 122 were eluted at 9.46, 9.76, 11.34, and 12.67 min, respectively. The quasi-molecular ion [M − H]− at m/z 499.1246 (C25H24O11) and the fragment ions [M − H]− at m/z 173.0441, 163.0389, 179.0337, 191.0551, and 135.0436 were consistent with a coumaroyl caffeoylquinic acid (pCoCQA) moiety. The absence of a base peak ion at m/z 173.0337 of compound 99 was consistent with 3C,5-pCoQA. Likewise, compounds 97, 112, and 122 were identified as 4-pCo,5CQA, trans-4-pCo,5CQA, and cis-4-pCo,5CQA, respectively. Compounds 101, 102, 103, 111, and 114, with the quasi-molecular ion [M − H]− at m/z 529.1351 (C26H26O12) and the fragment ions [M − H]− at m/z 173.0445 or 193.0496 were consistent with caffeoyl feruloylquinic acids (CFQAs). According to the MS2 data and retention times reported in the literature [13,22,24], compounds 101, 102, 103, 111, and 114 were identified as 3C,5FQA, 3F,5CQA, 3C,5FQA, 4C,5FQA, and 4F,5CQA, respectively.
Identification of Triacyl-Quinic Acids and Triacyl-Shikimic Acids
Compound 127 possessed a molecular ion [M − H]− at m/z 677.1512 (C34H30O15) and a fragment ion at m/z 353.0878, consistent with CQA. According to [22], compound 127 was identified as TriCQA.
Others
Compounds 1 and 2 were obtained at 0.93 and 1.11 min, with the molecular ion [M − H]− at m/z 353.1089 (C13H22O11) and fragment ions [M − H]− at m/z 173.0443, 191.0551, and 179.0549, indicating a quinic acid moiety. Considering the neutral loss of 162 Da, they could be considered as hexosides of quinic acid (QA-hexosides).
Compounds 3, 4, 5, 7, 8, 11, 12, 18, and 21 yielded a deprotonated ion [M − H]− at m/z 341.0878 (C15H18O9). Considering the neutral loss of 162 Da and the base peaks at m/z 135.0438 and 179.0339, they were identified as caffeic acids [25]. Hence, they might be considered as hexosides of caffeic acid (CA-hexosides).
2.3.3. Identification of Speculative Anthocyanins
The common anthocyanins in Cephalanthus tetrandrus (Roxb.) Ridsd. et Badh. F. are the glycosylated derivatives of pelargonidin (m/z 271.0601), cyanidin (m/z 287.0550), peonidin (m/z 301.0707), delphinidin (m/z 303.0499), and petunidin (m/z 317.0667).
Compounds 73 and 79, eluted at 7.98 and 8.38 min, possessed a similar molecular ion [M + H]+ at m/z 595.1657 (C27H31O15) and a fragment ion at m/z 287.0544 [M − 308.110]+, indicating the loss of one rutinose moiety. According to [26], compounds 73 and 78 were determined to be cyanidin-3-O-rutinoside isomers.
Compounds 26 and 48 were eluted at 5.14 and 6.02 min, respectively, with the quasi-molecular ion [M + H]+ at m/z 757.2186 (C33H41O20) and fragment ions [M + H]+ at m/z 287.0534, 449.1057, and 595.1647, indicating that they possessed a cyanidin moiety. Considering the neutral loss of 162 Da (757.2186 − 595.1647) and 308 Da (757.2186 − 449.1057), they were identified as cyanidin-O-rutinoside-O-galactoside [26,27].
Compound 69 was obtained at 7.51 min, with the quasi-molecular ion [M + H]+ at m/z 465.1028 (C21H21O12) and the fragment ion [M + H]+ at m/z 303.0494, indicating the neutral loss of 162 Da; hence, compound 69 corresponded to delphinidin-3-O-hexoside. Likewise, compounds 84, 89, 90, and 95 were determined to be petunidin-3-O-hexoside, petunidin-3-O-galactoside, petunidin-3-O-glucoside, and peonidin-3-O-hexoside, respectively [26].
Compounds 39 and 87 appeared at 5.68 and 8.68 min, respectively, with an identical molecular ion at m/z 289.0718 (C15H14O6) in negative mode and 291.0863 (C15H14O6) in positive mode, in addition to negative fragment ions at m/z 109.0282, 203.0704, and 245.0815 and positive fragment ions at m/z 139.0388, 123.0440, and 147.0439, in accordance with catechin and epicatechin [28,29].
Compound 64 possessed a molecular ion [M + H]+ at m/z 597.1450 (C26H29O16) and a fragment ion at m/z 303.0493 [M − 294.094]+, resulting from the loss of a xylosyl glucoside, which is characteristic of delphinidin-3-xylosylglucoside.
Compound 67 possessed a molecular ion [M + H]+ at m/z 449.1078 (C21H21O11) and an MS/MS fragment ion at m/z 303.0495 [M − 146.057]+, corresponding to the loss of a rhamnose moiety. According to [26], compound 67 was determined to be delphinidin-3-O-rhamnoside.
Compound 68 was eluted at 7.50 min, with the quasi-molecular ion at [M + H]+ at m/z 611.1607 (C27H31O16) and the fragment ion [M + H]+ at m/z 303.0493, indicating the presence of a delphinidin moiety. Considering the neutral loss of 162 Da, compound 68 was identified as delphinidin-3-O-rutinoside [26]. Likewise, compounds 82, 86, 93, and 98 were identified as petunidin-3-O-rutinoside, petunidin-3-O-rutinoside, peonidin-3-O-rutinoside, and pelargonidin-3-O-rutinoside, respectively [26].
2.3.4. Identification of Speculative Flavonoids
Identification of Flavonols
Compounds 36, 58, and 63 were eluted at 5.54, 6.63, and 6.89 min, respectively, with the quasi-molecular ion [M − H]− at m/z 771.1989 (C33H40O21) and fragment ions [M − H]− at m/z 301.0349, 463.0898, and 609.1459, respectively, indicating that they possessed a quercetin moiety. Considering the neutral loss of 162 Da (771.1989 − 609.1459) and 308 Da (771.1989 − 463.0898), they were identified as quercetin-O-glucosyl rutinoside. Similarly, the quasi-molecular ion [M − H]− at m/z 755.2040 (C33H40O20) of compound 60, with the loss of 454 Da (755.2040 − 301.0391), could be quercetin rhamnosyl rutinoside. The quasi-molecular ion [M − H]− at m/z 755.1829 (C36H36O18) of compounds 108, 113, 116, and 119, with fragment ions [M − H]− at m/z 301.0351 and 609.1461, as well as a neutral loss of 146 Da (755.1829 − 609.1461), were identified as quercetin coumaroyl rutinoside. Compound 62 showed a quasi-molecular ion at m/z 625.1410 (C27H30O17); considering a neutral loss of 162 Da (625.1410 − 463.0883), it could be quercetin-O-sophoroside. Compound 65 was found at 7.24 min, with the quasi-molecular ion [M − H]− at m/z 739.2091 (C33H40O19) and the fragment ion [M − H]− at m/z 285.0398 and 593.1513; the loss of 146 Da (739.2091 − 593.1513) and 308 Da (593.1513 − 285.0398) could correspond to kaempferol-O-rutinosyl rhamnoside. Likewise, compounds 123, 124, 129, 130, 131, and 132 were tentatively identified as kaempferol coumaroyl rutinoside.
Compounds 72 and 81 were eluted at 7.80 and 8.46 min, with the quasi-molecular ion [M − H]− at m/z 917.2357 (C42H46O23) and fragment ions [M − H]− at m/z 301.0343, 463.0869, 609.1456, and 755.2032, indicating that they possessed a quercetin moiety. Considering the neutral loss of 308 Da (917.2357 − 609.1456) and 146 Da (609.1456 − 463.0869 or 917.2357 − 755.2032), they could be quercetin caffeoyl rutinosyl rhamnoside. Likewise, the quasi-molecular ion [M − H]− at m/z 901.2408 (C42H46O22) of compounds 94, 96, 109, 120, and 125, which indicated a loss of 146 Da (901.2408 − 755.2032), could be quercetin coumaroyl rutinosyl rhamnoside.
Compound 74 appeared at 7.99 min, with the same quasi-molecular ion [M − H]− at m/z 593.1512 (C27H30O15) and the fragment ion [M − H]− at m/z 285.0394 as compound 80. Therefore, it was identified as a kaempferol-3-O-rutinoside isomer.
Compounds 83 and 85 were obtained at 8.49 and 8.67 min, respectively, with the quasi-molecular ion [M − H]− at m/z 623.1618 (C28H32O16) and the fragment ion [M − H]− at m/z 315.0502, indicating the loss of 308 Da; hence, they were identified as isorhamnetin-3-O-rutinoside by referring to the literature [30].
Compound 76 possessed a molecular ion [M − H]− at m/z 433.0776 (C20H18O11) and a fragment ion at m/z 301.0351 [M − 132.042]−, corresponding to the loss of an arabinose moiety; thus, compound 76 was identified as quercetin-O-arabinoside.
Compounds 77 and 88 possessed a similar molecular ion [M − H]− at m/z 447.0933 (C21H20O11) and a fragment ion at m/z 285.0387 [M − 162.052]−, indicating the loss of one glucose moiety. According to [31], compounds 77 and 88 were identified as astragalin isomers.
Compounds 92 and 100 possessed a molecular ion [M − H]− at m/z 447.0933 (C21H20O11) and a fragment ion at m/z 301.0341 [M − 146.057]−, corresponding to the loss of a rhamnose moiety; therefore, compounds 92 and 100 were identified as quercetin-O-rhamnoside.
Compound 133 possessed a molecular ion at m/z 269.0455 (C21H20O11) and fragment ions at m/z 117.0334 and 151.0029. Therefore, compound 133 was identified as apigenin.
Compound 134 eluted at 14.87 min, and it possessed a similar molecular ion [M − H]− at m/z 285.0405 (C15H10O6) and fragment ion at m/z 151.0021 to kaempferol. Accordingly, compound 134 was identified as a kaempferol isomer.
Identification of Flavones
Compound 121 possessed a molecular ion [M − H]− at m/z 283.0612 (C16H12O5) and fragment ions [M − H]− at m/z 268.0374, 269.0415, 151.0029, and 107.0128; thus, compound 121 was identified as genkwanin.
Compound 128 possessed a molecular ion [M − H]− at m/z 431.0984 (C21H20O10) and a fragment ion at m/z 269.0457. Therefore, compound 128 was identified as oroxin A.
Identification of Flavanones
Compounds 37 and 46 possessed a molecular ion [M − H]− at m/z 449.1089 (C21H22O11) and a fragment ion at m/z 287.0560 [M − 162.052]−, indicating the loss of a hexose moiety. According to [32], compounds 37 and 46 were identified as erodcyol-O-hexoside.
Identification of Flavanonols
Compounds 19 and 51 eluted at 4.48 and 6.32 min, respectively, with the quasi-molecular ion [M − H]− at m/z 465.1038 (C21H22O12) and the fragmentation ion [M − H]− at m/z 303.0507, resulting from the neutral loss of 162 Da (465.1038 − 303.0507) and corresponding to the loss of one hexose moiety. Therefore, compounds 19 and 51 were determined to be taxifolin galactoside and taxifolin glucoside, respectively.
Compound 66 possessed a molecular ion [M − H]− at m/z 303.0510 (C15H12O7) and fragment ions [M − H]− at m/z 125.0233, 153.0186, and 151.0026. Therefore, compound 66 was identified as taxifolin.
2.4. Pharmacological Activity of Constituents in CT
A total of 135 chemical constituents were identified in CT for the first time, including 67 chlorogenic acid derivatives, 48 flavonoids, and 20 anthocyanins. According to the literature, chlorogenic acid derivatives, including chlorogenic acid, isochlorogenic acid A, and isochlorogenic acid B, exhibit potent anti-inflammatory, antibacterial, antioxidant, analgesic, and antipyretic activities in vitro and in vivo (animal models) [33,34,35,36,37,38]. Quercetin has been reported to have anti-inflammatory, antioxidant, antibacterial, antitumor, and cardiovascular protective effects [39]. Kaempferol has antiosteoporosis and protective effects on damaged tissues, in addition to the above effects [40]. Procyanidins B1 and B2 have been reported to have anti-inflammatory, antibacterial, antioxidant, and other effects [41]. These compounds might be the effective constituents of CT, contributing to its pharmacological activity.
3. Materials and Methods
3.1. Chemicals and Reference Standards
MS-grade formic acid was purchased from Thermo Fisher Scientific (Carlsbad, CA, USA). Methanol and acetonitrile were of chromatographic grade, provided by Merck (Branchburg, NJ, USA). Water used as the mobile phase solvent was obtained from Watson Water (Guangzhou, China), and the ethanol used in the study was of analytical grade. The reference standards of procyanidin B1 (batch no. wkq19062802) and procyanidin B2 (batch no. wkq19042903) were obtained from Weikeqi Biological Technology Co., Ltd. (Chengdu, Sichuan, China). Reference standards of trans-3-caffeoylquinic acid (trans-3-CQA, neochlorogenic acid, X-014-170309), trans-4-caffeoylquinic acid (trans-4-CQA, cryptochlorogenic acid, Y-067-180320), trans-5-caffeoylquinic acid (trans-5-CQA, chlorogenic acid, L-007-171216), 3,5-dicaffeoylquinic acid (3,5-DiCQA, isochlorogenic acid A, Y-068-170903), 3,4-dicaffeoylquinic acid (3,4-DiCQA, isochlorogenic acid B, Y-069-180105), 4,5-dicaffeoylquinic acid (4,5-DiCQA, isochlorogenic acid C, Y-070-170515), isoquercitrin (Y-076-18106), kaempferol (S-014-171216), and naringenin (Y-030-190812) were provided by Chengdu Herbpurify Co., Ltd. (Chengdu, China). Reference standards of quercetin (AF8041802) and rutin (AF8032520) were provided by Chengdu Alfa Biotechnology Co., Ltd. (Chengdu, China). The reference standard of eriodictyol (PS1160-0025) was provided by Chengdu Push Bio-Technology Co., Ltd. (Chengdu, China). The reference standard of nicotiflorin (CFN99830) was provided by Wuhan Tianzhi Biotechnology Co., Ltd. (Wuhan, Hubei, China). The fresh leaves of CT were obtained from Leye County, Baise city, Guangxi province, and they were dried under vacuum conditions at 45 °C. The specimen (20201013) was stored at the School of Pharmaceutical Sciences, Hunan University of Medicine, Changsha, China.
3.2. Reference Standards and Sample Preparation
The dried powder of CT (5 g) was extracted under reflux in 100 mL of 70% aqueous ethanol for 1 h, and then the extracted solution was filtrated and dried under reduced pressure to yield a brown residue, which was dissolved in methanol. The sample was centrifuged at 12,000 rpm for 20 min. A volume of 2 µL was injected into an UHPLC-Q-exactive orbitrap MS for analysis. All reference standards were accurately weighed and dissolved in methanol before storing in a refrigerator at 4 °C until further analysis.
3.3. Instruments and Conditions
The instruments used for this study included a Thermo Q-exactive focus orbitrap MS connected to a Thermo Scientific Dionex Ultimate 3000 RS (Thermo Fisher Scientific, Carlsbad, CA, USA). Separation was performed on a Thermo Scientific Hypersil GOLDTM aQ (100 mm × 2.1 mm, 1.9 μm). The column temperature was kept at 35 °C, and the sample was maintained at 10 °C. The mobile phase was water with 0.1% formic acid (A) and acetonitrile (B). The gradient program was as follows: 0 min, 5% B; 2 min, 10% B; 5 min, 20% B; 10 min, 25% B; 12 min, 55% B; 20 min, 80% B; 25 min, 95% B; 26 min, 5% B; and 30 min, 5% B. MS analysis was performed in both positive and negative ionization modes using electrospray ionization (ESI) in the scan range of m/z 120–1000 at a resolution of 35,000. The source conditions were as follows: sheath gas, 30; auxiliary gas, 10; spray voltage, 3.0 kV for (−)-ESI and 3.5 kV for (+)-ESI; capillary temperature, 320 °C; auxiliary gas heater temperature, 350 °C. The MS1 spectra were acquired in full MS mode at a resolution of 35,000, whereas MS2 spectra were obtained by ddMS2 or parallel reaction monitoring (PRM) mode triggered by inclusion ions [12]. The NEC (normalized collision energy) was set as 30%, with 5.0 × e5 of the automatic gain control (AGC) target. Data were processed using Xcalibur™ version 4.1 and Compound Discoverer 3.0 (Thermo Fisher Scientific, Carlsbad, CA, USA).
3.4. Prediction of Expected Compounds
It is widely known that chemical constituents in the same category possess an identical carbon skeleton and homologous biosynthetic pathways. CGA analogues constitute a large family of esters formed between quinic acid or shikimic acid and one to four special residues, most commonly p-coumaric acid, caffeic acid, sinapic acid, and ferulic acid. Therefore, the molecular structure of the CGA derivatives can be predicted [11,26]. Likewise, flavonoids and anthocyanins can also be predicted. Quercetin and kaempferol are the carbon skeletons of flavonoids connected by hydroxyl (OH) and glycoside bonds; their structures differ in terms of the type and number of sugar units, e.g., glucose (C6H10O5), arabinose (C5H8O4), rhamnose (C6H10O4), rutinose (C12H20O9), and glucosyl rutinose (C18H30O14).
3.5. Establishment of Diagnostic Fragmentation Ions (DFIs) and Neutral Loss (NL)
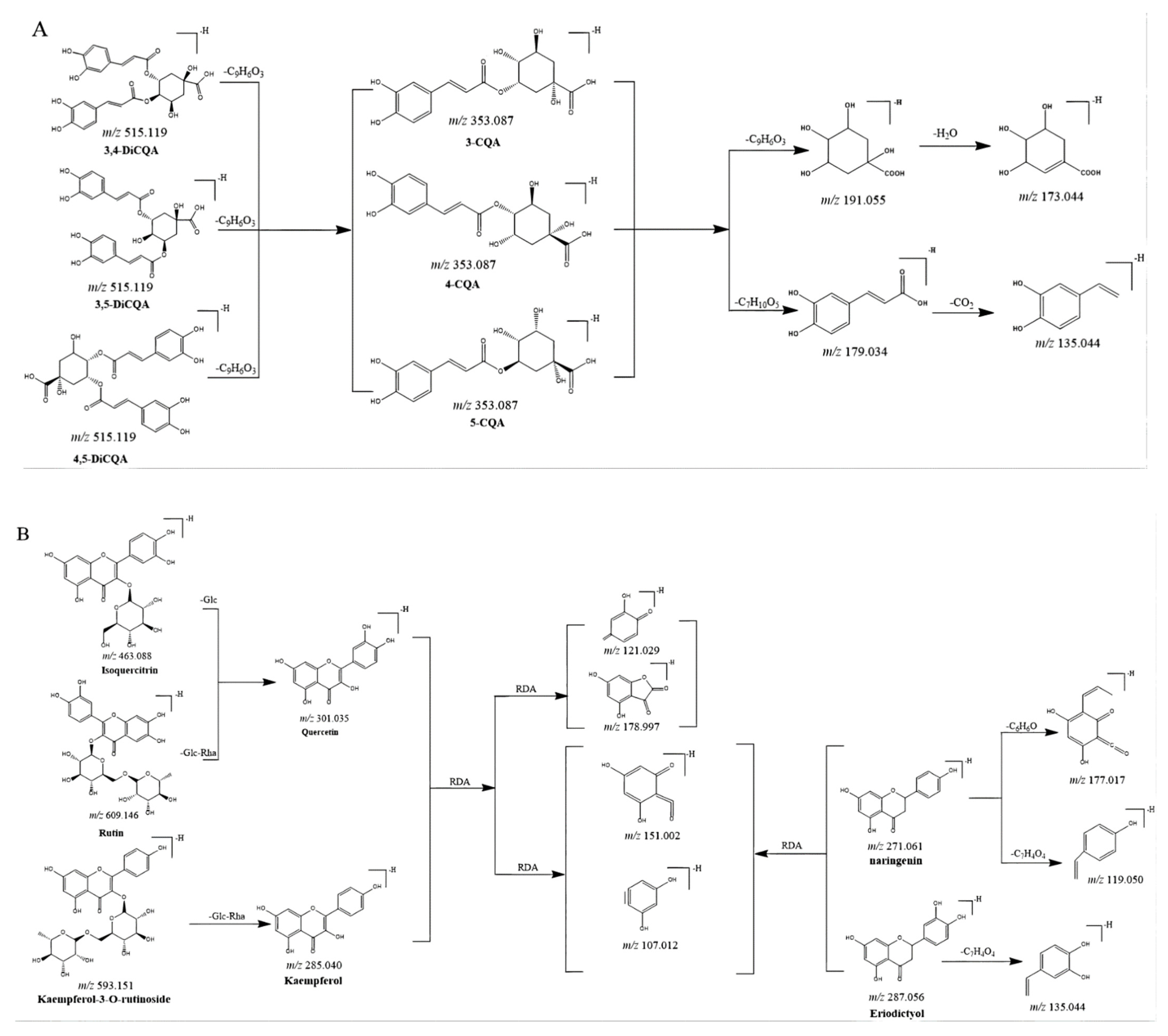
CGAs, anthocyanidins, and flavonoids with the same carbon skeleton were expected to have similar fragment ions. The fragment ion patterns of six CGAs, six flavonoids, and two anthocyanins were investigated using UHPLC-Q-exactive orbitrap MS in negative mode. The fragmentation pathway of CGAs is shown in Figure 2A. The common fragmentation ions were identified as 191.056 (C7H11O6), 173.045 (C7H10O5), 179.034 (C9H7O3), and 135.045 (C8H7O2), which could be considered diagnostic fragmentation ions. The neutral losses, including C9H6O3, C7H10O5, H2O, and CO2, are summarized in Figure 2A. Likewise, the diagnostic fragmentation ions (151.002, C7H3O4; 107.012, C6H3O2) and neutral losses (C7H4O4 and C6H10O5) of flavonoids are displayed in Figure 2B. The diagnostic fragmentation ions of 289.072 (C15H13O6), 407.077 (C22H15O8), 245.081 (C14H13O4), 125.023 (C6H5O3), and 161.023 (C9H5O3), along with neutral losses (C15H12O6, C8H10O4, C6H8O3, CO2, and C9H8O3), are shown in Figure 2C.
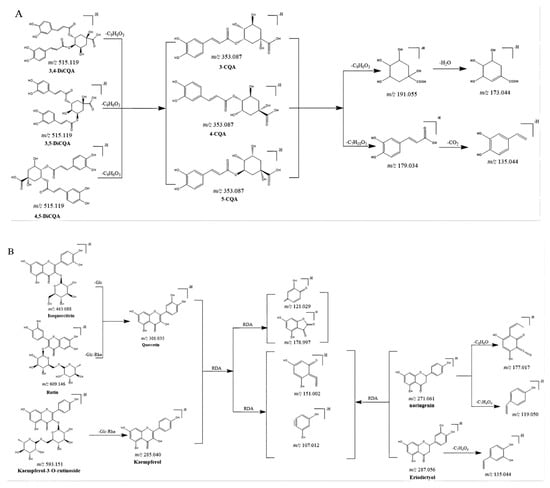
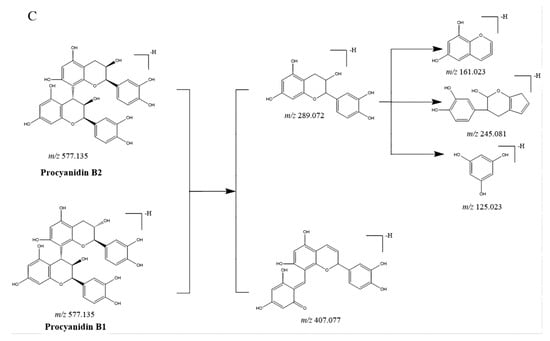
Figure 2.
Proposed selected fragmentation patterns of components identified in CT: CGAs (A); flavonoids (B); B-type procyanidin (C).
4. Conclusions
In this study, a rapid and effective method for identifying the chemical constituents of CT was developed using UHPLC-Q-exactive orbitrap combined with PRM; the compounds were predicted using DFI and NL techniques. A total of 135 compounds were identified, comprising 67 chlorogenic acid derivatives, 48 flavonoids, and 20 anthocyanins, all of which are reported for the first time in CT. These results expand the knowledge on the chemical composition of CT and provide a scientific basis for the subsequent elucidation of the medicinal substances present and their activities, enabling further development and utilization of this plant. Overall, the results lay the foundation for in-depth research on the pharmacodynamic basis of CT. Furthermore, this research strategy can be used for the characterization of various samples.
Author Contributions
S.-N.T., writing—original draft, investigation, and data curation; J.-B.Y., conceptualization, formal analysis, and validation; S.E., data curation; S.H. and J.-X.L., investigation and formal analysis; K.-Q.Y. and M.Z., resources; Q.L., software; L.S. and H.L., writing—review and editing, conceptualization, and supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Innovation and Entrepreneurship Training Program for undergraduates of Hunan University of Medicine (X202212214006) and China Ethnic Medical Association.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data will be provided upon request.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are available from the authors.
References
- Nanjing University of Chinese Medicine. Dictionary of Chinese Medicine; Shanghai Sci Technol Press: Shanghai, China, 2006; p. 653. ISBN 978-75-3238-271-2. [Google Scholar]
- Wang, X. Identification for Button in Pharmacognostica. J. Traditi. Chin. Med. 2007, 4, 86–103. [Google Scholar] [CrossRef]
- Ma, K.; Yang, C.B.; Huang, Y.Q.; Wu, X.W.; Jian, Y.C. Determination of high fructose amylose syrup in honey by solid phase extraction coupled with thin layer chromatography. China Flavor. 2021, 6, 140–143. [Google Scholar] [CrossRef]
- Tan, Y.Y.; Yang, C.Y.; Pan, Y.T. Improvement of thin-layer identification method of Nan Banlangen. J. Guangdong Chem. Ind. 2021, 9, 100–102. [Google Scholar] [CrossRef]
- Ferreyra, M.L.F.; Serra, P.; Casati, P. Recent advances on the roles of flavonoids as plant protective molecules after UV and high light exposure. J. Plant Physiol. 2021, 173, 736–749. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.C.; Peng, Q.R.; Yang, M.; Zhang, R.; Fu, Y.Y. Application of near infrared spectroscopy in honey detection. J. Sci. Technol. Food. Ind. 2020, 1, 334–341. [Google Scholar] [CrossRef]
- Peng, K.Z.; Yu, H.; Xia, M.; Liu, H.; Lan, C.J.; Wu, F.Y.; Wu, J. Study on the quality evaluation of hand ginseng herbs from different origins based on 1H-NMR metabolomi cs. J. Sichuan For. Sci. Technol. 2021, 3, 126–131. [Google Scholar] [CrossRef]
- Liang, G.Y.; Jiang, Y.M.; Zhou, M.; Xia, Y.; Li, C.Y. Determination of ethanol and acetic acid in fermented beverages by quantitative nuclear magnetic resonance hydrogen spectrometry. Food. Ferment. Ind. 2021, 10, 235–239. [Google Scholar] [CrossRef]
- Niu, J.; Cao, R.; Si, X.L.; Xin, E.D.; Zhang, Y.G.; Zhang, S.J.; Li, Y.F. Application of LC-MS in the analysis of flavonoids in natural products. Mod. China Med. 2020, 22, 1576–1579. [Google Scholar] [CrossRef]
- Hao, J.; Zhu, H.; Liu, S. Characterization of anthocyanins in fruit of Kadsura coccinea (Lem.) A.C. Smith by UPLC/Q-TOF-MS analysis and evaluation of stability of the major anthocyanins. J. Food. Chem. 2014, 7, 1312–1322. [Google Scholar] [CrossRef]
- Cai, W.; Li, K.L.; Xiong, P.; Gong, K.Y.; Zhu, L. A systematic strategy for rapid identification of chlorogenic acids derivatives in Duhaldea nervosa using UHPLC-Q-Exactive Orbitrap mass spectrometry. Arab. J. Chem. 2020, 13, 3751–3761. [Google Scholar] [CrossRef]
- Xiang, L.; Wei, J.; Tian, X.Y.; Wang, B.; Chan, W. Comprehensive analysis of acylcarnitine species in db/db mouse using a novel method of high-resolution parallel reaction monitoring reveals widespread metabolic dysfunction induced by diabetes. Anal. Chem. 2017, 89, 10368–10375. [Google Scholar] [CrossRef] [PubMed]
- Clifford, M.N.; Johnston, K.L.; Knight, S.; Kuhnert, N. Hierarchical scheme for LC-MSn identification of chlorogenic acids. J. Agric. Food. Chem. 2003, 51, 2900–2911. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Y.; Wang, Z.J.; Li, Y.; Liu, Y.; Cai, W. Astrategy for comprehensive identification of sequential constituents using ultra-high-performance liquid chromatography coupled with linear ion trap-Orbitrap mass spectrometer, application study on chlorogenic acids in Flos Lonicerae Japonicae. Talanta 2016, 47, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Clifford, M.N.; Kirkpatrick, J.; Kuhnert, N.; Roozendaal, H.; Salgado, P.R. LC-MSn analysis of the cis isomers of chlorogenic acids. Food. Chem. 2008, 106, 379–385. [Google Scholar] [CrossRef]
- Jaiswal, R.; Deshpande, S.; Kuhnert, N. Profiling the chlorogenic acids of Rudbeckia hirta, Helianthus tuberosus, Carlina acaulis and Symphyotrichum novae-angliae leavesby LC-MSn. Phytochem. Analysis 2011, 22, 432–444. [Google Scholar] [CrossRef]
- Jaiswal, R.; Halabi, E.A.; Karar, M.G.; Kuhnert, N. Identification andcharacterisation of the phenolics of Ilex glabra L. Gray (Aquifoliaceae) leaves by liquid chromatography tandem mass spectrometry. Phytochemistry 2014, 106, 141–155. [Google Scholar] [CrossRef]
- Jaiswal, R.; Matei, M.F.; Subedi, P.; Kuhnert, N. Does roasted coffee contain chlorogenic acid lactones or/and cinnamoylshikimate esters? Food. Res. Int. 2014, 61, 214–227. [Google Scholar] [CrossRef]
- Jaiswal, R.; Müller, H.; Müller, A.; Karar, M.G.E.; Kuhnert, N. Identification and characterization of chlorogenic acids, chlorogenic acid glycosides and flavonoids from Lonicera henryi L. (Caprifoliaceae) leaves by LC-MSn. Phytochemistry 2014, 108, 252–263. [Google Scholar] [CrossRef]
- Jaiswal, R.; Matei, M.F.; Golon, A.; Witt, M.; Kuhnert, N. Understanding the fate of chlorogenic acids in coffee roasting using mass spectrometry based targeted and non-targeted analytical strategies. Food. Funct. 2012, 3, 976–984. [Google Scholar] [CrossRef]
- Jaiswal, R.; Sovdat, T.; Vivan, F.; Kuhnert, N. Profiling and characterization by LC-MSn of the chlorogenic acids and hydroxycinnamoylshikimate esters in mate (Ilex paraguariensis). J. Agric. Food. Chem. 2010, 58, 5471–5484. [Google Scholar] [CrossRef]
- Liu, L.H.; Zhang, J.Y.; Zheng, B.J.; Guan, Y.; Wang, L.T. Rapid characterization of Chlorogenic Acids in Duhaldea nervosa based on UHPLC LTQ-Orbitrap-MS and mass spectral trees similarity filter technique. J. Sep. Sci. 2018, 41, 1764–1774. [Google Scholar] [CrossRef] [PubMed]
- Clifford, M.N.; Knight, S.; Kuhnert, N. Discriminating between the six isomers of dicaffeoylquinic acid by LC-MSn. J. Agric. Food. Chem. 2005, 53, 3821–3832. [Google Scholar] [CrossRef] [PubMed]
- Clifford, M.N.; Marks, S.; Knight, S.; Kuhnert, N. Characterization by LC-MSn of four new classes of pcoumaric acid-containing diacyl chlorogenic acids in green coffee beans. J. Agric. Food. Chem. 2006, 54, 4095–4101. [Google Scholar] [CrossRef] [PubMed]
- Gavrilova, V.; Kajdzanoska, M.; Gjamovski, V.; Stefova, M. Separation, Characterizationand quantification of phenolic compounds in blueberries and red and black currants byHPLC-DAD-ESI-MSn. J. Agric. Food. Chem. 2011, 59, 4009–4018. [Google Scholar] [CrossRef]
- Li, K.L.; Liu, L.H.; Xiong, P.; Tang, S.V.; Chen, H.X. Rapid Identification of Anthocyanin from the Epicarp of Kadsura Coccinea (Lem.) A.C. Smith by UHPLC-Q-Exactive Orbitrap Mass Spectrometry. Food. Anal. Methods 2021, 14, 2545–2555. [Google Scholar] [CrossRef]
- Zou, T.B.; Wang, D.L.; Guo, H.H.; Zhu, Y.N.; Luo, X.Q. Optimization of microwave-assisted extraction of anthocyanins from mulberry and identificationof anthocyanins in extractusing HPLC-ESI-MS. J. Food. Sci. 2012, 77, C46–C50. [Google Scholar] [CrossRef]
- Li, Q.; Luo, X.; He, S. Optimization for cellulase-microwave extraction process and its chemical composition of favonoizs substance from Adinandra nitida Merr.ex H.L.Lt. Sci. Technol. Food. Ind. 2020, 41, 15–22. [Google Scholar] [CrossRef]
- Jara, P.J.; Josep, L.T. Analysis of proanthocyanidins in almond blanch water by HPLC-ESI-QqQ-MS/MS and MALDI-TOF/TOF MS. J. Food. Res. Int. 2012, 49, 798–806. [Google Scholar] [CrossRef]
- Yang, Y.S.; Li, R.; Jiang, Z.T.; Liu, T. Analysis and identification of flavonoids in laurel leaves. Mod. Food Sci. Technol. 2016, 32, 270–275. [Google Scholar] [CrossRef]
- Chen, L.L.; Chen, C.H.; Zhang, X.X.; Wang, Y.; Wang, S.F. Identification of constituents in Gui-Zhi-Jia-Ge-Gen-Tang by LC-IT-MS combined with LC-Q-TOF-MS and elucidation of their metabolic networks in rat plasma after oral administration. Chin. J. Nat. Med. 2019, 17, 803–821. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.X. Component analysis and authenticity identification of Yunwu Tea on Lushan Mountain based on UPLC-QTOF-MS technology. Chin. J. Food. Sci. 2020, 20, 269–277. [Google Scholar] [CrossRef]
- Mao, Y.L. Chlorogenic Acid Prevents Ovariectomized Acquired Osteoporosis in Rats through the Shp2/PI3/Akt Signaling Pathway. Master’s Thesis, Nanchang University, Nanchang, China, 2016. [Google Scholar] [CrossRef]
- Zhang, B.R.; Ma, W.H.; Chen, B. Effect of Chlorogenic Acid on the Fever Induced by Endotoxin. J. Liaoning University. Tradl Chin. Med. 2012, 14, 229–231. [Google Scholar] [CrossRef]
- Santos, D. Evaluation of the anti-inflflammatory, analgesic and antipyretic activities of the natural polyphenol chlorogenic acid. Biol. Pharm. Bull. 2006, 29, 2236–2240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almeida, A.A.P. Antibacterial activity of coffee extracts and selected coffee chemical compounds against enterobacteria. J. Agricol. Food Chem. 2006, 54, 8738–8743. [Google Scholar] [CrossRef] [PubMed]
- Kono, Y. Iron chelation by chlorogenic acid as a natural antioxidant. Biosci. Biotechnol. Biochem. 1998, 62, 22–27. [Google Scholar] [CrossRef] [Green Version]
- Qi, H.; Shi, Y.T.; Wu, H.; Niu, C.Y.; Sun, X.Y.; Wang, K.W. Inhibition of temperature-sensitive TRPV3 channel by two natural isochlorogenic acid isomers for alleviation of dermatitis and chronic pruritus. Acta Pharm. Sin. B 2022, 12, 723–734. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, Y.Y.; Jiang, Q.C. Research progress on pharmacological action of quercetin. Spec. Econ. Anim. Plants 2020, 23, 24–28. [Google Scholar] [CrossRef]
- Lei, X.Q.; Chen, A.; Liu, Y.; He, J. Research progress on pharmacological action of kaempferol. Stud. Trace Elem. Health 2017, 34, 61–62. [Google Scholar]
- Martins, G.R.; Amaral, F.R.L.d.; Brum, F.L.; Mohana-Borges, R.; de Moura, S.S.T.; Ferreira, F.A.; Sangenito, L.S.; Santos, A.L.S.; Figueiredo, N.G.; da Silva, A.S. Chemical characterization, antioxidant and antimicrobial activities of açaí seed (Euterpe oleracea Mart.) extracts containing A and B-type procyanidins. LWT 2020, 132, 109830. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).