The Total Solubility of the Co-Solubilized PAHs with Similar Structures Indicated by NMR Chemical Shift
Abstract
1. Introduction
2. Results and Discussion
2.1. Solubilization of Phe, Ant and Pyr in Tween 80
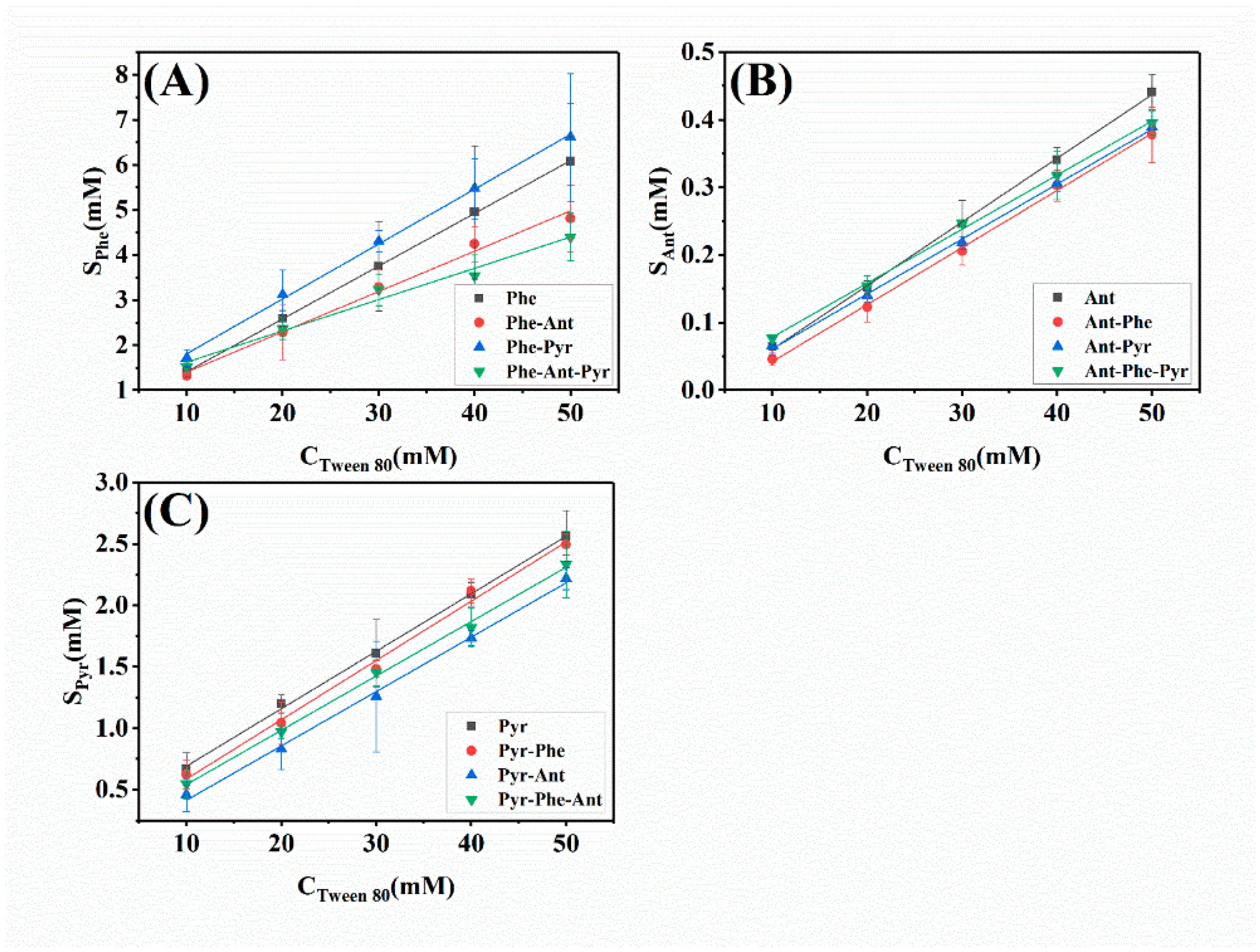
2.1.1. Apparent Solubility
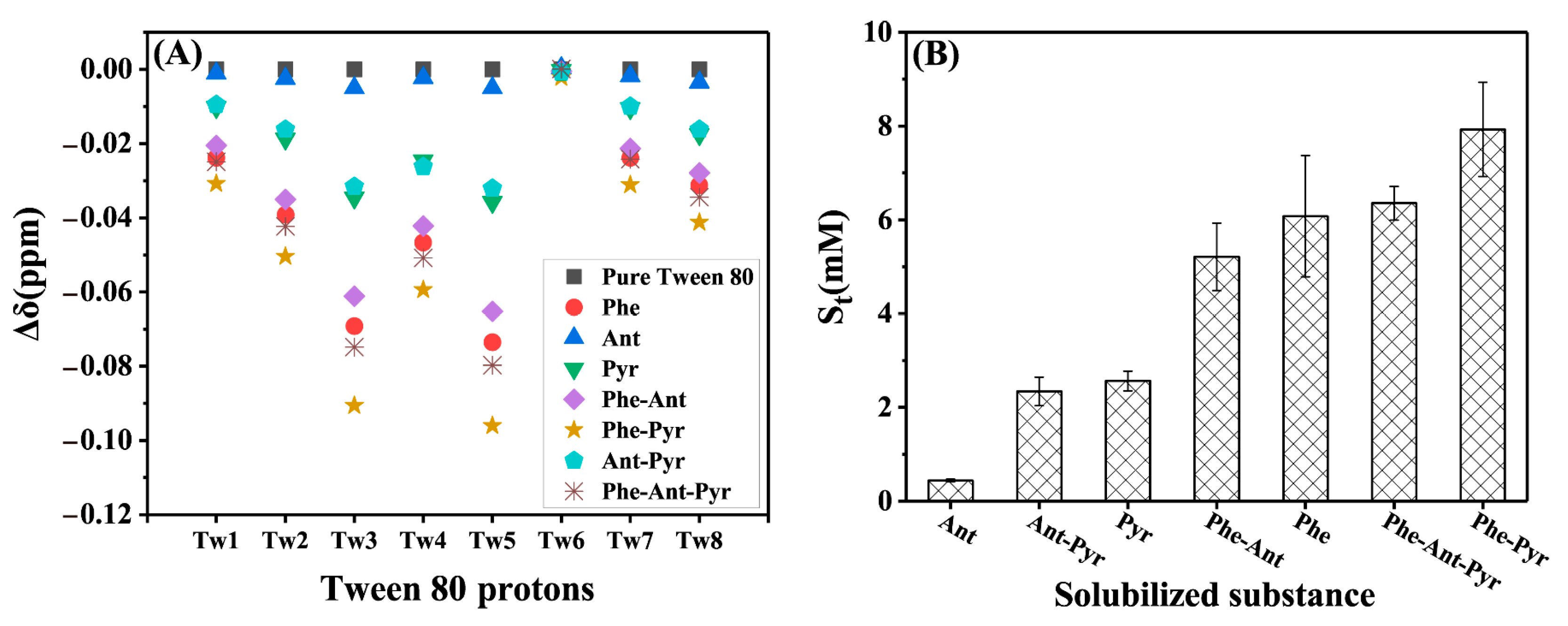
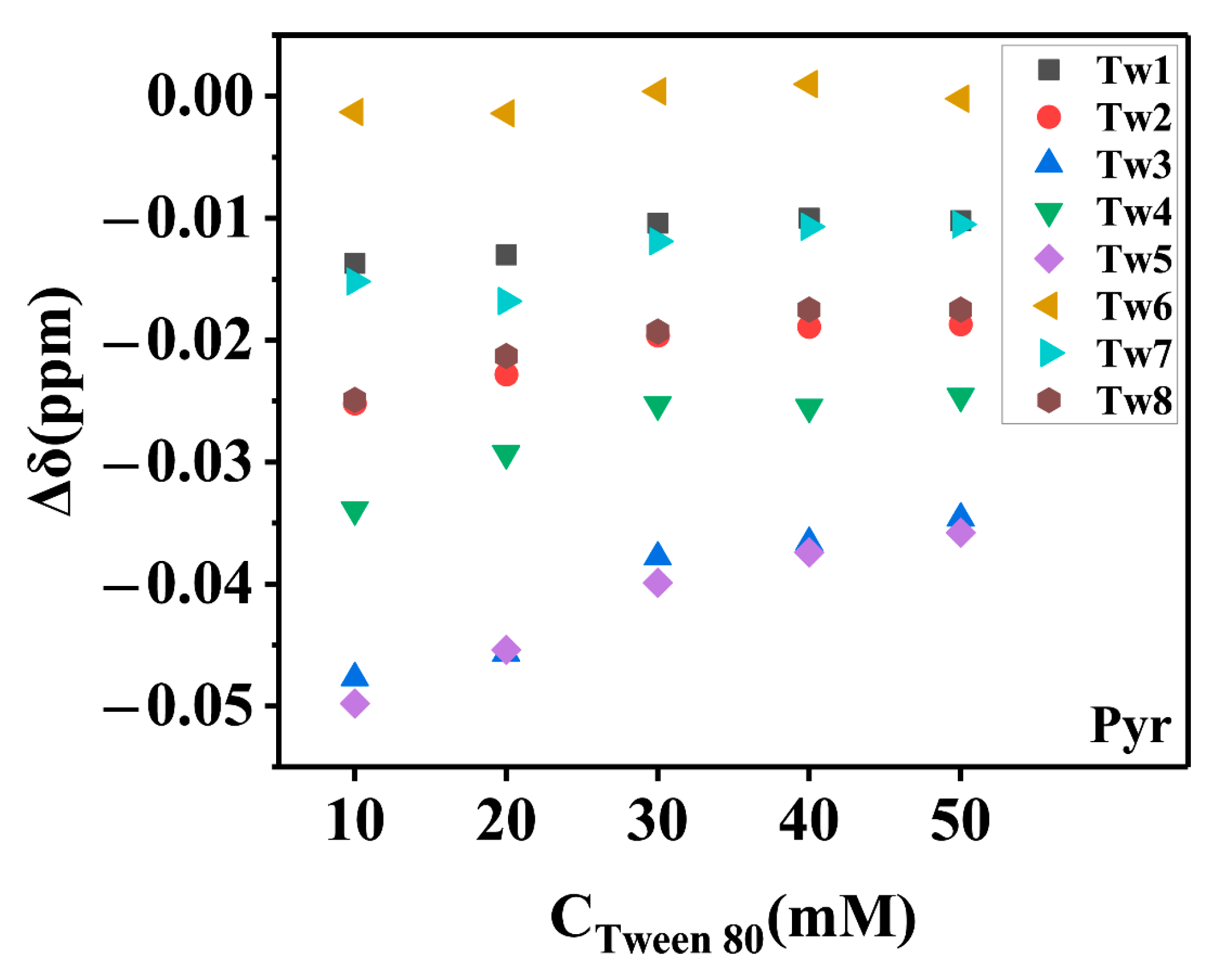
2.1.2. Solubilization Sites
2.1.3. The Total Solubility and Its Characterization by Chemical Shift Changes
2.1.4. Average Total Solubility and Its Characterization by Chemical Shift Changes
2.1.5. 2D ROESY
2.2. Solubilization of Phe, Ant and Pyr in SDS
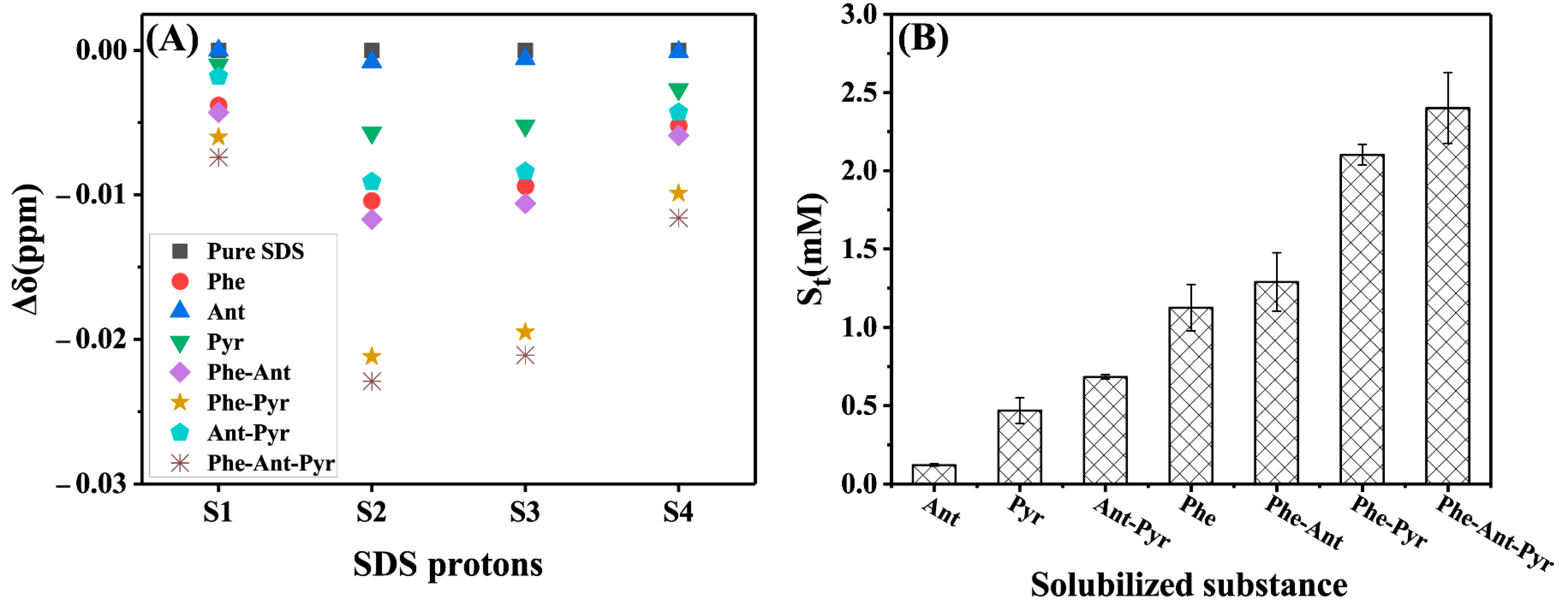
2.2.1. Apparent Solubility
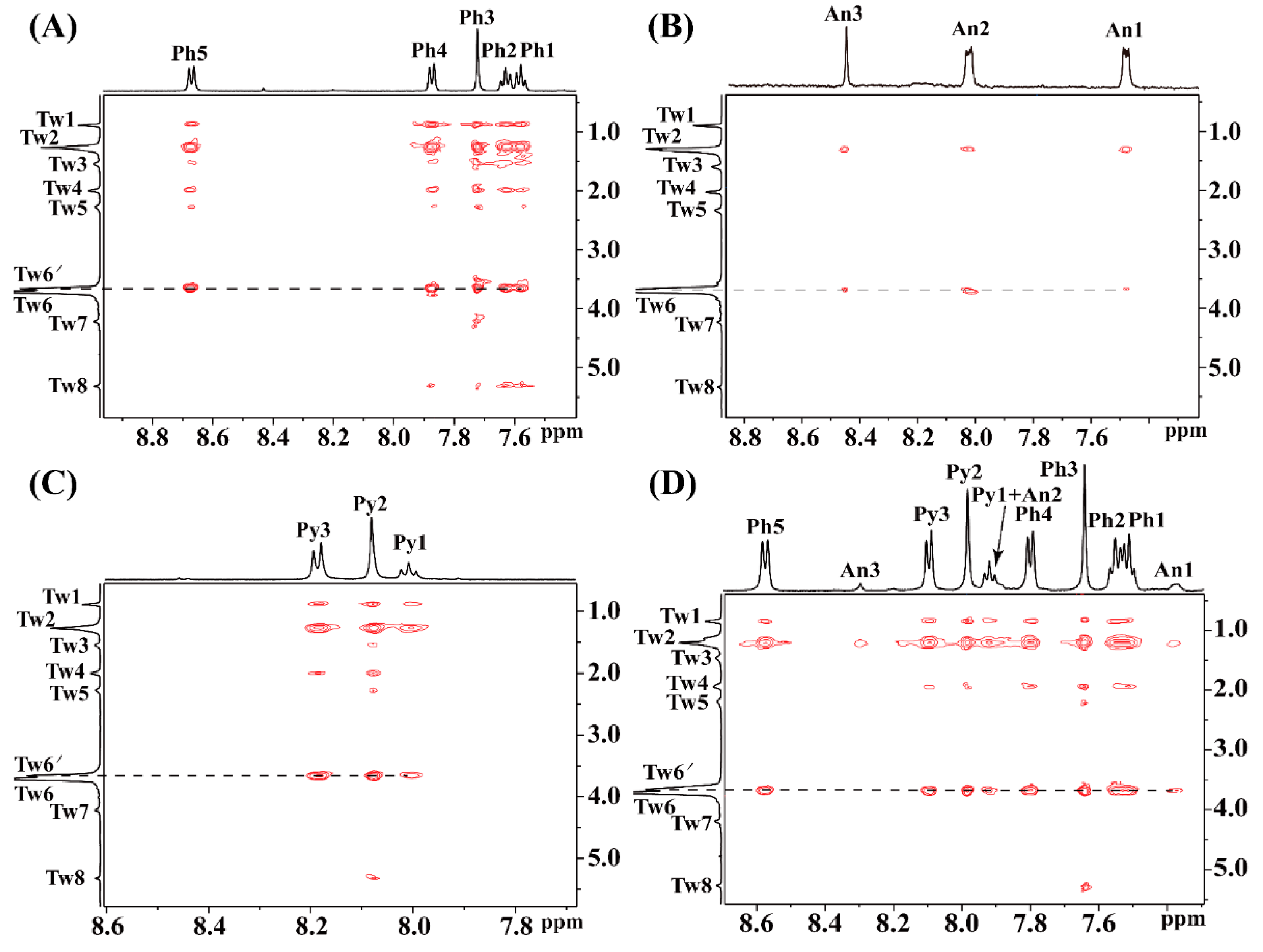
2.2.2. Solubilization Sites
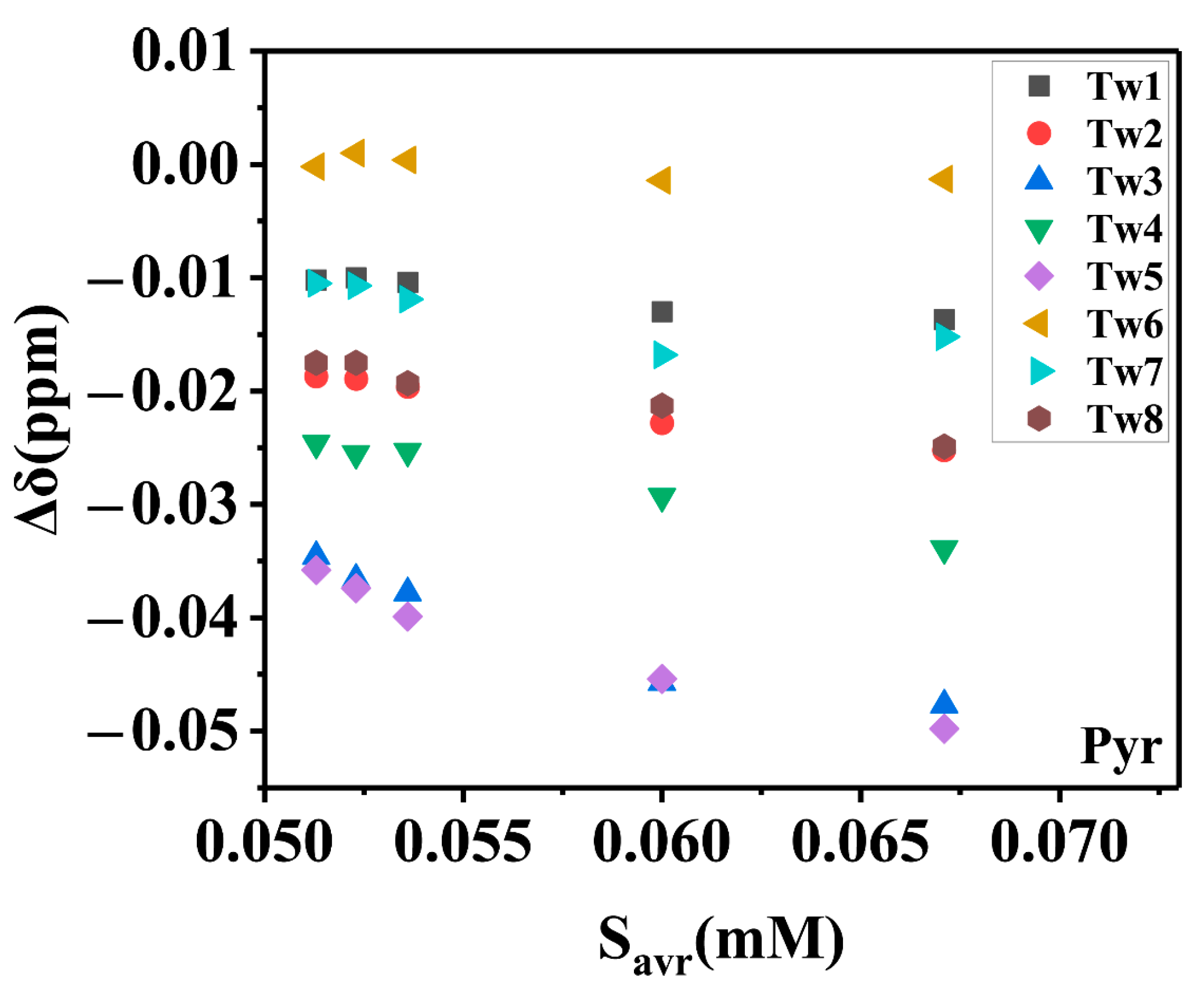
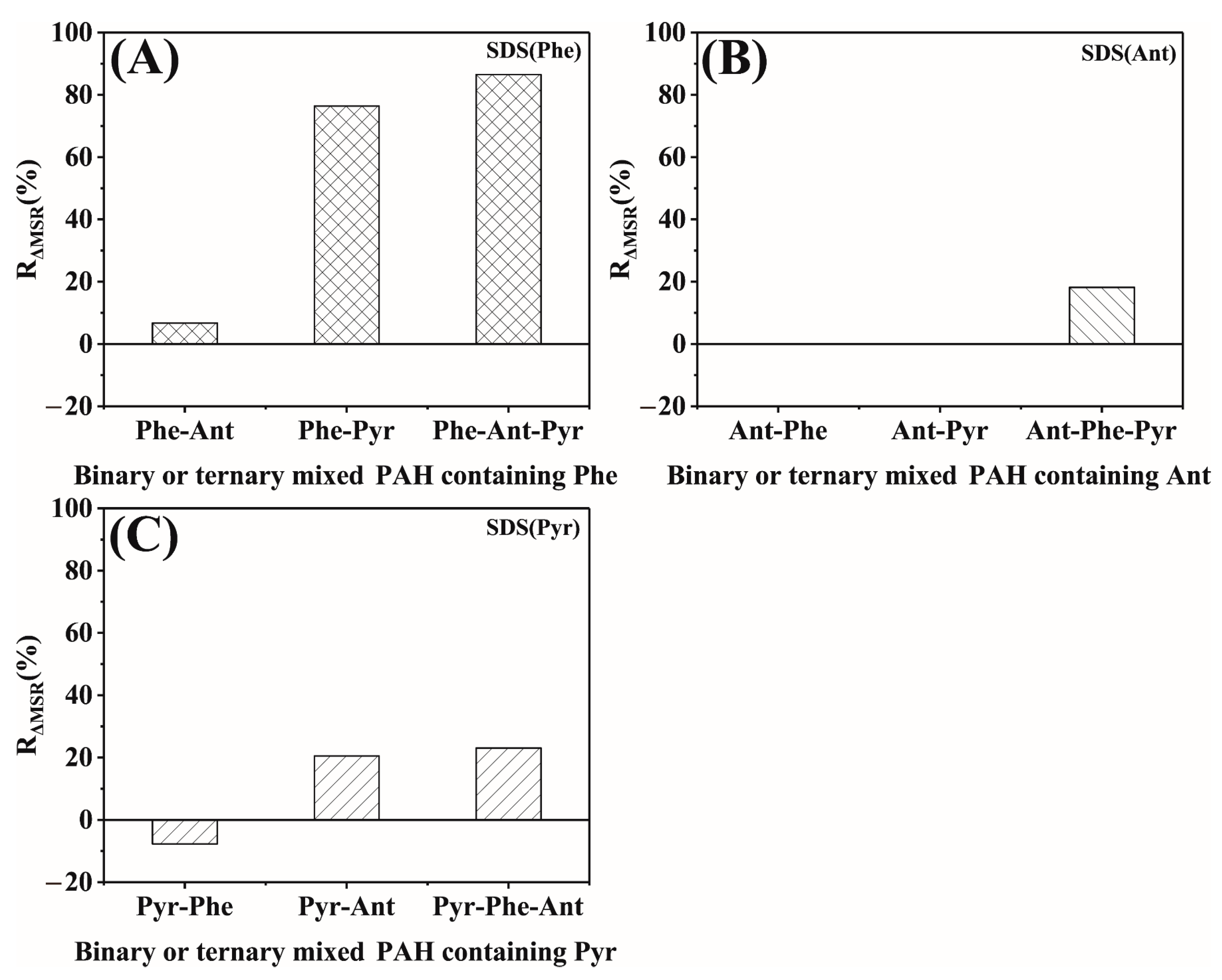
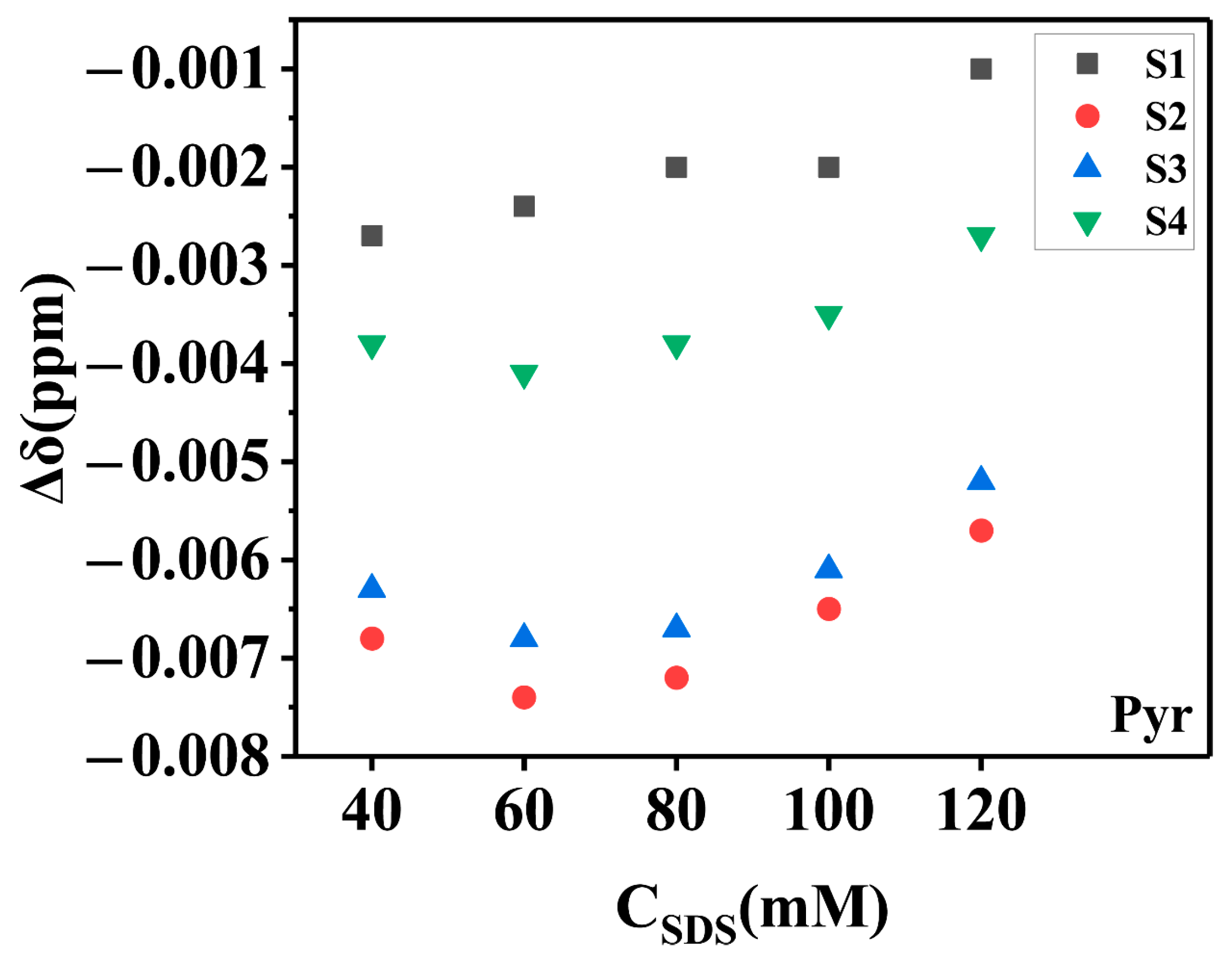
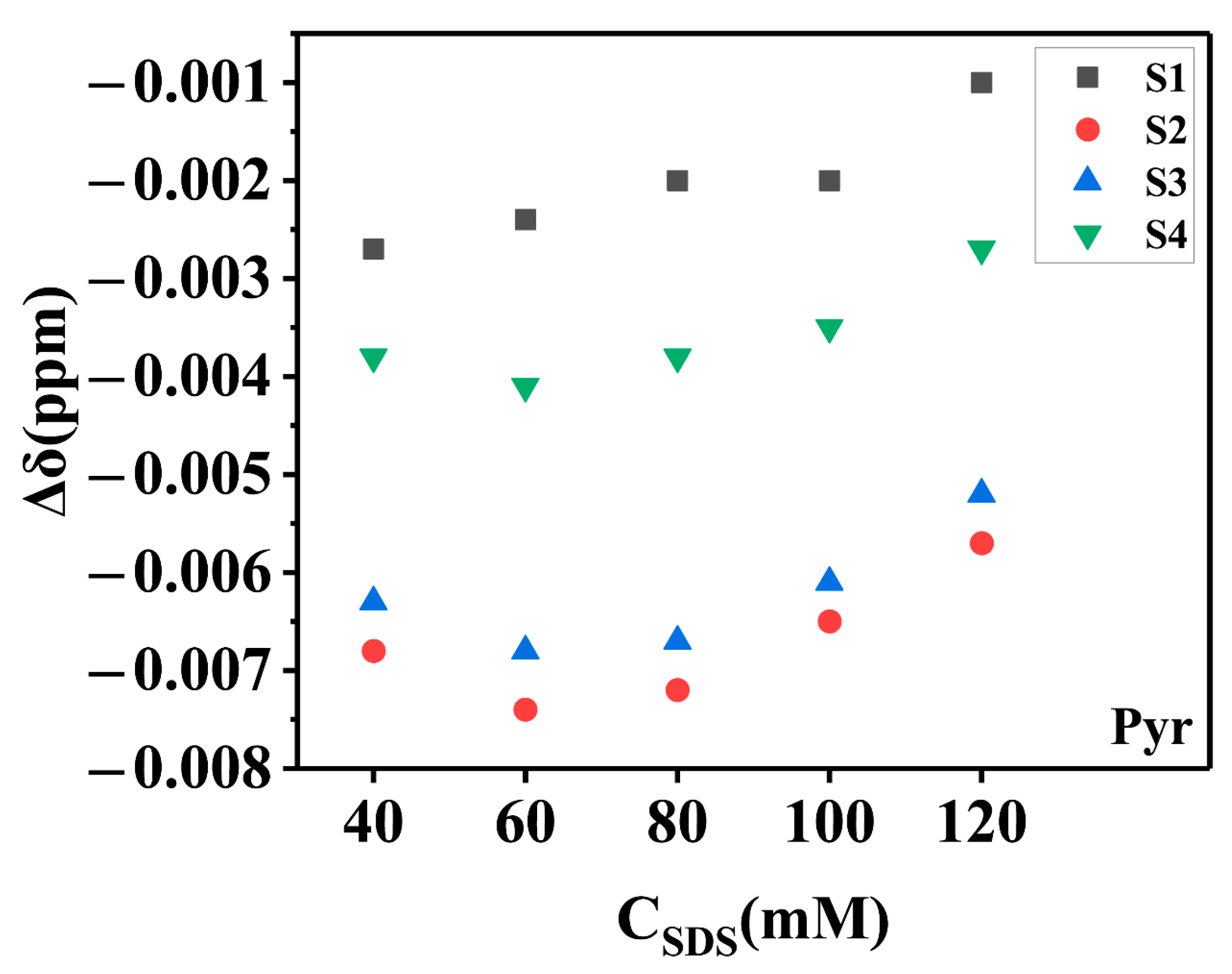
2.2.3. The Total Solubility and Its Characterization by Chemical Shift Changes
2.2.4. Average Total Solubility and Its Characterization by Chemical Shift Changes
2.2.5. 2D NOESY
2.3. General Mechanisms for the Relationship between the Chemical Shift Change and the Total Solubility
3. Materials and Methods
3.1. Materials
3.2. NMR Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Gallego, E.; Roca, F.J.; Perales, J.F.; Guardino, X.; Berenguer, M.J. VOCs and PAHs emissions from creosote-treated wood in a field storage area. Sci. Total Environ. 2008, 402, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Nzila, A. Biodegradation of high-molecular-weight polycyclic aromatic hydrocarbons under anaerobic conditions: Overview of studies, proposed pathways and future perspectives. Environ. Pollut. 2018, 239, 788–802. [Google Scholar] [CrossRef]
- Paria, S. Surfactant-enhanced remediation of organic contaminated soil and water. Adv. Colloid Interface Sci. 2008, 138, 24–58. [Google Scholar] [CrossRef]
- Kuppusamy, S.; Thavamani, P.; Venkateswarlu, K.; Lee, Y.B.; Naidu, R.; Megharaj, M. Remediation approaches for polycyclic aromatic hydrocarbons (PAHs) contaminated soils: Technological constraints, emerging trends and future directions. Chemosphere 2017, 168, 944–968. [Google Scholar] [CrossRef] [PubMed]
- Ogbonnaya, U.O.; Oyelami, A.O.; Umego, M.O.; Okere, U.V.; David, O.A.; Fasina, S.A.; Semple, K.T. Assessment of PAH contaminated land: Implementing a risk-based approach. Environ. Technol. Innovation 2017, 8, 84–95. [Google Scholar] [CrossRef]
- Liu, S.; Diebo, B.G.; Henry, J.K.; Smith, J.S.; Hostin, R.; Cunningham, M.E.; Mundis, G.; Ames, C.P.; Burton, D.; Bess, S.; et al. The benefit of nonoperative treatment for adult spinal deformity: Identifying predictors for reaching a minimal clinically important difference. Spine J. 2016, 16, 210–218. [Google Scholar] [CrossRef]
- Motorykin, O.; Matzke, M.M.; Waters, K.M.; Massey Simonich, S.L. Association of carcinogenic polycyclic aromatic hydrocarbon emissions and smoking with lung cancer mortality rates on a global scale. Environ. Sci. Technol. 2013, 47, 3410–3416. [Google Scholar] [CrossRef]
- Davie-Martin, C.L.; Stratton, K.G.; Teeguarden, J.G.; Waters, K.M.; Simonich, S.L.M. Implications of bioremediation of polycyclic aromatic hydrocarbon-contaminated soils for human health and cancer risk. Environ. Sci. Technol. 2017, 51, 9458–9468. [Google Scholar] [CrossRef]
- Song, W.; Li, J.; Zhang, W.; Hu, X.; Wang, L. An experimental study on the remediation of phenanthrene in soil using ultrasound and soil washing. Environ. Earth Sci. 2011, 66, 1487–1496. [Google Scholar] [CrossRef]
- Gan, S.; Lau, E.V.; Ng, H.K. Remediation of soils contaminated with polycyclic aromatic hydrocarbons (PAHs). J. Hazard. Mater. 2009, 172, 532–549. [Google Scholar] [CrossRef]
- Usman, M.; Chaudhary, A.; Biache, C.; Faure, P.; Hanna, K. Effect of thermal pre-treatment on the availability of PAHs for successive chemical oxidation in contaminated soils. Environ. Sci. Pollut. Res. 2016, 23, 1371–1380. [Google Scholar] [CrossRef]
- Cheng, M.; Zeng, G.M.; Huang, D.L.; Lai, C.; Xu, P.; Zhang, C.; Liu, Y. Hydroxyl radicals based advanced oxidation processes (AOPs) for remediation of soils contaminated with organic compounds: A review. Chem. Eng. J. 2016, 284, 582–598. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, X.; Zhou, X.; Waigi, M.G.; Gudda, F.O.; Zhang, C.; Ling, W. Promoted oxidation of polycyclic aromatic hydrocarbons in soils by dual persulfate/calcium peroxide system. Sci. Total Environ. 2021, 758, 143680. [Google Scholar] [CrossRef] [PubMed]
- Shahsavari, E.; Schwarz, A.; Aburto-Medina, A.; Ball, A.S. Biological degradation of polycyclic aromatic compounds (PAHs) in soil a current perspective. Curr. Pollut. Rep. 2019, 5, 84–92. [Google Scholar] [CrossRef]
- Bandowe, B.A.M.; Leimer, S.; Meusel, H.; Velescu, A.; Dassen, S.; Eisenhauer, N.; Hoffmann, T.; Oelmann, Y.; Wilcke, W. Plant diversity enhances the natural attenuation of polycyclic aromatic compounds (PAHs and oxygenated PAHs) in grassland soils. Soil Biol. Biochem. 2019, 129, 60–70. [Google Scholar] [CrossRef]
- Biache, C.; Ouali, S.; Cebron, A.; Lorgeoux, C.; Colombano, S.; Faure, P. Bioremediation of PAH-contamined soils: Consequences on formation and degradation of polar-polycyclic aromatic compounds and microbial community abundance. J. Hazard. Mater. 2017, 329, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, I.; Mukherji, S. Diverse effect of surfactants on pyrene biodegradation by a pseudomonas strain utilizing pyrene by cell surface hydrophobicity induction. Int. Biodeterior. Biodegrad. 2016, 108, 67–75. [Google Scholar] [CrossRef]
- Dell’Anno, F.; Sansone, C.; Ianora, A.; Dell’Anno, A. Biosurfactant-induced remediation of contaminated marine sediments: Current knowledge and future perspectives. Mar. Environ. Res. 2018, 137, 196–205. [Google Scholar] [CrossRef]
- Fatma, N.; Panda, M.; Kabir ud, D. A study on the solubilization of polycyclic aromatic hydrocarbons in gemini-conventional mixed surfactant systems by 1H NMR spectroscopy. Mater. Chem. Phys. 2020, 254, 123223. [Google Scholar] [CrossRef]
- Zhao, S.; Li, Y.; Cao, Z.; Wang, J. Sorption-desorption mechanisms and environmental friendliness of different surfactants in enhancing remediation of soil contaminated with polycyclic aromatic hydrocarbons. J. Soils Sediments 2020, 20, 2817–2828. [Google Scholar] [CrossRef]
- Patel, A.B.; Shaikh, S.; Jain, K.R.; Desai, C.; Madamwar, D. Polycyclic aromatic hydrocarbons: Sources, toxicity, and remediation approaches. Front. Microbiol. 2020, 11, 562813. [Google Scholar] [CrossRef] [PubMed]
- Lamichhane, S.; Bal Krishna, K.C.; Sarukkalige, R. Surfactant-enhanced remediation of polycyclic aromatic hydrocarbons: A review. J. Environ. Manage. 2017, 199, 46–61. [Google Scholar] [CrossRef] [PubMed]
- Long, J.; Li, L.; Jin, Y.; Sun, H.; Zheng, Y.; Tian, S. Synergistic solubilization of polycyclic aromatic hydrocarbons by mixed micelles composed of a photoresponsive surfactant and a conventional non-ionic surfactant. Sep. Purif. Technol. 2016, 160, 11–17. [Google Scholar] [CrossRef]
- Aryal, M.; Liakopoulou-Kyriakides, M. Biodegradation and kinetics of phenanthrene and pyrene in the presence of nonionic surfactants by arthrobacter strain Sphe3. Water Air Soil Pollut. 2013, 224, 1426. [Google Scholar] [CrossRef]
- Iglesias, O.; Sanromán, M.A.; Pazos, M. Surfactant-enhanced solubilization and simultaneous degradation of phenanthrene in marine sediment by Electro-Fenton treatment. Ind. Eng. Chem. Res. 2014, 53, 2917–2923. [Google Scholar] [CrossRef]
- Panda, M.; Fatma, N.; Kabir ud, D. Enhanced aqueous solubility of polycyclic aromatic hydrocarbons by green diester-linked cationic gemini surfactants and their binary solutions. J. Mol. Struct. 2016, 1115, 109–116. [Google Scholar] [CrossRef]
- Sales, P.S.; Fernandez, M.A. Synergism in the desorption of polycyclic aromatic hydrocarbons from soil models by mixed surfactant solutions. Environ. Sci. Pollut. Res. 2016, 23, 10158–10164. [Google Scholar] [CrossRef]
- Yadav, S.K.; Parikh, K.; Kumar, S. Mixed micelle formation of cationic gemini surfactant with anionic bile salt: A PAH solubilization study. Colloid Surf. A Physicochem. Eng. Asp. 2017, 522, 105–112. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Y.; Li, H. Synergistic solubilization of phenanthrene by mixed micelles composed of biosurfactants and a conventional non-ionic surfactant. Molecules 2020, 25, 4327. [Google Scholar] [CrossRef]
- Takeuchi, E.; Matsuoka, K.; Ishii, S.; Ishikawa, S.; Honda, C.; Endo, K. Solubilization of polycyclic aromatic hydrocarbons in C16E7 nonionic surfactant solutions. Colloid Surf. A Physicochem. Eng. Asp. 2014, 441, 133–139. [Google Scholar] [CrossRef]
- Singh, S.; Yadav, S.K.; Parikh, K.; Desai, A.; Dixit, S.; Kumar, S. Mixed micellization/clouding assisted solubilization of polycyclic aromatic hydrocarbon: Potential in environmental remediation. J. Mol. Liq. 2018, 272, 413–422. [Google Scholar] [CrossRef]
- Liang, X.; Zhang, M.; Guo, C.; Abel, S.; Yi, X.; Lu, G.; Yang, C.; Dang, Z. Competitive solubilization of low-molecular-weight polycyclic aromatic hydrocarbons mixtures in single and binary surfactant micelles. Chem. Eng. J. 2014, 244, 522–530. [Google Scholar] [CrossRef]
- Yang, X.; Lu, G.; She, B.; Liang, X.; Yin, R.; Guo, C.; Yi, X.; Dang, Z. Cosolubilization of 4,4′-dibromodiphenyl ether, naphthalene and pyrene mixtures in various surfactant micelles. Chem. Eng. J. 2015, 260, 74–82. [Google Scholar] [CrossRef]
- Liang, X.; Guo, C.; Liu, S.; Dang, Z.; Wei, Y.; Yi, X.; Abel, S. Cosolubilization of phenanthrene and pyrene in surfactant micelles: Experimental and atomistic simulations studies. J. Mol. Liq. 2018, 263, 1–9. [Google Scholar] [CrossRef]
- Ashraf, U.; Lone, M.S.; Masrat, R.; Shah, R.A.; Afzal, S.; Chat, O.A.; Dar, A.A. Co-solubilization of polycyclic aromatic hydrocarbon mixtures in aqueous micellar systems and its correlation with FRET for enhanced remediation processes. Chemosphere 2020, 242, 125160. [Google Scholar] [CrossRef]
- Bernardez, L.A. Investigation on the locus of solubilization of polycyclic aromatic hydrocarbons in non-ionic surfactant micelles with 1H NMR spectroscopy. Colloid Surf. A-Physicochem. Eng. Asp. 2008, 324, 71–78. [Google Scholar] [CrossRef]
- Edwards, D.A.; Luthy, R.G.; Liu, Z. Solubilization of polycyclic aromatic hydrocarbons in micellar nonionic surfactant solutions. Environ. Sci. Technol. 1991, 25, 127–133. [Google Scholar] [CrossRef]
- Peng, S.; Wu, W.; Chen, J. Removal of PAHs with surfactant-enhanced soil washing: Influencing factors and removal effectiveness. Chemosphere 2011, 82, 1173–1177. [Google Scholar] [CrossRef]
- Kuramochi, H.; Nakajima, D.; Goto, S.; Kawamoto, K.; Maeda, K. Water solubility of solid solution of phenanthrene and anthracene mixture. Polycycl. Aromat. Compd. 2006, 26, 299–312. [Google Scholar] [CrossRef]
- Pearlman, R.S.; Yalkowsky, S.H.; Banerjee, S. Water solubilities of polynuclear aromatic and heteroaromatic compounds. J. Phys. Chem. Ref. Data 1984, 13, 555–562. [Google Scholar] [CrossRef]
- Guha, S.; Jaffe, P.R.; Peters, C.A. Solubilization of PAH mixtures by a nonionic surfactant. Environ. Sci. Technol. 1998, 32, 930–935. [Google Scholar] [CrossRef]
- Haque, M.E.; Das, A.R.; Moulik, S.P. Mixed micelles of sodium deoxycholate and polyoxyethylene sorbitan monooleate (Tween 80). J. Colloid Interface Sci. 1999, 217, 1–7. [Google Scholar] [CrossRef] [PubMed]
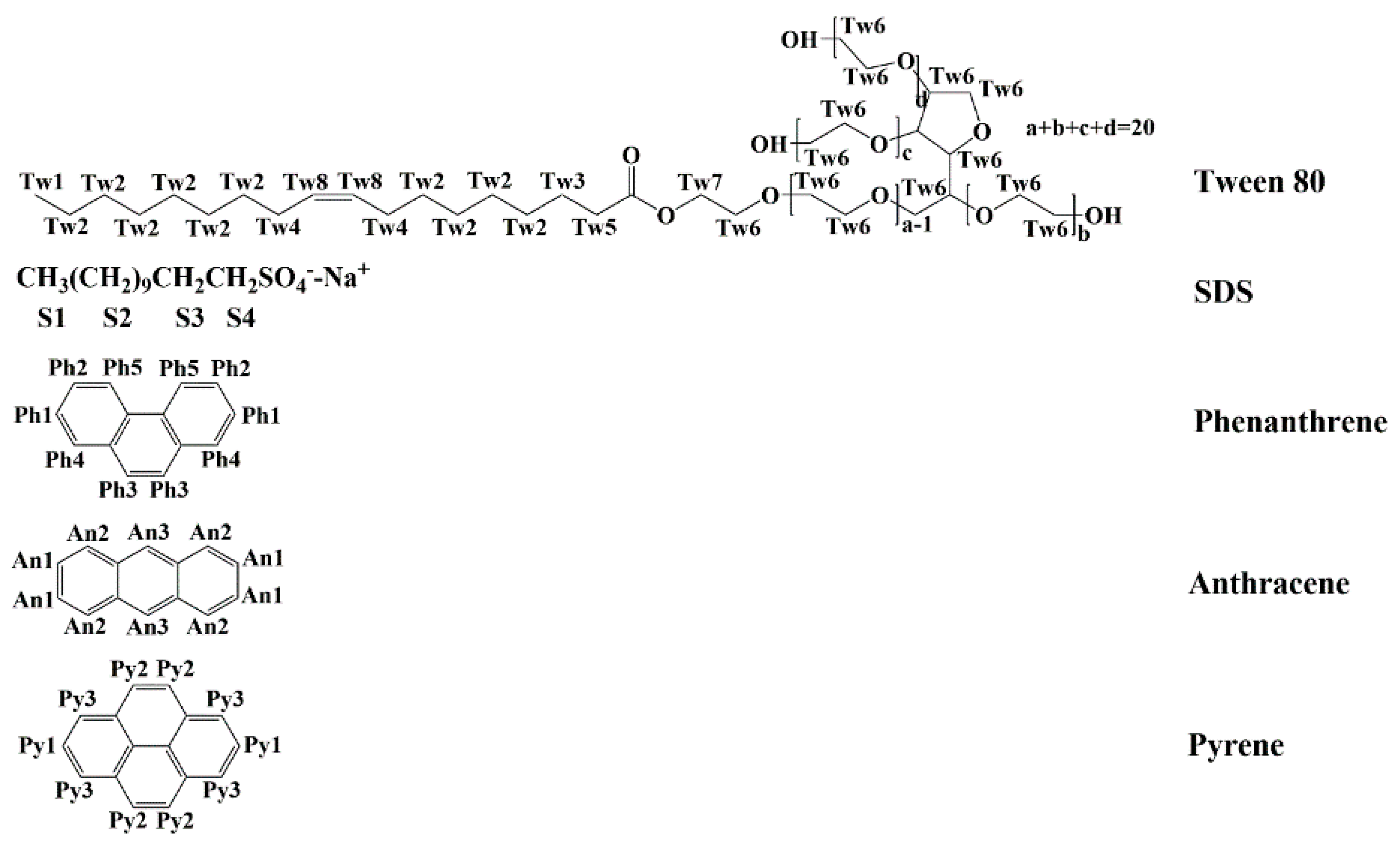



| Surfactant | MSR | MSR | MSR | MSR |
|---|---|---|---|---|
| Tween 80 | Phe | |||
| Phe (single) | Phe-Ant | Phe-Pyr | Phe-Ant-Pyr | |
| 0.1170 | 0.0894 | 0.1216 | 0.0694 | |
| Ant | ||||
| Ant (single) | Ant-Phe | Ant-Pyr | Ant-Phe-Pyr | |
| 0.0094 | 0.0084 | 0.0081 | 0.0080 | |
| Pyr | ||||
| Pyr (single) | Pyr-Phe | Pyr-Ant | Pyr-Ant-Phe | |
| 0.0468 | 0.0482 | 0.0442 | 0.0442 |
| Segment | Pure | Phe | Ant | Pyr | Phe-Ant | Phe-Pyr | Ant-Pyr | Phe-Ant-Pyr |
|---|---|---|---|---|---|---|---|---|
| Tw1 | 0.9046 | 0.8837 | 0.9035 | 0.8944 | 0.8806 | 0.8756 | 0.8951 | 0.8797 |
| Tw2 | 1.3033 | 1.2692 | 1.3008 | 1.2846 | 1.2639 | 1.2552 | 1.2871 | 1.2610 |
| Tw3 | 1.6087 | 1.5493 | 1.6037 | 1.5741 | 1.5416 | 1.5239 | 1.5771 | 1.5339 |
| Tw4 | 2.0411 | 1.9992 | 2.0388 | 2.0165 | 1.9944 | 1.9842 | 2.0149 | 1.9903 |
| Tw5 | 2.3426 | 2.2788 | 2.3376 | 2.3068 | 2.2710 | 2.2535 | 2.3105 | 2.2629 |
| Tw6 | 3.7172 | 3.7170 | 3.7176 | 3.7170 | 3.7156 | 3.7157 | 3.7164 | 3.7173 |
| Tw7 | 4.2301 | 4.2108 | 4.2283 | 4.2196 | 4.2065 | 4.2020 | 4.2201 | 4.2059 |
| Tw8 | 5.3435 | 5.3153 | 5.3400 | 5.3260 | 5.3118 | 5.3041 | 5.3273 | 5.3091 |
| Surfactant | MSR | MSR | MSR | MSR |
|---|---|---|---|---|
| SDS | Phe | |||
| Phe (single) | Phe-Ant | Phe-Pyr | Phe-Ant-Pyr | |
| 0.0089 | 0.0095 | 0.0157 | 0.0166 | |
| Ant | ||||
| Ant (single) | Ant-Phe | Ant-Pyr | Ant-Phe-Pyr | |
| 0.0011 | 0.0011 | 0.0011 | 0.0013 | |
| Pyr (single) | ||||
| Pyr | Pyr-Phe | Pyr-Ant | Pyr-Ant-Phe | |
| 0.0036 | 0.0033 | 0.0044 | 0.0045 |
| Segment | Pure | Phe | Ant | Pyr | Phe-Ant | Phe-Pyr | Ant-Pyr | Phe-Ant-Pyr |
|---|---|---|---|---|---|---|---|---|
| S1 | 0.8972 | 0.8934 | 0.8972 | 0.8962 | 0.8929 | 0.8912 | 0.8954 | 0.8898 |
| S2 | 1.3146 | 1.3042 | 1.3138 | 1.3089 | 1.3029 | 1.2934 | 1.3055 | 1.2917 |
| S3 | 1.6889 | 1.6795 | 1.6883 | 1.6837 | 1.6783 | 1.6694 | 1.6805 | 1.6678 |
| S4 | 4.0335 | 4.0283 | 4.0334 | 4.0308 | 4.0276 | 4.0236 | 4.0292 | 4.0219 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, T.; Hu, X.; Chen, Z.; Cui, X. The Total Solubility of the Co-Solubilized PAHs with Similar Structures Indicated by NMR Chemical Shift. Molecules 2021, 26, 2793. https://doi.org/10.3390/molecules26092793
Chen T, Hu X, Chen Z, Cui X. The Total Solubility of the Co-Solubilized PAHs with Similar Structures Indicated by NMR Chemical Shift. Molecules. 2021; 26(9):2793. https://doi.org/10.3390/molecules26092793
Chicago/Turabian StyleChen, Tao, Xin Hu, Zhong Chen, and Xiaohong Cui. 2021. "The Total Solubility of the Co-Solubilized PAHs with Similar Structures Indicated by NMR Chemical Shift" Molecules 26, no. 9: 2793. https://doi.org/10.3390/molecules26092793
APA StyleChen, T., Hu, X., Chen, Z., & Cui, X. (2021). The Total Solubility of the Co-Solubilized PAHs with Similar Structures Indicated by NMR Chemical Shift. Molecules, 26(9), 2793. https://doi.org/10.3390/molecules26092793






