Abstract
This study examined for the first time whether bee bread (BB, consisting of monofloral rape bee pollen) could alleviate lipid derangements and reduced bone quality in Zucker diabetic fatty (ZDF) rats, which are considered an appropriate animal model for type 2 diabetes mellitus (T2DM) investigation. Adult ZDF rats were segregated into four groups: lean non-diabetic rats (L group), obese diabetic rats untreated (C group), and those treated with the BB at two doses (500 and 700 mg/kg body weight, respectively, B1 and B2 groups) for 10 weeks. Significantly reduced levels of total cholesterol and triglyceride were recorded in the B2 group versus the C group. In both BB-treated groups, significantly increased relative volume of trabecular bone and trabecular thickness, enhanced density of secondary osteons, accelerated periosteal bone apposition, and improved blood flow were observed. A positive effect of higher dose of BB on femoral weight and cortical bone thickness was also demonstrated. Our results suggest a promising potential of BB to ameliorate T2DM-related complications associated with lipid and bone damages.
1. Introduction
Type 2 diabetes mellitus (T2DM) represents one of the most frequent public health problems with increasing prevalence worldwide. The World Health Organization states that the number of people with diabetes will double in the next ten years [1]. Generally, T2DM is a multifactorial chronic endocrine disorder [2] manifested by hyperglycaemia. This condition is caused by increased hepatic glucose production, lower insulin secretion, and impaired insulin action [3]. Subsequently, the lipid is used as an alternative resource of cellular energy, resulting in abnormalities of lipid metabolism. Hyperlipidaemia is characterised by serum lipids alterations, especially triglyceride (TG) increase and high-density lipoprotein (HDL) cholesterol decrease [4]. Another complication associated with T2DM represents diabetic bone disease. It is manifested by varied bone mineral density (BMD), abnormalities in skeletal microarchitecture, differences in bone metabolism, and lower bone strength. Chronic hyperglycaemia causes reduced expression of genes affecting the function of osteoblasts, downregulation of vitamin D receptors, enhanced bone marrow mesenchymal cells differentiation into adipocytes, increased production of advanced glycation end-products (AGEs) inhibiting bone remodelling, and higher oxidative stress [5,6]. All these conditions lead to altered bone morphology and increased risk of fractures.
Therapy for T2DM can be supported by various biological substances. Bee products are considered to be well-known functional foods. They contain a lot of proteins, sugars, essential amino acids, fatty acids, macro and microelements, and vitamins, [7] which are responsible for their high nutritional value [8]. Because of a presence of other bioactive compounds also having a beneficial effect on human health, they are useful tools for therapeutical approach as well [9]. In general, bee bread (BB), not a very well-known bee product, is the result of bee pollen fermentation in hives [10]. The bees (Apis mellifera) mix plant pollen with nectar or honey and their salivary enzymes [11] and fill the cells in hives with this mixture. Afterwards, anaerobic lactic fermentation begins [12] that decreases pH and enhances the bioavailability of nutrients [13,14]. Depending on geographical region, botanical origin, and storage conditions, the chemical composition of BB may differ. Salutary health impacts of BB have been rarely investigated thus far and they are conditioned by the presence of phytosterols, fatty acids, polyphenols and polysaccharides with anticancer properties, and antioxidant and immunological properties [15]. It is also regarded as a promising anti-microbial agent [7]. However, data showing the potential of BB to alleviate T2DM-reduced bone quality and hyperlipidaemia is missing. Zucker diabetic fatty (ZDF) rats are successfully used as a well-matched animal model for T2DM research [3]. They carry a spontaneous mutation in the leptin receptor gene and a defect in β-cell transcription that contributes to the diabetic phenotype [16]. ZDF rats show enhanced body weights by five weeks and their obesity gradually worsens with age [17].
This study was carried out to determine if BB could improve the diabetic lipid profile and impaired bone morphology using an in vivo animal model of obese Zucker diabetic rats. Femoral bone structure of BB-treated rats was assessed for the first time using both 3D and 2D imaging methods.
2. Results
2.1. Biochemical Analysis
The diabetic rats from all groups (C, B1, B2) had significantly elevated levels of blood glucose (BG), total cholesterol (TC), and TG as compared to lean rats (L group). A higher dose of BB (B2 group) significantly reduced TC and TG levels versus the C group. Moreover, decreased value for TC was recorded in the B1 group compared with the C group. Nonetheless, BG, blood insulin (BI), HDL cholesterol (HDLC), and low-density lipoprotein (LDL) cholesterol levels did not differ significantly in both the B1 and B2 groups versus the C group. The findings on the effect of BB on examined biochemical parameters in ZDF rats are summarized in Figure 1A–F.
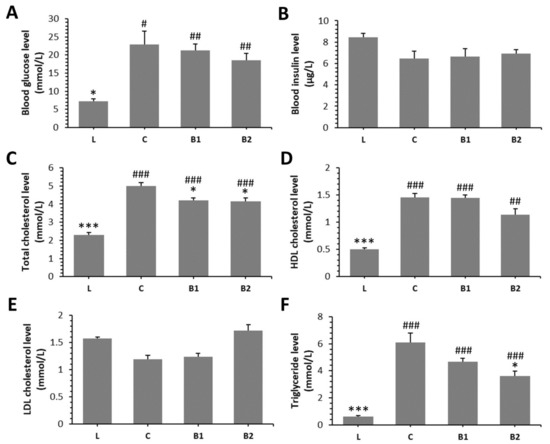
Figure 1.
Effects of bee bread (BB) on levels of blood glucose (A), blood insulin (B), total cholesterol (C), high-density lipoprotein (HDL) cholesterol (D), low-density lipoprotein (LDL) cholesterol (E), triglyceride (F) in Zucker diabetic fatty (ZDF) rats. * Significant differences compared with C group (p < 0.05), *** significant differences compared with the C group (p < 0.001), # significant changes in relation to the L group (p < 0.05), ## significant changes in relation to the L group (p < 0.01), and ### significant changes in relation to the L group (p < 0.001).
2.2. Macroscopical Analysis of Bones
The post-treatment body weight (BW) in both BB-treated groups was higher than that determined in lean rats. Significantly decreased femoral weight (FW) was observed in the C and B1 groups versus the L group. No significant difference in FW between L and B2 groups reflects a beneficial effect of a higher dose of BB on this parameter. However, total BW, femoral length (FL), and FW were non-significantly higher in the B1 and B2 groups as compared to the C group. The results are presented in Figure 2A–C.
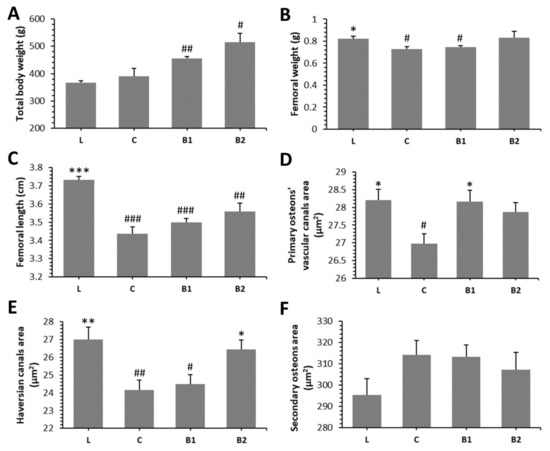
Figure 2.
Impacts of BB on macroscopical (A–C) and morphological 2D (D–F) bone parameters in ZDF rats. * Significant differences compared with the C group (p < 0.05), ** significant differences compared with the C group (p < 0.01), *** significant differences compared with the C group (p < 0.001), # significant changes in relation to the L group (p < 0.05), ## significant changes in relation to the L group (p < 0.01), and ### significant changes in relation to L group (p < 0.001).
2.3. Morphological 3D and 2D Analyses of Bones
Treatment with BB had an insignificant effect on relative bone volume (BV/TV) of cortical bone, cortical bone surface (BS), and BMD. Despite this, significantly decreased cortical bone thickness (Ct.Th.) was recorded in the C and B1 groups versus the L group. No significant changes between L and B2 groups insinuate a positive impact of a higher dose of BB on Ct.Th. The results are provided in Figure 3A–D. Figure 4A–D shows representative reconstructed 3D images of the cortical bone. Compared with diabetic control rats, rats from both the B1 and B2 groups had significantly elevated relative bone volume (BV/TV) of the trabecular bone and trabecular thickness (Tb.Th.). No significant impact of BB administration on trabecular number (Tb.N.), BMD, and trabecular bone surface (BS) was recorded. The data are shown in Figure 3E–I. Figure 4E–H illustrates representative reconstructed 3D images of the trabecular bone.
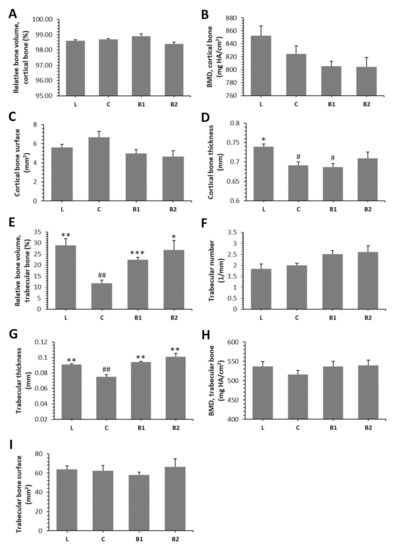
Figure 3.
Effects of BB on morphological 3D parameters of the cortical bone (A–D) and trabecular bone (E–I) in ZDF rats. BMD—bone mineral density. * Significant differences compared with the C group (p < 0.05), ** significant differences compared with the C group (p < 0.01), *** significant differences compared with the C group (p < 0.001), # significant changes in relation to the L group (p < 0.05), and ## significant changes in relation to L group (p < 0.01).
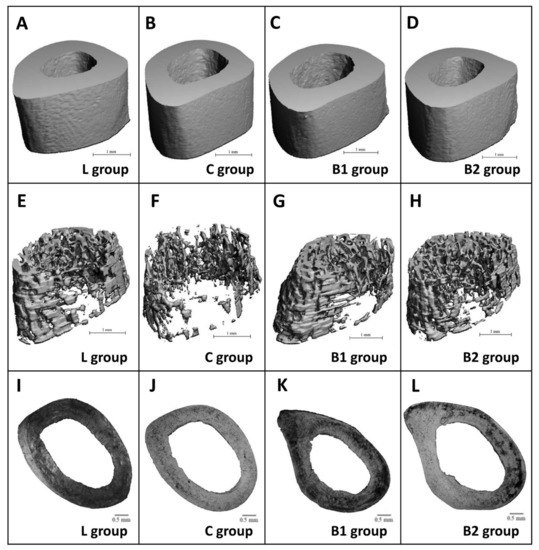
Figure 4.
Representative 3D images of cortical (A–D) and trabecular bone tissues (E–H), and representative 2D images of the cortical bone (I–L) in ZDF rats.
In general, non-vascular bone tissue formed both surfaces of the cortical bone in all groups studied. Near the endosteal surface and in the middle of substantia compacta, primary vascular radial bone tissue was observed. Several secondary osteons (SO) have also been identified in the middle part of the cortical bone. We recorded a higher density of SO and accelerated periosteal bone apposition in both BB-treated groups versus the C group (Figure 4I–L). Significantly higher areas of primary osteons’ vascular canals (POVC) were established in the B1 and L groups versus the C group. Compared with diabetic control rats, these from the B2 and L groups had significantly elevated areas of Haversian canals (HC). On the other hand, treatment with BB did not influence SO area. The data are summarized in Figure 2D–F.
3. Discussion
Bee products have been used as alternative medicine to treat various serious disorders including diabetes mellitus [18]. Our study, for the first time, examined the protective impacts of BB (formed by monofloral rape bee pollen) against T2DM-related complications consistent with lipid and bone disorders. Therefore, comparison with other studies using either identical bee product, animal model, or both, was not possible in this field of research. Only our findings related to BG, BI levels, and total BW of ZDF rats were discussed with published researches focused on BB and obese Zucker diabetic rats.
Capcarova et al. [19] state that ZDF rats receiving BB at a dose of 700 mg/kg BW (for 10 weeks) had significantly lower BG levels only in the pre-diabetic state versus the diabetic control group. At the end of the treatment, BG did not change significantly between treated and control groups, thus identical to our findings. Nevertheless, aforementioned decreases of BG during the experiment might also influence some biochemical and morphological parameters as documented in our study. We recorded non-significant reduction of BG levels in both B1 and B2 groups. Generally, lower BG levels and anti-diabetic activities of BB could be consistent with the presence of polyphenol compounds [20]. Among polyphenols, flavonoids represent the largest group. It is known that differences in flavonoid fraction may be conditioned by geographic profile. According to Tavdidishvili et al. [21], the main flavonoids were naringenin, rutin, and quercetin in Georgian BB samples. Sobral et al. [22] identified quercetin, myricetin, isorhamnetin, kaempferol, and herbacetrin glycoside derivatives as the most abundant phenolic compounds in BB samples from Portugal. In BB specimens from Romania, kaempferol, myricetin, and luteolin as the most numerous polyphenols were determined [23]. Similarly, Markiewicz-Zukowska et al. [24] reported kaempferol and apigenin as the main flavonoids in Polish BB. In BB samples from Ukraine, kaempferol, quercetin, apigenin, and naringenin were established [25]. According to Zhang et al. [26], major flavonoids in rape bee pollen ethanol extract included quercetin, kaempferol, and rutin. Onuekwuzu et al. [27] revealed that treatment with flavonoid and sitosterol-rich aqueous extract had hypoglycaemic effect and improved lipid abnormalities in alloxan-induced diabetic rats. Jung et al. [28] determined flavonoid derivates (also present in BB) as potential inhibitors of AGEs because glycation can lead to the onset of diabetic complications due to chronic hyperglycaemia.
In the study by Capcarova et al. [19], treatment with BB (700 mg/kg BW for 10 weeks) non-significantly increased BI levels in ZDF rats in contrast to the diabetic control group, which is in line with our findings. Since many flavonoids (including quercetin) found in BB scavenge reactive oxygen species (ROS) and might protect β cells from oxidative damage, they could also increase the circulating BI level. To support this hypothesis, quercetin (15 mg/kg/d intraperitoneally injected for 4 weeks) was determine to partially restore the streptozotocin (STZ)-induced insulin deficiency in rats [29].
Enhanced levels of TG, TC, HDLC, and LDL cholesterol (LDLC) were recorded in our diabetic control group versus the lean one, thus supporting the findings of Pang et al. [30] and Zhou et al. [31]. Our results revealed that a higher dose of BB reduces TG and TC levels, presumably due to hypotriglyceridaemic and hypocholesterolemic properties of BB. Significantly decreased TC and TG values were also observed in alloxan-induced diabetic rats supplemented with Nigerian honey (1.0 or 2.0 g/kg for 3 weeks) [32].
BB supplementation insignificantly increased total BW of ZDF rats in our study. The decrease in BW that occurs involuntarily, was set as a warning sign of diabetes. Although no significant differences in total BW were determined in B1 and B2 groups versus the C one, there is a tendency for higher BW in both treated groups. Capcarova et al. [19] also noted a negligible increase in post-treatment BW of ZDF rats receiving the same dose of BB (700 mg/kg BW for 10 weeks). In the study by Zaid et al. [33], no significant differences in total BW of ovariectomised (OVX) rats following Tualang honey administration were also reported. However, it is necessary to state that BB is richer in protein (up to 40%) than honey (about 0.5%). Similarly, treatment with bee pollen (0.2% for 90 d) had insignificant effect on total BW, FW, and FL of Wistar rats [34].
According to 3D imaging, supplementation with BB had more positive effects on the trabecular versus cortical bone morphology in ZDF rats. Zaid et al. [33] reported significant increase in BV/TV of trabecular bone, Tb.Th., and Tb.N. in OVX rats treated with Tualang honey (0.2 g/kg BW for 6 weeks). In addition, these rats showed more improvements in trabecular bone microarchitecture than the rats receiving 1% calcium in their drinking water. According to Tomaszewska et al. [35], bee pollen supplementation (10 g/kg of feed) enlarged trabecular BV/TV and maximal Tb.Th. in Japanese quails as well. The results by Yamaguchi et al. [36] reflect that bee pollen administration increases calcium content in femoral bones of rats, thus showing a beneficial impact on mineralization of the trabecular bone. In relation to the cortical bone, supplementation with Apis dorsata honey (2 g/kg BW for 3 months) can inhibit reduced Ct.Th. in OVX rats [37]. We also identified slightly improved Ct.Th. in the B2 group.
Overall, different effects of BB administration on the cortical and trabecular bone structure may be associated with their unequal levels of bone remodelling. The trabecular bone has a large area exposed to bone marrow and blood flow, and bone turnover is greater there. In the cortical bone, 2D imaging revealed accelerated periosteal bone formation and larger density of SO in both BB-treated groups. Generally, periosteal bone apposition represents an adaptive response to maintain bone strength during aging, while bone is resorbed in the sub-endocortical envelope [38]. The amount of periosteal apposition required to maintain bone strength during aging depends on adult bone morphology and tissue-modulus degradation rate. Furthermore, it is commonly known that enhanced density of smaller SO decelerates microdamage propagation [39] and is consistent with greater bone strength. Our results suggest that treatment with BB improved cortical bone strength via both aforementioned mechanisms.
We determined significantly increased sizes of POVC and HC in the B1 and B2 groups, respectively, versus the C group. This evidence could be associated with a vasodilation of blood vessels in both canals. According to Peng et al. [40], T2DM leads to the development of microangiopathy which is consistent with a vasoconstriction of blood vessels. Actually, Stabley et al. [41] identified vasoconstriction and impaired blood flow in long bones of ZDF rats. Peng et al. [42] revealed a reduced angiogenesis accompanied by a lower number of blood vessels in the femurs of STZ-induced diabetic mice. Generally, blood vessels within the POVC and HC pass above the medullary cavity, and through the trabeculae of the trabecular bone, thus providing oxygen, nutrients, hormones, or growth factors for both cortical and trabecular bone tissues [43]. In agreement with our results, BB supplementation had a beneficial effect on blood flow in the femoral bones of ZDF rats.
4. Materials and Methods
4.1. Ethics Statement
All experimental procedures were authorized under the number 2288/16-221 by the Ethical Committee and the State Veterinary and Food Institute of the Slovak Republic. Institutional and national guidelines for the care and use of animals, including EU Directive 2010/63/EU for animal experiments, were adequately followed.
4.2. Sample Preparation
The BB was provided by the Institute of Biodiversity Conservation and Biosafety of the Slovak University of Agriculture in Nitra (Slovak Republic). It consisted of monofloral rape (Brassica napus L.) bee pollen (Figure 5). Table 1 shows a chemical composition of BB used in our experiment. No toxic substances including heavy metals, pesticides, and mycotoxins were detected. BB was crushed, mixed with distilled water, and homogenized to be convenient for application by gastric gavage.
Figure 5.
Microphotography of BB used in our experiment.

Table 1.
Chemical composition of BB used in the experiment.
4.3. Animals
Adult male ZDF rats (n = 32) came from the breeding station of the Slovak Academy of Sciences and were bred at the Slovak University of Agriculture in Nitra (Slovakia). All animals were housed in pairs under standardized conditions with 12:12 h light-dark cycles and temperature of 22 ± 2 °C. They were fed a complete feed mixture for rats on an ad libitum basis and were assigned into 4 groups of 8 individuals each: lean (L group) consisted of non-diabetic rats, control (C group) included diabetic obese rats, and groups B1 and B2 consisted of ZDF treated with the BB at doses of 500 and 700 mg/kg BW, respectively, for 10 weeks. The exact doses of BB in both the B1 and B2 groups were applied directly into stomach by oral rodent gavage every day, whilst L and C groups received distilled water. The doses used were chosen according to the study by Capcarova et al. [19].
4.4. Biochemical Analysis
ZDF rats were sacrificed by intraperitoneal anaesthetic overdose of xylazine/zoletil mixture. Whole blood was processed and the levels of investigated parameters (BG, BI, TC, LDLC, HDLC, and TG) were subsequently examined. Table 2 illustrates all measured parameters and appropriate methods which were used to obtain them.

Table 2.
Measured parameters from biochemical and morphological 3D and 2D analyses.
4.5. Macroscopical Analysis of Bones
BW and BL of both femurs (n = 64) were measured. Furthermore, total BW of all ZDF rats was recorded.
4.6. Morphological 3D and 2D Analyses of Bones
Microstructural 3D analyses of femoral bones were performed by 3D imaging (micro-CT 50, Scanco Medical, Brüttisellen, Switzerland). Cortical bone structure was assessed in the region of interest (ROI) starting 12.5 mm from the end of the growth plate (distal end) and ending 2.0 mm. Evaluated parameters of the cortical bone (BV/TV, BMD, Ct.Th., and BS) are included in Table 2. Trabecular bone structure was analysed in the ROI starting 2.9 mm from the end of the growth plate and exceeding 2.0 mm. Examined parameters of the trabecular bone (BV/TV, Tb.N., Tb.Th., BMD, and BS) are shown in Table 2. High resolution scans with 14.8 µm voxel size were obtained in both ROIs, including scanning parameters 70 kVp, 200 mA, 300 ms, and 0.5 mm aluminium filter [44]. Thin sections from cortical bone tissue (2D imaging) were prepared according to Martiniakova et al. [45] and were evaluated by established classification systems [46,47]. Measured parameters included area of POVC (n = 796), HC (n = 329), and SO (n = 329). They were determined by Motic Images Plus 2.0 ML software (Motic China Group Co., Ltd., Nanjing, China).
4.7. Statistical Analysis
Statistical analysis was conducted using SPSS Statistics 26.0 software. The data were expressed as mean ± standard error of the mean. Differences in the parameters examined were detected by ANOVA with either Games–Howell’s, Tukey’s, or both, post hoc tests. Statistical significance was assessed at p < 0.05, p < 0.01, and p < 0.001.
5. Conclusions
Our study revealed that treatment with BB has a possible potential to improve lipid disorders and reduced bone morphology in ZDF rats. Therefore, it can be used as a nutritional supplement to ameliorate T2DM-related complications associated with hyperlipidaemia and diabetic bone disease. However, determination of the optimal dose and duration of the BB-therapy requires further experiments.
Author Contributions
Conceptualisation, M.M. and M.C.; methodology, A.K. and R.O.; software, J.B., V.K. and R.O.; validation, M.M., M.C. and R.O.; formal analysis, J.B., M.B. and V.M.; investigation, M.M., J.B., V.K., A.K., M.C. and R.O.; resources, M.M. and R.O.; data curation, M.B. and V.M.; writing—original draft preparation, M.M., J.B. and R.O.; writing—review and editing, M.M. and R.O.; visualisation, M.M., J.B., M.B. and R.O.; supervision, M.M., M.C. and R.O.; funding acquisition, M.M. and R.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Education, Science, Research and Sport of the Slovak Republic, grant numbers VEGA 1/0505/18 and VEGA 1/0444/20.
Institutional Review Board Statement
The study was conducted according to the Institutional and national guidelines for the care and use of animals, including EU Directive 2010/63/EU for animal experiments, and approved by the Ethical Committee and the State Veterinary and Food Institute of the Slovak Republic (protocol code 2288/16-221).
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are available from the authors.
References
- Milibari, A.A.; Matuure, E.Y.; Gadah, E.M. Prevalence, Determinants and Prevention of Type 2 Diabetes Mellitus (T2DM) in Arabic Countries: A Systematic Review Study. Health Sci. J. 2020, 14. [Google Scholar] [CrossRef]
- Al Mansour, M.A. The Prevalence and Risk Factors of Type 2 Diabetes Mellitus (DMT2) in a Semi-Urban Saudi Population. Int. J. Environ. Res. Public Health 2019, 17, 7. [Google Scholar] [CrossRef]
- Lin, Y.; Sun, Z. Current views on type 2 diabetes. J. Endocrinol. 2009, 204, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Vesa, C.M.; Popa, L.; Popa, A.R.; Rus, M.; Zaha, A.A.; Bungau, S.; Tit, D.M.; Aron, R.A.C.; Zaha, D.C. Current Data Regarding the Relationship between Type 2 Diabetes Mellitus and Cardiovascular Risk Factors. Diagnostics 2020, 10, 314. [Google Scholar] [CrossRef]
- Ghodsi, M.; Larijani, B.; Keshtkar, A.A.; Nasli-Esfahani, E.; Alatab, S.; Mohajeri-Tehrani, M.R. Mechanisms involved in altered bone metabolism in diabetes: A narrative review. J. Diabetes Metab. Disord. 2016, 15, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Moon, C.; Liu, J. The Effects of Type 1 vs. Type 2 Diabetes on Bone Metabolism. World J. Surg. Surg. Res. 2020, 3, 5. [Google Scholar]
- Didaras, N.A.; Karatasou, K.; Dimitriou, T.G.; Amoutzias, G.D.; Mossialos, D. Antimicrobial Activity of Bee-Collected Pollen and Beebread: State of the Art and Future Perspectives. Antibiotiotics 2020, 9, 811. [Google Scholar] [CrossRef] [PubMed]
- Yucel, B.; Topal, E.; Kosoglu, M. Bee Products as Functional Food. In Superfood and Functional Food An Overview of Their Processing and Utilization; IntechOpen: London, UK, 2017; p. 21. [Google Scholar]
- Sidor, E.; Dżugan, M. Drone Brood Homogenate as Natural Remedy for Treating Health Care Problem: A Scientific and Practical Approach. Molecules 2020, 25, 5699. [Google Scholar] [CrossRef]
- DeGrandi-Hoffman, G.; Eckholm, B.J.; Huang, M.H. A comparison of bee bread made by Africanized and European honey bees (Apis mellifera) and its effects on hemolymph protein titers. Apidologie 2012, 44, 52–63. [Google Scholar] [CrossRef]
- Ivanišová, E.; Kačániová, M.; Frančáková, H.; Petrová, J.; Hutková, J.; Brovarskyi, V.; Velychko, S.; Adamchuk, L.; Schubertová, Z.; Musilová, J. Bee bread - perspective source of bioactive compounds for future. Potravinarstvo Slovak J. Food Sci. 2015, 9, 592–598. [Google Scholar] [CrossRef]
- Barene, I.; Daberte, I.; Siksna, S. Investigation of bee bread and development of its dosage forms. Med. Teor. ir Prakt. 2014, 21, 16–22. [Google Scholar] [CrossRef]
- Habryka, C.; Kruczek, M.; Drygaś, B. Bee Products Used in Apitherapy. World Sci. News 2016, 48, 121–125. [Google Scholar]
- Urcan, A.C.; Al Marghitas, L.; Dezmirean, D.S.; Bobis, O.; Bonta, V.; I Muresan, C.; Margaoan, R. Chemical Composition and Biological Activities of Beebread—Review. Bull. Univ. Agric. Sci. Veter- Med. Cluj-Napoca. Anim. Sci. Biotechnol. 2017, 74, 6–14. [Google Scholar] [CrossRef]
- Mărgăoan, R.; Stranț, M.; Varadi, A.; Topal, E.; Yücel, B.; Cornea-Cipcigan, M.; Campos, M.G.; Vodnar, D.C. Bee Collected Pollen and Bee Bread: Bioactive Constituents and Health Benefits. Antioxidants 2019, 8, 568. [Google Scholar] [CrossRef]
- Ferreira, G.S.; Veening-Griffioen, D.H.; Boon, W.P.; Hooijmans, C.R.; Moors, E.H.; Schellekens, H.; Van Meer, P.J. Comparison of drug efficacy in two animal models of type 2 diabetes: A systematic review and meta-analysis. Eur. J. Pharmacol. 2020, 879, 173153. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, H. Effects of Propolis Extract and Propolis-Derived Compounds on Obesity and Diabetes: Knowledge from Cellular and Animal Models. Molecules 2019, 24, 4394. [Google Scholar] [CrossRef]
- Ali, A.M.; Kunugi, H. Apitherapy for Parkinson’s Disease: A Focus on the Effects of Propolis and Royal Jelly. Oxidative Med. Cell. Longev. 2020, 2020, 1–18. [Google Scholar] [CrossRef]
- Capcarova, M.; Kalafova, A.; Schwarzova, M.; Schneidgenova, M.; Prnova, M.S.; Svik, K.; Slovak, L.; Kisska, P.; Kovacik, A.; Brindza, J. Consumption of bee bread influences glycaemia and development of diabetes in obese spontaneous diabetic rats. Biologia 2020, 75, 705–711. [Google Scholar] [CrossRef]
- Shishehbor, F.; Azemi, M.E.; Zameni, D.; Saki, A. Inhibitory Effect of Hydroalcoholic Extracts of Barberry, Sour Cherry and Cornelian Cherry on α-Amylase and α-Glucosidase Activities. Int. J. Pharm. Res. Allied Sci. 2016, 5, 423–428. [Google Scholar]
- Tavdidishvili, D.; Khutsidze, T.; Pkhakadze, M.; Vanidze, M.; Kalandia, A. Flavonoids in Georgian Bee Bread and Bee Pollen. J. Chem. Chem. Eng. 2014, 8, 676–681. [Google Scholar] [CrossRef]
- Sobral, F.; Calhelha, R.C.; Barros, L.; Dueñas, M.; Tomás, A.; Santos-Buelga, C.; Vilas-Boas, M.; Ferreira, I.C.F.R. Flavonoid Composition and Antitumor Activity of Bee Bread Collected in Northeast Portugal. Molecules 2017, 22, 248. [Google Scholar] [CrossRef] [PubMed]
- Dranca, F.; Ursachi, F.; Oroian, M. Bee Bread: Physicochemical Characterization and Phenolic Content Extraction Optimization. Foods 2020, 9, 1358. [Google Scholar] [CrossRef] [PubMed]
- Markiewicz-Żukowska, R.; Naliwajko, S.K.; Bartosiuk, E.; Moskwa, J.; Isidorov, V.; Soroczyńska, J.; Borawska, M.H. Chemical composition and antioxidant activity of beebread, and its influence on the glioblastoma cell line (U87MG). J. Apic. Sci. 2013, 57, 147–157. [Google Scholar] [CrossRef]
- Isidorov, V.; Isidorova, A.; Sczczepaniak, L.; Czyżewska, U. Gas chromatographic–mass spectrometric investigation of the chemical composition of beebread. Food Chem. 2009, 115, 1056–1063. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, F.; Jamali, M.A.; Peng, Z. Antioxidant Enzyme Activities and Lipid Oxidation in Rape (Brassica campestris L.) Bee Pollen Added to Salami during Processing. Molecules 2016, 21, 1439. [Google Scholar] [CrossRef]
- Chidinma, I.C.; Chigozie, I.J. Anti-Diabetic Effect of a Flavonoid and Sitosterol - Rich Aqueous Extract ofPleurotus tuberregiumSclerotia in Alloxan-Induced Diabetic Rabbits. Endocr. Metab. Immune Disord. Drug Targets 2019, 19, 1148–1156. [Google Scholar] [CrossRef]
- Jung, H.A.; Park, J.J.; Islam, N.; Jin, S.E.; Min, B.-S.; Lee, J.-H.; Sohn, H.S.; Choi, J.S. Inhibitory activity of coumarins from Artemisia capillaris against advanced glycation endproduct formation. Arch. Pharmacal Res. 2012, 35, 1021–1035. [Google Scholar] [CrossRef]
- Coskun, O.; Kanter, M.; Korkmaz, A.; Oter, S. Quercetin, a flavonoid antioxidant, prevents and protects streptozotocin-induced oxidative stress and ?-cell damage in rat pancreas. Pharmacol. Res. 2005, 51, 117–123. [Google Scholar] [CrossRef]
- Pang, Y.-L.; Hu, J.-W.; Liu, G.-L.; Lu, S.-Y. Comparative medical characteristics of ZDF-T2DM rats during the course of development to late stage disease. Anim. Model. Exp. Med. 2018, 1, 203–211. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, H.; Man, F.; Guo, Z.; Xu, J.; Yan, W.; Li, J.; Pan, Q.; Wang, W. Sitagliptin Protects Cardiac Function by Reducing Nitroxidative Stress and Promoting Autophagy in Zucker Diabetic Fatty (ZDF) Rats. Cardiovasc. Drugs Ther. 2018, 32, 541–552. [Google Scholar] [CrossRef]
- Erejuwa, O.O.; Nwobodo, N.N.; Akpan, J.L.; Okorie, U.A.; Ezeonu, C.T.; Ezeokpo, B.C.; Nwadike, K.I.; Erhiano, E.; Wahab, M.S.A.; Sulaiman, S.A. Nigerian Honey Ameliorates Hyperglycemia and Dyslipidemia in Alloxan-Induced Diabetic Rats. Nutrients 2016, 8, 95. [Google Scholar] [CrossRef]
- Zaid, S.S.M.; Sulaiman, S.A.; Othman, N.H.; Soelaiman, I.-N.; Shuid, A.N.; Mohamad, N.; Muhamad, N. Protective effects of Tualang honey on bone structure in experimental postmenopausal rats. Clinics 2012, 67, 779–784. [Google Scholar] [CrossRef]
- Martiniaková, M.; Bobonova, I.; Omelka, R.; Duranova, H.; Babosová, R.; Stawarz, R.; Toman, R. Low administration of bee pollen in the diet affects qualitative histological characteristics of bone in male rats. Potravinarstvo Slovak J. Food Sci. 2014, 8, 277–283. [Google Scholar] [CrossRef]
- Tomaszewska, E.; Knaga, S.; Dobrowolski, P.; Lamorski, K.; Jabłoński, M.; Tomczyk-Warunek, A.; Kadhim, M.J.; Hułas-Stasiak, M.; Borsuk, G.; Muszyński, S. The effect of bee pollen on bone biomechanical strength and trabecular bone histomorphometry in tibia of young Japanese quail (Coturnix japonica). PLoS ONE 2020, 15, e0230240. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M.; Hamamoto, R.; Uchiyama, S.; Ishiyama, K.; Hashimoto, K. Anabolic Effects of Bee Pollen Cistus ladaniferus Extract on Bone Components in the Femoral-Diaphyseal and -Metaphyseal Tissues of Rats in Vitro and in Vivo. J. Health Sci. 2006, 52, 43–49. [Google Scholar] [CrossRef]
- Yudaniayanti, I.S.; Primarizky, H.; Nangoi, L.; Yuliani, G.A. Protective effects of honey by bees (Apis dorsata) on decreased cortical thickness and bone impact strength of ovariohysterectomized rats as models for menopause. Veter World 2019, 12, 868–876. [Google Scholar] [CrossRef]
- Jepsen, K.J.; Andarawis-Puri, N. The amount of periosteal apposition required to maintain bone strength during aging depends on adult bone morphology and tissue-modulus degradation rate. J. Bone Miner. Res. 2012, 27, 1916–1926. [Google Scholar] [CrossRef]
- Bell, K.L.; Loveridge, N.; Reeve, J.; Thomas, C.D.; Feik, S.A.; Clement, J.G. Super-osteons (remodeling clusters) in the cortex of the femoral shaft: Influence of age and gender. Anat. Rec. Adv. Integr. Anat. Evol. Biol. 2001, 264, 378–386. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Hui, K.; Hao, C.; Peng, Z.; Gao, Q.X.; Jin, Q.; Lei, G.; Min, J.; Qi, Z.; Bo, C.; et al. Low bone turnover and reduced angiogenesis in streptozotocin-induced osteoporotic mice. Connect. Tissue Res. 2016, 57, 277–289. [Google Scholar] [CrossRef]
- Stabley, J.N.; Prisby, R.D.; Behnke, B.J.; Delp, M.D. Type 2 diabetes alters bone and marrow blood flow and vascular control mechanisms in the ZDF rat. J. Endocrinol. 2015, 225, 47–58. [Google Scholar] [CrossRef]
- Peng, J.; Qu, H.; Peng, J.; Luo, T.-Y.; Lv, F.-J.; Chen, L.; Wang, Z.-N.; Ouyang, Y.; Cheng, Q.-F. Abnormal spontaneous brain activity in type 2 diabetes with and without microangiopathy revealed by regional homogeneity. Eur. J. Radiol. 2016, 85, 607–615. [Google Scholar] [CrossRef]
- Filipowska, J.; Tomaszewski, K.A.; Niedźwiedzki, Ł.; Walocha, J.A.; Niedźwiedzki, T. The role of vasculature in bone development, regeneration and proper systemic functioning. Angiogenesis 2017, 20, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Omelka, R.; Martiniakova, M.; Svik, K.; Slovak, L.; Payer, J.; Oppenbergerova, I.; Kovacova, V.; Babikova, M.; Soltesova-Prnova, M. The effects of eggshell calcium (Biomin H®) and its combinations with alfacalcidol (1α-hydroxyvitamin D3) and menaquinone-7 (vitamin K2) on ovariectomy-induced bone loss in a rat model of osteoporosis. J. Anim. Physiol. Anim. Nutr. 2021, 105, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Martiniaková, M.; Boboňová, I.; Omelka, R.; Grosskopf, B.; Stawarz, R.; Toman, R. Structural changes in femoral bone tissue of rats after subchronic peroral exposure to selenium. Acta Veter Scand. 2013, 55, 8. [Google Scholar] [CrossRef] [PubMed]
- De Ricqles, A.J.; Meunier, F.J.; Castanet, J.; Francillon-Vieillot, H. Comparative microstructure of bone. In Bone Matrix and Bone Specific Products; CRC Press, Inc.: Boca Raton, FL, USA, 1991; Volume 3, pp. 1–78. [Google Scholar]
- Enlow, D.H.; Brown, S.O. A Comparative Histological Study of Fossil and Recent Bone Tissues. Part I. Tex. J. Sci. 1956, 8, 405–412. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).