Investigation the Effects of Green-Synthesized Copper Nanoparticles on the Performance of Activated Carbon-Chitosan-Alginate for the Removal of Cr(VI) from Aqueous Solution
Abstract
1. Introduction
2. Results
2.1. Investigation of the Materials’ Properties
2.1.1. Fourier Transform Infrared Spectroscopy Study
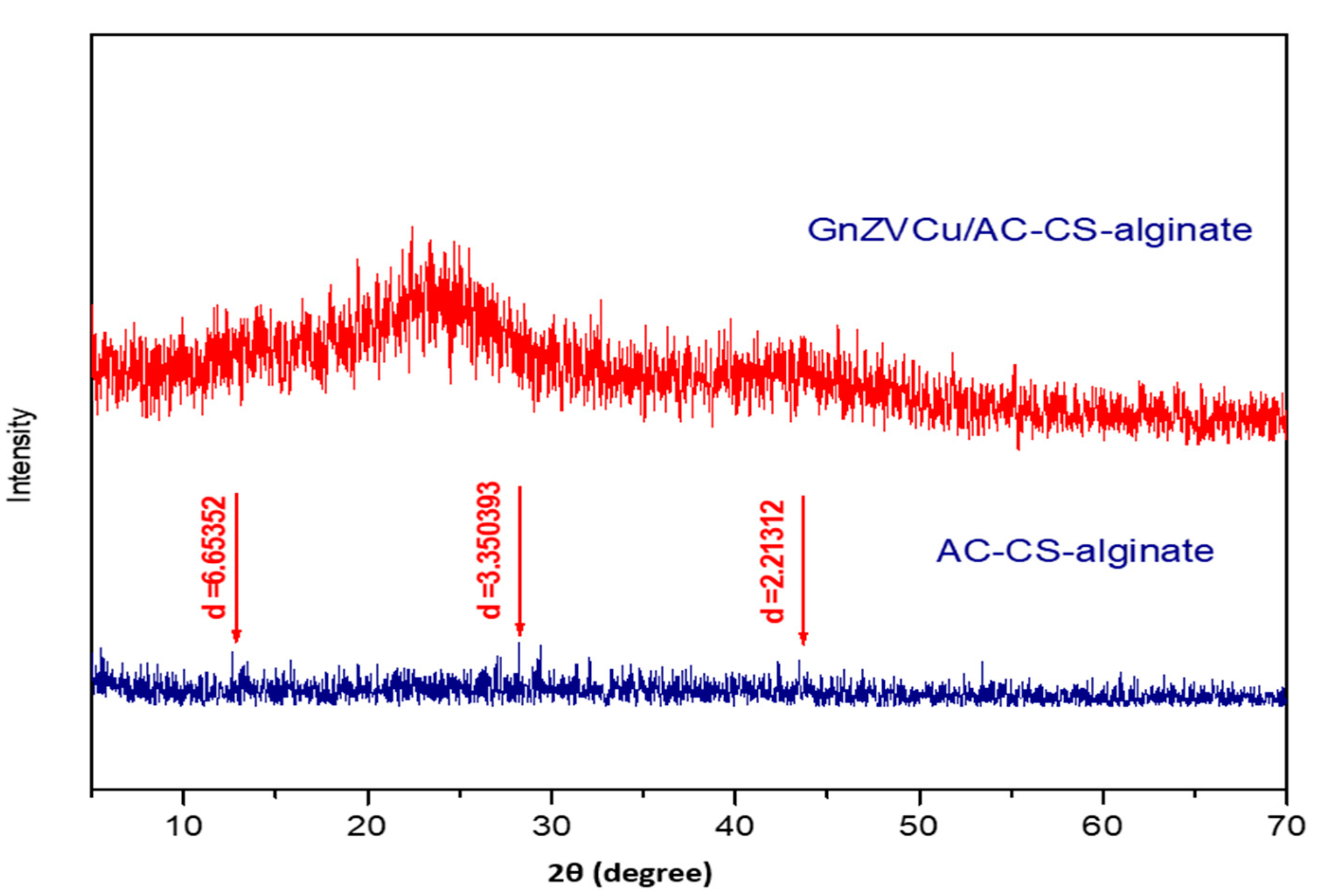
2.1.2. X-ray Diffraction Study
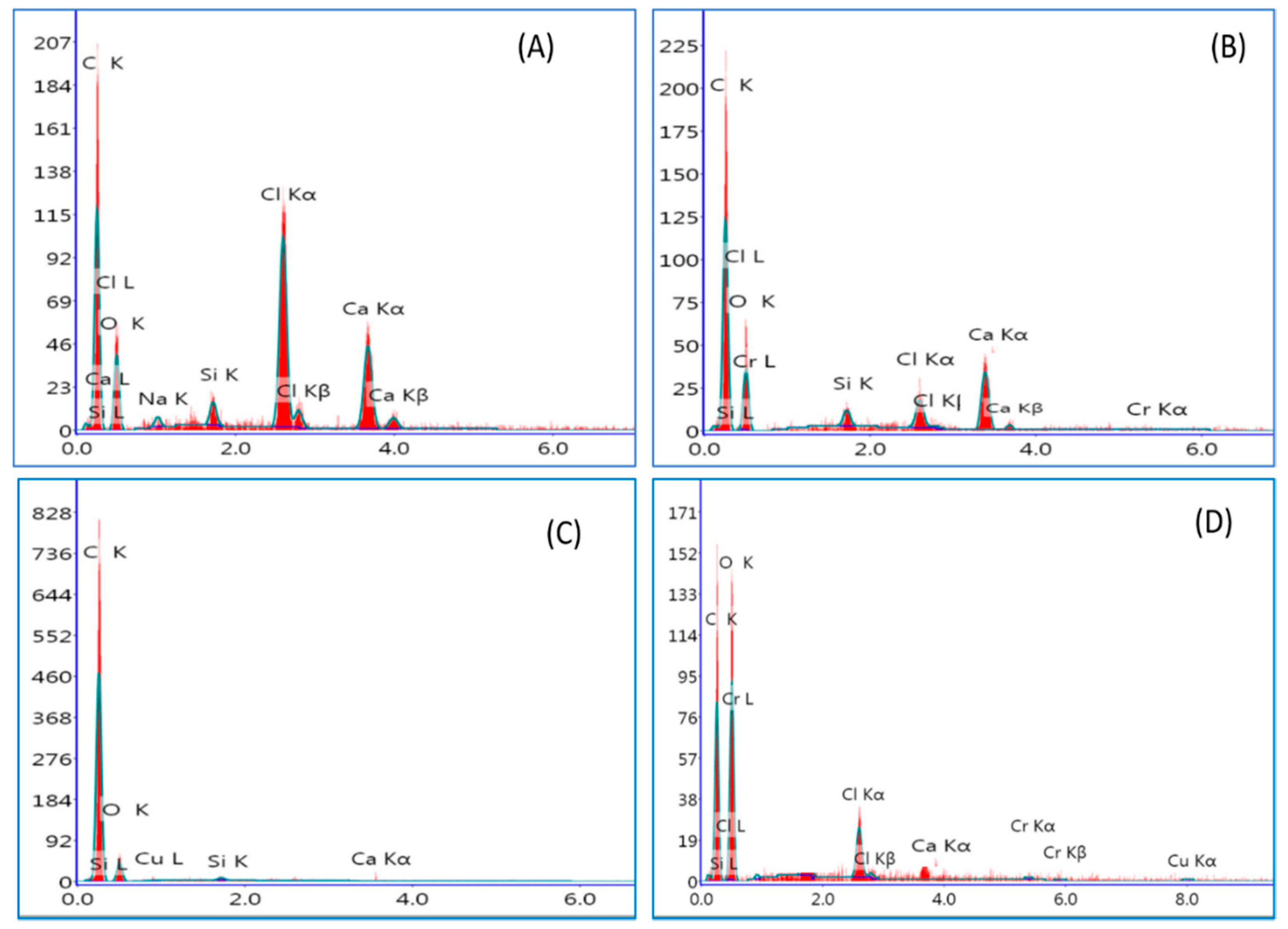
2.1.3. Morphology and Elemental Composition of the Materials
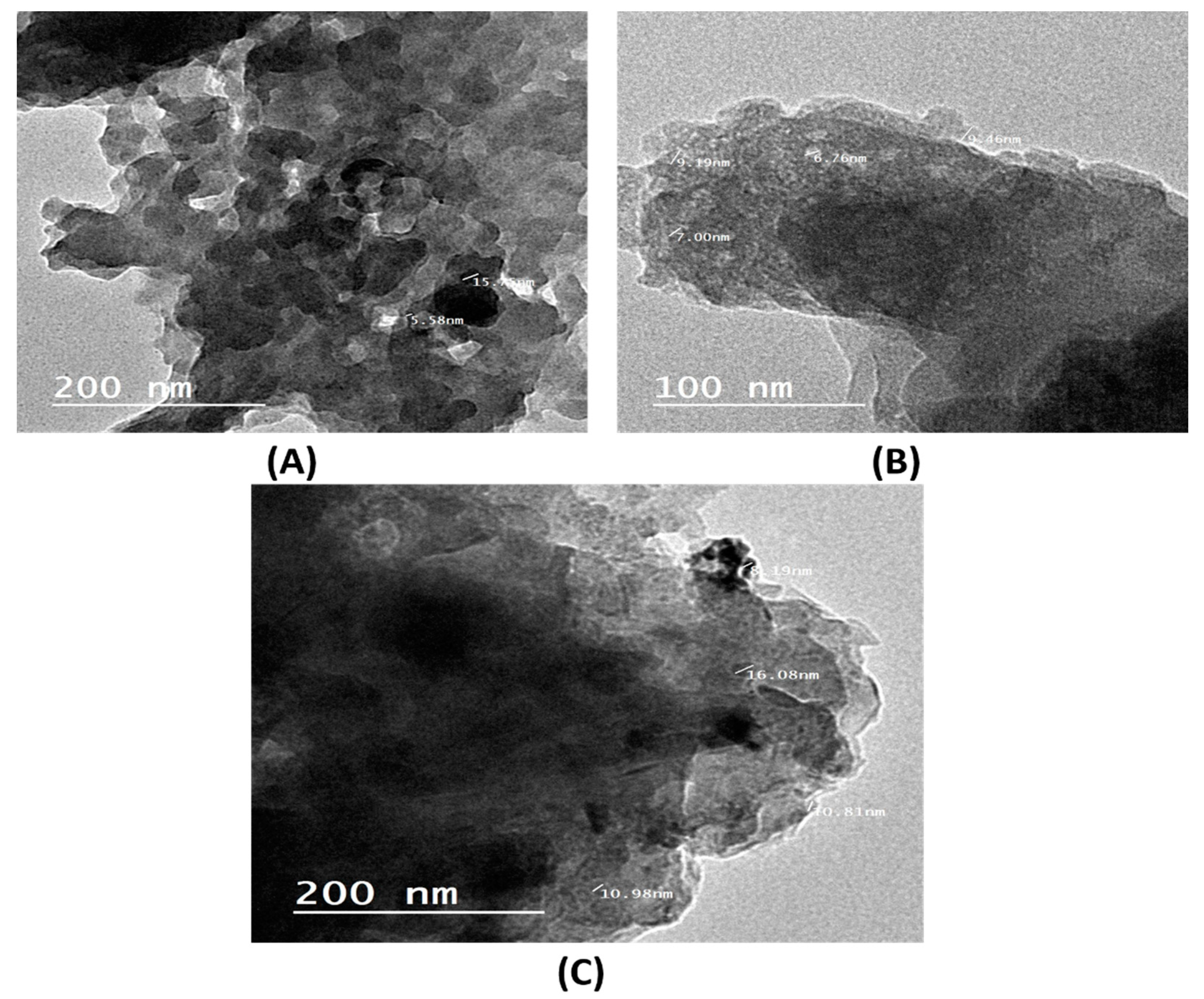
2.1.4. TEM Analysis
2.2. Performance of the Nano-Composites in the Removal of Cr+6
2.2.1. Effect of pH on the Nanocomposite Performance
2.2.2. Contact Time Effect
2.3. Effect of Cr (VI) Ion Concentration
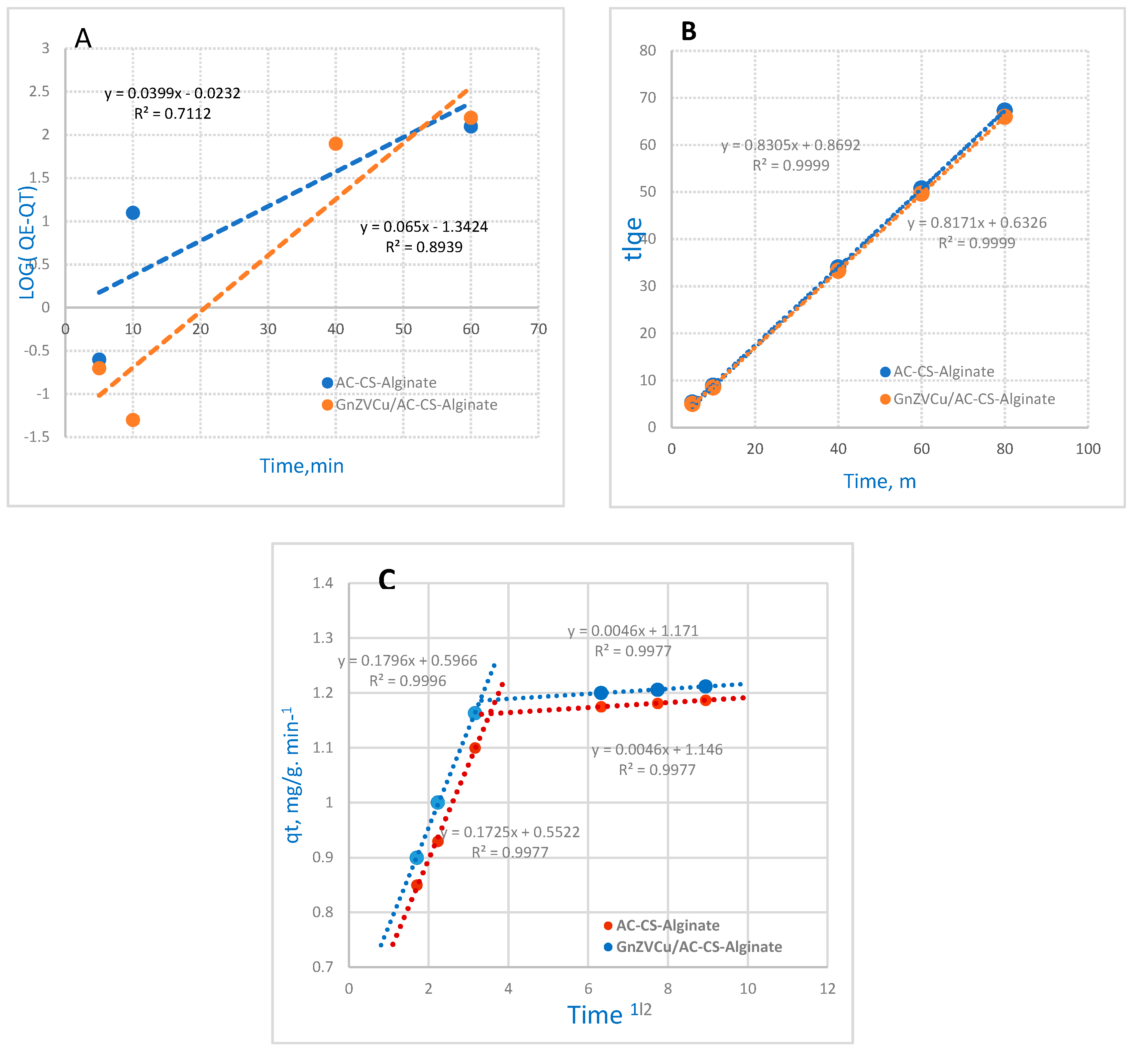
2.4. Kinetic Models
2.4.1. Pseudo-First Order Reaction Kinetics
2.4.2. Pseudo-Second-Order Reaction Kinetics
2.4.3. Mories–Weber Equation
2.5. Isotherm Model
2.6. The (D-R) Isotherm
2.7. Sorption Thermodynamics
3. Materials and Methods
3.1. Materials
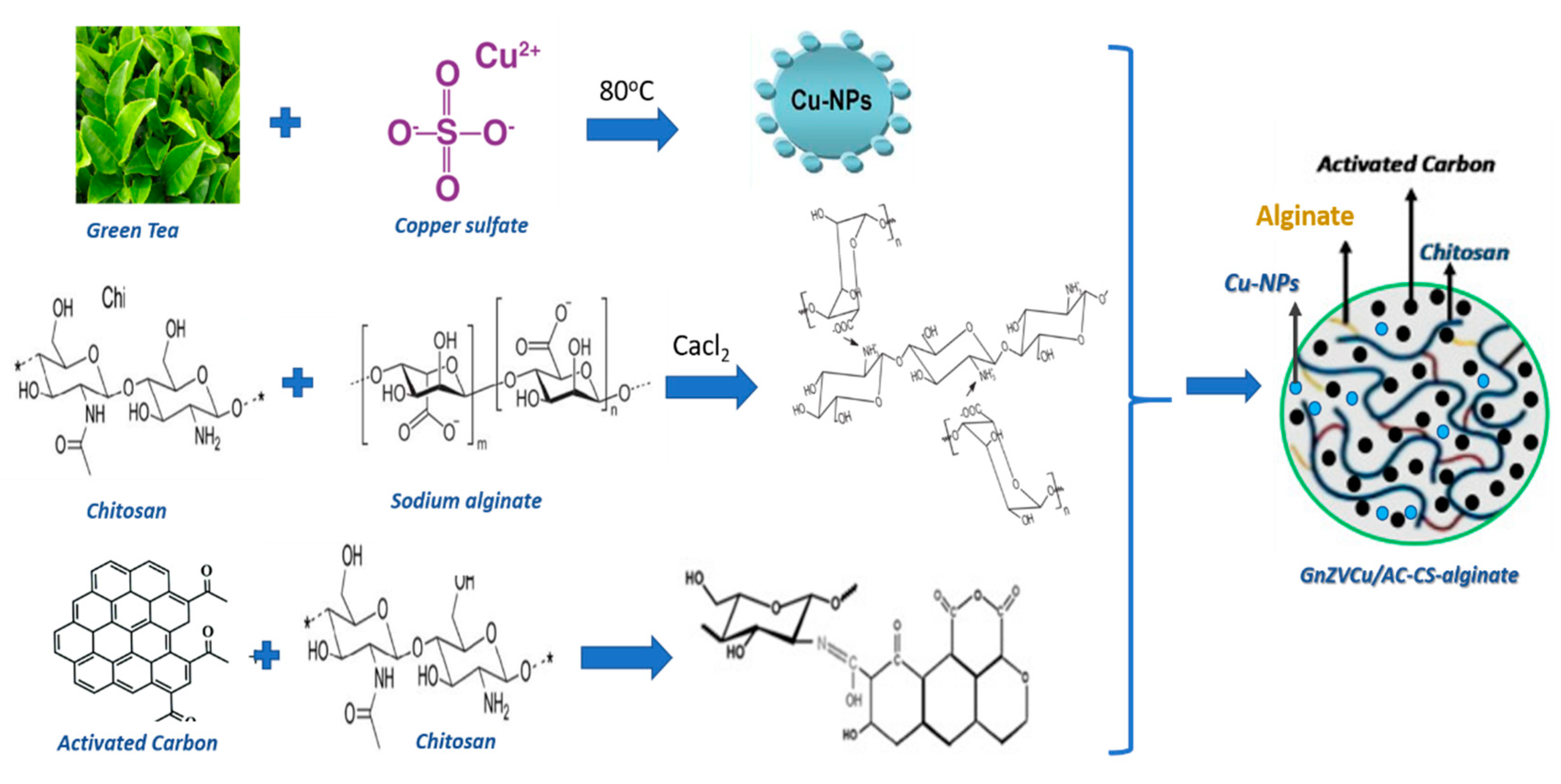
3.1.1. Synthesis of Copper Nanoparticles
3.1.2. Preparation of GnZVCu/AC-CS-Alginate Nanocomposites
3.2. The Method of Chromium (VI) Analysis
3.3. The Nanocomposites Surface Characterization
3.3.1. Instruments
3.3.2. Adsorption Examinations of Hexavalent Chromium Cr+6
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Arancibia-Miranda, N.; Baltazar, S.E.; García, A.; Muñoz-Lira, D.; Sepúlveda, P.; Rubio, M.A.; Altbir, D. Nanoscale zero valent supported by Zeolite and Montmorillonite: Template effect of the removal of lead ion from an aqueous solution. J. Hazard. Mater. 2016, 301, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Zuo-Jiang, S.; He, Y.; Sun, Q.; Wang, Y.; Liu, W.; Sun, S.; Chen, K. A facile, versatile approach to hydroxyl-anchored metal oxides with high Cr(VI) adsorption performance in water treatment. R. Soc. Open Sci. 2016, 3, 160524. [Google Scholar] [CrossRef]
- Mohseni-Bandpi, A.; Kakavandi, B.; Kalantary, R.R.; Azari, A.; Keramati, A. Development of a novel magnetite–chitosan composite for the removal of fluoride from drinking water: Adsorption modeling and optimization. RSC Adv. 2015, 5, 73279–73289. [Google Scholar] [CrossRef]
- Rakhunde, R.; Deshpande, L.; Juneja, H.D. Chemical Speciation of Chromium in Water: A Review. Crit. Rev. Environ. Sci. Technol. 2012, 42, 776–810. [Google Scholar] [CrossRef]
- Shi, L.N.; Zhang, X.; Chen, Z.L. Removal ofchromium (VI) from wastewater usingbentonite-supported nanoscale zero-valent iron. Water Res. 2011, 45, 886–892. [Google Scholar] [CrossRef]
- Metters, J.P.; Kadara, R.O.; Banks, C.E. Electroanalytical sensing of chromium(iii) and (vi) utilising gold screen printed macro electrodes. Analyst 2012, 137, 896–902. [Google Scholar] [CrossRef]
- Zimmermann, A.C.; Mecabô, A.; Fagundes, T.; Rodrigues, C.A. Adsorption of Cr(VI) using Fe-crosslinked chitosan complex (Ch-Fe). J. Hazard. Mater. 2010, 179, 192–196. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.K.; Wang, H. Cr(VI) removal by combined redox reactions and adsorption using pectin-stabilized nanoscale ze-ro-valent iron for simulated chromium contaminated water. RSC Adv. 2015, 5, 65068–65073. [Google Scholar] [CrossRef]
- Fang, J.; Gu, Z.M.; Gang, D.C.; Liu, C.X.; Ilton, E.; Deng, B.L. Cr(VI) removal from aqueous solution by activated carbon coat-edwith quaternized poly(4-vinylpyridine). Environ. Sci. Technol. 2007, 41, 4748–4753. [Google Scholar] [CrossRef]
- Keshmirizadeh, E.; Yousefi, S.; Rofouei, M.K. An investigation on the new operational parameter effective in Cr(VI) removal efficiency: A study on electrocoagulation by alternating pulse current. J. Hazard. Mater. 2011, 190, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Lo, I.M.C. Synthesis of mesoporous magnetic gamma-Fe2O3 and its application to Cr(VI) removal from contaminated water. Water Res. 2009, 43, 3727–3734. [Google Scholar] [CrossRef]
- Li, L.; Iqbal, J.; Zhu, Y.; Zhang, P.; Chen, W.; Bhatnagar, A.; Du, Y. Chitosan/Ag-hydroxyapatite nanocomposite beads as a potential adsorbent for the efficient removal of toxic aquatic pollutants. Int. J. Biol. Macromol. 2018, 120, 1752–1759. [Google Scholar] [CrossRef]
- Ahmed, E.S.; Moustafa, H.Y.; El-Masry, A.M.; Hassan, S.A. Natural and synthetic polymers for water treatment against dis-solved pharmaceuticals. J. Apply. Polym. Sci. 2014, 131, 40458–40468. [Google Scholar] [CrossRef]
- Borges, O.; Borchard, G.; Verhoef, J.C.; de Sousa, A.; Junginger, H.E. Preparation of coated nanoparticles for a new mucosal vaccine delivery system. Int. J. Pharm. 2005, 299, 155–166. [Google Scholar] [CrossRef]
- Khoushab, F.; Yamabhai, M. Chitin Research Revisited. Mar. Drugs 2010, 8, 1988–2012. [Google Scholar] [CrossRef]
- Hacer, D. Preparation and characterization of calcium alginate-based composite adsorbents for the removal of Cd, Hg, and Pb ions from aqueous solution. Toxicol. Environ. Chem. 2012, 94, 3. [Google Scholar]
- Motwani, S.K.; Chopra, S.; Talegaonkar, S.; Kohli, K.; Ahmad, F.J.; Khar, R.K. Chitosan–sodium alginate nanoparticles as submicroscopic reservoirs for ocular delivery: Formulation, optimisation and in vitro characterisation. Eur. J. Pharm. Biopharm. 2008, 68, 513–525. [Google Scholar] [CrossRef] [PubMed]
- Ali, J.; Ahmed, A.; Mohd, S. Mesoporous Crosslinked Chitosan-Activated Charcoal Composite for the Removal of Thionine Cationic Dye: Comprehensive Adsorption and Mechanism Study. J. Polym Environ. 2020, 28, 1095–1105. [Google Scholar]
- Nasrullah, A.; Bhat, A.; Naeem, A.; Isa, M.H.; Danish, M. High surface area mesoporous activated carbon-alginate beads for efficient removal of methylene blue. Int. J. Biol. Macromol. 2018, 107, 1792–1799. [Google Scholar] [CrossRef]
- Huang, C.-C.; Lo, S.-L.; Lien, H.-L. Zero-valent copper nanoparticles for effective dechlorination of dichloromethane using sodium borohydride as a reductant. Chem. Eng. J. 2012, 203, 95–100. [Google Scholar] [CrossRef]
- Shu, J.; Cheng, S.; Xia, H.; Zhang, L.; Peng, J.; Li, C.; Zhang, S. Copper loaded on activated carbon as an efficient adsorbent for removal of methylene blue. RSC Adv. 2017, 7, 14395–14405. [Google Scholar] [CrossRef]
- Wang, S.; Vincent, T.; Roux, J.-C.; Faur, C.; Guibal, E. Pd(II) and Pt(IV) sorption using alginate and algal-based beads. Chem. Eng. J. 2017, 313, 567–579. [Google Scholar] [CrossRef]
- Vijayalakshmi, K.; Devi, B.M.; Latha, S.; Gomathi, T.; Sudha, P.; Venkatesan, J.; Anil, S. Batch adsorption and desorption studies on the removal of lead (II) from aqueous solution using nanochitosan/sodium alginate/microcrystalline cellulose beads. Int. J. Biol. Macromol. 2017, 104, 1483–1494. [Google Scholar] [CrossRef] [PubMed]
- Abdelmalek, B.E.; Sila, A.; Haddar, A.; Bougatef, A.; Ayadi, M.A. β-Chitin and chitosan from squid gladius: Biological activities of chitosan and its application as clarifying agent for apple juice. Int. J. Biol. Macromol. 2017, 104, 953–962. [Google Scholar] [CrossRef] [PubMed]
- Afshar, H.A.; Ghaee, A. Preparation of aminated chitosan/alginate scaffold containing halloysite nanotubes with improved cell attachment. Carbohydr. Polym. 2016, 151, 1120–1131. [Google Scholar] [CrossRef] [PubMed]
- Kavaklı, C.; Barsbay, M.; Tilki, S.; Güven, O.; Kavaklı, P.A. Activation of Polyethylene/Polypropylene Nonwoven Fabric by Radiation-Induced Grafting for the Removal of Cr(VI) from Aqueous Solutions. Water Air Soil Pollut. 2016, 227, 473. [Google Scholar] [CrossRef]
- Hassan, A.; Abdel-Mohsen, A.; Fouda, M.M. Comparative study of calcium alginate, activated carbon, and their composite beads on methylene blue adsorption. Carbohydr. Polym. 2014, 102, 192–198. [Google Scholar] [CrossRef]
- Badruddoza, A.Z.M.; Tay, A.S.H.; Tan, P.Y.; Hidajat, K.; Uddin, M.S. Carboxymethyl-bcyclodextrin conjugated magnetic nanoparticles as nano-adsorbents for removal of copper ions: Synthesis and adsorption studies. J. Hazard. Mater. 2011, 185, 1177–1186. [Google Scholar] [CrossRef]
- Garg, V.; Gupta, R.; Kumar, R. Adsorption of chromium from aqueous solution on treated sawdust. Bioresour. Technol. 2004, 92, 79–81. [Google Scholar] [CrossRef]
- Selvi, K.; Pattabhi, S.; Kadirvelu, K. Removal of Cr(VI) from aqueous solution by adsorption onto activated carbon. Bioresour. Technol. 2001, 80, 87–89. [Google Scholar] [CrossRef]
- Barrera-Díaz, C.E.; Lugo-Lugo, V.; Bilyeu, B. A review of chemical, electrochemical and biological methods for aqueous Cr(VI) reduction. J. Hazard. Mater. 2012, 223-224, 1–12. [Google Scholar] [CrossRef]
- Muhammad, S.; Alaadin, A.B.; Muhammad, N.A. Electrocoagulation for the treatment of Wastewater for reuse in irrigation and plantation (Report). J. Basic Appl. 2011, 7, 11–20. [Google Scholar]
- Millar, G.J.; Couperthwaite, S.J.; Dawes, L.A.; Thompson, S.; Spencer, J. Activated alumina for the removal of fuoride ions from high alkalinity groundwater: New insights from equilibrium and column studies with multicomponent solutions. Sep. Purif. Technol. 2017, 187, 14–24. [Google Scholar] [CrossRef]
- Lagergren, S. Zurtheorie der sogenannten adsorption gel sterstoffe. Sven. Vetensk. Handl. 1898, 24, 1–39. [Google Scholar]
- Ho, Y.; McKay, G. Pseudo-second order model for sorption processes. Process. Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Aljeboree, M.; Alshirifi, N.; Alkaim, F. Kinetics and equilibrium study for the adsorption of textile dyes on coconut shell activated carbon. Arab. J. Chem. 2017, 10, 3381. [Google Scholar] [CrossRef]
- Elwakeel, K.; El-Bindary, A.; Kouta, E.; Guibal, E. Functionalization of polyacrylonitrile/Na-Y-zeolite composite with amidoxime groups for the sorption of Cu(II), Cd(II) and Pb(II) metal ions. Chem. Eng. J. 2018, 332, 727–736. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. The Kinetics of Sorption of Divalent Metal Ions onto Sphagnum Moss Peat. Water Res. 2000, 34, 735–742. [Google Scholar] [CrossRef]
- Meenakshi, S.; Viswanathan, N. Identification of selective ion-exchange resin for fluoride sorption. J. Colloid Interface Sci. 2007, 308, 438–450. [Google Scholar] [CrossRef]
- Israa, I.N.; Hilal, W.S. Adsorption of Eriochrom Black T Azo Dye onto Nanosized Anatase TiO2. J. Environ. Eng. Sci. 2015, 2, 86–92. [Google Scholar]
- Ho, Y.-S. Effect of pH on lead removal from water using tree fern as the sorbent. Bioresour. Technol. 2005, 96, 1292–1296. [Google Scholar] [CrossRef]
- Veliev, E.V.; Öztürk, T.; Veli, S.; Fatullayev, A.G. Application of diffusion model for adsorption of azo reactrive dye on pumice. Pol. J. Environ. Stud. 2006, 15, 347–353. [Google Scholar]
- Daneshvar, N.; Salari, D.; Aber, S. Chromium adsorption and Cr(VI) reduction to trivalent chromium in aqueous solutions by soya cake. J. Hazard. Mater. 2002, 94, 49–61. [Google Scholar] [CrossRef]
- Freundlich, H.M.F. Uber die adsorption in losungen. Z. Phys. Chem. 1906, 57, 385–470. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Özcan, A.; Öncü, E.M.; Özcan, A.S. Kinetics, isotherm and thermodynamic studies of adsorption of Acid Blue 193 from aqueous solutions onto natural sepiolite. Colloids Surf. A 2006, 277, 90–97. [Google Scholar] [CrossRef]
- Elgarahy, A.M.; Elwakeel, K.Z.; Elshoubaky, G.A.; Mohammad, S.H. Microwave-accelerated sorption of cationic dyes onto green marine algal biomass. Environ. Sci. Pollut. Res. 2019, 26, 22704–22722. [Google Scholar] [CrossRef] [PubMed]
- Salvestrini, S.; Leone, V.; Iovino, P.; Canzano, S.; Capasso, S. Considerations about the correct evaluation of sorption ther-modynamic parameters from equilibrium isotherms. J. Chem. Thermodyn. 2014, 68, 310–316. [Google Scholar] [CrossRef]
- Wentong, Z.; Jing, Z.; Wei, W.; Lirong, M.; Jianjun, Z.; Jimin, X. Comparative study of modified/non-modified aluminum and silica aerogels for anionic dye adsorption performance. RSC Adv. 2018, 8, 29129. [Google Scholar]
- Haleemat, I.; Folahan, A.; Olalekan, S.F.; Bhekumusa, J.X. Adsorption of Cr (VI) on synthetic hematite (α-Fe2O3) nanopar-ticles of different morphologies. Korean J. Chem. Eng. 2014, 31, 142–154. [Google Scholar]
- Shujauddin, K.; Lei, Z.; Aimin, L.; Muhammad, I.; Xiaojuan, Z. Microwave-assisted hydrothermal carbonization of furfural residue for adsorption of Cr(VI): Adsorption and kinetic study. Pol. J. Environ. Stud. 2020, 29, 1671–1681. [Google Scholar]
- Ihsanullah; Al-Khaldi, F.A.; Abu-Sharkh, B.; Abulkibash, A.M.; Qureshi, M.I.; Laoui, T.; Atieh, M.A. Effect of acid modification on adsorption of hexavalent chromium (Cr(VI)) from aqueous solution by activated carbon and carbon nanotubes. Desalination Water Treat. 2016, 57, 7232–7244. [Google Scholar] [CrossRef]
- Shahrina, S.; Lau, W.J.; Goha, P.S.; Jaafara, J.; Ismaila, A.F. Adsorptive Removal of Cr(VI) and Cu(II) Ions from Water Solution using Graphene Oxide–Manganese Ferrite (GMF) Nanomaterials. Int. J. Eng. 2018, 31, 1341–1346. [Google Scholar]
- Pâmela, B.; Amanda, D.; Eduardo, C.D.; Valter, A.B.; Alexandre, T.P. Adsorption and removal of chromium (VI) contained in aqueous solutions using a chitosan-based hydrogel. Environ. Sci. Pollut. Res. 2019, 26, 28481–28489. [Google Scholar]
- Yousef, A.; Hossein, E.; Rauf, F. Enhancement removal of Cr (VI) ion using magnetically modified MgO nanoparticles. Mater. Res. Express. 2019, 6, 125513. [Google Scholar]
- Malwade, K.; Lataye, D.; Mhaisalkar, V.; Kurwadkar, S.; Ramirez, D. Adsorption of hexavalent chromium onto activated carbon derived from Leucaena leucocephala waste sawdust: Kinetics, equilibrium and thermodynamics. Int. J. Environ. Sci. Technol. 2016, 13, 2107–2116. [Google Scholar] [CrossRef]
- Baghani, A.; Hossein, M.; Gholami, M.; Rastkari, N.; Delikhoon, M. One-pot synthesis, characterization and adsorption studies of amine-functionalized magnetite nanoparticles for removal of Cr(VI) and Ni(II) ions from aqueousmsolution: Ki-netic, isotherm and thermodynamic studies. J. Environ. Health Sci. Eng. 2016, 11, 1–12. [Google Scholar]
- Padmavathy, K.; Madhub, G.; Haseena, P. A study on effects of pH, adsorbent dosage, time, initial concentration and ad-sorption isotherm study for the removal of hexavalent chromium (Cr(VI)) from wastewater by magnetite nanoparticles. Procedia Technol. 2016, 24, 585–594. [Google Scholar] [CrossRef]
- Huang, Z.-N.; Wang, X.-L.; Yang, D.-S. Adsorption of Cr(VI) in wastewater using magnetic multi-wall carbon nanotubes. Water Sci. Eng. 2015, 8, 226–232. [Google Scholar] [CrossRef]
- Elfeky, S.A.; Mahmoud, S.E.; Youssef, A.F. Applications of CTAB modified magnetic nanoparticles for removal of chromium (VI) from contaminated water. J. Adv. Res. 2017, 8, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Asghar, M.A.; Zahir, E.; Shahid, S.M.; Khan, M.N.; Iqbal, J.; Walker, G. Iron, copper and silver nanoparticles: Green synthesis using green and black tea leaves extracts and evaluation of antibacterial, antifungal and aflatoxin B1 adsorption activity. LWT 2018, 90, 98–107. [Google Scholar] [CrossRef]
- Jibran, I.; Noor, S.S.; Murtaza, S.; Muhammad, I.; Nawshad, M.; Fares, M.H.; Sara, A.A.; Javed, A.K.; Zia, H.K.; Amit, B.; et al. Synergistic effects of activated carbon and nano-zerovalent copper on the performance of hydroxyapatite-alginate beads for the removal of As+3 from aqueous solution. J. Clean. Prod. 2019, 235, 875–886. [Google Scholar]
- Mohamed Aly-Eldeen, A.; Abeer El-Sayed, A.M.; Dalia, M.S.A.; El Zokm, M.G. The uptake of Eriochrome Black T dye from aqueous solutions utilizing waste activated sludge: Adsorption process optimization using factorial design. EJABF 2018, 44, 179–186. [Google Scholar]
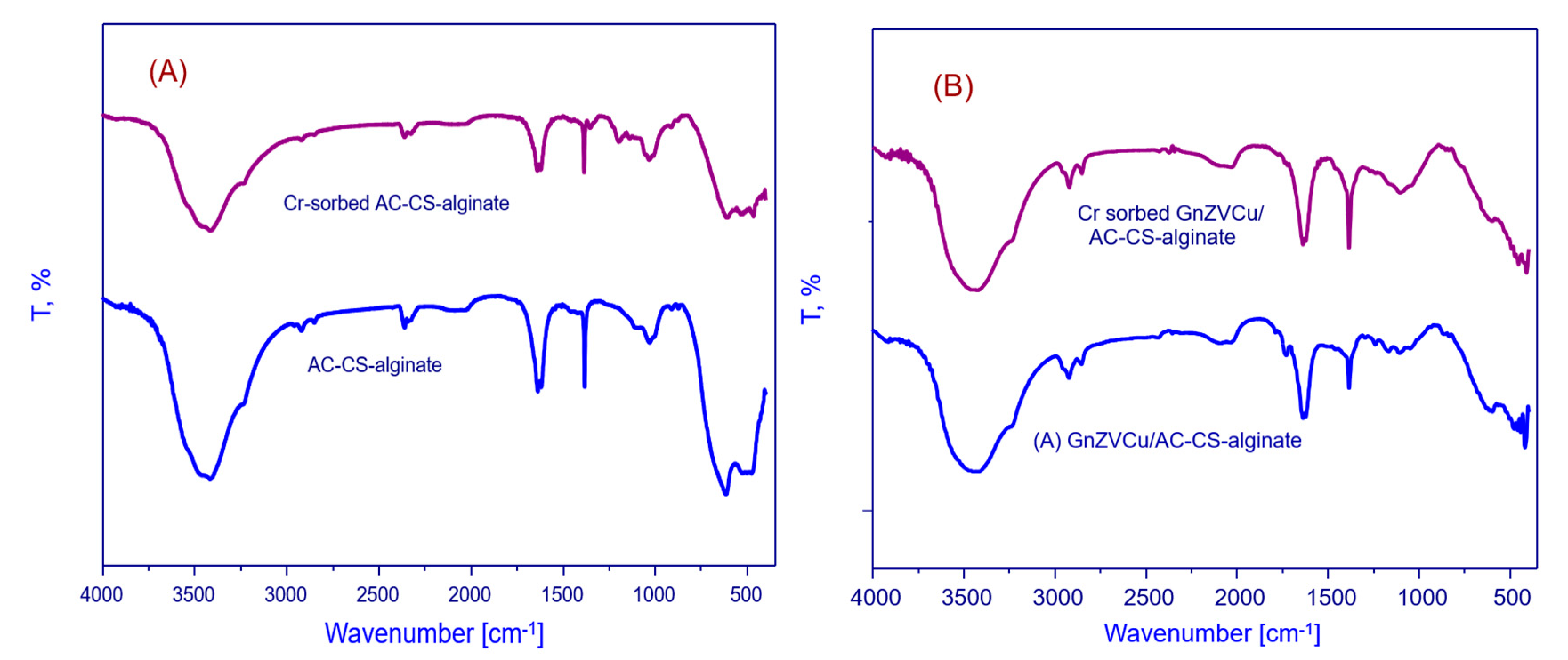



| S. No | Frequency (cm−1) | Functional Group |
|---|---|---|
| 1 | 3426 cm−1 | OH or NH2 stretching |
| 2 | 1637 cm−1 | COO stretching |
| 3 | 1619 cm−1 | C-C stretching |
| 4 | 1334 cm−1 | O-H bending |
| 5 | 1031 cm−1 | C-O stretching |
| Kinetic Models | Parameter | (AC-CS-Alginate) | (GnZVCu/AC-CS-Alginate) |
|---|---|---|---|
| PFOR | qe, exp (mg g−1) | 1.187 | 1.212 |
| qe, cal (mg g−1) | 0.9479 | 0.0454 | |
| Kads (min−1) | 1.0962 | 1.1614 | |
| R2 | 0.7112 | 0.8939 | |
| PSOR | qe, cal (mg g−1) | 1.2041 | 1.2233 |
| K2 (g mg−1 min−1) | 0.9555 | 1.2925 | |
| R2 | 0.9999 | 0.9999 | |
| Mories–Weber | Kd (mg g−1 min0.5) | 0.1725 | 0.1796 |
| R2 | 0.9977 | 0.9996 |
| Kinetic Isotherm | Parameter | (AC-CS-Alginate) | (GnZVCu/AC-CS-Alginate) |
|---|---|---|---|
| Langmuir | qe, exp (mg g−1) | 1.187 | 1.212 |
| qe, cal (mg g−1) | 5.467 | 5.810 | |
| KL (L mg−1) | 0.365 | 0.513 | |
| R2 | 0.990 | 0.991 | |
| Freundlich | KF (moln−1 Ln g−1) | 1.54 | 1.84 |
| N | 2.24 | 2.260 | |
| R2 | 0.9918 | 0.9981 | |
| D-R model | E (kJ mol−1) | 0.7593 | 0.7383 |
| q(D-R) (mg g−1) | 2.705 | 2.768 | |
| R2 | 0.9932 | 0.9983 |
| Parameter | T (K) | A% | LnKL | ∆Ho (KJ.mol−1) | ∆So (J.mol−1.K−1) | ∆Go (kJ.mol−1) | R2 |
|---|---|---|---|---|---|---|---|
| Cr+6/(AC-CS-Alginate) | 300 | 94 | 0.672 | −23.393 | −70.729 | −1.619 | 0.9917 |
| 313 | 90.5 | 0.174 | −0.455 | ||||
| 323 | 87 | −0.178 | 0.481 | ||||
| Cr+6/GnZVCu/AC-CS-Alginate | 300 | 96 | 1.098 | −21.461 | −61.633 | −2.747 | 0.9993 |
| 313 | 94 | 0.672 | −1.754 | ||||
| 323 | 91.7 | 0.322 | −0.867 |
| Metal | Adsorbent | qe (mg/g) | Reference |
|---|---|---|---|
| Cr (VI) | Modified activated carbon | 18.51 | [53] |
| Graphene oxide–manganese ferrite (GMF) nanomaterials | 34.02 | [54] | |
| Chitosan-based hydrogel | 93.03 | [55] | |
| Synthesize dMgO/Fe3O4 nanocomposite | 23.90 | [56] | |
| Activated carbon from Leucaena leucocepnala | 13.85 | [57] | |
| Fe3O4-NH2 (amino functionalized magnetic nano-adsorbent | 232.51 | [58] | |
| Magnetite nanoparticles | 3.810 | [59] | |
| Magnetic multiwall carbon nanotubes | 16.23 | [60] | |
| Fe3O4 nanoparticles capped with cetyltrimethylammonium bromide | 18.50 | [61] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, I.A.; Hussein, H.S.; Ragab, A.H.; AlMasoud, N.; Ghfar, A.A. Investigation the Effects of Green-Synthesized Copper Nanoparticles on the Performance of Activated Carbon-Chitosan-Alginate for the Removal of Cr(VI) from Aqueous Solution. Molecules 2021, 26, 2617. https://doi.org/10.3390/molecules26092617
Ahmed IA, Hussein HS, Ragab AH, AlMasoud N, Ghfar AA. Investigation the Effects of Green-Synthesized Copper Nanoparticles on the Performance of Activated Carbon-Chitosan-Alginate for the Removal of Cr(VI) from Aqueous Solution. Molecules. 2021; 26(9):2617. https://doi.org/10.3390/molecules26092617
Chicago/Turabian StyleAhmed, Inas A., Hala S. Hussein, Ahmed H. Ragab, Najla AlMasoud, and Ayman A. Ghfar. 2021. "Investigation the Effects of Green-Synthesized Copper Nanoparticles on the Performance of Activated Carbon-Chitosan-Alginate for the Removal of Cr(VI) from Aqueous Solution" Molecules 26, no. 9: 2617. https://doi.org/10.3390/molecules26092617
APA StyleAhmed, I. A., Hussein, H. S., Ragab, A. H., AlMasoud, N., & Ghfar, A. A. (2021). Investigation the Effects of Green-Synthesized Copper Nanoparticles on the Performance of Activated Carbon-Chitosan-Alginate for the Removal of Cr(VI) from Aqueous Solution. Molecules, 26(9), 2617. https://doi.org/10.3390/molecules26092617






