Polymorphism, Halogen Bonding, and Chalcogen Bonding in the Diiodine Adducts of 1,3- and 1,4-Dithiane
Abstract
:1. Introduction
2. Results and Discussion
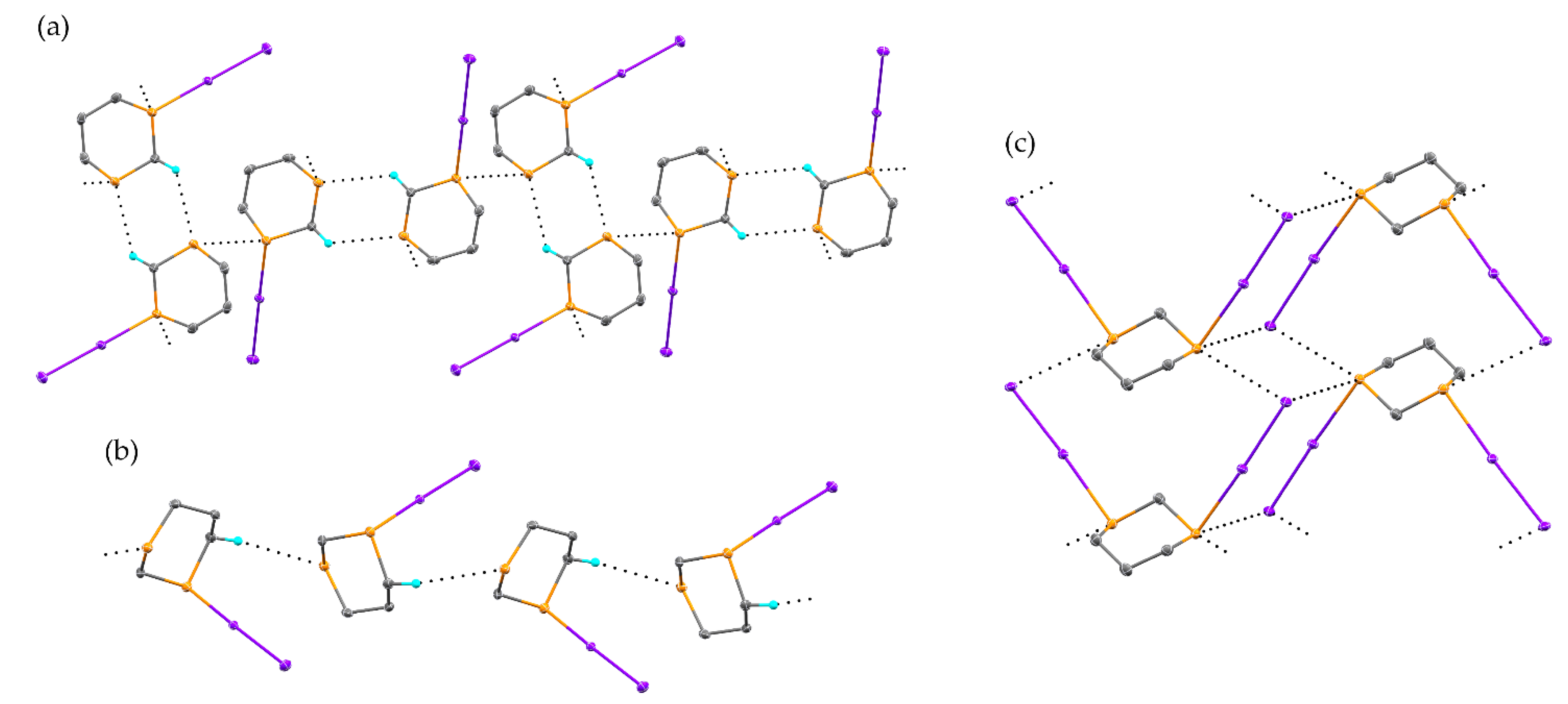
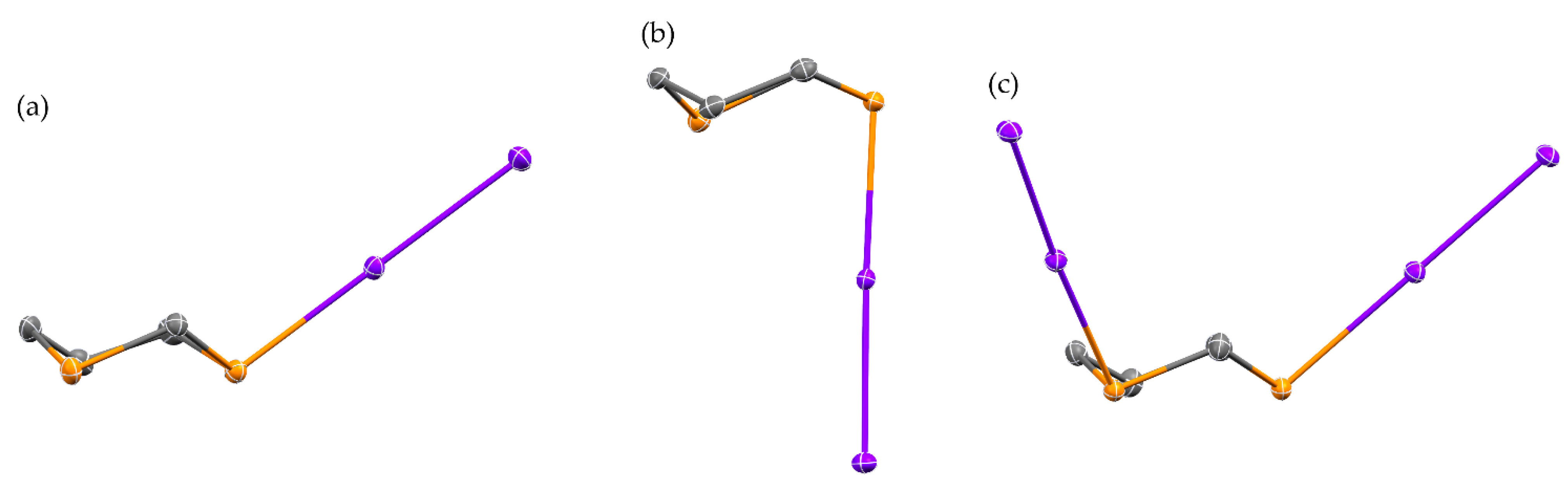
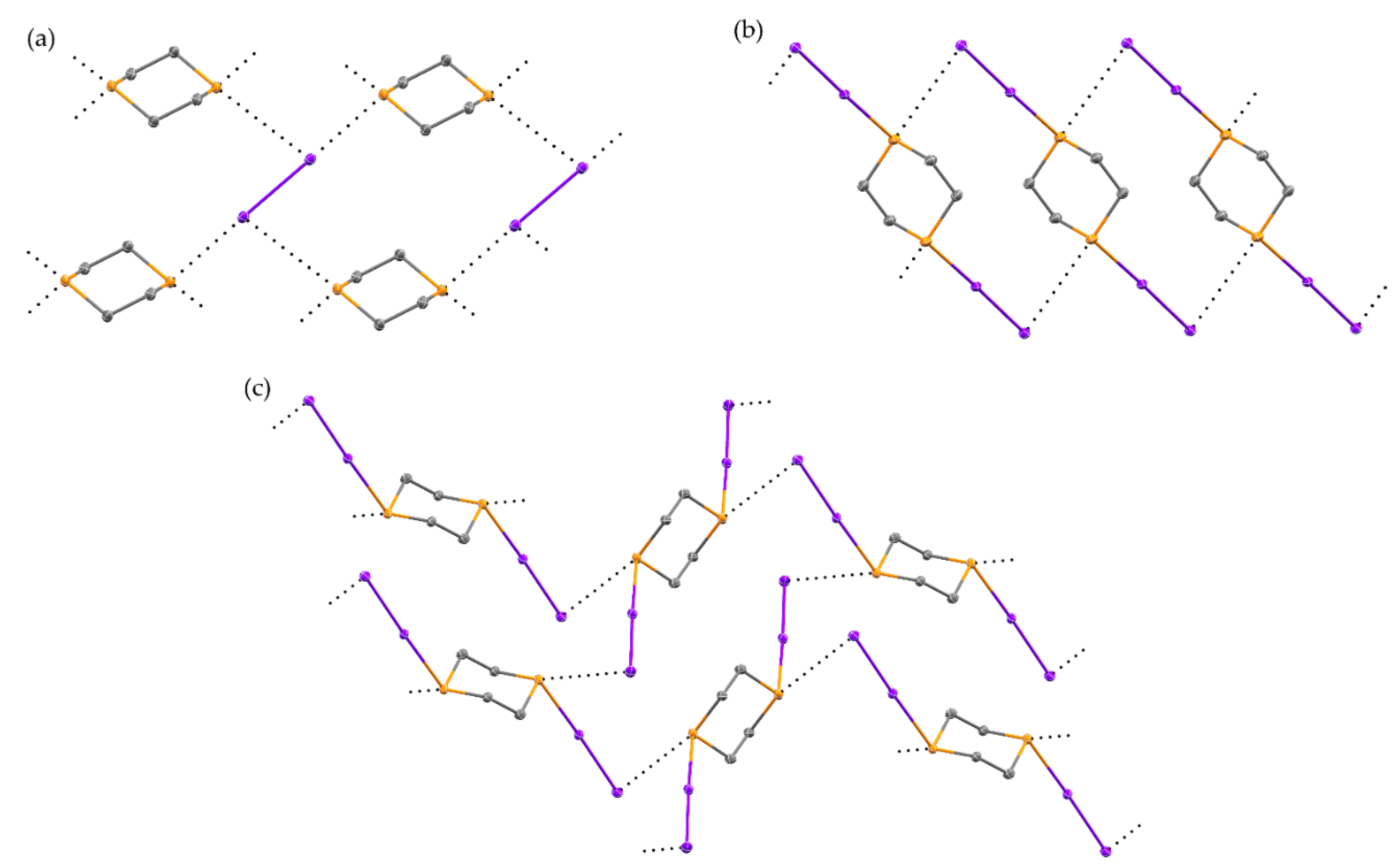
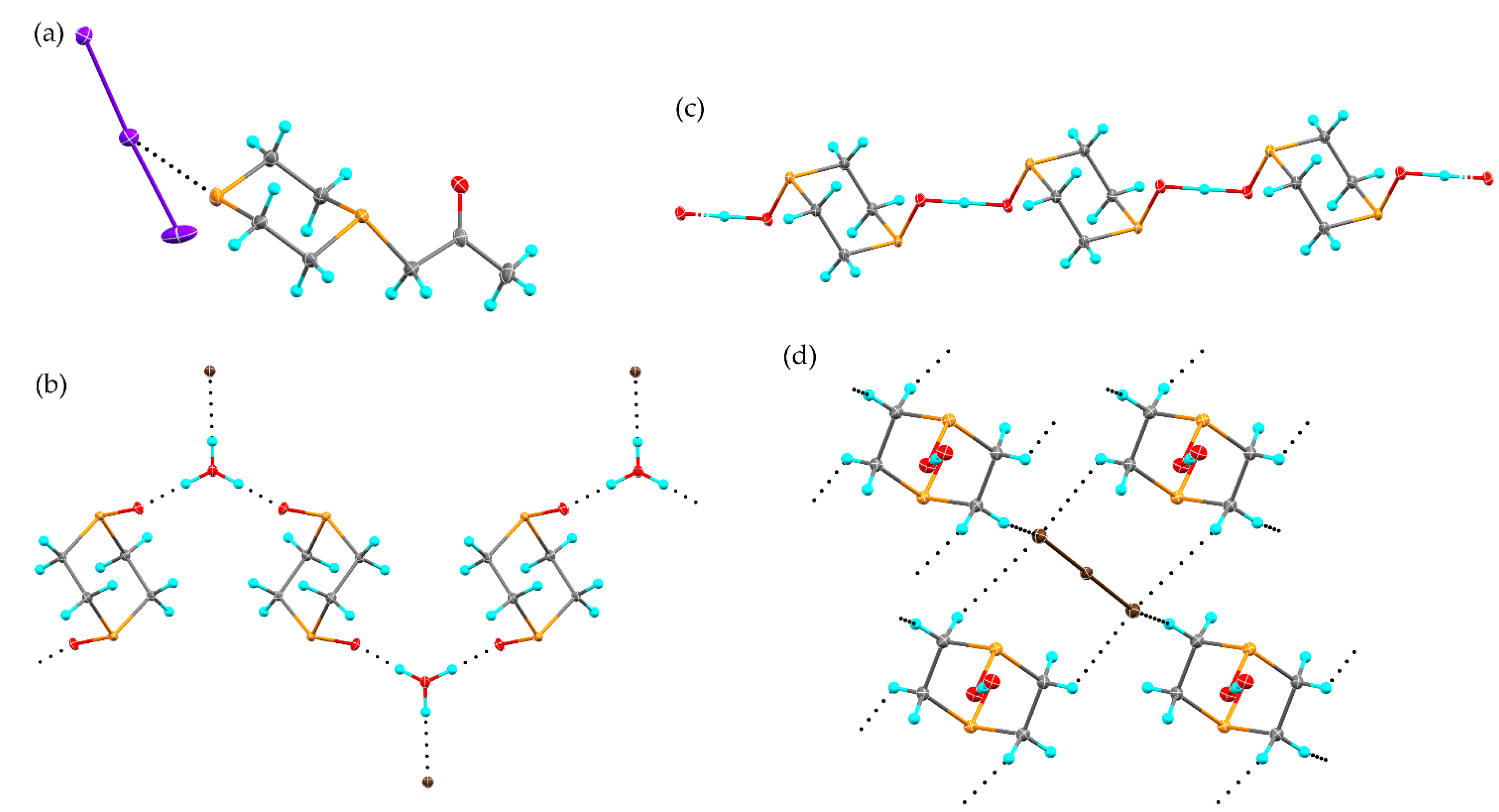
2.1. Reaction of 1,3-Dithiane with I2
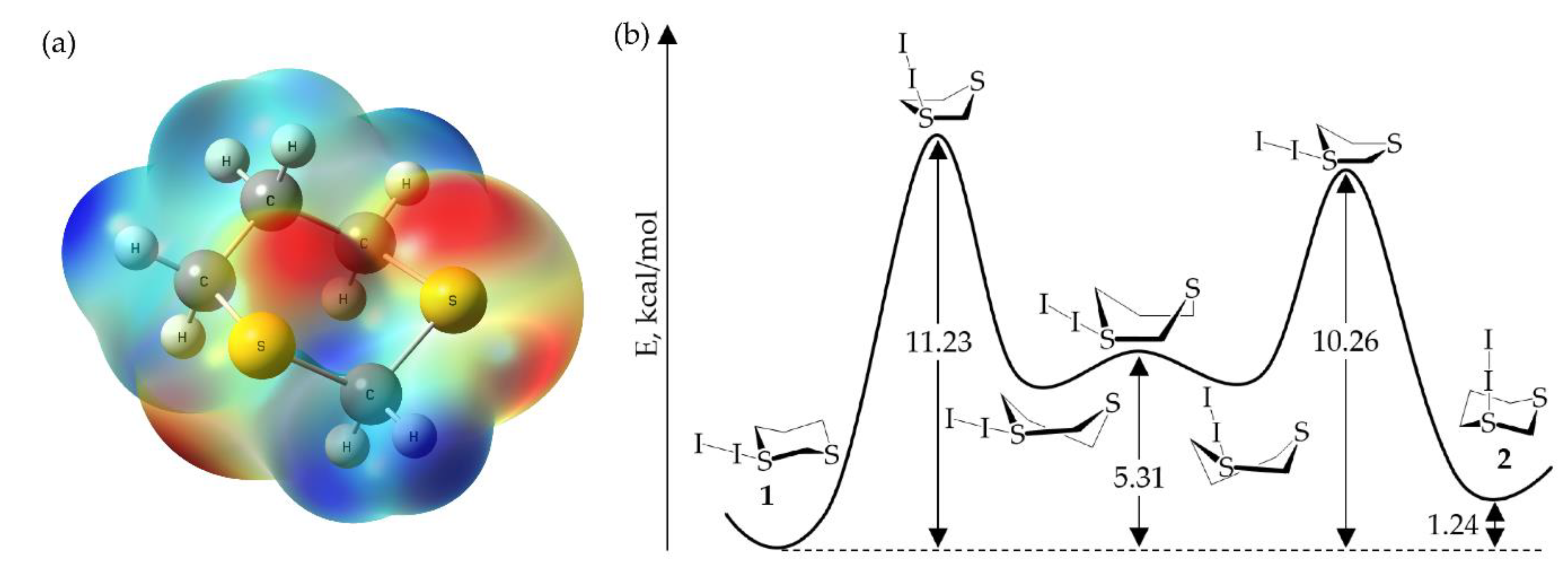
2.2. Computational Investigation of S-Diiodo-1,3-Dithiane Ring Flip
2.3. Reaction of 1,4-Dithiane with I2
2.4. Other Dihalogen Promoted Reactions of 1,4-Dithiane
3. Materials and Methods
3.1. Materials
3.2. Thermal Analysis
3.3. Single-Crystal X-ray Analysis
3.4. Computational Study of 1 and 2
3.5. Synthesis of Cocrystals
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Cavallo, G.; Metrangolo, P.; Milani, R.; Pilati, T.; Priimagi, A.; Resnati, G.; Terraneo, G. The halogen bond. Chem. Rev. 2016, 116, 2478–2601. [Google Scholar] [CrossRef] [Green Version]
- Vogel, L.; Wonner, P.; Huber, S.M. Chalcogen Bonding: An Overview. Angew. Chem. Int. Ed. 2019, 58, 1880–1891. [Google Scholar] [CrossRef]
- Aakeroy, C.B.; Bryce, D.L.; Desiraju, G.R.; Frontera, A.; Legon, A.C.; Nicotra, F.; Rissanen, K.; Schneiner, S.; Terraneo, G.; Metrangolo, P.; et al. Definition of the chalcogen bond. Pure Appl. Chem. 2018, 91, 4–13. [Google Scholar]
- Desiraju, G.R.; Shing Ho, P.; Kloo, L.; Legon, A.C.; Marquardt, R.; Metrangolo, P.; Politzer, P.; Resnati, G.; Rissanen, K. Definition of the halogen bond (IUPAC recommendations 2013). Pure Appl. Chem. 2013, 85, 1711–1713. [Google Scholar] [CrossRef]
- Politzer, P.; Murray, J.S.; Clark, T. Halogen bonding: An electrostatically-driven highly directional noncovalent interaction. Phys. Chem. Chem. Phys. 2010, 12, 7748–7757. [Google Scholar] [CrossRef]
- Kolář, M.; Hostaš, J.; Hobza, P. The strength and directionality of a halogen bond are co-determined by the magnitude and size of the σ-hole. Phys. Chem. Chem. Phys. 2014, 16, 9987–9996. [Google Scholar] [CrossRef]
- Knight, F.R.; Fuller, A.L.; Bühl, M.; Slawin, A.M.Z.; Woollins, J.D. Hypervalent adducts of chalcogen-containing peri -substituted naphthalenes; Reactions of sulfur, selenium, and tellurium with dihalogens. Inorg. Chem. 2010, 49, 7577–7596. [Google Scholar] [CrossRef] [Green Version]
- Brezgunova, M.E.; Aubert, E.; Dahaoui, S.; Fertey, P.; Lebègue, S.; Jelsch, C.; Ángyán, J.G.; Espinosa, E. Charge density analysis and topological properties of Hal 3-synthons and their comparison with competing hydrogen bonds. Cryst. Growth Des. 2012, 12, 5373–5386. [Google Scholar] [CrossRef]
- Lu, Y.; Shi, T.; Wang, Y.; Yang, H.; Yan, X.; Luo, X.; Jiang, H.; Zhu, W. Halogen bonding—A novel interaction for rational drug design? J. Med. Chem. 2009, 52, 2854–2862. [Google Scholar] [CrossRef]
- Bhattacharya, A.A.; Curry, S.; Franks, N.P. Binding of the general anesthetics propofol and halothane to human serum albumin: High resolution crystal structures. J. Biol. Chem. 2000, 275, 38731–38738. [Google Scholar] [CrossRef] [Green Version]
- Rowlinson, S.W.; Kiefer, J.R.; Prusakiewicz, J.J.; Pawlitz, J.L.; Kozak, K.R.; Kalgutkar, A.S.; Stallings, W.C.; Kurumbail, R.G.; Marnett, L.J. A Novel Mechanism of Cyclooxygenase-2 Inhibition Involving Interactions with Ser-530 and Tyr-385. J. Biol. Chem. 2003, 278, 45763–45769. [Google Scholar] [CrossRef] [Green Version]
- Peloquin, A.J.; Kobra, K.; McMillen, C.D.; Iacono, S.T.; Pennington, W.T. Isolation of hydrazine oxidation products via halogen bonding: C–I bond scission and crystal polymorphism. CrystEngComm 2021, 23, 419–426. [Google Scholar] [CrossRef]
- Peloquin, A.; McMillen, C.D.; Iacono, S.T.; Pennington, W.T. Crystal Engineering Using Polyiodide Halogen and Chalcogen Bonding to Isolate the Phenothiazinium Radical Cation and its Rare Dimer, 10-(3-phenothiazinylidene)phenothiazinium. Chem. A Eur. J. 2021, 27, 8398–8405. [Google Scholar] [CrossRef] [PubMed]
- Benz, S.; López-Andarias, J.; Mareda, J.; Sakai, N.; Matile, S. Catalysis with Chalcogen Bonds. Angew. Chem. Int. Ed. 2017, 56, 812–815. [Google Scholar] [CrossRef]
- Chao, G.Y.; McCullough, J.D. The refinement of the structure of the complex of iodine with 1,4 dithiane, C4H8S2.2I2. Acta Crystallogr. 1960, 13, 727–732. [Google Scholar] [CrossRef]
- Arca, M.; Cristiani, F.; Devillanova, F.A.; Garau, A.; Isaia, F.; Lippolis, V.; Verani, G.; Demartin, F. Reactivity of 1,3,5-trithiacyclohexane and 1,3,5- triselenacyclohexane towards molecular diiodine. Crystal structures of the diiodine adducts. Polyhedron 1997, 16, 1983–1991. [Google Scholar] [CrossRef]
- Blake, A.J.; Devillanova, F.A.; Garau, A.; Gilby, L.M.; Gould, R.O.; Isaia, F.; Lippolis, V.; Parsons, S.; Radek, C.; Schröder, M. Thioether-iodine charge-transfer complexes. Synthesis and low-temperature single-crystal structures of complexes of penta-, hexa-and octa-dentate homoleptic thioether macrocycles. J. Chem. Soc. Dalt. Trans. 1998, 12, 2037–2046. [Google Scholar] [CrossRef]
- Blake, A.J.; Li, W.S.; Lippolis, V.; Schröder, M. 1,4,8,11-Tetrakis(diiodine)-1,4,8,11-tetrathiacyclotetradecane. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 1997, 53, 886–888. [Google Scholar] [CrossRef]
- Blake, A.J.; Cristiani, F.; Devillanova, F.A.; Garau, A.; Gilby, L.M.; Gould, R.O.; Isaia, F.; Lippolis, V.; Parsons, S.; Radek, C.; et al. Structural and solution studies of diiodine charge-transfer complexes of thioether crowns. Dalt. Trans. 1997, 8, 1337–1346. [Google Scholar] [CrossRef]
- Hamed, M.M.A.; Mohamed, M.B.; Mahmoud, M.R. Charge-Transfer Molecular Complexes of Thianes with σ and π Electron Acceptors. Bull. Chem. Soc. Jpn. 1994, 67, 2006–2010. [Google Scholar] [CrossRef]
- Klaboe, P. The Raman Spectra of Some Iodine, Bromine, and Iodine Monochloride Charge-Transfer Complexes in Solution. J. Am. Chem. Soc. 1967, 89, 3667–3676. [Google Scholar] [CrossRef]
- Hendra, P.J.; Sadasivan, N. The far infra-red spectra of iodine complexes of 1:4-diselenan and 1:4-dithian. Spectrochim. Acta 1965, 21, 1127–1133. [Google Scholar] [CrossRef]
- McCullough, J.D.; Chao, Y.; Zuccaro, D.E. The structure of the compounds of iodine with 1,4 diselenane and 1,4 dithiane. Acta Crystallogr. 1959, 12, 815–816. [Google Scholar] [CrossRef]
- Freeman, F.; Le, K.T. A computational study of conformations and conformers of 1,3-dithiane (1,3-dithiacyclohexane). J. Phys. Chem. A 2003, 107, 2908–2918. [Google Scholar] [CrossRef]
- Freeman, F.; Derek, E. A computational study of conformational interconversions in 1,4-dithiacyclohexane (1,4-dithiane). J. Comput. Chem. 2003, 24, 909–919. [Google Scholar] [CrossRef]
- Notario, R.; Roux, M.V.; Cuevas, G.; Cárdenas, J.; Leyva, V.; Juaristi, E. Computational study of 1,3-dithiane 1,1-dioxide (1,3-dithiane sulfone). Description of the inversion process and manifestation of stereoelectronic effects on 1JC-H coupling constants. J. Phys. Chem. A 2006, 110, 7703–7712. [Google Scholar] [CrossRef]
- Murray, J.S.; Lane, P.; Politzer, P. Expansion of the σ-hole concept. J. Mol. Model. 2009, 15, 723–729. [Google Scholar] [CrossRef]
- Politzer, P.; Murray, J.S.; Clark, T. Halogen bonding and other σ-hole interactions: A perspective. Phys. Chem. Chem. Phys. 2013, 15, 11178–11189. [Google Scholar] [CrossRef]
- Politzer, P.; Murray, J.S. σ-Hole interactions: Perspectives and misconceptions. Crystals 2017, 7, 212. [Google Scholar] [CrossRef] [Green Version]
- Mccullough, J.D. Knobler, C.; Baker, C.; Hope, H. The Crystal and Molecular Structure of the Iodine Monobromide Complex of 1,4-Dithiane, C4H8S2-2IBr. Inorg. Chem. 1971, 10, 697–700. [Google Scholar] [CrossRef]
- Krivenchuk, V.E. Oximes of dialkyl- and alkyleneacetonylsulfonium bromides. Khimiko-Farmatsevticheskii Zhurnal 1970, 4, 18–22. [Google Scholar]
- Bell, E.V.; Bennet, G.M. s-trans-Isomerism of Disulphoxides. J. Chem. Soc. 1927, 1798–1803. [Google Scholar] [CrossRef]
- Takemura, A.; McAllister, L.J.; Karadakov, P.B.; Pridmore, N.E.; Whitwood, A.C.; Bruce, D.W. Competition and cooperation: Hydrogen and halogen bonding in co-crystals involving 4-iodotetrafluorobenzoic acid, 4-iodotetrafluorophenol and 4-bromotetrafluorophenol. CrystEngComm 2014, 16, 4254–4264. [Google Scholar] [CrossRef]
- Shearer, H.M.M. The Structure of the a-Modification of 1:4-Dithiane-1:4-Dioxide. J. Chem. Soc. 1959, 1394–1397. [Google Scholar] [CrossRef]
- APEX3; Bruker AXS: Madison, WI, USA, 2015.
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Bourhis, L.J.; Dolomanov, O.V.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. The anatomy of a comprehensive constrained, restrained refinement program for the modern computing environment—Olex2 dissected. Acta Crystallogr. Sect. A 2015, 71, 59–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09 B.01; Gaussian Inc.: Wallingford, CT, USA, 2010. [Google Scholar]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other function. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar] [CrossRef] [Green Version]
- Woon, D.E.; Dunning, T.H. Gaussian basis sets for use in correlated molecular calculations. V. Core-valence basis sets for boron through neon. J. Chem. Phys. 1995, 103, 4572–4585. [Google Scholar] [CrossRef] [Green Version]
- Dunning, T.H. Gaussian basis sets for use in correlated molecular calculations. V. Core-valence basis sets for neon through hydrogen. J. Chem. Phys. 1989, 90, 1007–1023. [Google Scholar] [CrossRef]
- Wadt, W.R.; Hay, P.J. Ab initio effective core potentials for molecular calculations. Potentials for main group elements Na to Bi. J. Chem. Phys. 1985, 82, 284–298. [Google Scholar] [CrossRef]
| Crystal | dS-I | dI-I | θS-I-I | θring-I-Ia |
|---|---|---|---|---|
| 1 | 2.7124(4) | 2.85193(18) | 179.438(9) | 52.29(3) |
| 2 | 2.7465(6) | 2.8364(2) | 176.579(12) | 85.45(4) |
| 3 | 2.8135(9) | 2.8072(4) | 177.140(18) | 40.70(6) |
| 2.8329(9) | 2.7874(4) | 176.229(18) | 49.07(7) | |
| 4 | 3.0813(7) | 2.7827(4) | 179.074(12) | 38.25(5) |
| 5 | 2.7891(6) | 2.8117(3) | 178.576(11) | 42.78(5) |
| 6 | 2.8036(7) | 2.8031(3) | 177.426(16) | 47.40(5) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peloquin, A.J.; Alapati, S.; McMillen, C.D.; Hanks, T.W.; Pennington, W.T. Polymorphism, Halogen Bonding, and Chalcogen Bonding in the Diiodine Adducts of 1,3- and 1,4-Dithiane. Molecules 2021, 26, 4985. https://doi.org/10.3390/molecules26164985
Peloquin AJ, Alapati S, McMillen CD, Hanks TW, Pennington WT. Polymorphism, Halogen Bonding, and Chalcogen Bonding in the Diiodine Adducts of 1,3- and 1,4-Dithiane. Molecules. 2021; 26(16):4985. https://doi.org/10.3390/molecules26164985
Chicago/Turabian StylePeloquin, Andrew J., Srikar Alapati, Colin D. McMillen, Timothy W. Hanks, and William T. Pennington. 2021. "Polymorphism, Halogen Bonding, and Chalcogen Bonding in the Diiodine Adducts of 1,3- and 1,4-Dithiane" Molecules 26, no. 16: 4985. https://doi.org/10.3390/molecules26164985
APA StylePeloquin, A. J., Alapati, S., McMillen, C. D., Hanks, T. W., & Pennington, W. T. (2021). Polymorphism, Halogen Bonding, and Chalcogen Bonding in the Diiodine Adducts of 1,3- and 1,4-Dithiane. Molecules, 26(16), 4985. https://doi.org/10.3390/molecules26164985






