Abstract
A simple flow injection FlameAAS for lead determination with an alginate-polyurethane composite (ALG-PUC) monolithic in-valve column has been developed. The ALG-PUC monolithic rod was prepared by mixing methylene diphenyl diisocyanate with polyol and sodium alginate with the ratio of 2:1:1 by weight for a 5 min polymerization reaction. It was then put into a column (0.8 cm i.d × 11 cm length) situated in a switching valve for the FI set up. A single standard calibration could be obtained by plotting the loaded µg Pb2+ vs. FI response (absorbances). The loaded µg Pb2+ is calculated: μg Pb2+ = FRload × LT × CPb2+, where the FR load is the flow rate of the loading analyte solution (mL min−1), LT is the loading time (min), and CPb2+ is the Pb2+ concentration (µg mL−1). A linear calibration equation was obtained: FI response (absorbances) = 0.0018 [µg Pb2+] + 0.0032, R2 = 0.9927 for 1–150 µg Pb2+, and RSD of less than 20% was also obtained. Application of the developed procedure has been demonstrated in real samples.
1. Introduction
Lead is a highly toxic element that can be found in the environment. For chronic exposure, it can harm and affect humas health, for example, it can cause nephritis of the kidney, brain damage, and central nervous system disorders [1]. Atomic absorption spectrometry (AAS) is among one of the various methods for the determination of lead due to its availability in laboratories and high selectivity in the detection of Pb2+ [2,3,4]. Other techniques employed as standard methods include, electrothermal atomic absorption, inductively coupled plasma, inductively coupled plasma/mass spectrometry, anodic stripping voltammetry, and colorimetric employing dithizone [5]. Adsorptive stripping voltammetry (AdSV) provides good sensitivity (ng mL−1 or lower [6,7,8]) and could be used for multi-elements analysis [9,10,11]. ICP-MS or ICP-AES also provides similar information [12,13,14,15]. The cost of operation in addition to the cost of the instrument itself would be relatively high, especially in less developed places. A well-trained person is needed for its operation. A flame atomic absorption spectrometer (FlameAAS) is commonly available in most laboratories. There have been a number of works applying the flow techniques to FlameAAS to enhance performance [16,17,18,19,20,21]. In our research group, we have employed it with polyurethane foam (PUF) for sample pretreatment because of its various advantages [22,23]. The use of PUF with flow injection analysis (FIA) has been reported as modified-PUF powder for ions adsorption in online systems [20,21,24,25,26], but some disadvantages such as high pressure when the system was used over a long period of time and inconvenience when packing the material into the column were encountered. For the modification of PUF with alginate, it has shown selectivity toward Pb2+, durability, and reusability [27]. Advantages obtained using a monolithic PUF rod have been observed in previous work on SDS assay [28]. Instead of using the previously reported alginate-polyurethane composite (ALG-PUC) in powder form as a batch procedure for removal of lead, the grafting of alginate and PUF was synthesized as a modified ALG-PUC monolithic column situated in-valve during FI set up. It is expected that a long system running time with low back pressure was beneficial due to the high porosity of ALG-PUC, and it was easy to fabricate. From our previous experiences, using an in-valve column for FI setup made the determination of Pb2+ while employing single standard calibration possible, even without using a monolith column [29]. This work aimed to prove that the use of a monolithic ALG-PUC in-valve column with single standard calibration approach can enhance the performance of FlameAAS in lead determination, although with limitations the in availability of the instrument components.
2. Results and Discussion
2.1. Synthesis of Polyurethane Foam (PUF) and Alginate-Polyurethane Composite (ALG-PUC) and Their Characteristics
The chemical structure of PUF consists of diisocyanate groups from MDI and hydroxyl groups from polyol [-MDI-Polyol-MDI-Polyol-]n, while the components of ALG-PUC are the diisocyanate groups from the MDI and hydroxyl groups from polyol and alginate [-MDI-Polyol-MDI-ALG-]n (Figure 1). The characterization of PUF and ALG-PUC was investigated using IR spectra (see Figure S1) and SEM images (see Figure S2). Alginate might have a role as a hydroxyl group and may bond with diisocyanate groups. In contrast ALG-PUC is whiter and has a higher porosity than PUF (Figure S3). Alginate appears on the binding side of the carboxylic group (-COO−) for the sorption of Pb2+ as an ion-association, which is highly selective with Pb2+ ions when the pH is higher than 3.96 [27]. The ratio of the carboxylic group and Pb2+ ions was 2:1 [27]. Monolithic ALG-PUC could only be used for at least 75 cycles, as the absorbance would decrease significantly after that.
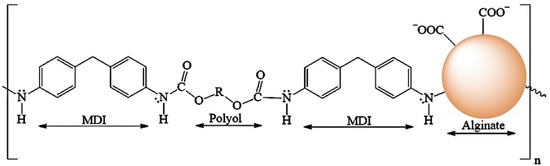
Figure 1.
The estimated ALG-PUC structure.
2.2. Study of Elution Profile of Monolithic ALG-PUC Packed In-Valve-FI
The elution of a monolithic ALG-PUC column for Pb2+ was studied. An in-valve column (0.8 cm i.d × 11 cm length) packed with monolithic ALG-PUC was situated in a switching valve of the FI set up (see in the Section 3.1). A 10 µg mL−1 Pb2+ solution was passed through the column for 3 min. The residual and unsorbed Pb2+ was cleaned with water. The column was eluted with 2 mol L−1 nitric acid. The experiments for a 5 min loading time were also performed. The obtained elution profiles are illustrated in Figure 2. It can be seen that an eluent (2 mol L−1 nitric acid) volume of 4 mL (flow rate 5 mL min−1, with less than 1 min) could practically quantitatively elute the loaded Pb2+. The peak maxima were observed in the same position. This indicated that the peak height could be used for the FI-response corresponding to absorbance due to the sorbed amount of lead.
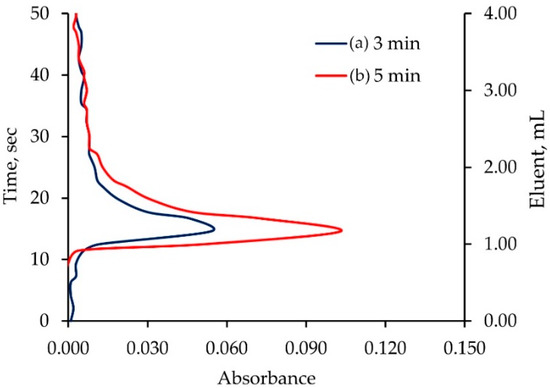
Figure 2.
Elution profiles of loaded Pb2+ on the monolithic ALG-PUC in-valve column situated in FI set up: (a) 3 min and (b) 5 min loading time.
2.3. Effect of Nitric Acid Concentration
A 60 µg of Pb2+ was loaded into the monolithic ALG-PUC column. Elution using different concentrations (0.1–2.5 mol L−1) of nitric acid was studied. The results are illustrated in Figure 3. A concentration of 2 mol L−1 or above yielded the maximum elution of Pb2+. This could be because the H+ from nitric acid would replace Pb2+ at the binding site in the connection with carboxyl group (-COO−) of alginate [27].
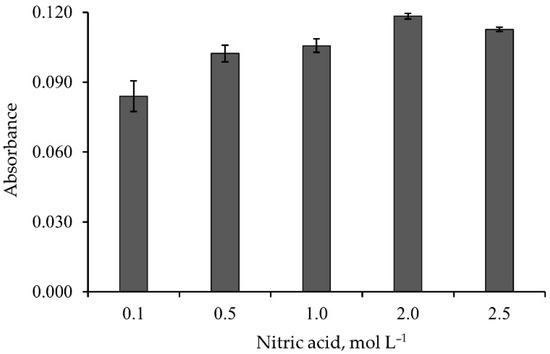
Figure 3.
Effect of nitric acid concentration on elution of the loaded Pb2+.
2.4. Single Standard Calibration
A series of Pb2+ solutions (2.0–10.0 µg mL−1) were passed into the column with various loading times (2–15 min) and under the same loading flow rate (1 mL min−1) and elution condition (2 mol L−1 nitric acid, 5 mL min−1). Linear calibration graphs based on each loading time were obtained (plot of absorbance vs. Pb2+ concentration), as displayed in Figure 4. It can be seen that the longer the loading time, the higher the calibration slope.
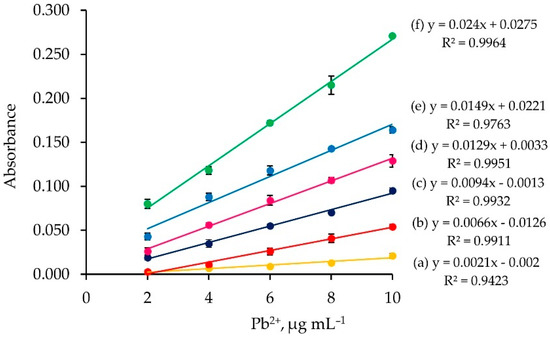
Figure 4.
Calibration graphs obtained for different loading times: (a) 2 min; (b) 3 min; (c) 5 min; (d) 7 min; (e) 10 min; and (f) 15 min.
One can calculate the microgram of loaded Pb2+ by µg Pb2+ = FRload × LT × CPb2+, where FRload is the flow rate of loading analyte solution (mL min−1), LT is the loading time (min), and CPb2+ is the Pb2+ concentration (µg mL−1). It can be observed from Figure 4 that the same amount of loaded Pb2+ exhibits the same absorbance values.
A single calibration approach was investigated. Solutions of different Pb2+ concentrations (0.1–10.0 µg mL−1) were percolated into the monolithic ALG-PUC packed in-valve of the FI set up for different loading times (2–15 min) with a loading flow rate of 1 mL min−1 and with the same elution condition as before. The results are presented in Table 1 and Figure 5. As expected, the same microgram of Pb2+ (from the calculation described previously) provided practically the same FI response (absorbances), for example, for the condition of F, 20 µg Pb2+ obtained from either using Pb2+ 2 or 4 µg mL−1 with a loading time of 5 or 10 min, respectively, provided FI responses (absorbances) of 0.035 ± 0.004 and 0.043 ± 0.004. Similarly, for the conditions M, 60 µg Pb2+ resulted from using either 4 or 6 µg mL−1 Pb2+ with a 10 or 15 min loading time produced 0.118 ± 0.005 and 0.118 ± 0.004 FI responses, respectively. A single standard calibration can be then obtained by plotting the calculated loaded µg Pb2+ vs. FI response (absorbances), as illustrated in Figure 6, with linear calibration equation being FI response (absorbances) = 0.0018 [µg Pb2+] + 0.0032, R2 = 0.993 for 1–150 µg Pb2+. The relative standard deviation was less than 20%. If a loading higher than 150 µg Pb2+ was applied, the saturation of the ALG-PUC binding site was situated. With limitations of the instrumentation that was available to our lab group explained above, we needed to set up a system using only one single injection valve and a peristatic pump. With that setup, which was described earlier in the manuscript, the same calculated µg Pb2+ (from flow rate, loading time, and concentration) was loaded onto the column and provided the same FI response, leading to the validity of the single standard calibration. With the setup (only very basic components were available for our work), to result in the applicability of the single standard calibration approach, the loading time needed to be 5 min or longer (if a time shorter than 5 min, there would be an error due to switching the valve for flow lines, see Table S1), while the flow rate was fixed at 1 mL min−1. Considering for loading time of 5 min for 1 µg Pb2+ (calculated), which was the last point of the calibration, a 1 mL min−1 flow rate, would result in 0.2 µg mL−1 Pb2+ of loading solution. This would reflect the limit of quantitation. The sensitivity obtained by the setup could be improved if the system could be composed of better-quality components: a peristatic pump and a three way valve with automation control. The concept should further be developed for automation using a sequential injection system. It should be noted that using the single standard approach for a given set of conditions (fixed flow rate), if loading a sample solution with a given loading time produced a FI response lower than the lowest point of the linear calibration, the sample solution could be reloaded with more appropriate time to produce an FI response within the linear range. Similarly, if loading a sample solution resulted in a higher FI response, a shorter reloading time would provide a FI response within the linear range.

Table 1.
Correlation involving the use of single standard calibration.
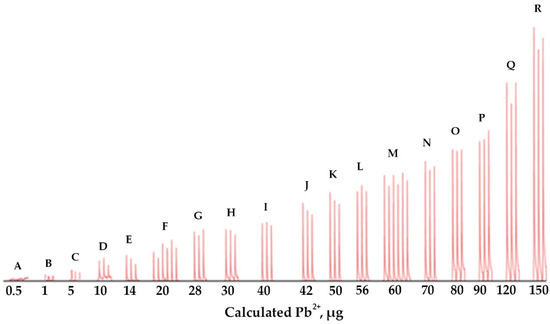
Figure 5.
FI responses due to various amounts of Pb2+. (See Table 1).
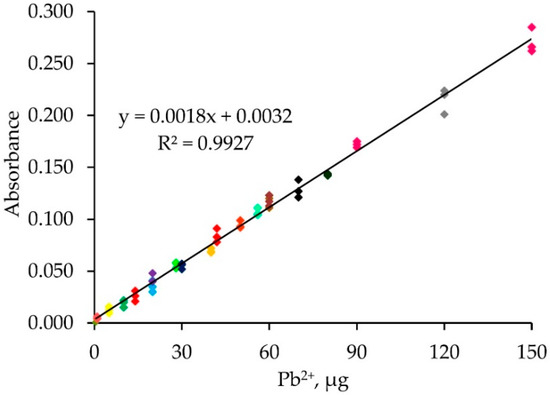
Figure 6.
The single standard calibration graph.
2.5. Lead Assay in Samples of Aqueous Sulfuric Acid Solutions Contained in Acid Batteries
The developed method was applied to determine the amount of Pb2+ in samples of aqueous sulfuric acid solutions contained in acid batteries. The results of the recovery studies are represented in Table 2.

Table 2.
Lead content in samples of aqueous sulfuric acid solutions contained in acid batteries.
3. Materials and Methods
3.1. Apparatus
In this work, an atomic absorption spectrometer (PerkinElmer, AAnalyst 800, Waltham, MA, USA) was previously used. Unfortunately, due to the accidental failure of this FlameAAS, the change to the new one was inevitable. For this reason, an atomic absorption spectrometer (Hitachi, ZA 3300, Tokyo, Japan) was selected and tested for the experimental conditions before use. The instrument provided similar results. A Fourier-transform infrared spectrometer (Thermo Scientific, Nicolet 6700, Waltham, MA, USA) and scanning electron microscope, SEM, (JEOL, JSM-6610 LV, Tokyo, Japan) were to characterize the synthesized monolithic ALG-PUC column. Six-port valves (Rheodyne, 7125, Berkeley, CL, USA), a peristaltic pump (Cole-Parmer, Masterflex L/S, Vernon Hills, IL, USA) for Pump 1 and a peristaltic pump (Cole-Parmer, Masterflex L/X, Vernon Hills, IL, USA) for Pump 2 were included. A digital pH meter (METTLER TOLEDO, Greifensee, Switzerland) was for pH adjustment.
Due to the use of a single six-port valve, the FI setup with an in-valve column for the determination of lead using FlameAAS was designed as shown in Figure 7. Water used as a carrier was passed through Pump 2 into the column at a flow of 5 mL min−1, it then passed through the FlameAAS, while a standard/sample solution was passed through Pump 1 into the six-port valve, finishing as waste (period d in Figure S4). Next, the Pb2+ standard or sample solution was delivered with a 1 mL min−1 flow rate to the in-valve monolithic ALG-PUC column via Pump 1 with a certain loading time (period a in Figure S4). Pump 2 would deliver water to wash the residual and unsorbed Pb2+ with a flow rate of 5 mL min−1 for 1 min (period b in Figure S4). At that stage, the valve was in the injection position. Water from Pump 1 passed the six-port valve to waste. The valve was then changed into the loading position (period x1 in Figure S4). The eluent from Pump 2 passed into the six-port valve. After that, the valve was changed to its injection position (period c in Figure S4), the sorbed Pb2+ was eluted from the column using 2 mol L−1 nitric acid via Pump 2 with a 5 mL min−1 flow rate for 1 min and absorbance was detected by the FlameAAS. The valve was changed to loading position. The eluent line was switched to water (period x2 in Figure S4). The washing step was applied (period d was repeated).
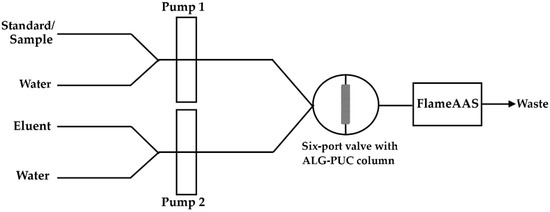
Figure 7.
FI-FlameAAS system.
3.2. Reagents and Materials
All of the reagents used in this work were of analytical grade. Deionized water made of the volume of the solutions. Working solutions of Pb2+ were prepared from 1000 µg mL−1 Pb2+ stock solution (Loba chemie, Mumbai, India).
3.3. Preparation of Monolithic ALG-PUC Rod
The mixing of methylene diphenyl diisocyanate (MDI; IRPC, Rayong, Thailand) with polyol (polyether; IRPC, Rayong, Thailand) and sodium alginate in the ratio of 2:1:1 by weight and polymerization was completed within 5 min to obtain a bulk product in a 500 mL beaker. A monolithic ALG-PUC rod was obtained by pressing a plastic rod (0.8 cm i.d × 11 cm length) into the bulk product. The obtained monolithic rod was about 0.1 g in weight (see Figure S3) and was put into an acrylic rod (0.8 cm i.d × 11 cm length) that had ferules at the two ends with tubing connecting it into the flow system.
3.4. Sample Preparation
Each of the aqueous sulfuric acid solutions contained in acid batteries was filtrated and pipetted 10.00 mL. After that, the adjustment to pH 4 by 10 M NaOH was applied. Finally, deionized water was added to the treated sample to add volume up to 50.00 mL.
4. Conclusions
The grafting of alginate and PUF was synthesized as a modified alginate-polyurethane composite (ALG-PUC) monolithic column situated in-valve of an FI set up with FlameAAS. With the setup (only very basic components of which were available for our work), applicability of the single standard calibration approach was possible. Although the sensitivity obtained from the system was not comparable with some other relatively expensive instruments, it demonstrated some benefits compared to the use of conventional FlameAAS alone, notably, that FlameAAS is commonly available in laboratories.
Supplementary Materials
The following are available online, Figure S1: IR spectra of sodium alginate (purple), PUF (blue) and ALG-PUC (green), Figure S2: SEM images with two different magnifications: (a1) PUF, 1000x; (a2) PUF, 5000x; (b1) ALG-PUC, 1000x; and (b2) ALG-PUC, 5000x (each sample was cut into a small piece, attached to carbon tape and received a sputtering coat with gold), Figure S3: Photos of: (a) PUF; (b) ALG-PUC; and (c) monolithic ALG-PUC rod, Figure S4: Full diagram of the work sequence, with each sample having a work loop of: a = loading time (varying as desire); b = washing time (1 min); c = eluting time (1 min); d = cleaning time (3 min); x1 and x2 = waiting time (0.5 min each), Table S1: Experimental results with loading time lower than 5 min.
Author Contributions
Conceptualization, M.V. and K.G.; experimental work, P.I.N.A.; data analysis and data evaluation; M.V., K.G., P.I.N.A., C.Y., and K.K. (Kullapon Kesonkan); writing—original draft preparation; P.I.N.A., M.V., and K.G.; writing—review and editing, M.V., K.G., P.I.N.A., K.K. (Kanokwan Kiwfo), C.Y., and K.K. (Kullapon Kesonkan); supervision, M.V., K.G., N.T., and H.M.; funding acquisition, M.V. and K.G. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Science Achievement Scholarship of Thailand (SAST), the TRF Distinguished Research Professor Award Grant (DPG6080002 to Kate Grudpan) and Center of Excellence for Innovation in Analytical Science and Technology, Chiang Mai University.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All the data are reported in this manuscript and Supplementary Materials.
Acknowledgments
The Department of Chemistry, Faculty of Science, King Mongkut’s University of Technology Thonburi, the Science Achievement Scholarship of Thailand (SAST), the Petchra Pra Jom Klao Research Scholarship from King Mongkut’s University of Technology Thonburi and the TRF Distinguished Research Professor Award Grant (DPG6080002) as well as Chiang Mai University are acknowledged. Siripat Suteerapataranon is thanked for the useful discussions.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Blanchet-Chouinard, G.; Larivière, D. Determination of Pb in environmental samples after cloud point extraction using crown ether. Talanta 2018, 179, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Soylak, M.; Narin, I.; Bezerra, M.d.A.; Ferreira, S.L.C. Factorial design in the optimization of preconcentration procedure for lead determination by FAAS. Talanta 2005, 65, 895–899. [Google Scholar] [CrossRef] [PubMed]
- Adamczyk-Szabela, D.; Lisowska, K.; Romanowska-Duda, Z.; Wolf, W.M. Associated effects of cadmium and copper alter the heavy metals uptake by melissa officinalis. Molecules 2019, 24, 2458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Habila, M.A.; AlMasoud, N.; Alomar, T.S.; AlOthman, Z.A.; Yilmaz, E.; Soylak, M. Deep eutectic solvent-based microextraction of lead(II) traces from water and aqueous extracts before FAAS measurements. Molecules 2020, 25, 4794. [Google Scholar] [CrossRef]
- Gottler, R.A. Part 3000 metals. In Standard Methods for the Examination of Water and Wastewater, 23rd ed.; American Public Health Association: Washington, DC, USA, 2017; pp. 3-15–3-83. [Google Scholar]
- Zayats, G.D.; Meryan, V.T.; Revenco, M.D.; Chiugureanu, D.G. Determination of the lead by adsorptive stripping voltammetry in the presence of 2,2′-dipyridyl-2,4-dioxybenzoic acid molecular complex. Anal. Lett. 2002, 35, 577–584. [Google Scholar] [CrossRef]
- Arancibia, V.; Nagles, E.; Cornejo, S. Determination of lead in the presence of morin-5′-sulfonic acid and sodium dodecyl sulfate by adsorptive stripping voltammetry. Talanta 2009, 80, 184–188. [Google Scholar] [CrossRef]
- Baś, B.; Jakubowska, M. The renovated silver ring electrode in determination of lead traces by differential pulse anodic stripping voltammetry. Anal. Chim. Acta 2008, 615, 39–46. [Google Scholar] [CrossRef]
- Pardi, H.; Deswati, D.; Suyani, H.; Edelwis, T.W. Simultaneous determination of Cu, Pb, Cd, and Zn in tap water samples in the presence of alizarin: Differential pulse adsorptive stripping voltammetry. Anal. Bioanal. Electrochem. 2017, 9, 969–981. [Google Scholar]
- Deswati, D.; Pardi, H.; Suyani, H.; Rahmi, I. Adsorptive stripping voltammetry for the simultaneous determination of Cd, Cu, Cr, and Pb in water samples using fluorexon: An optimization single factor. Anal. Bioanal. Electrochem. 2018, 10, 1491–1505. [Google Scholar]
- Martos, D.L.M.; Jost, C.L. Sequential determination of five heavy metal ions in Brazilian phosphate fertilizers and surface waters by stripping voltammetry. Int. J. Environ. Sci. Technol. 2019, 16, 6535–6546. [Google Scholar] [CrossRef]
- Wuilloud, R.G.; Acevedo, H.A.; Vazquez, F.A.; Martinez, L.D. Determination of lead in drinking water by ICP-AES with ultrasonic nebulization and flow-injection on-line preconcentration using an amberlite XAD-16 resin. Anal. Lett. 2002, 35, 1649–1665. [Google Scholar] [CrossRef]
- Zougagh, M.; de Torres, A.G.; Alonso, E.V.; Pavón, J.M.C. Automatic on line preconcentration and determination of lead in water by ICP-AES using a TS-microcolumn. Talanta 2004, 62, 503–510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreira, S.L.C.; Ferreira, H.S.; Matos, G.D.; Anunciacao, D.S.; dos Santos, W.N.L.; Campos, V.P.; de Andrade, J.B.; Welz, B.; Araujo, R.G.O.; Curtius, A.J.; et al. Critical evaluation of analytical procedures for the determination of lead in seawater. Appl. Spectrosc. Rev. 2012, 47, 633–653. [Google Scholar] [CrossRef]
- Rahmi, D.; Zhu, Y.; Fujimori, E.; Umemura, T.; Haraguchi, H. Multielement determination of trace metals in seawater by ICP-MS with aid of down-sized chelating resin-packed minicolumn for preconcentration. Talanta 2007, 72, 600–606. [Google Scholar] [CrossRef] [PubMed]
- Sooksamiti, P.; Geckeis, H.; Grudpan, K. Determination of lead in soil samples by in-valve solid-phase extraction–flow injection flame atomic absorption spectrometry. Analyst 1996, 121, 1413–1417. [Google Scholar] [CrossRef]
- Fang, Z.; Xu, S.; Bai, X. A new flow injection single-standard calibration method for flame atomic absorption spectrometry based on dilution by microsample dispersion. Anal. Chim. Acta 1996, 326, 49–55. [Google Scholar] [CrossRef]
- Praveen, R.S.; Naidu, G.R.K.; Prasada Rao, T. Dithiocarbamate functionalized or surface sorbed Merrifield resin beads as column materials for on line flow injection–flame atomic absorption spectrometry determination of lead. Anal. Chim. Acta 2007, 600, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Elçi, L.; Arslan, Z.; Tyson, J.F. Determination of lead in wine and rum samples by flow injection-hydride generation-atomic absorption spectrometry. J. Hazard. Mater. 2009, 162, 880–885. [Google Scholar] [CrossRef] [Green Version]
- Lemos, V.A.; Ferreira, S.L.C. On-line preconcentration system for lead determination in seafood samples by flame atomic absorption spectrometry using polyurethane foam loaded with 2-(2-Benzothiazolylazo)-2-P-Cresol. Anal. Chim. Acta 2001, 441, 281–289. [Google Scholar] [CrossRef]
- Dos Santos, W.L.; dos Santos, C.M.M.; Costa, J.L.O.; Andrade, H.M.C.; Ferreira, S.L.C. Multivariate optimization and validation studies in on-line pre-concentration system for lead determination in drinking water and saline waste from oil refinery. Microchem. J. 2004, 77, 123–129. [Google Scholar] [CrossRef]
- Vongboot, M.; Suesoonthon, M. Removal of copper and iron by polyurethane foam column in FIA system for the determination of nickel in pierced ring. Talanta 2015, 131, 325–329. [Google Scholar] [CrossRef] [PubMed]
- Yeerum, C.; Issarangkura Na Ayutthaya, P.; Kesonkan, K.; Chaiyakhan, A.; Vongboot, M. Down-scaling sample preparation using polyurethane foam and colorimetric technique for the chromium assay in accessories. Anal. Sci. 2020, 36, 1137–1140. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Azeem, S.M.; Bader, N.R.; Kuss, H.M.; El-Shahat, M.F. Determination of total iron in food samples after flow injection preconcentration on polyurethane foam functionalized with N,N-bis(salicylidene)-1,3-propanediamine. Food Chem. 2013, 138, 1641–1647. [Google Scholar] [CrossRef] [PubMed]
- De Sousa, J.M.; Couto, M.T.; Cassella, R.J. Polyurethane foam functionalized with phenylfluorone for online preconcentration and determination of copper and cadmium in water samples by flame atomic absorption spectrometry. Microchem. J. 2018, 138, 92–97. [Google Scholar] [CrossRef]
- Abdel Azeem, S.M.; Hanafi, H.A.; El-Nadi, Y.; El-Shahat, M.F. Separation of nickel and cadmium from aqueous solutions by flow injection preconcentration onto cadion functionalized polyurethane foam. Microchem. J. 2021, 166, 106192. [Google Scholar] [CrossRef]
- Sone, H.; Fugetsu, B.; Tanaka, S. Selective elimination of lead(II) ions by alginate/polyurethane composite foams. J. Hazard. Mater. 2009, 162, 423–429. [Google Scholar] [CrossRef]
- Yeerum, C.; Wongwilai, W.; Grudpan, K.; Vongboot, M. Green assay of anionic surfactant via ion-association with methylene blue sorbed on polyurethane foam monolithic rod and using a smartphone. Talanta 2018, 190, 85–88. [Google Scholar] [CrossRef]
- Tanikkul, S.; Jakmunee, J.; Lapanantnoppakhun, S.; Rayanakorna, M.; Sooksamiti, P.; Synovec, R.E.; Christian, G.D.; Grudpan, K. Flow injection in-valve-mini-column pretreatment combined with ion chromatography for cadmium, lead and zinc determination. Talanta 2004, 64, 1241–1246. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).