Determination of Lead Employing Simple Flow Injection AAS with Monolithic Alginate-Polyurethane Composite Packed In-Valve Column
Abstract
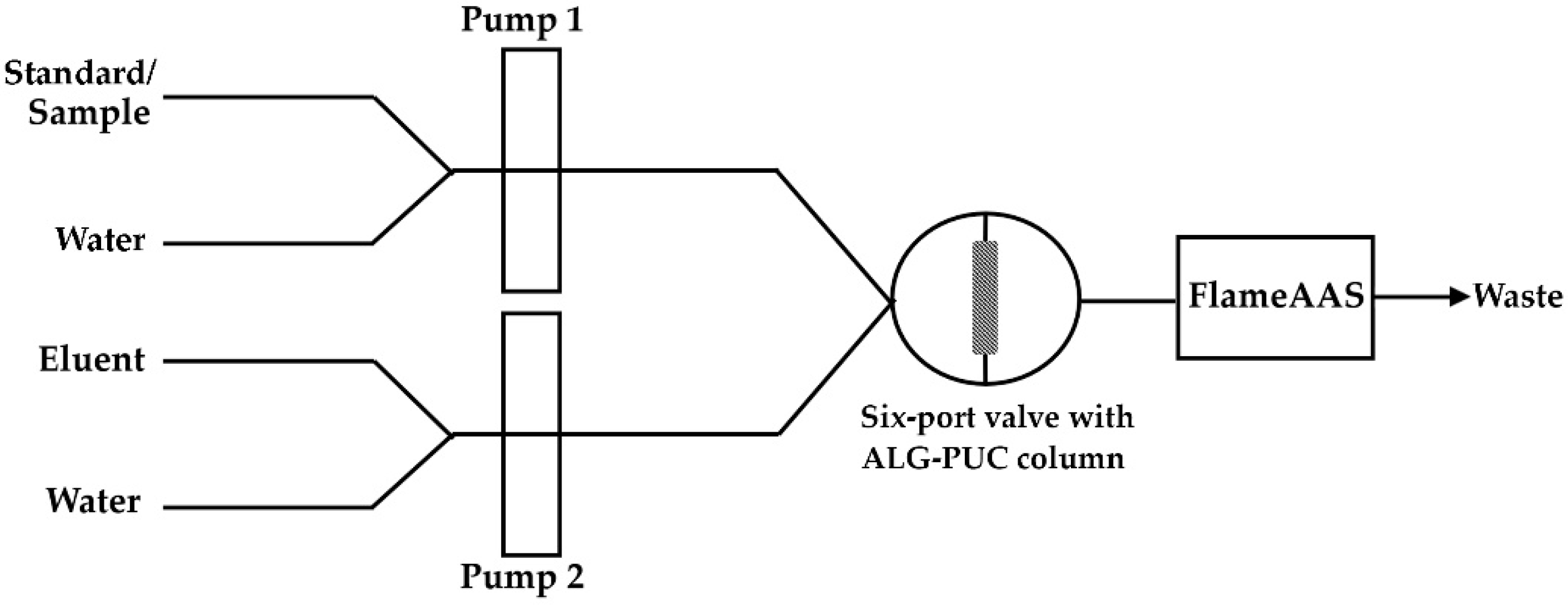
:1. Introduction
2. Results and Discussion
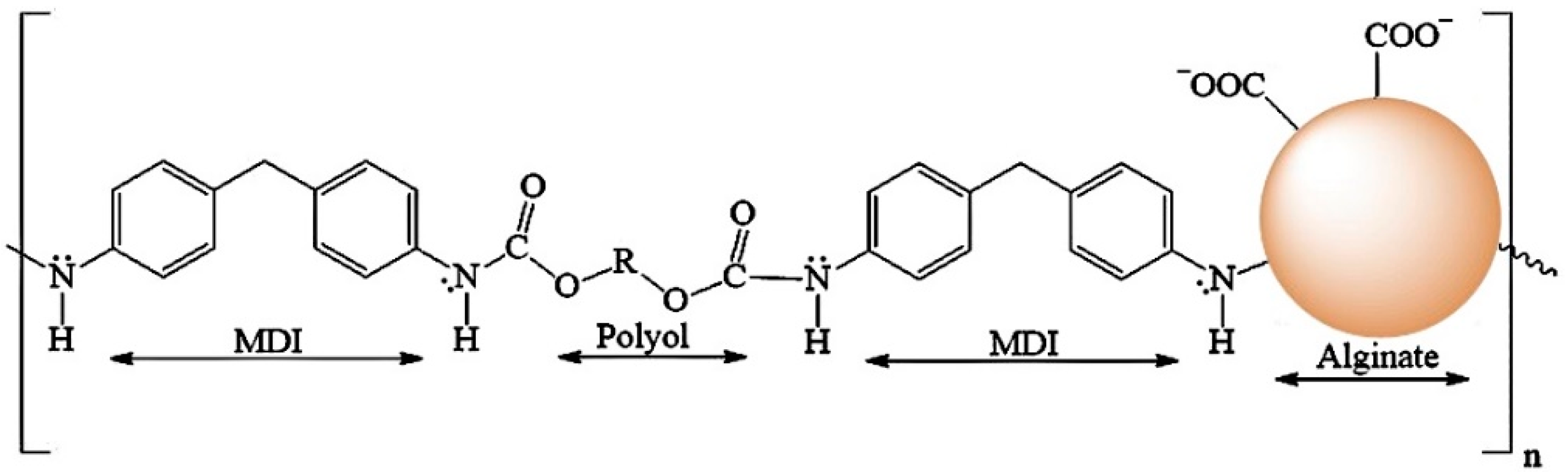
2.1. Synthesis of Polyurethane Foam (PUF) and Alginate-Polyurethane Composite (ALG-PUC) and Their Characteristics
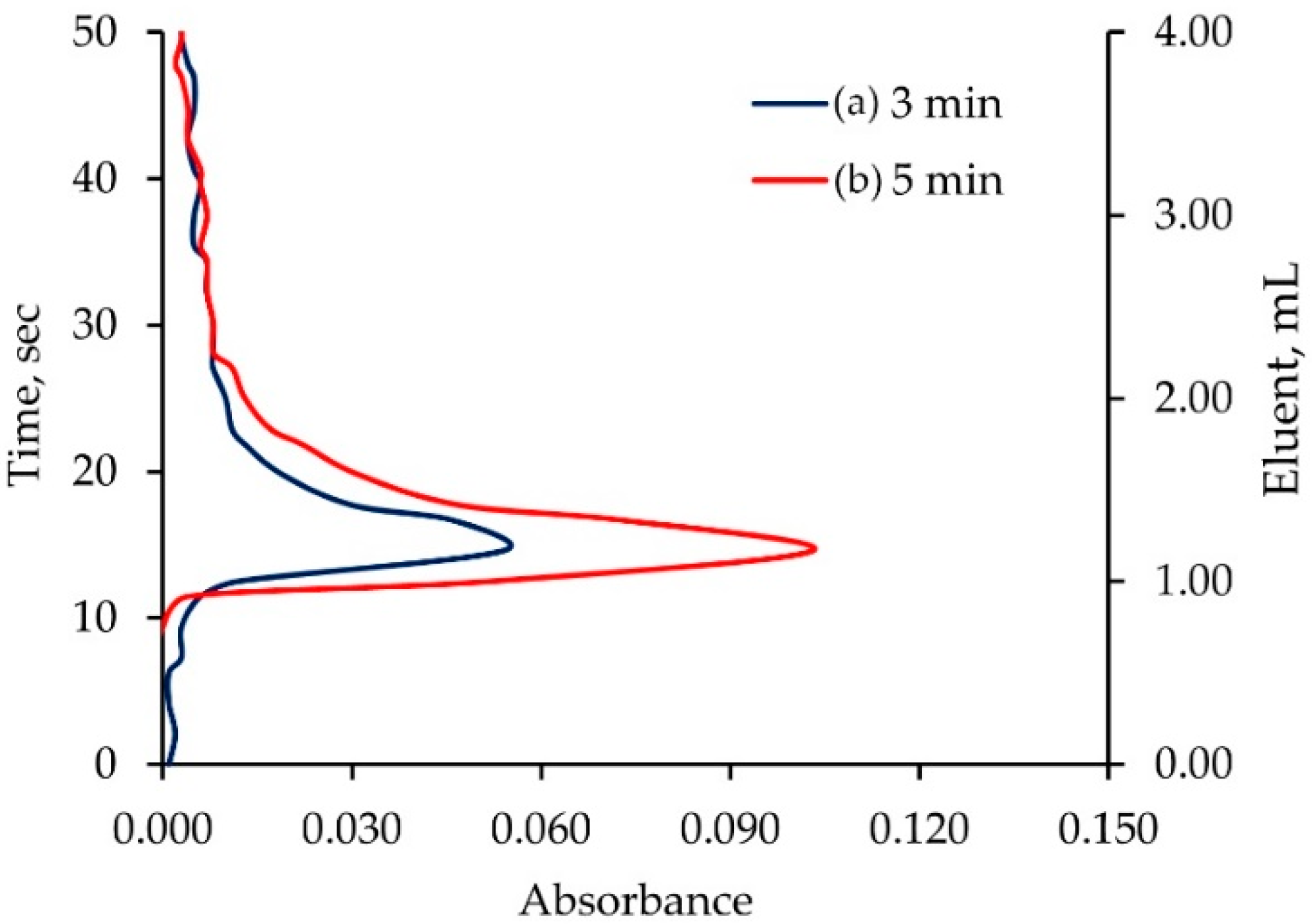
2.2. Study of Elution Profile of Monolithic ALG-PUC Packed In-Valve-FI
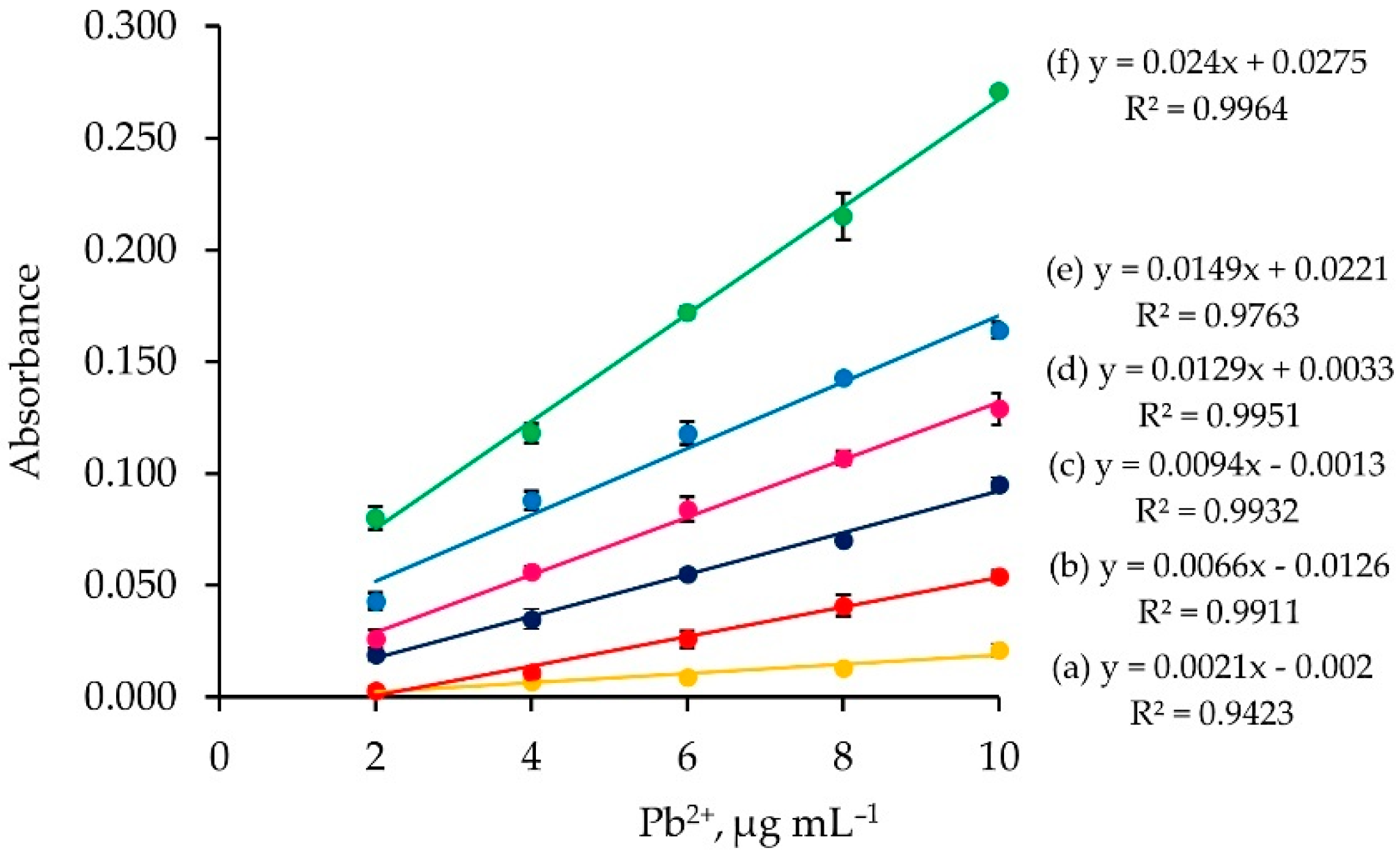
2.3. Effect of Nitric Acid Concentration
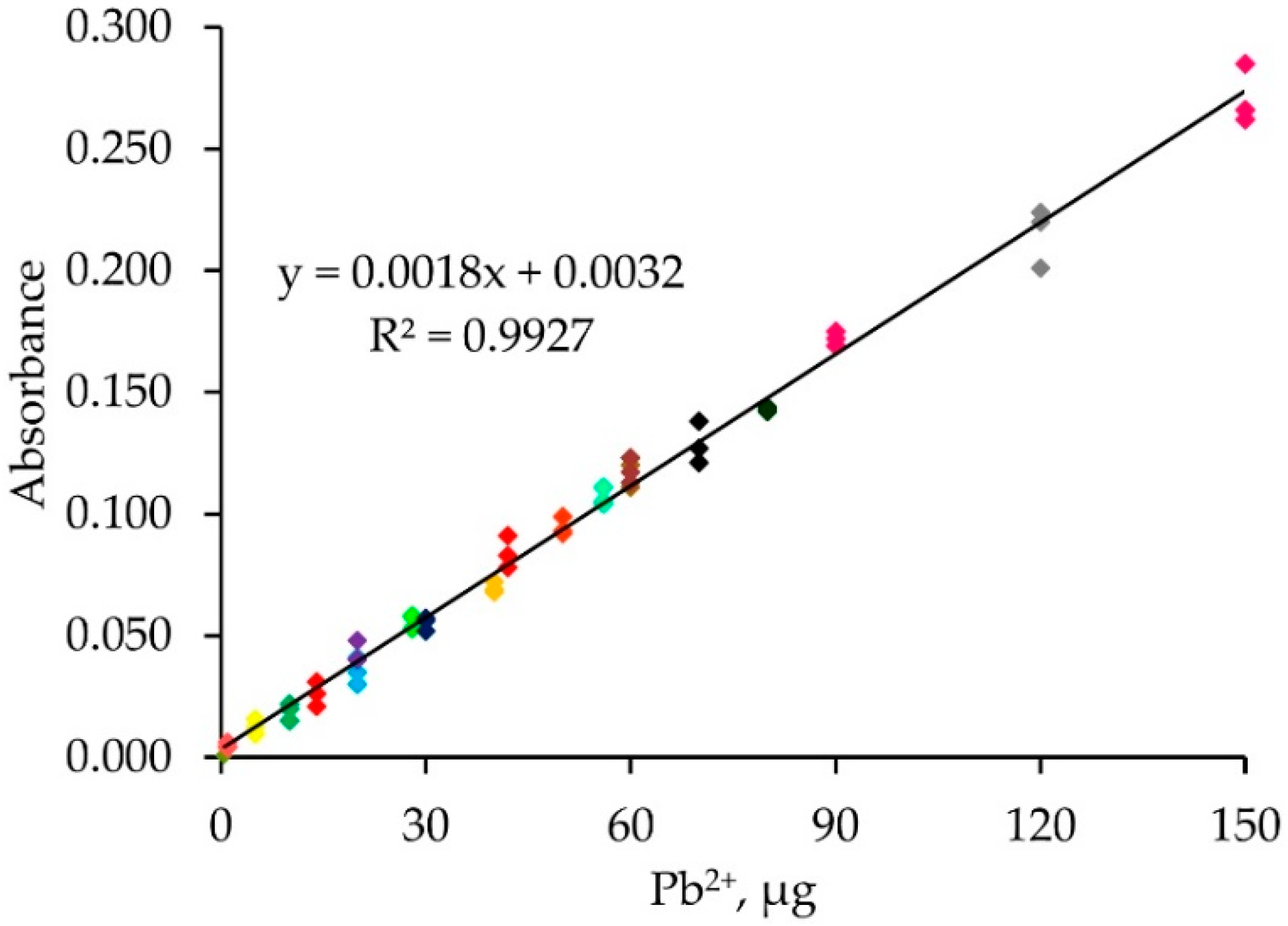
2.4. Single Standard Calibration
2.5. Lead Assay in Samples of Aqueous Sulfuric Acid Solutions Contained in Acid Batteries
3. Materials and Methods
3.1. Apparatus
3.2. Reagents and Materials
3.3. Preparation of Monolithic ALG-PUC Rod
3.4. Sample Preparation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blanchet-Chouinard, G.; Larivière, D. Determination of Pb in environmental samples after cloud point extraction using crown ether. Talanta 2018, 179, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Soylak, M.; Narin, I.; Bezerra, M.d.A.; Ferreira, S.L.C. Factorial design in the optimization of preconcentration procedure for lead determination by FAAS. Talanta 2005, 65, 895–899. [Google Scholar] [CrossRef] [PubMed]
- Adamczyk-Szabela, D.; Lisowska, K.; Romanowska-Duda, Z.; Wolf, W.M. Associated effects of cadmium and copper alter the heavy metals uptake by melissa officinalis. Molecules 2019, 24, 2458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Habila, M.A.; AlMasoud, N.; Alomar, T.S.; AlOthman, Z.A.; Yilmaz, E.; Soylak, M. Deep eutectic solvent-based microextraction of lead(II) traces from water and aqueous extracts before FAAS measurements. Molecules 2020, 25, 4794. [Google Scholar] [CrossRef]
- Gottler, R.A. Part 3000 metals. In Standard Methods for the Examination of Water and Wastewater, 23rd ed.; American Public Health Association: Washington, DC, USA, 2017; pp. 3-15–3-83. [Google Scholar]
- Zayats, G.D.; Meryan, V.T.; Revenco, M.D.; Chiugureanu, D.G. Determination of the lead by adsorptive stripping voltammetry in the presence of 2,2′-dipyridyl-2,4-dioxybenzoic acid molecular complex. Anal. Lett. 2002, 35, 577–584. [Google Scholar] [CrossRef]
- Arancibia, V.; Nagles, E.; Cornejo, S. Determination of lead in the presence of morin-5′-sulfonic acid and sodium dodecyl sulfate by adsorptive stripping voltammetry. Talanta 2009, 80, 184–188. [Google Scholar] [CrossRef]
- Baś, B.; Jakubowska, M. The renovated silver ring electrode in determination of lead traces by differential pulse anodic stripping voltammetry. Anal. Chim. Acta 2008, 615, 39–46. [Google Scholar] [CrossRef]
- Pardi, H.; Deswati, D.; Suyani, H.; Edelwis, T.W. Simultaneous determination of Cu, Pb, Cd, and Zn in tap water samples in the presence of alizarin: Differential pulse adsorptive stripping voltammetry. Anal. Bioanal. Electrochem. 2017, 9, 969–981. [Google Scholar]
- Deswati, D.; Pardi, H.; Suyani, H.; Rahmi, I. Adsorptive stripping voltammetry for the simultaneous determination of Cd, Cu, Cr, and Pb in water samples using fluorexon: An optimization single factor. Anal. Bioanal. Electrochem. 2018, 10, 1491–1505. [Google Scholar]
- Martos, D.L.M.; Jost, C.L. Sequential determination of five heavy metal ions in Brazilian phosphate fertilizers and surface waters by stripping voltammetry. Int. J. Environ. Sci. Technol. 2019, 16, 6535–6546. [Google Scholar] [CrossRef]
- Wuilloud, R.G.; Acevedo, H.A.; Vazquez, F.A.; Martinez, L.D. Determination of lead in drinking water by ICP-AES with ultrasonic nebulization and flow-injection on-line preconcentration using an amberlite XAD-16 resin. Anal. Lett. 2002, 35, 1649–1665. [Google Scholar] [CrossRef]
- Zougagh, M.; de Torres, A.G.; Alonso, E.V.; Pavón, J.M.C. Automatic on line preconcentration and determination of lead in water by ICP-AES using a TS-microcolumn. Talanta 2004, 62, 503–510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreira, S.L.C.; Ferreira, H.S.; Matos, G.D.; Anunciacao, D.S.; dos Santos, W.N.L.; Campos, V.P.; de Andrade, J.B.; Welz, B.; Araujo, R.G.O.; Curtius, A.J.; et al. Critical evaluation of analytical procedures for the determination of lead in seawater. Appl. Spectrosc. Rev. 2012, 47, 633–653. [Google Scholar] [CrossRef]
- Rahmi, D.; Zhu, Y.; Fujimori, E.; Umemura, T.; Haraguchi, H. Multielement determination of trace metals in seawater by ICP-MS with aid of down-sized chelating resin-packed minicolumn for preconcentration. Talanta 2007, 72, 600–606. [Google Scholar] [CrossRef] [PubMed]
- Sooksamiti, P.; Geckeis, H.; Grudpan, K. Determination of lead in soil samples by in-valve solid-phase extraction–flow injection flame atomic absorption spectrometry. Analyst 1996, 121, 1413–1417. [Google Scholar] [CrossRef]
- Fang, Z.; Xu, S.; Bai, X. A new flow injection single-standard calibration method for flame atomic absorption spectrometry based on dilution by microsample dispersion. Anal. Chim. Acta 1996, 326, 49–55. [Google Scholar] [CrossRef]
- Praveen, R.S.; Naidu, G.R.K.; Prasada Rao, T. Dithiocarbamate functionalized or surface sorbed Merrifield resin beads as column materials for on line flow injection–flame atomic absorption spectrometry determination of lead. Anal. Chim. Acta 2007, 600, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Elçi, L.; Arslan, Z.; Tyson, J.F. Determination of lead in wine and rum samples by flow injection-hydride generation-atomic absorption spectrometry. J. Hazard. Mater. 2009, 162, 880–885. [Google Scholar] [CrossRef] [Green Version]
- Lemos, V.A.; Ferreira, S.L.C. On-line preconcentration system for lead determination in seafood samples by flame atomic absorption spectrometry using polyurethane foam loaded with 2-(2-Benzothiazolylazo)-2-P-Cresol. Anal. Chim. Acta 2001, 441, 281–289. [Google Scholar] [CrossRef]
- Dos Santos, W.L.; dos Santos, C.M.M.; Costa, J.L.O.; Andrade, H.M.C.; Ferreira, S.L.C. Multivariate optimization and validation studies in on-line pre-concentration system for lead determination in drinking water and saline waste from oil refinery. Microchem. J. 2004, 77, 123–129. [Google Scholar] [CrossRef]
- Vongboot, M.; Suesoonthon, M. Removal of copper and iron by polyurethane foam column in FIA system for the determination of nickel in pierced ring. Talanta 2015, 131, 325–329. [Google Scholar] [CrossRef] [PubMed]
- Yeerum, C.; Issarangkura Na Ayutthaya, P.; Kesonkan, K.; Chaiyakhan, A.; Vongboot, M. Down-scaling sample preparation using polyurethane foam and colorimetric technique for the chromium assay in accessories. Anal. Sci. 2020, 36, 1137–1140. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Azeem, S.M.; Bader, N.R.; Kuss, H.M.; El-Shahat, M.F. Determination of total iron in food samples after flow injection preconcentration on polyurethane foam functionalized with N,N-bis(salicylidene)-1,3-propanediamine. Food Chem. 2013, 138, 1641–1647. [Google Scholar] [CrossRef] [PubMed]
- De Sousa, J.M.; Couto, M.T.; Cassella, R.J. Polyurethane foam functionalized with phenylfluorone for online preconcentration and determination of copper and cadmium in water samples by flame atomic absorption spectrometry. Microchem. J. 2018, 138, 92–97. [Google Scholar] [CrossRef]
- Abdel Azeem, S.M.; Hanafi, H.A.; El-Nadi, Y.; El-Shahat, M.F. Separation of nickel and cadmium from aqueous solutions by flow injection preconcentration onto cadion functionalized polyurethane foam. Microchem. J. 2021, 166, 106192. [Google Scholar] [CrossRef]
- Sone, H.; Fugetsu, B.; Tanaka, S. Selective elimination of lead(II) ions by alginate/polyurethane composite foams. J. Hazard. Mater. 2009, 162, 423–429. [Google Scholar] [CrossRef]
- Yeerum, C.; Wongwilai, W.; Grudpan, K.; Vongboot, M. Green assay of anionic surfactant via ion-association with methylene blue sorbed on polyurethane foam monolithic rod and using a smartphone. Talanta 2018, 190, 85–88. [Google Scholar] [CrossRef]
- Tanikkul, S.; Jakmunee, J.; Lapanantnoppakhun, S.; Rayanakorna, M.; Sooksamiti, P.; Synovec, R.E.; Christian, G.D.; Grudpan, K. Flow injection in-valve-mini-column pretreatment combined with ion chromatography for cadmium, lead and zinc determination. Talanta 2004, 64, 1241–1246. [Google Scholar] [CrossRef]


| Condition 1 | Pb2+ (µg mL−1) | Loading Time (min) | Calculated µg Pb2+ | FI Response (Absorbances) | |||
|---|---|---|---|---|---|---|---|
| I | II | III | Average | ||||
| A | 0.1 | 5 | 0.5 | 0.000 | 0.001 | 0.001 | 0.001 ± 0.000 |
| B | 0.2 | 5 | 1 | 0.006 | 0.004 | 0.004 | 0.004 ± 0.001 |
| C | 1.0 | 5 | 5 | 0.016 | 0.012 | 0.009 | 0.013 ± 0.003 |
| D | 2.0 | 5 | 10 | 0.020 | 0.022 | 0.015 | 0.019 ± 0.003 |
| E | 2.0 | 7 | 14 | 0.031 | 0.026 | 0.021 | 0.026 ± 0.004 |
| F | 2.0 | 10 | 20 | 0.035 | 0.030 | 0.041 | 0.035 ± 0.004 |
| F | 4.0 | 5 | 20 | 0.040 | 0.048 | 0.040 | 0.043 ± 0.004 |
| G | 4.0 | 7 | 28 | 0.057 | 0.053 | 0.058 | 0.056 ± 0.002 |
| H | 6.0 | 5 | 30 | 0.057 | 0.056 | 0.052 | 0.055 ± 0.002 |
| I | 8.0 | 5 | 40 | 0.069 | 0.072 | 0.068 | 0.070 ± 0.002 |
| J | 6.0 | 7 | 42 | 0.091 | 0.083 | 0.078 | 0.084 ± 0.005 |
| K | 10.0 | 5 | 50 | 0.099 | 0.093 | 0.092 | 0.095 ± 0.003 |
| L | 8.0 | 7 | 56 | 0.105 | 0.111 | 0.104 | 0.107 ± 0.003 |
| M | 4.0 | 15 | 60 | 0.120 | 0.111 | 0.123 | 0.118 ± 0.005 |
| M | 6.0 | 10 | 60 | 0.113 | 0.123 | 0.117 | 0.118 ± 0.004 |
| N | 10.0 | 7 | 70 | 0.138 | 0.121 | 0.127 | 0.129 ± 0.007 |
| O | 8.0 | 10 | 80 | 0.144 | 0.143 | 0.142 | 0.143 ± 0.001 |
| P | 6.0 | 15 | 90 | 0.175 | 0.172 | 0.169 | 0.172 ± 0.002 |
| Q | 8.0 | 15 | 120 | 0.224 | 0.201 | 0.220 | 0.215 ± 0.010 |
| R | 10.0 | 15 | 150 | 0.285 | 0.262 | 0.266 | 0.271 ± 0.010 |
| Sample | Pb2+ (µg mL−1) | |||
|---|---|---|---|---|
| Added | Found | Recovery (%) | Content in Sample 1 | |
| S1 | 0.0 | 0.4 ± 0.1 | - | 2.0 ± 0.2 |
| 2.0 | 2.5 ± 0.1 | 105 | ||
| S2 | 0.0 | 1.0 ± 0.1 | - | 5.0 ± 0.5 |
| 2.0 | 3.0 ± 0.1 | 100 | ||
| S3 | 0.0 | 0.4 ± 0.1 | - | 2.0 ± 0.2 |
| 2.0 | 2.6 ± 0.1 | 110 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Issarangkura Na Ayutthaya, P.; Yeerum, C.; Kesonkan, K.; Kiwfo, K.; Grudpan, K.; Teshima, N.; Murakami, H.; Vongboot, M. Determination of Lead Employing Simple Flow Injection AAS with Monolithic Alginate-Polyurethane Composite Packed In-Valve Column. Molecules 2021, 26, 4397. https://doi.org/10.3390/molecules26154397
Issarangkura Na Ayutthaya P, Yeerum C, Kesonkan K, Kiwfo K, Grudpan K, Teshima N, Murakami H, Vongboot M. Determination of Lead Employing Simple Flow Injection AAS with Monolithic Alginate-Polyurethane Composite Packed In-Valve Column. Molecules. 2021; 26(15):4397. https://doi.org/10.3390/molecules26154397
Chicago/Turabian StyleIssarangkura Na Ayutthaya, Piyanat, Chonnipa Yeerum, Kullapon Kesonkan, Kanokwan Kiwfo, Kate Grudpan, Norio Teshima, Hiroya Murakami, and Monnapat Vongboot. 2021. "Determination of Lead Employing Simple Flow Injection AAS with Monolithic Alginate-Polyurethane Composite Packed In-Valve Column" Molecules 26, no. 15: 4397. https://doi.org/10.3390/molecules26154397
APA StyleIssarangkura Na Ayutthaya, P., Yeerum, C., Kesonkan, K., Kiwfo, K., Grudpan, K., Teshima, N., Murakami, H., & Vongboot, M. (2021). Determination of Lead Employing Simple Flow Injection AAS with Monolithic Alginate-Polyurethane Composite Packed In-Valve Column. Molecules, 26(15), 4397. https://doi.org/10.3390/molecules26154397








