Flavonoids: A Myth or a Reality for Cancer Therapy?
Abstract
1. Introduction
1.1. Biosynthetic Pathway of Flavonoids in Plants
1.2. Role of Flavonoids in Plants
1.3. Flavonoids and Biotechnology
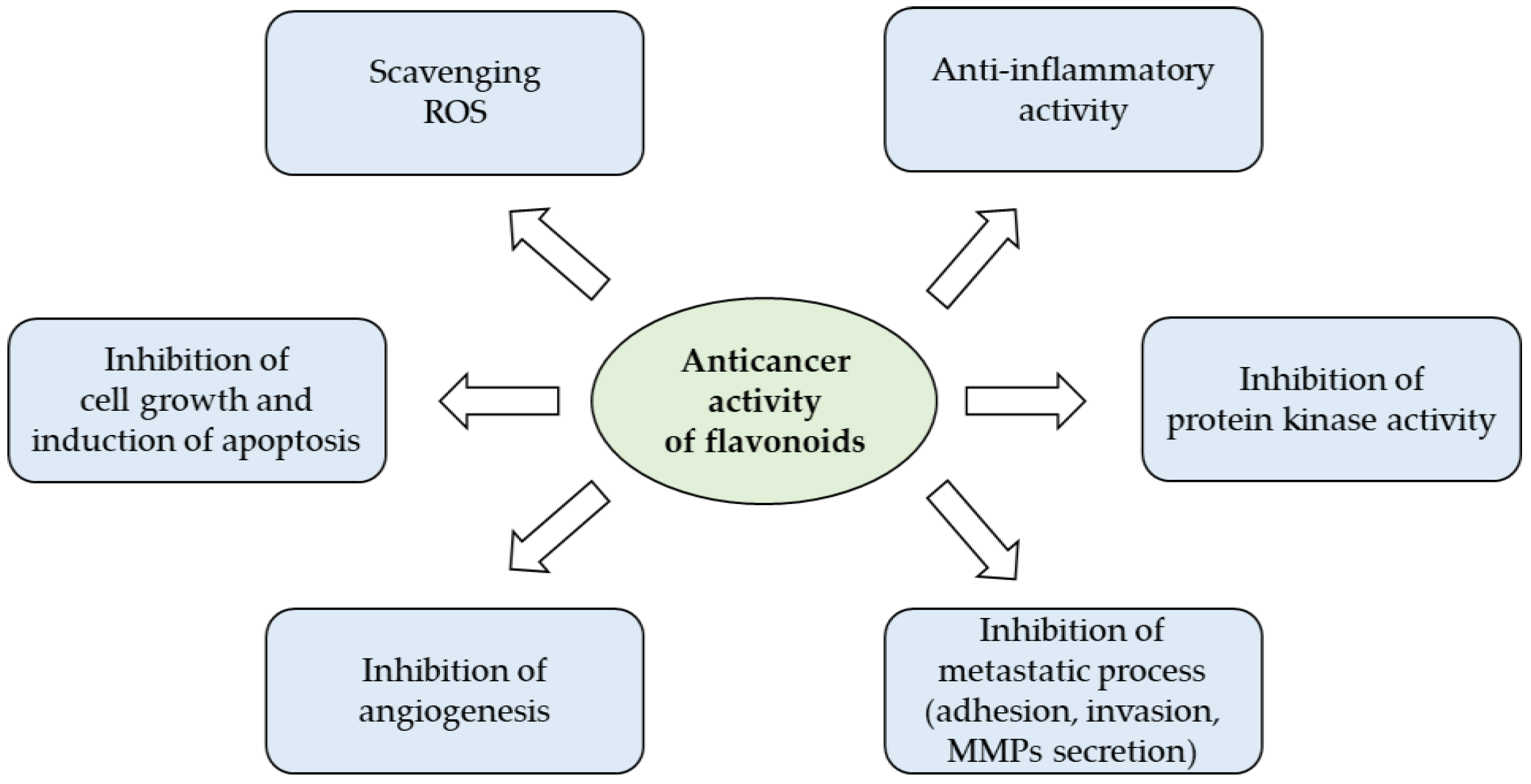
2. Cancer Preventive Activities of Flavonoids
2.1. Flavonoids and Chronic Inflammation
2.2. Flavonoids and Oxidative Stress
2.3. Flavonoids and Apoptosis/Autophagy
2.4. Flavonoids Targeting Cancer Stem Cells
2.5. Anti-Angiogenic and Anti-Metastatic Properties of Flavonoids
2.6. Flavonoids and Cancer Cell Differentiation
2.7. Flavonoids to Improve Sensitivity to Chemotherapy
3. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Steck, S.E.; Murphy, E.A. Dietary patterns and cancer risk. Nat. Rev. Cancer. 2020, 20, 125–138. [Google Scholar] [CrossRef]
- Marai, J.P.J.; Deavours, B.; Dixon, R.A.; Ferreira, D. The Stereochemistry of Flavonoids. In The Science of Flavonoids; Springer: New York, NY, USA, 2007; pp. 1–35. [Google Scholar]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef]
- Middleton, E. The flavonoids. Trends Pharmacol. Sci. 1984, 5, 335–338. [Google Scholar]
- Xiong, Y.; Zhang, P.; Warner, R.D.; Fang, Z. 3-Deoxyanthocyanidin Colorant: Nature, Health, Synthesis, and Food Applications. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1533–1549. [Google Scholar] [CrossRef] [PubMed]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef]
- Hostetler, G.L.; Ralston, R.A.; Schwartz, S.J. Flavones: Food Sources, Bioavailability, Metabolism, and Bioactivity. Adv. Nutr. 2017, 8, 423–435. [Google Scholar] [CrossRef]
- Aherne, S.A.; O’Brien, N.M. Dietary flavonols: Chemistry, food content, and metabolism. Nutrition 2002, 18, 75–81. [Google Scholar] [CrossRef]
- Mazur, W.M.; Duke, J.A.; Wähälä, K.; Rasku, S.; Adlercreutz, H. Isoflavonoids and lignans in legumes: Nutritional and health aspects in humans. Nutr. Biochem. 1998, 9, 193–200. [Google Scholar] [CrossRef]
- Hammerstone, F.J.; Lazarus, S.A.; Schmitz, H.H. Procyanidin Content and Variation in Some Commonly Consumed Foods. J. Nutr. 2020, 130, 2086S–2092S. [Google Scholar] [CrossRef]
- Navarro, M.; Moreira, I.; Arnaez, E.; Quesada, S.; Azofeifa, G.; Alvarado, D.; Monagas, M.J. Proanthocyanidin Characterization, Antioxidant and Cytotoxic Activities of Three Plants Commonly Used in Traditional Medicine in Costa Rica: Petiveria alliaceae L., Phyllanthus niruri L. and Senna reticulataWilld. Plants 2017, 6, 50. [Google Scholar] [CrossRef]
- Buer, C.S.; Imin, N.; Djordjevic, M.A. Flavonoids: New roles for old molecules. J. Integr. Plant Biol. 2010, 52, 98–111. [Google Scholar] [CrossRef]
- Weston, L.A.; Mathesius, U. Flavonoids: Their Structure, Biosynthesis and Role in the Rhizosphere, Including Allelopathy. J. Chem. Ecol. 2013, 39, 283–297. [Google Scholar] [CrossRef]
- Santos-Buelga, C.; San Feliciano, A. Flavonoids: From Structure to Health Issues. Molecules 2017, 22, 477. [Google Scholar] [CrossRef]
- Ross, J.A.; Kasum, C.M. Dietary flavonoids: Bioavailability, metabolic effects, and safety. Annu. Rev. Nutr. 2002, 22, 19–34. [Google Scholar] [CrossRef]
- Crozier, A.; Del Rio, D.; Clifford, M.N. Bioavailability of dietary flavonoids and phenolic compounds. Mol. Aspects Med. 2010, 31, 446–467. [Google Scholar] [CrossRef]
- Thilakarathna, S.H.; Rupasinghe, H.P. Flavonoid Bioavailability and Attempts for Bioavailability Enhancement. Nutrients 2013, 5, 3367–3387. [Google Scholar] [CrossRef]
- Landete, J.M. Updated knowledge about polyphenols: Functions, bioavailability, metabolism, and health. Crit. Rev. Food Sci. Nutr. 2012, 52, 936–948. [Google Scholar] [CrossRef] [PubMed]
- Pei, R.; Liu, X.; Bolling, B. Flavonoids and gut health. Curr. Opin. Biotechnol. 2020, 61, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Pollastri, S.; Tattini, M. Flavonols: Old compounds for old roles. Ann. Bot. 2011, 108, 1225–1233. [Google Scholar] [CrossRef] [PubMed]
- Winkel-Shirley, B. Flavonoid Biosynthesis. A Colorful Model for Genetics, Biochemistry, Cell Biology, and Biotechnology. Plant Physiol. 2001, 126, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Averesch, N.J.H.; Krömer, J.O. Metabolic engineering of the shikimate pathway for production of aromatics and derived compounds—Present and future strain construction strategies. Front. Bioeng. Biotechnol. 2018, 6, 32. [Google Scholar] [CrossRef]
- Vogt, T. Phenylpropanoid biosynthesis. Mol. Plant 2010, 3, 2–20. [Google Scholar] [CrossRef]
- Winkel, B.S.J. Metabolic channeling in plants. Ann. Rev. Plant Biol. 2004, 55, 85–107. [Google Scholar] [CrossRef]
- Zhao, J.; Dixon, R.A. The ‘ins’ and ‘outs’ of flavonoid transport. Trends Plant Sci. 2010, 15, 72–80. [Google Scholar] [CrossRef]
- Griesbach, R. Biochemistry and genetics of flower color. Plant Breed Rev. 2005, 25, 89–114. [Google Scholar]
- Pourcel, L.; Routaboul, J.M.; Cheynier, V.; Lepiniec, L.; Debeaujon, L. Flavonoid oxidation in plants: From biochemical properties to physiological functions. Trends Plant Sci. 2007, 12, 29–36. [Google Scholar] [CrossRef]
- Agati, G.; Azzarello, E.; Pollastri, S.; Tattini, M. Flavonoids as antioxidants in plants: Location and functional significance. Plant Sci. 2012, 196, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Parida, A.K.; Das, A.B. Salt tolerance and salinity effects on plants: A review. Ecotoxicol. Environ. Saf. 2005, 60, 324–349. [Google Scholar] [CrossRef] [PubMed]
- Forni, C.; Duca, D.; Glick, B.R. Mechanisms of plant response to salt and drought stress and their alteration by rhizobacteria. Plant Soil 2017, 410, 335–356. [Google Scholar] [CrossRef]
- Halder, M.; Sarkar, S.; Jha, S. Elicitation: A biotechnological tool for enhanced production of secondary metabolites in hairy root cultures. Eng. Life Sci. 2019, 19, 880–895. [Google Scholar] [CrossRef]
- Forni, C.; Frattarelli, A.; Lentini, A.; Beninati, S.; Lucioli, S.; Caboni, E. Assessment of the antiproliferative activity on murine melanoma cells of extracts from elicited cell suspensions of strawberry, strawberry tree, blackberry and red raspberry. Plant Biosyst. 2016, 150, 1233–1239. [Google Scholar] [CrossRef]
- Lucioli, S.; Di Bari, C.; Nota, P.; Frattarelli, A.; Forni, C.; Caboni, E. Methyl jasmonate promotes anthocyanins production in Prunus salicina × Prunus persica in vitro shoot cultures. Plant Biosyst. 2017, 151, 788–791. [Google Scholar] [CrossRef]
- Amer, A. Biotechnology approaches for in vitro production of flavonoids. J. Microbiol. Biotechnol. Food Sci. 2018, 7, 457–468. [Google Scholar] [CrossRef]
- Lucioli, S.; Pastorino, F.; Nota, P.; Ballan, G.; Frattarelli, A.; Fabbri, A.; Forni, C.; Caboni, E. Extracts from Cell Suspension Cultures of Strawberry (Fragaria x ananassa Duch): Phytochemical Characterization and Cytotoxic Effects on Human Cancer Cells. Molecules 2019, 24, 1738. [Google Scholar] [CrossRef]
- Smetanska, I.; Stahl, U.; Donalies, U.E.B.; Nevoigt, E. Production of Secondary Metabolites Using Plant Cell Cultures Food Biotechnology; Springer: Berlin/Heidelberg, Germany, 2008; Volume 111, pp. 187–228. [Google Scholar] [CrossRef]
- Naik, P.M.; Al-Khayri, J.M. Abiotic and biotic elicitors-role in secondary metabolites production through in vitro culture of medicinal plants. In Abiotic and Biotic Stress in Plants—Recent Advances and Future Perspectives; Shanker, A.K., Shankar, C., Eds.; InTech: Rijeka, Croatia, 2016; pp. 247–277. [Google Scholar]
- Gupta, O.P.; Karkute, S.G.; Banerjee, S.; Meena, N.L.; Dahuja, A. Contemporary Understanding of miRNA-Based Regulation of Secondary Metabolites Biosynthesis in Plants. Front. Plant Sci. 2017, 8, 374. [Google Scholar] [CrossRef]
- Rodríguez-García, C.; Sánchez-Quesada, C.; Gaforio, J.J. Dietary Flavonoids as cancer chemopreventive agents: An updated review of human studies. Antioxidants 2019, 8, 137. [Google Scholar] [CrossRef]
- Patil, V.M.; Masand, N. Anticancer Potential of Flavonoids: Chemistry, Biological Activities, and Future Perspectives. In Studies in Natural Products Chemistry; Elsevier B.V.: Amsterdam, The Netherlands, 2018; Volume 59, pp. 401–430. [Google Scholar] [CrossRef]
- Greten, F.R.; Grivennikov, S.I. Inflammation and Cancer: Triggers, Mechanisms, and Consequences. Immunity 2019, 16, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Na, B.K.; Pak, J.H.; Hong, S.J. Clonorchis sinensis and clonorchiasis. Acta Trop 2020, 203, 105309. [Google Scholar] [CrossRef] [PubMed]
- Baj, J.; Korona-Głowniak, I.; Forma, A.; Maani, A.; Sitarz, E.; Rahnama-Hezavah, M.; Radzikowska, E.; Portincasa, P. Mechanisms of the Epithelial–Mesenchymal Transition and Tumor Microenvironment in Helicobacter Pylori-Induced Gastric Cancer. Cells 2020, 9, 1055. [Google Scholar] [CrossRef]
- Mak, L.Y.; Cruz-Ramòn, V.; Chinchilla-López, P.; Torres, H.A.; Lo Conte, N.K.; Rice, J.P.; Foxhall, L.E.; Sturgis, E.M.; Merrill, J.K.; Bailey, H.H.; et al. Global Epidemiology, Prevention, and Management of Hepatocellular Carcinoma. Am. Soc. Clin. Oncol. Educ. Book 2018, 23, 62–279. [Google Scholar] [CrossRef]
- McGuigan, A.; Kelly, P.; Turkington, R.C.; Jones, C.; Coleman, H.G.; McCain, R.S. Pancreatic cancer: A review of clinical diagnosis, epidemiology, treatment and outcomes. World J. Gastroenterol. 2018, 24, 4846–4861. [Google Scholar] [CrossRef] [PubMed]
- Que, J.; Garman, K.S.; Souza, R.F.; Spechler, S.J. Pathogenesis and Cells of Origin of Barrett’s Esophagus. Gastroenterology 2019, 157, 349–364. [Google Scholar] [CrossRef]
- Chaturvedi, G.; Gupta, A.K.; Das, S.; Gohil, A.J.; Lamba, S. Marjolin Ulcer: An Observational Epidemiological Study from a Tertiary Care Centre in India. Ann. Plast. Surg. 2019, 83, 518–522. [Google Scholar] [CrossRef]
- Klebe, S.; Leigh, J.; Henderson, D.W.; Nurminen, M. Asbestos, Smoking and Lung Cancer: An Update. Int. J. Environ. Res. Public Health 2019, 17, 258. [Google Scholar] [CrossRef]
- Qu, Y.L.; Liu, J.; Zhang, L.X.; Wu, C.M.; Chu, A.J.; Wen, B.L.; Ma, C.; Yan, X.Y.; Zhang, X.; Wang, D.M.; et al. Asthma and the risk of lung cancer: A meta-analysis. Oncotarget 2017, 8, 11614–11620. [Google Scholar] [CrossRef] [PubMed]
- Haenen, C.C.P.; Buurma, A.A.J.; Genders, R.E.; Quint, K.D. Squamous cell carcinoma arising in hypertrophic lichen planus. BMJ Case Rep. 2018, 2018. [Google Scholar] [CrossRef] [PubMed]
- Hakenberg, O.W.; Dräger, D.L.; Erbersdobler, A.; Naumann, C.M.; Jünemann, K.P.; Protzel, C. The Diagnosis and Treatment of Penile Cancer. Dtsch. Arztebl. Int. 2018, 115, 646–652. [Google Scholar] [CrossRef] [PubMed]
- Savant, S.S.; Sriramkumar, S.; O’Hagan, H.M. The Role of Inflammation and Inflammatory Mediators in the Development, Progression, Metastasis, and Chemoresistance of Epithelial Ovarian Cancer. Cancers 2018, 10, 251. [Google Scholar] [CrossRef]
- Sfanos, K.S.; Yegnasubramanian, S.; Nelson, W.G.; De Marzo, A.M. The inflammatory microenvironment and microbiome in prostate cancer development. Nat. Rev. Urol. 2018, 15, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Islam, J.; Shree, A.; Vafa, A.; Afzal, S.M.; Sultana, S. Taxifolin ameliorates Benzo [a] pyrene-induced lung injury possibly via stimulating the Nrf2 signalling pathway. Int. Immunopharmacol. 2021, 96, 107566. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Zhu, X.; Wang, Q.; Li, X.; Wang, E.; Zhao, Q.; Wang, Q.; Cao, H. The anti-tumor effect of taxifolin on lung cancer via suppressing stemness and epithelial-mesenchymal transition in vitro and oncogenesis in nude mice. Ann. Transl. Med. 2020, 8, 590. [Google Scholar] [CrossRef]
- Chen, M.; Li, J.; Liu, X.; Song, Z.; Han, S.; Shi, R.; Zhang, X. Chrysin prevents lipopolysaccharide-induced acute lung injury in mice by suppressing the IRE1alpha/TXNIP/NLRP3 pathway. Pulm. Pharmacol. Ther. 2021, 68, 102018. [Google Scholar] [CrossRef] [PubMed]
- Yuvaraj, S.; Ramprasath, T.; Saravanan, B.; Vasudevan, V.; Sasikumar, S.; Selvam, G.S. Chrysin attenuates high-fat-diet-induced myocardial oxidative stress via upregulating eNOS and Nrf2 target genes in rats. Mol. Cell. Biochem. 2021, in press. [Google Scholar] [CrossRef]
- Raina, R.; Afroze, N.; Kedhari, M.S.; Haque, S.; Bajbouj, K.; Hamad, M.; Hussain, A. Chrysin inhibits propagation of HeLa cells by attenuating cell survival and inducing apoptotic pathways. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 2206–2220. [Google Scholar]
- Zhang, Z.; Shi, J.; Yang, T.; Liu, T.; Zhang, K. Management of aggressive fibromatosis. Oncol Lett. 2021, 21, 43. [Google Scholar]
- Kontomanolis, E.N.; Koutras, A.; Syllaios, A.; Schizas, D.; Mastoraki, A.; Garmpis, N.; Diakosavvas, M.; Angelou, K.; Tsatsaris, G.; Pagkalos, A.; et al. Role of Oncogenes and Tumor-suppressor Genes in Carcinogenesis: A Review. Anticancer Res. 2020, 40, 6009–6015. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.; Gupta, S.C.; Tyagi, A.K. Reactive oxygen species (ROS) and cancer: Role of antioxidative nutraceuticals. Cancer Lett. 2017, 387, 95–105. [Google Scholar] [CrossRef]
- Moloney, J.N.; Cotter, T.G. ROS signalling in the biology of cancer. Semin. Cell Dev. Biol. 2018, 80, 50–64. [Google Scholar] [CrossRef] [PubMed]
- Galadari, S.; Rahman, A.; Pallichankandy, S.; Thayyullathil, F. Reactive oxygen species and cancer paradox: To promote or to suppress? Free Radic. Biol. Med. 2017, 104, 144–164. [Google Scholar] [CrossRef]
- Kanno, S.; Tomizawa, A.; Hiura, T.; Osanai, Y.; Shouji, A.; Ujibe, M.; Ohtake, T.; Kimura, K.; Ishikawa, M. Inhibitory effects of naringenin on tumor growth in human cancer cell lines and sarcoma S-180-implanted mice. Biol. Pharm. Bull. 2005, 28, 527–530. [Google Scholar] [CrossRef] [PubMed]
- Arul, D.; Subramanian, P. Naringenin (citrus flavonone) induces growth inhibition, cell cycle arrest and apoptosis in human hepatocellular carcinoma cells. Pathol. Oncol. Res. 2013, 19, 763–770. [Google Scholar] [CrossRef]
- Yen, H.R.; Liu, C.J.; Yeh, C.C. Naringenin suppresses TPA-induced tumor invasion by suppressing multiple signal transduction pathways in human hepatocellular carcinoma cells. Chem. Biol. Interact. 2015, 235, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Bao, L.; Liu, F.; Guo, H.B.; Li, Y.; Tan, B.B.; Zhang, W.X.; Peng, Y.H. Naringenin inhibits proliferation, migration, and invasion as well as induces apoptosis of gastric cancer SGC7901 cell line by downregulation of AKT pathway. Tumour. Biol. 2016, 37, 11365–11374. [Google Scholar] [CrossRef] [PubMed]
- Torricelli, P.; Elia, A.C.; Magara, G.; Feriotto, G.; Forni, C.; Borromeo, I.; De Martino, A.; Tabolacci, C.; Mischiati, C.; Beninati, S. Reduction of oxidative stress and ornithine decarboxylase expression in a human prostate cancer cell line PC-3 by a combined treatment with alpha-tocopherol and naringenin. Amino Acids 2021, 53, 63–72. [Google Scholar] [CrossRef]
- Rebello, C.J.; Beyl, R.A.; Lertora, J.J.L.; Greenway, F.L.; Ravussin, E.; Ribnicky, D.M.; Poulev, A.; Kennedy, B.J.; Castro, H.F.; Campagna, S.R.; et al. Safety and pharmacokinetics of naringenin: A randomized, controlled, single-ascending-dose clinical trial. Diabetes Obes. Metab. 2020, 22, 91–98. [Google Scholar] [CrossRef]
- Pistritto, G.; Trisciuoglio, D.; Ceci, C.; Garufi, A.; D’Orazi, G. Apoptosis as anticancer mechanism: Function and dysfunction of its modulators and targeted therapeutic strategies. Aging 2016, 8, 603–619. [Google Scholar] [CrossRef] [PubMed]
- Strasser, A.; Vaux, D.L. Cell Death in the Origin and Treatment of Cancer. Mol. Cell 2020, 78, 1045–1054. [Google Scholar] [CrossRef]
- Chan, E.W.C.; Wong, S.K.; Chan, H.T. Casticin from Vitex species: A short review on its anticancer and anti-inflammatory properties. J. Integr. Med. 2018, 16, 147–152. [Google Scholar] [CrossRef]
- Liu, X.; Jiang, Q.; Liu, H.; Luo, S. Vitexin induces apoptosis through mitochondrial pathway and PI3K/Akt/mTOR signaling in human non-small cell lung cancer A549 cells. Biol. Res. 2019, 52, 7. [Google Scholar] [CrossRef]
- Teekaraman, D.; Elayapillai, S.P.; Viswanathan, M.P.; Jagadeesan, A. Quercetin inhibits human metastatic ovarian cancer cell growth and modulates components of the intrinsic apoptotic pathway in PA-1 cell line. Chem. Biol. Interact. 2019, 300, 91–100. [Google Scholar] [CrossRef]
- Perez-Montoyo, H. Therapeutic Potential of Autophagy Modulation in Cholangiocarcinoma. Cells 2020, 9, 614. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Shamaladevi, N.; Jayaprakasha, G.K.; Patil, B.S.; Lokeshwar, B.L. Polyphenol-rich extract of Pimenta dioica berries (Allspice) kills breast cancer cells by autophagy and delays growth of triple negative breast cancer in athymic mice. Oncotarget 2015, 6, 16379–16395. [Google Scholar] [CrossRef]
- Han, B.; Yu, Y.Q.; Yang, Q.L.; Shen, C.Y.; Wang, X.J. Kaempferol induces autophagic cell death of hepatocellular carcinoma cells via activating AMPK signaling. Oncotarget 2017, 8, 86227–86239. [Google Scholar] [CrossRef] [PubMed]
- Křížová, L.; Dadáková, K.; Kašparovská, J.; Kašparovský, T. Isoflavones. Molecules 2019, 24, 1076. [Google Scholar] [CrossRef] [PubMed]
- Moharil, R.B.; Dive, A.; Khandekar, S.; Bodhade, A. Cancer stem cells: An insight. J. Oral. Maxillofac. Pathol. 2017, 21, 463. [Google Scholar] [CrossRef]
- Cianciosi, D.; Varela-Lopez, A.; Forbes-Hernandez, T.Y.; Gasparrini, M.; Afrin, S.; Reboredo-Rodriguez, P.; Zhang, J.; Quiles, J.L.; Nabavi, S.F.; Battino, M.; et al. Targeting molecular pathways in cancer stem cells by natural bioactive compounds. Pharmacol. Res. 2018, 135, 150–165. [Google Scholar] [CrossRef] [PubMed]
- Hermawan, A.; Ikawati, M.; Jenie, R.I.; Khumaira, A.; Putri, H.; Nurhayati, I.P.; Angraini, S.M.; Muflikhasari, H.A. Identification of potential therapeutic target of naringenin in breast cancer stem cells inhibition by bioinformatics and in vitro studies. Saudi Pharm. J. 2021, 29, 12–26. [Google Scholar] [CrossRef]
- Kim, B.; Jung, N.; Lee, S.; Sohng, J.K.; Jung, H.J. Apigenin Inhibits Cancer Stem Cell-Like Phenotypes in Human Glioblastoma Cells via Suppression of c-Met Signaling. Phytother. Res. 2016, 30, 1833–1840. [Google Scholar] [CrossRef]
- Erdogan, S.; Turkekul, K.; Serttas, R.; Erdogan, Z. The natural flavonoid apigenin sensitizes human CD44(+) prostate cancer stem cells to cisplatin therapy. Biomed. Pharmacother. 2017, 88, 210–217. [Google Scholar] [CrossRef]
- Li, Y.W.; Xu, J.; Zhu, G.Y.; Huang, Z.J.; Lu, Y.; Li, X.Q.; Wang, N.; Zhang, F.X. Apigenin suppresses the stem cell-like properties of triple-negative breast cancer cells by inhibiting YAP/TAZ activity. Cell Death Discov. 2018, 4, 105. [Google Scholar] [CrossRef]
- Tu, D.G.; Lin, W.T.; Yu, C.C.; Lee, S.S.; Peng, C.Y.; Lin, T.; Yu, C.H. Chemotherapeutic effects of luteolin on radio-sensitivity enhancement and interleukin-6/signal transducer and activator of transcription 3 signaling repression of oral cancer stem cells. J. Formos. Med. Assoc. 2016, 115, 1032–1038. [Google Scholar] [CrossRef]
- Rauf, A.; Imran, M.; Khan, I.A.; Ur-Rehman, M.; Gilani, S.A.; Mehmood, Z.; Mubarak, M.S. Anticancer potential of quercetin: A comprehensive review. Phytother. Res. 2018, 32, 2109–2130. [Google Scholar] [CrossRef]
- Zhou, W.; Kallifatidis, G.; Baumann, B.; Rausch, V.; Mattern, J.; Gladkich, J.; Giese, N.; Moldenhauer, G.; Wirth, T.; Büchler, M.W.; et al. Dietary polyphenol quercetin targets pancreatic cancer stem cells. Int. J. Oncol. 2010, 37, 551–561. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Yang, L.; Li, S.; Ye, D.; Yang, L.; Liu, Q.; Zhao, Z.; Cai, Q.; Tan, J.; Li, X. Quercetin Inhibits Breast Cancer Stem Cells via Downregulation of Aldehyde Dehydrogenase 1A1 (ALDH1A1), Chemokine Receptor Type 4 (CXCR4), Mucin 1 (MUC1), and Epithelial Cell Adhesion Molecule (EpCAM). Med. Sci. Monit. 2018, 24, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Si, Y.; Wang, Z.; Wang, J.; Guo, Y.; Zhang, X. Quercetin inhibits the growth of human gastric cancer stem cells by inducing mitochondrial-dependent apoptosis through the inhibition of PI3K/Akt signaling. Int. J. Mol. Med. 2016, 38, 619–626. [Google Scholar] [CrossRef]
- Viallard, C.; Larrivée, B. Tumor angiogenesis and vascular normalization: Alternative therapeutic targets. Angiogenesis 2017, 20, 409–426. [Google Scholar] [CrossRef] [PubMed]
- Ramjiawan, R.R.; Griffioen, A.W.; Duda, D.G. Anti-angiogenesis for cancer revisited: Is there a role for combinations with immunotherapy? Angiogenesis 2017, 20, 185–204. [Google Scholar] [CrossRef] [PubMed]
- Suvarna, V.; Murahari, M.; Khan, T.; Chaubey, P.; Sangave, P. Phytochemicals and PI3K Inhibitors in Cancer—An Insight. Front. Pharmacol. 2017, 8, 916. [Google Scholar] [CrossRef]
- Mirossay, L.; Varinská, L.; Mojžiš, J. Antiangiogenic Effect of Flavonoids and Chalcones: An Update. Int. J. Mol. Sci. 2017, 19, 27. [Google Scholar] [CrossRef] [PubMed]
- Chin, H.K.; Horng, C.T.; Liu, Y.S.; Lu, C.C.; Su, C.Y.; Chen, P.S.; Chiu, H.Y.; Tsai, F.J.; Shieh, P.C.; Yang, J.S. Kaempferol inhibits angiogenic ability by targeting VEGF receptor-2 and downregulating the PI3K/AKT, MEK and ERK pathways in VEGF-stimulated human umbilical vein endothelial cells. Oncol. Rep. 2018, 39, 2351–2357. [Google Scholar] [CrossRef]
- Kikuchi, H.; Yuan, B.; Hu, X.; Okazaki, M. Chemopreventive and anticancer activity of flavonoids and its possibility for clinical use by combining with conventional chemotherapeutic agents. Am. J. Cancer Res. 2019, 9, 1517–1535. [Google Scholar] [PubMed]
- Li, H.; Chen, C. Quercetin Has Antimetastatic Effects on Gastric Cancer Cells via the Interruption of uPA/uPAR Function by Modulating NF-kappab, PKC-delta, ERK1/2, and AMPKalpha. Integr. Cancer Ther. 2018, 17, 511–523. [Google Scholar] [CrossRef]
- Yao, X.; Jiang, W.; Yu, D.; Yan, Z. Luteolin inhibits proliferation and induces apoptosis of human melanoma cells in vivo and in vitro by suppressing MMP-2 and MMP-9 through the PI3K/AKT pathway. Food Funct. 2019, 10, 703–712. [Google Scholar] [CrossRef]
- Provenzano, B.; Lentini, A.; Tatti, R.; De Martino, A.; Borromeo, I.; Mischiati, C.; Feriotto, G.; Forni, C.; Tabolacci, C.; Beninati, S. Evaluation of polyamines as marker of melanoma cell proliferation and differentiation by an improved high-performance liquid chromatographic method. Amino Acids 2019, 51, 1623–1631. [Google Scholar] [CrossRef]
- Forni, C.; Braglia, R.; Lentini, A.; Nuccetelli, M.; Provenzano, B.; Tabolacci, C.; Beninati, S. Role of transglutaminase 2 in quercetin-induced differentiation of B16-F10 murine melanoma cells. Amino Acids 2009, 36, 731–738. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, C.H.; Grandits, A.M.; Purton, L.E.; Sill, H.; Wieser, R. All-trans retinoic acid in non-promyelocytic acute myeloid leukemia: Driver lesion dependent effects on leukemic stem cells. Cell Cycle 2020, 19, 2573–2588. [Google Scholar] [CrossRef]
- Moradzadeh, M.; Roustazadeh, A.; Tabarraei, A.; Erfanian, S.; Sahebkar, A. Epigallocatechin-3-gallate enhances differentiation of acute promyelocytic leukemia cells via inhibition of PML-RARalpha and HDAC1. Phytother. Res. 2018, 32, 471–479. [Google Scholar] [CrossRef]
- Yang, H.; Hui, H.; Wang, Q.; Li, H.; Zhao, K.; Zhou, Y.; Zhu, Y.; Wang, X.; You, Q.; Guo, Q.; et al. Wogonin induces cell cycle arrest and erythroid differentiation in imatinib-resistant K562 cells and primary CML cells. Oncotarget 2020, 11, 300–301. [Google Scholar] [CrossRef]
- Tomko, A.M.; Whynot, E.G.; Ellis, L.D.; Dupré, D.J. Anti-Cancer Potential of Cannabinoids, Terpenes, and Flavonoids Present in Cannabis. Cancers 2020, 12, 1985. [Google Scholar] [CrossRef]
- Lin, Y.; Sun, H.; Dang, Y.; Li, Z. Isoliquiritigenin inhibits the proliferation and induces the differentiation of human glioma stem cells. Oncol. Rep. 2018, 39, 687–694. [Google Scholar] [CrossRef] [PubMed]
- He, M.H.; Zhang, Q.; Shu, G.; Lin, J.C.; Zhao, L.; Liang, X.X.; Yin, L.; Shi, F.; Fu, H.L.; Yuan, Z.X. Dihydromyricetin sensitizes human acute myeloid leukemia cells to retinoic acid-induced myeloid differentiation by activating STAT1. Biochem. Biophys. Res. Commun. 2018, 495, 1702–1707. [Google Scholar] [CrossRef] [PubMed]
- Feriotto, G.; Tagliati, F.; Giriolo, R.; Casciano, F.; Tabolacci, C.; Beninati, S.; Khan, M.T.H.; Mischiati, C. Caffeic Acid Enhances the Anti-Leukemic Effect of Imatinib on Chronic Myeloid Leukemia Cells and Triggers Apoptosis in Cells Sensitive and Resistant to Imatinib. Int. J. Mol. Sci. 2021, 22, 1644. [Google Scholar] [CrossRef] [PubMed]
- Yuan, B.; Okusumi, S.; Yoshino, Y.; Moriyama, C.; Tanaka, S.; Hirano, T.; Takagi, N.; Toyoda, H. Delphinidin induces cytotoxicity and potentiates cytocidal effect in combination with arsenite in an acute promyelocytic leukemia NB4 cell line. Oncol. Rep. 2015, 34, 431–438. [Google Scholar] [CrossRef]
- Sang, D.P.; Li, R.J.; Lan, Q. Quercetin sensitizes human glioblastoma cells to temozolomide in vitro via inhibition of Hsp27. Acta Pharmacol. Sin. 2014, 35, 832–838. [Google Scholar] [CrossRef] [PubMed]
- Johnston, G.A. Flavonoid nutraceuticals and ionotropic receptors for the inhibitory neurotransmitter GABA. Neurochem. Int. 2015, 89, 120–125. [Google Scholar] [CrossRef]
- Desai, V.; Jain, A.; Shaghaghi, H.; Summer, R.; Lai, J.C.K.; Bhushan, A. Combination of Biochanin A and Temozolomide Impairs Tumor Growth by Modulating Cell Metabolism in Glioblastoma Multiforme. Anticancer. Res. 2019, 39, 57–66. [Google Scholar] [CrossRef]
- Palko-Labuz, A.; Sroda-Pomianek, K.; Wesolowska, O.; Kostrzewa-Suslow, E.; Uryga, A.; Michalak, K. MDR reversal and pro-apoptotic effects of statins and statins combined with flavonoids in colon cancer cells. Biomed. Pharmacother. 2019, 109, 1511–1522. [Google Scholar] [CrossRef]
- La, X.; Zhang, L.; Li, Z.; Li, H.; Yang, Y. (−)-Epigallocatechin gallate (EGCG) enhances the sensitivity of colorectal cancer cells to 5-FU by inhibiting GRP78/NF-kappaB/miR-155-5p/MDR1 pathway. J. Agric. Food Chem. 2019, 67, 2510–2518. [Google Scholar] [CrossRef]
- Azevedo-Martins, J.M.; Rabelo-Santos, S.H.; Do Amaral Westin, M.C.; Zeferino, L.C. Tumoral and stromal expression of MMP-2, MMP-9, MMP-14, TIMP-1, TIMP-2, and VEGF-A in cervical cancer patient survival: A competing risk analysis. BMC Cancer 2020, 20, 660. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Wang, Y.; Zhang, Y.; Wan, X.; Li, J.; Liu, K.; Wang, F.; Liu, K.; Liu, Q.; Yang, C.; et al. Anti-cancer activities of tea epigallocatechin-3-gallate in breast cancer patients under radiotherapy. Curr. Mol. Med. 2012, 12, 163–176. [Google Scholar] [CrossRef]
- Qian, J.; Xia, M.; Liu, W.; Li, L.; Yang, J.; Mei, Y.; Meng, Q.; Xie, Y. Glabridin resensitizes p-glycoprotein-overexpressing multidrug-resistant cancer cells to conventional chemotherapeutic agents. Eur. J. Pharmacol. 2019, 852, 231–243. [Google Scholar] [CrossRef] [PubMed]
- Kundur, S.; Prayag, A.; Selvakumar, P.; Nguyen, H.; McKee, L.; Cruz, C.; Srinivasan, A.; Shoyele, S.; Lakshmikuttyamma, A. Synergistic anticancer action of quercetin and curcumin against triple-negative breast cancer cell lines. J. Cell Physiol. 2019, 234, 11103–11108. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.Y.; Manh Hung, L.V.; Unno, T.; Cho, S.K. Nobiletin Enhances Chemosensitivity to Adriamycin through Modulation of the Akt/GSK3beta/beta(-)Catenin/MYCN/MRP1 Signaling Pathway in A549 Human Non-Small-Cell Lung Cancer Cells. Nutrients 2018, 10, 1829. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Tian, D.; Liao, X.; Zhang, Y.; Xiao, J.; Chen, W.; Liu, Q.; Chen, Y.; Li, D.; Zhu, L.; et al. Apigenin combined with gefitinib blocks autophagy flux and induces apoptotic cell death through inhibition of HIF-1alpha, c-Myc, p-EGFR, and glucose metabolism in EGFR L858R+T790M-mutated H1975 cells. Front. Pharmacol. 2019, 10, 260. [Google Scholar] [CrossRef]
- Erdogan, S.; Doganlar, O.; Doganlar, Z.B.; Turkekul, K. Naringin sensitizes human prostate cancer cells to paclitaxel therapy. Prostate Int. 2018, 6, 126–135. [Google Scholar] [CrossRef]



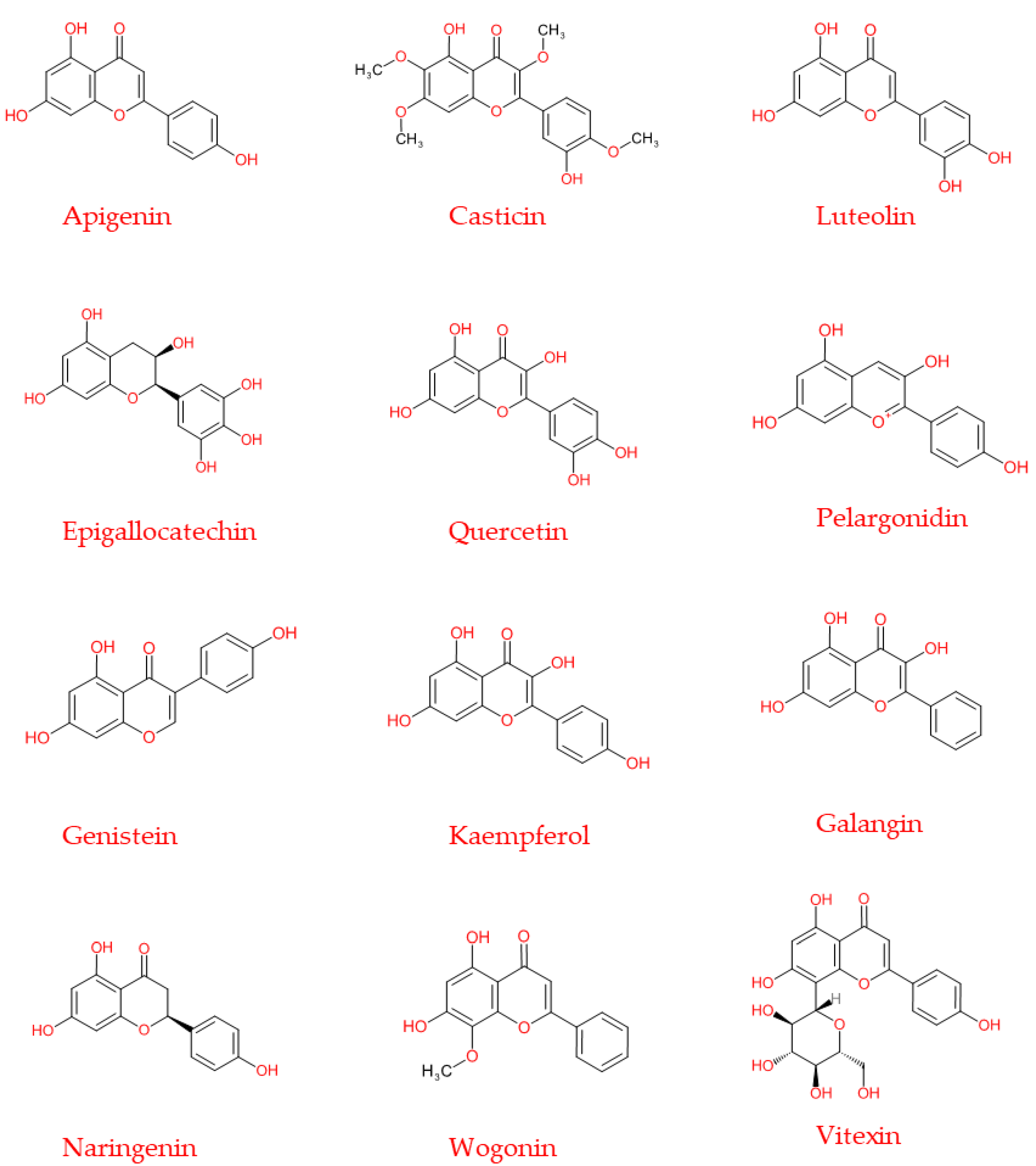
| Classes | Representative Flavonoids | Food Sources | Functions | Information | References |
|---|---|---|---|---|---|
| 3-Deoxyanthocyanidins | Apigeninidin, luteolinidin | Sorghum, purple corn | Pigmentation, antioxidant | Anthocyanidins without hydroxyl in carbon 3 of C ring | [5] |
| Anthocyanins | Cyanidin-3-O-glucoside, peonidin-3-O-glucoside | Blackberry, blueberry, cherry, strawberry | UV protection, pollinators and seed disperser attraction | 3-glycoside form of anthocyanidins | [6] |
| Flavones | Apigenin, luteolin | Celery, green peppers, parsley, peppermint, thyme | Natural pesticides in plants, nodulation, UV protection | Double bond between C2 and C3, no substitution in C3 position | [7] |
| Flavonols | Kaempferol, quercetin, myricetin, rutin | Apple, blueberries, broccoli, cabbage, cherries, garlic, onion, tea, red wine | Pigmentation, UV protection, male fertility, signaling | Double bond between C2 and C3, ketone group on C4, hydroxyl on C3 can be glycosylated | [8] |
| Isoflavonoids | Daidzein, genistein, glycitein | Legumes | Nodulation, defense | 3-phenylchromen-4-one backbone | [9] |
| Proanthocyanidins | Condensed Tannins | Cocoa beans, apples, red wine | Pigmentation, pathogens and predator defense | Oligomeric flavonoids (catechin and epicatechin oligomers) | [10,11] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Forni, C.; Rossi, M.; Borromeo, I.; Feriotto, G.; Platamone, G.; Tabolacci, C.; Mischiati, C.; Beninati, S. Flavonoids: A Myth or a Reality for Cancer Therapy? Molecules 2021, 26, 3583. https://doi.org/10.3390/molecules26123583
Forni C, Rossi M, Borromeo I, Feriotto G, Platamone G, Tabolacci C, Mischiati C, Beninati S. Flavonoids: A Myth or a Reality for Cancer Therapy? Molecules. 2021; 26(12):3583. https://doi.org/10.3390/molecules26123583
Chicago/Turabian StyleForni, Cinzia, Massimiliano Rossi, Ilaria Borromeo, Giordana Feriotto, Giovambattista Platamone, Claudio Tabolacci, Carlo Mischiati, and Simone Beninati. 2021. "Flavonoids: A Myth or a Reality for Cancer Therapy?" Molecules 26, no. 12: 3583. https://doi.org/10.3390/molecules26123583
APA StyleForni, C., Rossi, M., Borromeo, I., Feriotto, G., Platamone, G., Tabolacci, C., Mischiati, C., & Beninati, S. (2021). Flavonoids: A Myth or a Reality for Cancer Therapy? Molecules, 26(12), 3583. https://doi.org/10.3390/molecules26123583









