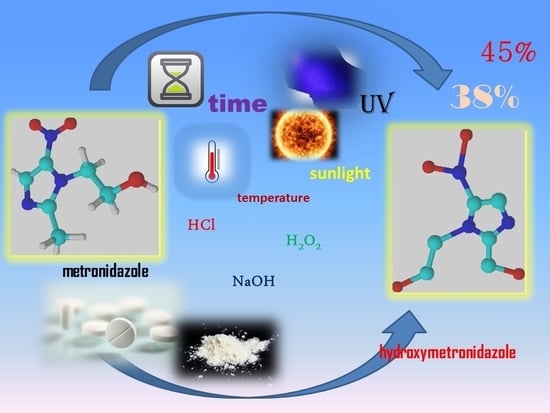
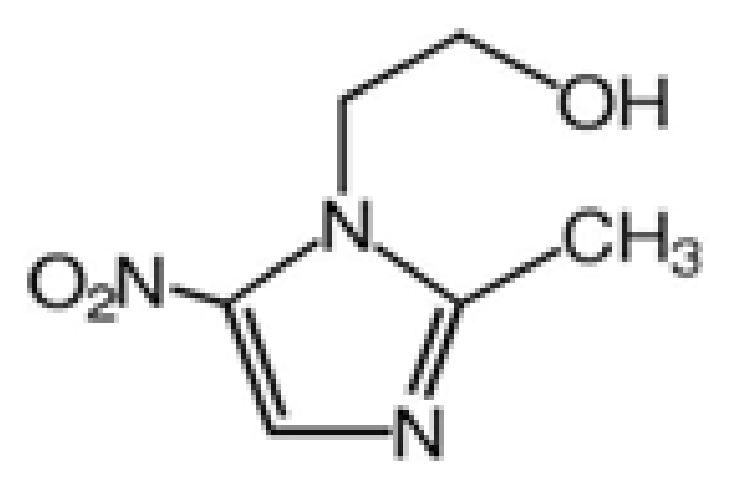
Stability of Metronidazole and Its Complexes with Silver(I) Salts under Various Stress Conditions
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. General Chemicals and Reagents
4.2. Optimization of Analysis Conditions
4.3. Validation of the Analytical Method
4.4. Stability Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Thomas, G. Medicinal Chemistry; Wiley: Hoboken, NJ, USA, 2008. [Google Scholar]
- Dingsdag, S.A.; Hunter, N. Metronidazole: An update on metabolism, structure–cytotoxicity and resistance mechanisms. J. Antimicrob. Chemother. 2018, 73, 265–279. [Google Scholar] [CrossRef]
- Karta Charakterystyki Produktu Leczniczego (Summary of Product Characteristics Card) Metronidazol Polpharma 250 mg tabletki, Zakłady Farmaceutyczne POLPHARMA S.A. 2016. Available online: https://rejestrymedyczne.ezdrowie.gov.pl/rpl/search/public (accessed on 10 June 2021).
- Bhangale, C.J.; Wagh, V.D. Development and validation of stability indicating HPTLC method for the determination of metronidazole in bulk and dosage form. Pravara J. Sci. Technol. 2017, 1, 3–11. [Google Scholar]
- Buczko, W.; Danysz, A. Kompendium Farmakologii i Farmakoterapii (Compendium of Pharmacology and Pharmacotherapy); Wyd. Edra Urban & Partner: Wrocław, Poland, 2016. [Google Scholar]
- Al-Khodira, F.A.I.; Refat, M.S. Investigation of coordination ability of Mn(II), Fe(III), Co(II), Ni(II), and Cu(II) with metronidazole, the antiprotozoal drug, in alkaline media: Synthesis and spectroscopic studies. Rus. J. Gen. Chem. 2017, 87, 873–879. [Google Scholar] [CrossRef]
- Habib, M.J.; Asker, A.F. Complex formation between metronidazole and sodium urate: Effect on photodegradation of metronidazole. Pharm. Res. 1989, 6, 58–61. [Google Scholar] [CrossRef] [PubMed]
- Andersen, F.M.; Bundgaard, H. Inclusion complexation of metronidazole benzoate with β-cyclodextrin and its depression of anhydrate-hydrate transition in aqueous suspensions. Int. J. Pharm. 1984, 19, 189–197. [Google Scholar] [CrossRef]
- Athar, F.; Husain, K.; Abid, M.; Agarwal, S.M.; Coles, S.J.; Hursthouse, M.B.; Maurya, M.R.; Azam, A. Synthesis and anti-amoebic activity of gold(I), ruthenium(II), and copper(II) complexes of metronidazole. Chem. Biodivers. 2005, 2, 1320–1330. [Google Scholar] [CrossRef] [PubMed]
- LaRusso, N.F.; Tomasz, M.; Müller, M.; Lipman, R. Interaction of metronidazole with nucleic acids in vitro. Mol. Pharmacol. 1977, 13, 872–882. [Google Scholar] [PubMed]
- Lansdown, A.B. Silver in health care: Antimicrobial effects and safety in use. Curr. Probl. Dermatol. 2006, 33, 17–34. [Google Scholar]
- Medici, S.; Peana, M.; Crisponi, G.; Nurchi, V.M.; Lachowicz, J.I.; Remelli, M.; Zoroddu, M.A. Silver coordination compounds: A new horizon in medicine. Coord. Chem. Rev. 2016, 327, 349–359. [Google Scholar] [CrossRef]
- Gad, S.C. Pharmaceutical Manufacturing Handbook; Wiley-Interscience: Hoboken, NJ, USA, 2008. [Google Scholar]
- Kalinowska-Lis, U.; Felczak, A.; Chęcińska, L.; Zawadzka, K.; Patyna, E.; Lisowska, K.; Ochocki, J. Synthesis, characterization and antimicrobial activity of water-soluble silver(I) complexes of metronidazole drug and selected counter-ions. Dalton Trans. 2015, 44, 8178–8189. [Google Scholar] [CrossRef] [PubMed]
- Radko, L.; Stypuła-Trębas, S.; Posyniak, A.; Żyro, D.; Ochocki, J. Silver(I) complexes of the pharmaceutical agents metronidazole and 4-hydroxymethylpyridine: Comparison of cytotoxic profile for potential clinical application. Molecules 2019, 24, 1949. [Google Scholar] [CrossRef]
- Żyro, D.; Śliwińska, A.; Szymczak-Pajor, I.; Stręk, M.; Ochocki, J. Light Stability, Pro-apoptotic and genotoxic properties of silver(I) complexes of metronidazole and 4-hydroxymethylpyridine against pancreatic cancer cells in vitro. Cancers 2020, 12, 3848. [Google Scholar] [CrossRef] [PubMed]
- Shkarlat, G.L.; Tyutyunnik, Y.D.; Klimenko, L.Y.; Zhuravel, I.O. Application of HPLC and GLC in the analysis of metronidazole. Top. Iss. New Drugs Dev. 2016, 1, 219. [Google Scholar]
- Maher, H.M.; Youssef, R.M. Development of validated chromatographic methods for the simultaneous determination of metronidazole and spiramycin in tablets. Chromatographia 2009, 69, 345–350. [Google Scholar] [CrossRef]
- Verma, P.; Namboodiry, V.; Mishra, S.; Bhagwat, A.; Bhoir, S. A Stability indicating HPLC method for the determination of metronidazole using ecofriendly solvent as mobile phase component. Int. J. Pharm. Pharm. Sci. 2013, 5, 496–501. [Google Scholar]
- Elkhoudary, M.M.; Abdel Salam, R.A.; Hadad, G.M. Development and optimization of HPLC analysis of metronidazole, diloxanide, spiramycin and cliquinol in pharmaceutical dosage forms using experimental design. J. Chromatogr. Sci. 2016, 54, 1701–1712. [Google Scholar] [CrossRef][Green Version]
- Ali, N.W.; Gamal, M.; Abdelkawy, M. Chromatographic methods for simultaneous determination of diiodohydroxyquinoline and metronidazole in their binary mixture. Pak. J. Pharm. Sci. 2013, 26, 865–871. [Google Scholar] [PubMed]
- Elkady, E.F.; Mahrouse, M.A. Reversed-phase ion-pair HPLC and TLC-densitometric methods for the simultaneous determination of ciprofloxacin hydrochloride and metronidazole in tablets. Chromatographia 2011, 73, 297–305. [Google Scholar] [CrossRef]
- Zhang, D.; Armour, E.; Sherma, J. Development of quantitative HPTLC–densitometry methods following a model approach for transfer of TLC screening methods for pharmaceutical products of cefixime, cefuroxime axetil, cephalexin·H2O, ciprofloxacin HCl, levofloxacin, and metronidazole. Acta Chromatogr. 2017, 29, 484–486. [Google Scholar] [CrossRef]
- Meshram, D.B.; Bagade, S.B.; Tajne, M.R. TLC–densitometric analysis of clotrimazole and metronidazole in combined dosage forms. J. Planar Chromatogr. 2008, 21, 277–282. [Google Scholar] [CrossRef]
- Abdelaleem, E.A.; Abdelwahab, N.S. Simultaneous determination of some antiprotozoal drugs in their binary and ternary mixtures with mebeverine hydrochloride in different dosage forms. J. Liq. Chromatogr. Relat. Technol. 2013, 36, 1528–1539. [Google Scholar] [CrossRef]
- Meshram, D.B.; Bagade, S.B.; Tajne, M.R. Simultaneous determination of metronidazole and miconazole nitrate in gel by HPTLC. Pak. J. Pharm. Sci. 2009, 22, 323–328. [Google Scholar]
- Ashour, S.; Kattan, N. Simultaneous determination of miconazole nitrate and metronidazole in different pharmaceutical dosage forms by gas chromatography and flame ionization detector (GC-FID). Int. J. Biomed. Sci. 2010, 6, 13–18. [Google Scholar]
- Nayak, S.; Goupale, D.C.; Dubey, A.; Vipin, S. Comparative stability study of metronidazole in aqueous and non aqueous vehicle. J. Appl. Pharm. 2011, 3, 295–300. [Google Scholar] [CrossRef]
- Shemer, H.; Kunukcu, Y.K.; Linden, K.G. Degradation of the pharmaceutical metronidazole via UV, Fenton and photo-Fenton processes. Chemosphere 2006, 63, 269–276. [Google Scholar] [CrossRef]
- Johansen, M.; Larsen, C. Stability and kinetics of hydrolysis of metronidazole monosuccinate in aqueous solution and in plasma. Int. J. Pharm. 1984, 21, 201–209. [Google Scholar] [CrossRef]
- Ofokansi, K.; Uzor, P.F. Stability studies and degradation kinetics of some commercially available metronidazole suspensions in Nigeria. J. Pharm. Appl. Sci. 2011, 8, 1379–1386. [Google Scholar]
- Aleanizy, F.S.; Al-Eid, H.; Tahir, E.E.; Alqahtani, F.; Al-Gohar, O. Stability and in vitro dissolution studies of metronidazole tablets and infusions. Dissolut. Technol. 2017, 24, 22–27. [Google Scholar] [CrossRef]
- Shaheen, S.M. Accelerated stability study of metronidazole infusion 100 mL. J. Teach. Assoc. 2005, 18, 118–121. [Google Scholar] [CrossRef]
- De Souza, N.A.B.; Medeiros, A.C.D.; Santos, A.F.O.; Macêdo, R.O. Thermal stability of metronidazole drug and tablets. J. Therm. Anal. Calorim. 2003, 72, 535–538. [Google Scholar] [CrossRef]
- Donnelly, R.F.; Ying, J. Stability of metronidazole suspensions. Int. J. Pharm. Compd. 2015, 19, 248–251. [Google Scholar] [PubMed]
- Nogueira, R.; Rocha, W.F.C.; Silva, T.E.; Rego, E.C.P.; Moreira, G.F.; Wollinger, W.; Rodrigues, J.M. Development studies of a new metronidazole certified reference material. J. Brazil. Chem. Soc. 2012, 23, 435–444. [Google Scholar] [CrossRef]
- Wu, Y.; Fassihi, R. Stability of metronidazole, tetracycline HCl and famotidine alone and in combination. Int. J. Pharm. 2005, 290, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Waszczykowska, A.; Żyro, D.; Jurowski, P.; Ochocki, J. Effect of treatment with silver(I) complex of metronidazole on ocular rosacea: Design and formulation of new silver drug with potent antimicrobial activity. J. Trace Elem. Med. Biol. 2020, 61, 126531. [Google Scholar] [CrossRef]
- Waszczykowska, A.; Żyro, D.; Ochocki, J.; Jurowski, P. Clinical Application and Efficacy of Silver Drug in Ophthalmology: A Literature Review and New Formulation of EYE Drops with Drug Silver (I) Complex of Metronidazole with Improved Dosage Form. Biomedicines 2021, 9, 210. [Google Scholar] [CrossRef] [PubMed]
- Loftsson, T. Drug Stability for Pharmaceutical Scientists; Elsevier Books: Edinburgh, UK, 2013. [Google Scholar]
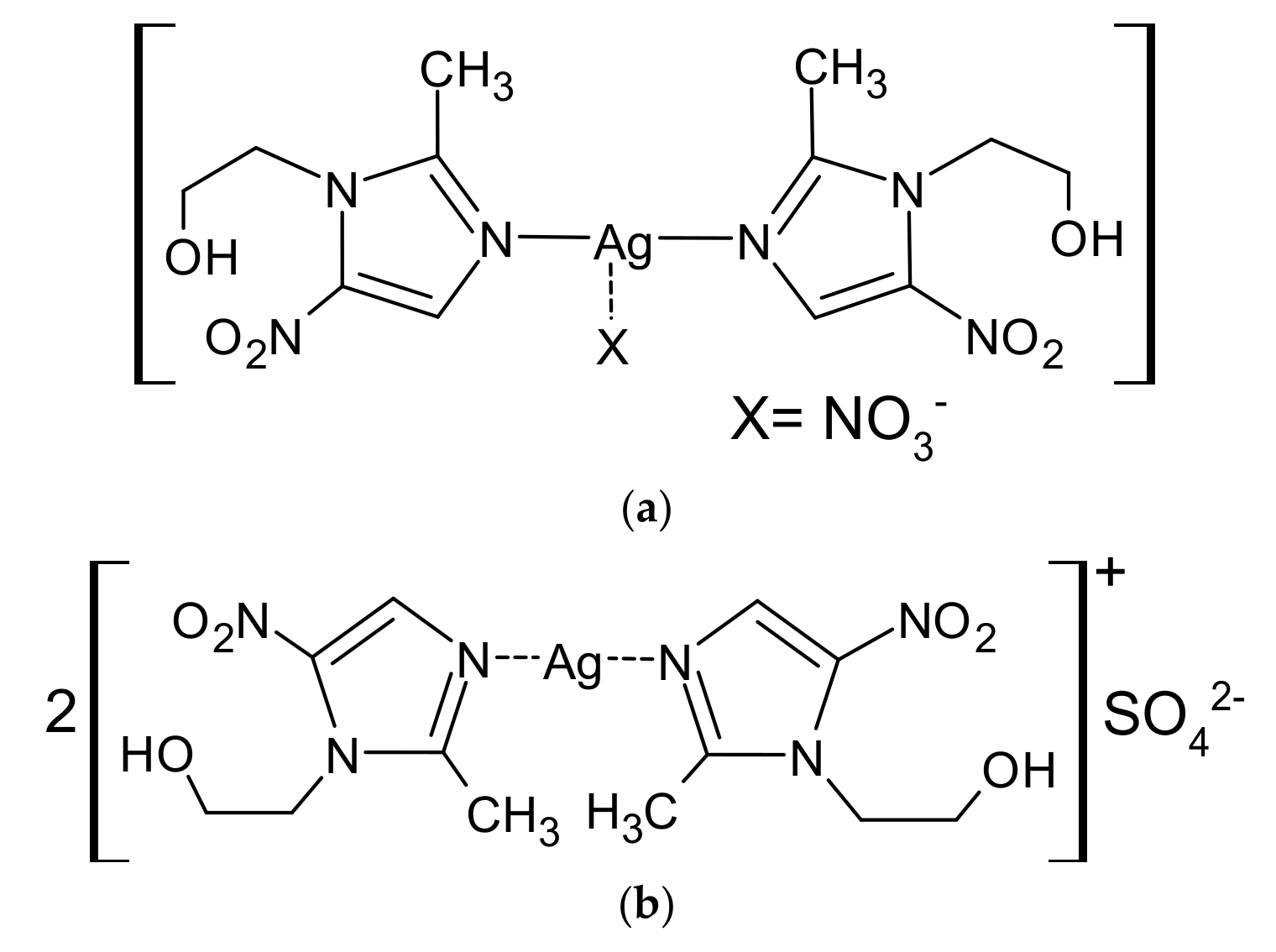
- Żyro, D.; Stręk, M.; Śliwińska, A.; Chęcińska, L.; Kusz, J.; Ochocki, J. Synthesis, Spectroscopy and Biological Evaluation of Silver(I) Complexes with Metronidazole. X-Ray Crystal Structure of [Ag2(MTZ)4]SO4. Medical drugs for humans. Modern issues of pharmacotherapy and prescription of medicine, part 1. In Proceedings of the IV International Scientific and Practical Conference, Kharkiv, Ukraine, 12–13 March 2020; pp. 35–43. [Google Scholar]
- ICH Guide Q2 (R1). Validation of Analytical Procedures-Text and Methodology. 2005. Available online: https://www.ich.org/page/quality-guidelines (accessed on 10 June 2021).
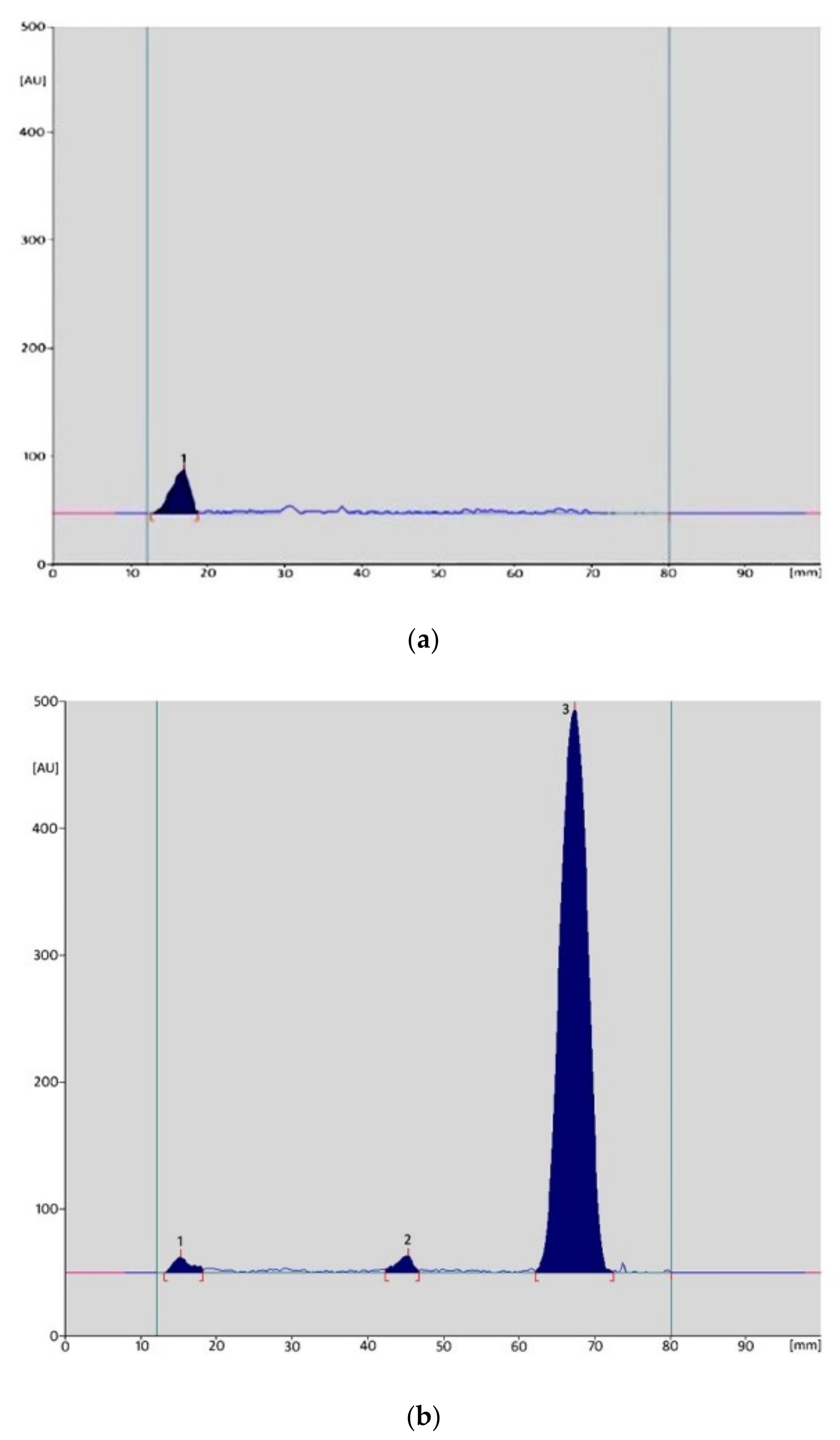
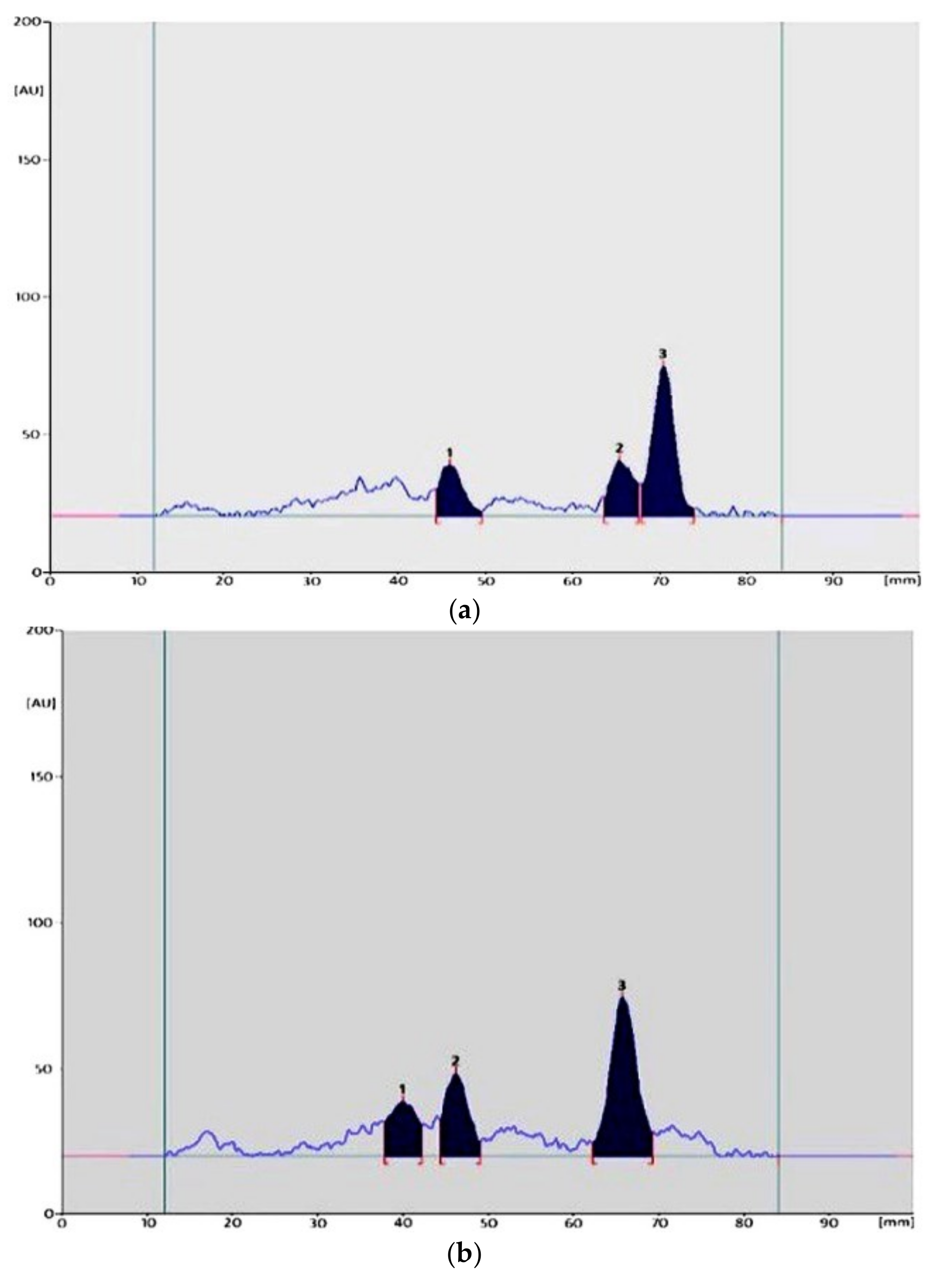

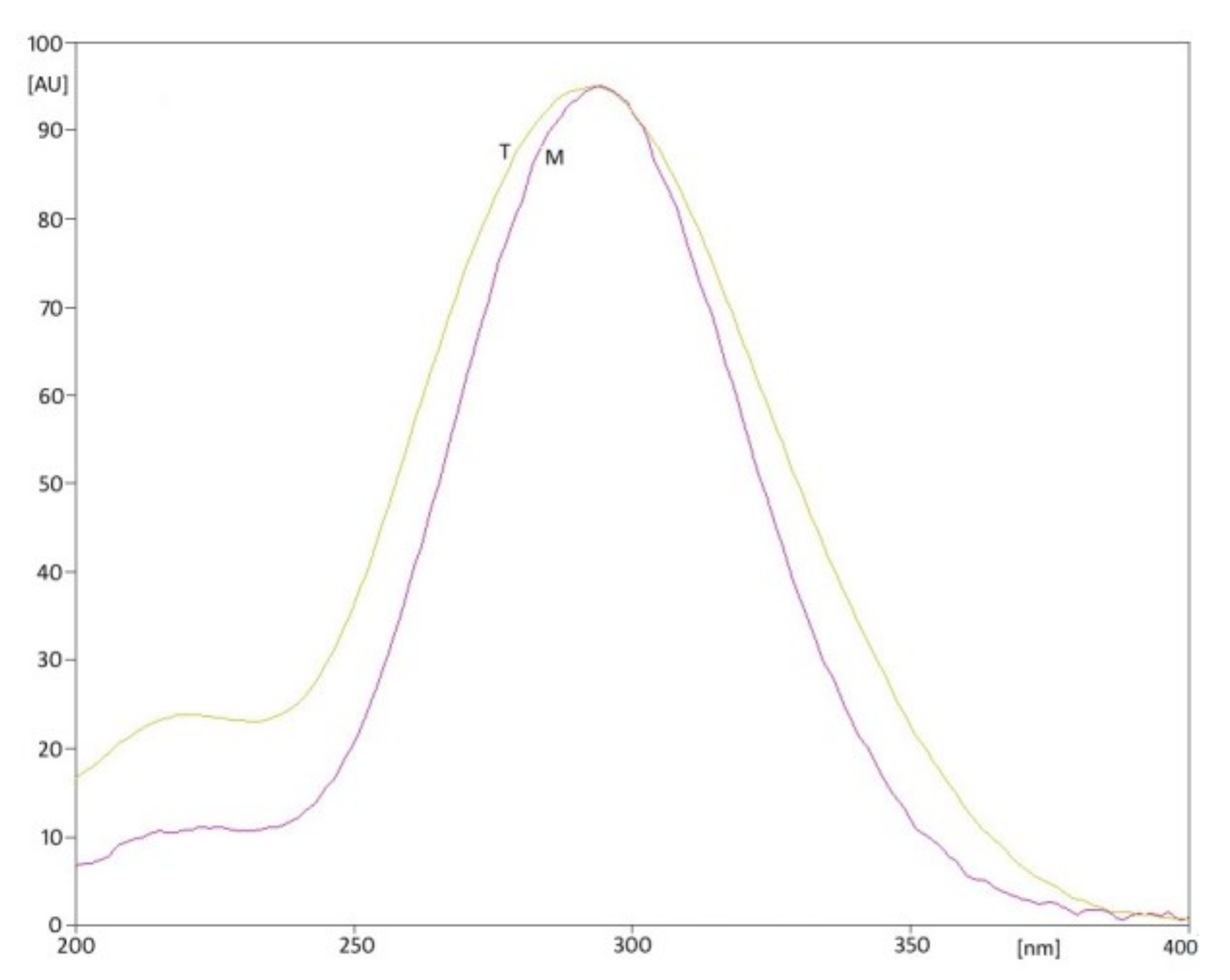
| Parameter | Values | |
|---|---|---|
| Linearity | 1.05—4.62 µg/spot P = 6854.9 + 3460.4·c r = 0.9830 Sa = 322.7 Sb = 999.2 Se = 1044.1 | |
| LOQ | 2.89 µg/spot | |
| LOD | 0.87 µg/spot | |
| Range of the method | 2.89—4.62 µg/spot | |
| Precision | SD = 134.85 RSD% = 0.71 | |
| Accuracy | 80% | = 99.07 SD = 0.26 RSD% = 0.27 |
| 100% | = 99.89 SD = 0.86 RSD% = 0.86 | |
| 120% | = 100.45 SD = 0.61 RSD% = 0.61 | |
| Solution | Parameter | MeOH | 3M HCl | 1M HCl | 0.5M HCl | 0.1M HCl | 0.1M NaOH | 0.5M NaOH | 1M NaOH | 3M NaOH | H2O2 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 30 °C | |||||||||||
| Standard substance | k | 0.0152 | 0.0226 | 0.0243 | 0.0187 | 0.0161 | 0.0239 | 0.0500 | 0.1900 | 0.3100 | 0.0200 |
| t0.5 | 45.59 | 30.66 | 28.52 | 37.06 | 43.04 | 29.00 | 13.86 | 3.65 | 2.24 | 34.65 | |
| t0.1 | 6.93 | 4.66 | 4.34 | 5.64 | 6.55 | 4.41 | 2.12 | 0.55 | 0.34 | 5.27 | |
| Complex I | k | 0.0135 | 0.0204 | 0.0335 | 0.0096 | 0.0100 | 0.0152 | 0.0491 | 0.1300 | 0.3750 | 0.0250 |
| t0.5 | 51.33 | 33.97 | 20.69 | 72.19 | 69.30 | 45.59 | 14.11 | 5.33 | 1.85 | 27.72 | |
| t0.1 | 7.81 | 5.17 | 3.15 | 10.98 | 10.54 | 6.93 | 2.15 | 0.81 | 0.28 | 4.22 | |
| Complex II | k | 0.0099 | 0.0217 | 0.0396 | 0.0096 | 0.0118 | 0.0224 | 0.1084 | 0.3894 | 0.6108 | 0.0259 |
| t0.5 | 69.93 | 31.98 | 17.52 | 72.26 | 58.68 | 30.98 | 6.39 | 1.78 | 1.13 | 26.79 | |
| t0.1 | 10.63 | 4.86 | 2.66 | 10.98 | 8.92 | 4.71 | 0.97 | 0.27 | 0.17 | 4.07 | |
| Tablets | k | 0.0174 | 0.0383 | 0.0435 | 0.0513 | 0.0057 | 0.0213 | 0.0574 | 0.3725 | 0.3800 | 0.0225 |
| t0.5 | 39.83 | 18.09 | 15.93 | 13.51 | 121.58 | 32.54 | 12.07 | 1.86 | 1.82 | 30.80 | |
| t0.1 | 6.06 | 2.75 | 2.42 | 2.05 | 18.49 | 4.95 | 1.84 | 0.28 | 0.28 | 4.68 | |
| 60 °C | |||||||||||
| Standard substance | k | 0.0261 | 0.0674 | 0.0352 | 0.0230 | 0.0213 | 0.1361 | 0.3100 | 1.8500 | - | 0.0375 |
| t0.5 | 26.55 | 10.28 | 19.69 | 30.13 | 32.54 | 5.09 | 2.24 | 0.37 | - | 18.48 | |
| t0.1 | 4.04 | 1.56 | 2.99 | 4.58 | 4.95 | 0.77 | 0.34 | 0.06 | - | 2.81 | |
| Complex I | k | 0.0191 | 0.0657 | 0.0439 | 0.0204 | 0.0109 | 0.0813 | 0.3350 | 2.7100 | - | 0.0275 |
| t0.5 | 36.28 | 10.55 | 15.79 | 33.97 | 63.58 | 8.52 | 2.07 | 0.26 | - | 25.20 | |
| t0.1 | 5.52 | 1.60 | 2.40 | 5.17 | 9.67 | 1.30 | 0.31 | 0.04 | - | 3.83 | |
| Complex II | k | 0.0205 | 0.0698 | 0.0457 | 0.0448 | 0.0191 | 0.1306 | 0.8442 | 2.1347 | - | 0.1127 |
| t0.5 | 33.82 | 9.94 | 15.17 | 15.49 | 36.29 | 5.31 | 0.82 | 0.32 | - | 6.15 | |
| t0.1 | 5.14 | 1.51 | 2.30 | 2.35 | 5.51 | 0.81 | 0.12 | 0.05 | - | 0.93 | |
| Tablets | k | 0.0226 | 0.0691 | 0.0522 | 0.0657 | 0.0178 | 0.2000 | 1.1200 | 2.53 | - | 0.0250 |
| t0.5 | 30.66 | 10.03 | 13.28 | 10.55 | 38.93 | 3.47 | 0.62 | 0.27 | - | 27.72 | |
| t0.1 | 4.66 | 1.53 | 2.02 | 1.60 | 5.92 | 0.53 | 0.09 | 0.04 | - | 4.22 | |
| 90 °C | |||||||||||
| Standard substance | k | 0.0439 | 0.0830 | 0.0652 | 0.0287 | 0.0261 | 0.9900 | 1.7500 | - | - | 0.0550 |
| t0.5 | 15.79 | 8.35 | 10.63 | 24.15 | 26.55 | 0.70 | 0.40 | - | - | 12.60 | |
| t0.1 | 2.40 | 1.27 | 1.62 | 3.67 | 4.04 | 0.11 | 0.06 | - | - | 1.92 | |
| Complex I | k | 0.0343 | 0.0691 | 0.1078 | 0.0670 | 0.0178 | 1.4300 | 2.4500 | - | - | 0.0400 |
| t0.5 | 20.20 | 10.03 | 6.43 | 10.34 | 38.93 | 0.48 | 0.28 | - | - | 17.33 | |
| t0.1 | 3.07 | 1.53 | 0.98 | 1.57 | 5.92 | 0.07 | 0.04 | - | - | 2.62 | |
| Complex II | k | 0.0358 | 0.0792 | 0.0581 | 0.0551 | 0.0255 | 0.6294 | 1.9614 | - | - | 0.2275 |
| t0.5 | 19.35 | 8.75 | 11.92 | 12.59 | 27.23 | 1.10 | 0.35 | - | - | 3.05 | |
| t0.1 | 2.94 | 1.33 | 1.81 | 1.91 | 4.14 | 0.17 | 0.05 | - | - | 0.46 | |
| Tablets | k | 0.0339 | 0.0678 | 0.0622 | 0.0752 | 0.0200 | 3.2800 | 3.5300 | - | - | 0.0250 |
| t0.5 | 20.44 | 10.22 | 11.14 | 9.22 | 34.65 | 0.21 | 0.20 | - | - | 27.72 | |
| t0.1 | 3.11 | 1.55 | 1.69 | 1.40 | 5.27 | 0.03 | 0.03 | - | - | 4.22 | |
| Radiation | Parameter | Substance | Complex I | Complex II | Tablets | |
|---|---|---|---|---|---|---|
| UV | k | 0.5583 | 0.2583 | 0.6087 | 0.3883 | |
| t0.5 | 1.24 | 2.68 | 1.14 | 1.78 | ||
| t0.1 | 0.19 | 0.41 | 0.17 | 0.27 | ||
| Sunlight | Solutions | k | 0.0467 | 0.0450 | 0.0251 | 0.0650 |
| t0.5 | 14.84 | 15.40 | 27.61 | 10.66 | ||
| t0.1 | 2.26 | 2.34 | 4.20 | 1.62 | ||
| Solid state | k | 0.0013 | 0.0014 | 0.0074 | 0.0036 | |
| t0.5 | 533.08 | 495.00 | 93.67 | 192.5 | ||
| t0.1 | 81.08 | 75.29 | 14.23 | 29.28 | ||
| Control | ||||||
| UV | k | 0.0200 | 0.0183 | 0.0251 | 0.0367 | |
| t0.5 | 34.65 | 37.87 | 27.61 | 18.88 | ||
| t0.1 | 5.27 | 5.76 | 4.20 | 2.87 | ||
| Sunlight | Solutions | k | 0.0217 | 0.0200 | 0.0235 | 0.0233 |
| t0.5 | 31.94 | 34.65 | 29.54 | 29.74 | ||
| t0.1 | 4.86 | 5.27 | 4.49 | 4.52 | ||
| Solid state | k | 0.0002 | 0.0002 | 0.0001 | 0.0003 | |
| t0.5 | 3465.00 | 3465.00 | 6753.73 | 2310.00 | ||
| t0.1 | 527.00 | 527.00 | 1026.22 | 351.33 | ||
| Solution | Equations | r | Ea [J/mol] |
|---|---|---|---|
| Standard substance | |||
| MeOH | logk = 0.9328−0.8352 · 1/T | 0.9996 | 16,183.26 |
| 3M HCl | logk = 1.8824−1.0550 · 1/T | 0.9480 | 19,849.41 |
| 1M HCl | logk = 0.8641−0.7560 · 1/T | 0.9822 | 15,059.71 |
| 0.5M HCl | logk = −0.5991−0.3440 · 1/T | 0.9966 | 6536.26 |
| 0.1M HCl | logk = −0.5264−0.3824 · 1/T | 0.9996 | 7371.54 |
| 0.1M NaOH | logk = 7.9886−2.9240 · 1/T | 0.9956 | 56,819.34 |
| 0.5M NaOH | logk = 7.9068−2.7950 · 1/T | 0.9993 | 54,248.64 |
| H2O2 | logk = 0.9600−0.8033 · 1/T | 0.9970 | 15,435.33 |
| Complex I | |||
| MeOH | logk = 0.5410−0.7374 · 1/T | 0.9788 | 14,227.71 |
| 3M HCl | logk = 1.6369−0.9879 · 1/T | 0.9057 | 18,615.45 |
| 1M HCl | logk = 1.4218−0.8912 · 1/T | 0.9341 | 17,832.89 |
| 0.5M HCl | logk = 2.9912−1.5310 · 1/T | 0.9837 | 29,645.84 |
| 0.1M HCl | logk = −0.5641−0.4440 · 1/T | 0.9104 | 8798.15 |
| 0.1M NaOH | logk = 9.8273−3.5620 · 1/T | 0.9795 | 69,335.86 |
| 0.5M NaOH | logk = 8.8320−3.0810 · 1/T | 0.9982 | 59,659.80 |
| H2O2 | logk = −0.4459−0.3560 · 1/T | 0.9265 | 7171.47 |
| Complex II | |||
| MeOH | logk = 1.3067−1.0011 · 1/T | 0.9998 | 19,425.12 |
| 3M HCl | logk = 1.8293−1.0396 · 1/T | 0.9311 | 19,602.68 |
| 1M HCl | logk = −0.4581−0.2879 · 1/T | 0.9809 | 5819.35 |
| 0.5M HCl | logk = 2.7098−1.4099 · 1/T | 0.9350 | 26,426.23 |
| 0.1M HCl | logk = 0.1263−0.6209 · 1/T | 0.9966 | 11,609.09 |
| 0.1M NaOH | logk = 7.0361−2.6341 · 1/T | 0.9999 | 50,457.27 |
| 0.5M NaOH | logk = 6.6784−2.2956 · 1/T | 0.9824 | 43,777.28 |
| H2O2 | logk = 3.9259−1.6473 · 1/T | 0.9922 | 32,872.42 |
| Tablets | |||
| MeOH | logk = −0.0520−0.5220 · 1/T | 0.9818 | 10,176.46 |
| 3M HCl | logk = 0.1622−0.4681 · 1/T | 0.9751 | 8714.21 |
| 1M HCl | logk = −0.4612−0.2725 · 1/T | 0.9999 | 5456.29 |
| 0.5M HCl | logk = −0.2585−0.3110 · 1/T | 0.9933 | 5835.70 |
| 0.1M HCl | logk = 1.1299−1.0030 · 1/T | 0.9259 | 19,153.25 |
| 0.1M NaOH | logk = 11.3210−3.9570 · 1/T | 0.9930 | 76,854.51 |
| 0.5M NaOH | logk = 9.7085−3.2890 · 1/T | 0.9806 | 62,849.16 |
| H2O2 | logk = −1.3350−0.0934 · 1/T | 0.8910 | 1607.62 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Starek, M.; Dąbrowska, M.; Chebda, J.; Żyro, D.; Ochocki, J. Stability of Metronidazole and Its Complexes with Silver(I) Salts under Various Stress Conditions. Molecules 2021, 26, 3582. https://doi.org/10.3390/molecules26123582
Starek M, Dąbrowska M, Chebda J, Żyro D, Ochocki J. Stability of Metronidazole and Its Complexes with Silver(I) Salts under Various Stress Conditions. Molecules. 2021; 26(12):3582. https://doi.org/10.3390/molecules26123582
Chicago/Turabian StyleStarek, Małgorzata, Monika Dąbrowska, Joanna Chebda, Dominik Żyro, and Justyn Ochocki. 2021. "Stability of Metronidazole and Its Complexes with Silver(I) Salts under Various Stress Conditions" Molecules 26, no. 12: 3582. https://doi.org/10.3390/molecules26123582
APA StyleStarek, M., Dąbrowska, M., Chebda, J., Żyro, D., & Ochocki, J. (2021). Stability of Metronidazole and Its Complexes with Silver(I) Salts under Various Stress Conditions. Molecules, 26(12), 3582. https://doi.org/10.3390/molecules26123582