Abstract
A second generation of 4-aminoquinoline- and 8-aminoquinoline-based tetrazoles and lactams were synthesized via the Staudinger and Ugi multicomponent reactions. These compounds were subsequently evaluated in vitro for their potential antiplasmodium activity against a multidrug-resistant K1 strain and for their antitrypanosomal activity against a cultured T. b. rhodesiense STIB900 strain. Several of these compounds (4a–g) displayed good antiplasmodium activities (IC50 = 0.20–0.62 µM) that were comparable to the reference drugs, while their antitrypanosomal activity was moderate (<20 µM). Compound 4e was 2-fold more active than primaquine and was also the most active (IC50 = 7.01 µM) against T. b. rhodesiense and also exhibited excellent aqueous solubility (>200 µM) at pH 7.
1. Introduction
Neglected tropical and infectious diseases caused by protozoan organisms such as kinetoplastid and plasmodium parasites pose a great danger to human lives. Key among these protozoan infections are the human African trypanosomiasis (HAT), also known as sleeping sickness, caused by two Trypanosoma brucei subspecies (i.e., Trypanosoma brucei rhodesiense (T. b. rhodesiense) and Trypanosoma brucie gambiense (T. brucie gambiense)) and malaria caused by five plasmodium species, with Plasmodium falciparum (P. falciparum) being the most virulent [1,2,3,4]. HAT is fatal if left untreated, and T. b. gambiense accounts for about 95–97% of all cases while T. b. rhodesiense accounts for only 3–5% [5]. In 2018 HAT and malaria had over 229 million reported cases and 405,000 deaths, the majority being attributed to the latter [6,7]. However, the number of reported cases and deaths have been dropping significantly due, in part, to the World Health Organization (WHO) goals of eliminating both HAT and malaria [8,9].
Currently, there are no vaccines against HAT and malaria, even though both have been around for over a century. There are very few drugs available for treating HAT, and their use depends on the causative species and the stage, the disease [2,10]. Furthermore, the drawbacks of drugs such as melarsoprol, pentamidine, suramin and their combinations are the need to be administered intravenously (IV), toxicity and the ever-present threat of drug resistance emerging [11,12,13,14]. Thus, there is an urgent need for the development of a pipeline of new drugs against this disease. On the other hand, resistant forms of the plasmodium parasite are threatening the mainstay of malaria chemotherapy, artemisinin [15,16,17,18]. Therefore, there is also an urgent need for continuously developing newer drugs that will be efficacious against all forms of the plasmodia parasite.
Previously we reported a series of 4-aminoquinoline- based tetrazole compounds that were more efficacious, against both the chloroquine (CQ)-sensitive and -resistant strains, than the reference drug CQ [19,20]. Herein we report a limited and focused number of the second generation of compounds where the tetrazole moiety is replaced by a β-lactam ring and the 4-amino-7chloroquinoline ring is replaced by primaquine, an 8-aminoquinoline (Figure 1).
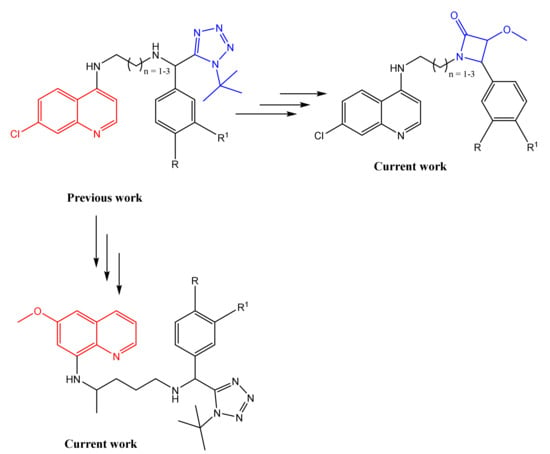
Figure 1.
Rationale for the current work.
In the last decade and a half, a number of literature reports have highlighted the use of the β-lactam moiety in antiprotozoal research, especially as antiplasmodium agents [21,22,23,24,25,26,27]. D’hooge et al. reported a series of nontoxic cis-β-lactams that exhibited IC50 values in the micromolar range against the CQ-sensitive D10 strain [22] and Singh et al. also reported a series of β-lactams that exhibited low micromolar potency against three P. falciparum strains (D10, K1 and W2) [23]. On the other hand, Kumar and coworkers also reported a number of 4-amino-7chloroquinoline-β-lactam hybrids that possessed antiplasmodium activity in the low micromolar to nanomolar range against the CQ-resistant W2 strain [24,25]. These hybrids were synthesized by coupling the 4-amino-7chloroquinoline moieties to separately prepared β-lactams. In our current work, the 4-amino-7chloroquinoline-β-lactam hybrids are prepared via the Staudinger reaction [22,28], where the 4-amino-7chloroquinoline plays the role of an amine input in the reaction. Our group also previously reported the antitrypanosomal activity of gamma- and delta-lactams [29], and to our knowledge, there has not been any literature report of the evaluation of β-lactam-quinoline hybrids as antitrypanosomal agents. This is in view of the fact that antimalarial 8-aminoquinolines, i.e., primaquine and tafenoquine, have been reported to show promising antitrypanosomal activity [30,31,32].
2. Results and Discussion
2.1. Chemistry
The synthesis of the 4-amino-7chloroquinoline tethered β-lactams commenced by reacting key diamines 2a–c [33] with various aromatic aldehydes (1a–e) to give Schiff-bases 3a–g, which were dried under vacuum prior to use in the next step without any further purification (Scheme 1). The Staudinger reaction of the Schiff-bases with the methoxy acetyl chloride exclusively furnished the cis β-lactam products (4a–g) in 4–30% yields and over 90% purity except for 4f that still contained starting Schiff base traces even after extensive purification. The cis configuration was assigned based on the 1H NMR model proposed by Xu and coworkers [34,35]. According to the model, when the J-coupling constant between the two protons on the adjacent chiral carbons of the β-lactam ring is between 4 and 6 ppm, then the configuration is cis, while between 2 and 3 ppm indicates trans. The same aromatic aldehydes (1a–e) used in β-lactams synthesis were also subjected to the isocyanide-based Ugi multicomponent reaction involving TMSN3 and commercially available primaquine diphosphate salt (5) following the method as reported by our group [19,36,37,38] (Scheme 1). The desired target compounds (6a–e) were obtained in 21–50% yields as 1:1 diastereomeric mixtures due to the fact that the primaquine salt used in this study is racemic. Attempts to separate/resolve the diastereomeric mixture using HPLC was unsuccessful owing to the fact these diastereomeric mixtures coalesce into a single sharp peak in the HPLC chromatograms (Figures S1–S5) irrespective of whether the gradient of the mobile phase or pH is varied. Thus, for subsequent biological studies, these 1:1 diastereomeric mixtures were used without being separated/resolved. All the synthesized target compounds were fully characterized using routine spectroscopic methods (NMR, IR, MS) (see Table S1 and NMR Figures in supplementary material) NMR, IR, MS) and their yields and HPLC purities can be found in Table S1 in the supplementary material.
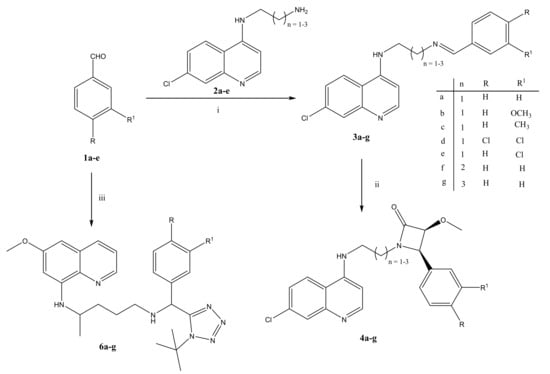
Scheme 1.
Reagents and conditions: (i) MeOH, rt, 24 h; (ii) methoxy acetyl chloride, Et3N, DCM, N2, −78 °C (0.5 h), then overnight for 18 h at room temperature; (iii) Primaquine diphosphate salt (4), Et3N, TMSN3, t-Butyl isocyanide, MeOH, 40 °C, 24 h.
2.2. In Vitro Antiprotozoan Activities
All the synthesized compounds were evaluated in vitro for their antiplasmodium (against the multidrug K1 resistant strain) and antitrypanosomal activity (against the cultured T. b. brucei rhodesiense STIB900 parasite strain). Chloroquine, primaquine, melarsoprol and podophyllotoxin were used as a positive control, and the results are tabulated in Table 1. Generally, all β-lactam based compounds were more potent than primaquine-based tetrazoles. Furthermore, all the β-Lactam based compounds also showed superior antiplasmodium activity compared to primaquine, with the most active 3,4-dichloro substituted 4d (IC50 = 0.20 µM) being 3-fold more active than primaquine and equipotent to chloroquine. The β-lactam based compounds with the butyl spacer (IC50 = 0.31 µM) was the most favored compound to the ones with the ethyl (IC50 = 0.47 µM) and propyl spacers (IC50 = 0.57 µM). None of the primaquine-based tetrazoles had better antiplasmodium activity than the two reference drugs, with the most active, 3,4-dichloro substituted 6d (IC50 = 1.31 µM), being 2-fold less active than primaquine and 6-fold less active than chloroquine. In terms of electronic effects, compounds with an aromatic ring containing moderately electron-withdrawing groups (EWG) (Cl or diCl) substitutions fared better than those contain electron-donating groups (EDG) (Me and OCH3) in both sets of compounds. The selective indices of some of the active β-lactam-based compounds were closer to or greater than 100, indicating that these compounds are more selective towards the chloroquine-resistant Plasmodium falciparum parasite. Disappointingly, none of these compounds were as potent as the first-generation compounds against the K1 strain.

Table 1.
In vitro antiplasmodium and antitrypanosomal activity, and cytotoxicity of the synthesized target compounds.
Against T. b. brucei rhodesiense, all the tested compounds showed moderate activity, and none of them had superior activity than the reference drug, melarsoprol. Compounds 4e (IC50 = 7.01 µM) and 6b (IC50 = 14.16 µM) were the most active in the β-lactam and tetrazole series, respectively. Unlike in the case of activity against Plasmodium falciparum, the compound with the propyl spacer (IC50 = 16.09 µM) was more active than compounds with ethyl (IC50 = 53.16 µM) and butyl spacers (IC50 = 20.00 µM). In terms of electronic effects, compounds containing EWG in the β-lactam series were more active than those containing EDG. However, in the tetrazole series, no electronic effect trend could be delineated since all the compounds had comparable activities.
2.3. Solubility of Selected Compounds
A limited number of compounds from each series were selected for assessment of their aqueous kinetic solubility at pH 7.0:4a, 4e and 4g from the β-lactam series and 6c–e from the tetrazole series (Table 2). These compounds were selected based on their good antiplasmodium (4a, 4e and 4g), promising antitrypanosomal (4d and 6c–e) and excellent cytotoxicity (4a and 6d) profiles. The β-lactam series of compounds generally exhibited excellent solubility (>200 µM) compared to the tetrazole series compounds (<20 µM).

Table 2.
Aqueous kinetic solubility of selected compounds.
3. Materials and Methods
3.1. General Information
All chemical reagents used were supplied by either Sigma-Aldrich® (Johannesburg, South Africa) or Merck (Johannesburg, South Africa) and were used without further purification. Unless otherwise stated, all the solvents used were anhydrous and were purchased from Sigma-Aldrich, with the exception of THF and diethyl ether, which were dried in-house prior to use. Chromatographic solvents such as Ethyl acetate, dichloromethane and Hexane were purchased from Kimix (Cape Town, South Africa) or Protea Chemicals (Cape Town, South Africa) and were distilled prior to use. HPLC grade methanol, acetonitrile and formic acid (98–100%) were purchased from Sigma-Aldrich. Ultrafiltered water was purified by a Millipore Synergy water purification system (Microsep, Tygervalley, South Africa) and was used as such. The progress of the reactions was monitored by thin-layer chromatography (TLC) using Merck PF254 aluminum-backed precoated silica gel plates and viewed under ultraviolet (UV) light, and where necessary, visualized using iodine vapor. Product purification on the flash column chromatography was carried out using Merck Kieselgel 60:70–230 mesh. Both analytical and preparative HPLC were performed on a modular Waters HPLC system consisting of a 2767 sample manager, 2545 quaternary gradient pump, 1500 series column heater and a 2998 photodiode array detector (PDA) with a Prep 2998 flow-cell. Waters Xbridge C18, 4.6 × 150 mm column with 5 μm particles was used for the analytical scale HPLC analysis and a Waters Xbridge C18, OBD 19 × 250 mm preparative HPLC column was used for the purifications. Xbridge C18, 5 μm, 4.6 × 20 mm and Xbridge C18, 5 μm, 19 × 10 mm guard columns were connected to the inlets of the analytical and preparative HPLC columns, respectively (Microsep, Tygervalley, South Africa).
Melting points were determined on a Reichert-Jung Thermovar hot-stage microscope and are uncorrected. Mass spectra were obtained by flow-injection (5 mM NH4 formate pH 3 in H2O:ACN, no column used) on an AB SCIEX 4000 QTRAP Hybrid triple quadrupole linear ion trap mass spectrometer, coupled with an Agilent 1200 Rapid Resolution (600 bar) HPLC system consisting of a binary pump, degasser, autosampler and temperature-controlled column compartment (SCIEX, Johannesburg, South Africa). Infra-red spectra were recorded on a PerkinElmer Spectrum 100 FT-IR spectrometer (Perkin Elmer SA, Midrand, South Africa) in the 4000–450 cm−1 range, with samples dissolved in dichloromethane solution or placed in KBr discs. NMR spectra were recorded on Bruker 400 MHz (Bruker, Bremen, Germany) and/or Varian Unity 400 MHz and/or Varian Mercury 300 MHz spectrometers (Agilent Technologies, CA, USA). All chemical shifts are reported in ppm and were referenced using solvent signals (2.50 and 39.4 ppm for DMSO-d6 and 7.6 and 77.0 ppm for CDCl3). Chemical shifts (δ) are recorded in parts per million (ppm). Coupling constants, J, are measured in Hertz (Hz) and rounded off to one decimal place. Abbreviations used in the assignment of the 1H NMR spectra are as follows: br (broad), d (doublet), dd (doublet of doublets), m (multiplets), s (singlet) and t (triplet). 13C NMR chemical shifts are listed without assignment to specific carbon atoms.
3.2. Synthetic Procedures
3.2.1. General Procedure for the β-Lactam Based Compounds
Quinoline diamine 2a–c (1.0 mmol) and various aldehydes 1a–e (1.0 mmol) were stirred in anhydrous methanol at room temperature for 24 h to afford Schiff-based 3a–g that were subsequently used, after drying in vacuum for 4 h, in the next step without any further purification. Dry DCM (5 mL), followed by Et3N (3.0 eq), was added to the Schiff-based (1.0 eq), and the resulting mixtures were stirred for 30 min at −78 °C under an inert N2 atmosphere. Thereafter, methoxy acetyl chloride (1.5 eq) in dry DCM (2 mL) was added to the mixtures dropwise, mixtures allowed to warm to room temperature and further stirred overnight for 18 h at room temperature. On completion, saturated NaHCO3 was then added to the mixtures and the organic layers extracted with DCM. The combined organic extracts were then washed with brine and water, dried over anhydrous MgSO4 and solvent removed in vacuo to yield crude products, which were purified by column chromatography to exclusively give the cis β-lactam based compounds:
1-[2-(7-Chloroquinolin-4-ylamino)ethyl]-3-methoxy-4-phenylazetidin-2-one4a, as a white solid (55 mg, 30%); m. p. 142–146 °C, Rf (DCM: MeOH, 95: 0.5%) 0.21; IR νmax (DCM)/cm−1 1744 (C = O), 1620 (Ar C = C), 1264 (C-O ester); δH (400 MHz; CDCl3) 8.49 (1H, d, J 5.4 Hz, H2), 8.00 (1H, d, J 2.1 Hz, H8), 7.84 (1H, d, J 9.0 Hz, H5), 7.42 (1H, dd, J 9.0 and 2.1 Hz, H6), 7.35 (5H, m, ArH), 6.26 (1H, d, J 5.4 Hz, H3), 4.97 (1H, d, J 4.4 Hz, H1′), 4.74 (1H, d, J 4.4 Hz, H2′), 3.54 (2H, m, H9), 3.46 (2H, m, H10), 3.12 (3H, s, H6′); δC (101 MHz; CDCl3) 168.9, 151.5, 149.8, 148.8, 135.3, 133.2, 129.1, 128.7 (2C), 128.4, 128.3 (2C), 125.8, 121.9, 117.3, 98.5, 85.4, 63.3, 58.2, 42.4 and 41.1; LCMS m/z 382 [M + H]+, HPLC purity: 95.6%; tr’ = 8.37 min.
1-[2-(7-Chloroquinolin-4-ylamino)ethyl]-3-methoxy-4-(4-methoxyphenyl)azetidin-2-one4b, as a yellow solid (44.6 mg, 9%); m. p. 144–147 °C, Rf (DCM: MeOH, 95: 0.5%) 0.21; IR νmax (DCM)/cm−1 1746 (C = O), 1610 (Ar C = C), 1267 (C-O ester); δH (400 MHz; CDCl3) 8.48 (1H, d, J 5.5 Hz, H2), 7.98 (1H, s, H8), 7.84 (1H, d, J 8.9 Hz, H5), 7.41 (1H, d, J 9.0, H6), 7.24 (2H, d, J 8.6 Hz, 2 × H4′), 6.86 (2H, d, J 8.6 Hz, 2 × H3′), 6.45 (1H, br s, NH), 6.25 (1H, d, J 5.5 Hz, H3), 4.74 (1H, d, J 4.4 Hz, H1′), 4.71 (1H, d, J 4.4 Hz, H2′), 3.79 (3H, s, H5′), 3.52 (2H, t, J 4.6 Hz, H9), 3.47 (1H, m, H10a), 3.41 (1H, m, H10b), 2.92 (3H, s, H6′); δC (101 MHz; CDCl3) 169.0, 160.3, 151.1, 150.1, 148.3, 135.5, 129.7 (2C), 127.9, 125.9, 124.8, 122.0, 117.2, 114.1 (2C), 98.4, 85.3, 62.9, 58.2, 55.3, 42.5 and 40.9; LCMS m/z 412 [M + H]+, HPLC purity: 95.6%; tr’ = 8.43 min.
1-[2-(7-Chloroquinolin-4-ylamino)ethyl]-3-methoxy-4-p-tolylazetidin-2-one4c, as a yellow solid (21 mg, 6%); m. p. 78–82 °C, Rf (DCM: MeOH, 95: 0.5%) 0.31; IR νmax (DCM)/cm−1 1744 (C = O), 1604 (Ar C = C), 12,652 (C-O ester); δH (400 MHz; CDCl3) 8.49 (1H, d, J 1.3 Hz, H8), 8.04 (1H, d, J 5.7 Hz, H2), 7.88 (1H, d, J 9.0 Hz, H5), 7.41 (1H, dd, J 9.0 and 1.3 Hz, H6), 7.20 (2H, d, J 8.0 Hz, 2 × H4′), 7.14 (2H, d, J 8.0 Hz, 2 × H3′), 6.78 (1H, br s, NH), 6.24 (1H, d, J 5.7 Hz, H3), 4.77 (1H, d, J 4.3 Hz, H1′), 4.73 (1H, d, J 4.3 Hz, H2′), 3.52 (2H, m, H9), 3.47 (2H, m, H10), 3.13 (3H, s, H6′), 2.35 (3H, s, H5′); δC (101 MHz; CDCl3) 169.0, 150.7, 150.1, 147.1, 139.1, 136.0, 129.4 (2C), 128.9, 128.4 (2C), 126.8, 126.1, 122.3, 117.0, 98.2, 85.3, 63.1, 58.2, 42.5, 40.9 and 21.6; LCMS m/z 396 [M + H]+, HPLC purity: 94.6%; tr’ = 8.29 min.
1-[2-(7-Chloroquinolin-4-ylamino)ethyl]-4-(3,4-dichlorophenyl)-3-methoxyazetidin-2-one4d, as a pale-yellow solid (17.3 g, 4%); m. p. 152–156 °C, Rf (DCM: MeOH, 95: 0.5%) 0.16; IR νmax (DCM)/cm−1 1739 (C = O), 1601 (Ar C = C), 1277 (C-O ester); δH (400 MHz; CDCl3) 8.45 (1H, d, J 5.6 Hz, H2), 7.96 (1H, d, J 2.1 Hz, H8), 7.89 (1H, d, J 9.0 Hz, H5), 7.39 (3H, m, ArH), 7.16 (1H, dd, J 9.0 and 2.1 Hz, H6), 6.74 (1H, br s, NH), 6.25 (1H, d, J 5.6 Hz, H3), 4.78 (1H, d, J 4.4 Hz, H1′), 4.74 (1H, d, J 4.4 Hz, H2′), 3.49 (2H, m, H9), 3.43 (2H, m, H10), 3.18 (3H, s, H6′); δC (101 MHz; CDCl3) 168.6, 150.5, 150.1, 147.2, 136.0, 133.7, 133.2, 130.6, 130.4, 127.6, 127.0, 126.2, 126.1, 122.2, 117.0, 98.3, 85.5, 62.1, 58.6, 42.2 and 41.0; LCMS m/z 450 [M + H]+, HPLC purity: 99.5%; tr’ = 8.81 min.
1-[2-(7-Chloroquinolin-4-ylamino)ethyl]-4-(4-chlorophenyl)-3-methoxyazetidin-2-one4e, as a pale-yellow solid (20.3 mg, 14%); m. p. 135–138 °C, Rf (DCM: MeOH, 95: 0.5%) 0.14; IR νmax (DCM)/cm−1 1740 (C = O), 1598 (Ar C = C), 1265 (C-O ester); δH (400 MHz; CDCl3) 8.46 (1H, d, J 5.3 Hz, H2), 7.95 (1H, d, J 1.9 Hz, H8), 7.85 (1H, d, J 9.0 Hz, H5), 7.39 (1H, dd, J 9.0 and 1.9 Hz, H6), 7.31 (2H, d, J 8.5 Hz, 2 × H4′), 7.24 (2H, d, J 8.5 Hz, 2 × H3′), 6.47 (1H, br s, NH), 6.24 (1H, d, J 5.3 Hz, H3), 4.77 (1H, d, J 4.3 Hz, H1′), 4.72 (1H, d, J 4.3 Hz, H2′), 3.54 (1H, m, H10a), 3.47 (3H, m, H9 and H10b), 3.13 (3H, s, H6′); δC (101 MHz; CDCl3) 168.7, 151.2, 150.0, 148.4, 135.4, 135.1, 131.2, 129.7 (2C), 128.9 (2C), 128.0, 125.9, 122.0, 117.2, 98.5, 85.4, 62.6, 58.3, 42.3 and 41.0; LCMS m/z 416 [M + H]+, HPLC purity: 99.2%; tr’ = 8.63 min.
1-[3-(7-Chloroquinolin-4-ylamino)propyl]-3-methoxy-4-phenylazetidin-2-one4f, as a yellow paste (19.6 mg, 5%); Rf (DCM: MeOH, 95: 0.5%) 0.41; IR νmax (DCM)/cm−1 1745 (C = O), 1609 (Ar C = C), 1269 (C-O ester); δH (300 MHz; CDCl3) 8.59 (1H, d, J 5.4 Hz, H2), 8.11–8.13 (2H, m, H5 and H8), 7.99 (1H, d, J 9.0 Hz, H6), 7.47–7.52 (5H, m, ArH), 6.51 (1H, br s, NH), 6.46 (1H, d, J 5.4 Hz, H3), 4.85 (1H, d, J 4.4 Hz, H1′), 4.81 (1H, d, J 4.4 Hz, H2′), 3.60 (3H, s, H6′), 3.49 (2H, m, H11), 3.28 (2H, m, H9), 1.97 (2H, m, H10; δC (75 MHz; CDCl3) 169.2, 155.2, 147.5, 140.1, 136.8, 136.2, 129.0, 128.7, 128.5, 128.1, 126.6, 125.9, 124.3, 122.1, 117.5, 98.5, 85.1, 61.9, 57.8, 42.1, 40.5 and 26.7; LCMS m/z 397 [M + H]+.
1-[4-(7-Chloroquinolin-4-ylamino)butyl]-3-methoxy-4-phenylazetidin-2-one4g, as a yellow solid (28 mg, 9%); m. p. 86–89 °C, Rf (DCM: MeOH, 95: 0.5%) 0.31; IR νmax (DCM)/cm−1 1739 (C = O), 1599 (Ar C = C), 1258 (C-O ester); δH (400 MHz; CDCl3) 8.39 (1H, d, J 9.0 Hz, H5), 8.28 (1H, d, J 5.6 Hz, H2), 8.05 (1H, s, H8), 7.92 (1H, br s, NH), 7.36 (6H, m, H6 and ArH), 6.39 (1H, d, J 5.6 Hz, H3), 4.76 (1H, d, J 4.3 Hz, H1′), 4.73 (1H, d, J 4.3 Hz, H2′), 3.44 (4H, m, H9 and H12), 3.09 (3H, s, H6′), 1.92 (2H, m, H10), 1.72 (2H, m, H11); δC (101 MHz; CDCl3) 167.7, 153.5, 145.6, 142.7, 137.7, 133.5, 128.8, 128.5 (2C), 128.4, 126.7, 126.2, 124.1, 123.1, 116.3, 98.1, 85.4, 62.4, 58.1, 43.2, 40.9, 25.6 and 25.1; LCMS m/z 411 [M + H]+, HPLC purity: 98.0%; tr’ = 9.12 min.
3.2.2. General Procedure for the Tetrazole-Based Compounds
The procedure reported previously by Tukulula et al. [19,36,37,38] was followed. Briefly, the commercially available racemic primaquine diphosphate salt 4 (1.1 mmol) was reacted, in Ugi multicomponent reaction conditions, with aromatic aldehydes 1a–e (1.1 mmol), TMSN3 (1.1 mmol) and tert-butyl isocyanide (1.1 mmol) in the presence of triethylamine (4.0 eq) at 40 °C for 24 h to afford crude desired compounds as unresolved racemic 1:1 diastereomeric mixtures 6a–e. These the diastereomeric mixtures were then purified by both column chromatography and by HPLC with no success in resolving/separating them:
N-{5-[(1-tert-butyl-1H-tetrazol-5-yl)(phenyl)methylamino]pentan-2-yl}-6-methoxyquinolin-8-amine6a, as a thick yellow oil (108.4 mg, 21%); Rf (DCM: acetone, 95: 05%) 0.50; IR νmax (DCM)/cm−1 1615 (Ar C = C), 1385 (N = N), 1315 (C = N), 1266 (C-N), 1254 (C-O ester); δH (300 MHz; CDCl3) 8.47 (1H, m, H2), 7.88 (1H, dd, J 8.3 and 1.6 Hz, H4), 7.25 (6H, m, ArH and H3), 6.29 (1H, d, J 2.3 Hz, H7), 6.23 (1H, d, J 2.5 Hz, H5), 5.99 (1H, t, J 8.6 Hz, NH), 5.22 (1H, d, J 6.7 Hz, H14), 3.85 (3H, s, OCH3), 3.56 (1H, m, H9), 2.53 (2H, m, H13), 1.63 (4H, m, H11 and H12), 1.55 (9H, d, J 4.04 Hz, 3 × H18), 1.25 (3H, d, J 6.4 Hz, H10); δC (75 MHz; CDCl3) 159.5, 155.7, 145.0, 144.2, 138.9, 135.4, 134.7, 129.9, 128.9 (2C), 128.2, 128.0 (2C), 121.8, 96.6, 91.6, 61.2, 59.0, 55.2, 55.1, 47.9, 34.2, 30.0 (3C), 26.5 and 20.5; LCMS m/z 474 [M + H]+; HPLC purity: 99.3%; tr’ = 16.53 min.
N-{5-[(1-tert-butyl-1H-tetrazol-5-yl)(methoxyphenyl)methylamino]pentan-2-yl}-6-methoxyquinolin-8-amine6b, as a yellow oil (219.6 mg, 40%); Rf (DCM: acetone, 95: 05%) 0.54; IR νmax (DCM)/cm−1 1615 (Ar C = C), 1387 (N = N), 1269 (C = N), 1266 (C-N), 1217 (C-O ester); δH (400 MHz; CDCl3) 8.50 (1H, m, H2), 7.91 (1H, dd, J 8.3 and 1.8 Hz, H4), 7.29 (1H, dd, J 8.2 and 4.2 Hz, H3), 7.18 (2H, m, 2 × H16), 6.83 (2H, m, 2 × H15), 6.32 (1H, d, J 1.7 Hz, H7), 6.26 (1H, d, J 1.7 Hz, H5), 6.03 (1H, m, NH), 5.20 (1H, d, J 9.0 Hz, H14), 3.85 (3H, d, J 1.4 Hz, OCH3), 3.77 (3H, d, J 1.0 Hz, H17), 3.61 (1H, m, H9), 2.54 (2H, m, H13), 1.67 (4H, m, H11 and H12), 1.57 (9H, d, J 5.3 Hz, 3 × H18), 1.28 (3H, d, J 6.4 Hz, H10); δC (101 MHz; CDCl3) 159.4, 155.9, 145.1, 144.2 (2C), 135.4, 134.7 (2C), 131.0, 129.9, 129.2 (2C), 121.8, 114.3 (2C), 96.6, 91.6, 61.1, 58.3, 55.2, 47.9 (2C), 34.2, 30.0 (3C), 26.5 and 20.5; LCMS m/z 505 [M + H]+; HPLC purity: 99.5%; tr’ = 16.39 min.
N-{5-[(1-tert-butyl-1H-tetrazol-5-yl)(p-tolyl)methylamino]pentan-2-yl}-6-methoxyquinolin-8-amine6c, as a thick yellow oil (265.9 mg, 50%); Rf (DCM: acetone, 95: 05%) 0.51; IR νmax (DCM)/cm−1 1617 (Ar C = C), 1390 (N = N), 1325 (C = N), 1260 (C-N), 1232 (C-O ester); δH (400 MHz; CDCl3) 8.53 (1H, m, H2), 7.93 (1H, dd, J 8.3 and 1.7 Hz, H4), 7.31 (1H, dd, J 8.3 and 4.2 Hz, H3), 7.12–7.18 (4H, m, 2 × H16 and 2 × H15), 6.34 (1H, d, J 2.4 Hz, H7), 6.28 (1H, d, J 2.5 Hz, H5), 6.04 (1H, m, NH), 5.29 (1H, d, J 8.2 Hz, H14), 3.91 (3H, d, J 1.3 Hz, OCH3), 3.62 (1H, m, H9), 2.57 (2H, m, H13), 2.33 (3H, s, H17), 1.69 (4H, m, H11 and H12), 1.60 (9H, d, J 5.4 Hz, 3 × H18), 1.25 (3H, d, J 6.4 Hz, H10); δC (101 MHz; CDCl3) 159.5, 155.9, 145.1, 144.2 (2C), 138.0, 135.9, 135.2, 134.7, 129.9, 129.6 (2C), 127.9 (2C), 121.8, 96.6, 91.6, 61.2, 58.7, 55.2, 47.9, 34.2, 30.1 (3C), 26.5, 21.0 and 20.5; LCMS m/z 488 [M + H]+; HPLC purity: 99.4%; tr’ = 16.99 min.
N-{5-[(1-tert-butyl-1H-tetrazol-5-yl)(3,4-dichlorophenyl)methylamino]pentan-2-yl}-6-methoxyquinolin-8-amine6d, as a thick yellow oil (100.5 mg, 17%); Rf (DCM: acetone, 95: 0.5%) 0.29; IR νmax (DCM)/cm−1 1609 (Ar C = C), 1383 (N = N), 1330 (C = N), 1264 (C-N), 1245 (C-O ester); δH (400 MHz; CDCl3) 8.53 (1H, m, H2), 7.94 (1H, dd, J 8.3 and 1.6 Hz, H4), 7.42 (1H, d, J 2.1 Hz, H15), 7.42 (1H, d, J 2.1 Hz, H16), 7.38 (1H, s, H17), 7.32 (1H, dd, J 8.2 and 4.2 Hz, H3), 6.35 (1H, d, J 1.9 Hz, H7), 6.28 (1H, d, J 2.2 Hz, H5), 6.03 (1H, m, NH), 5.22 (1H, d, J 9.6 Hz, H14), 3.91 (3H, d, J 1.4 Hz, OCH3), 3.62 (1H, m, H9), 2.56 (2H, m, H13), 1.69 (4H, m, H11 and H12), 1.63 (9H, d, J 12.1 Hz, 3 × H18), 1.25 (3H, d, J 6.4 Hz, H10); δC (101 MHz; CDCl3) 159.5, 155.0, 145.0, 144.3, 139.1, 135.4, 134.7, 132.6, 130.8, 130.1, 130.0, 129.9, 127.3, 121.8, 96.6, 91.6, 61.3, 57.8, 55.2, 47.9, 34.0, 33.7, 30.0 (3C), 26.5 and 20.8; LCMS m/z 542.0 [M + H]+; HPLC purity: 99.5%; tr’ = 18.28 min.
N-{5-[(1-tert-butyl-1H-tetrazol-5-yl)(4-chlorophenyl)methylamino]pentan-2-yl}-6-methoxyquinolin-8-amine6e, as a thick yellow oil (228.5 mg, 41%); Rf (DCM: acetone, 95: 0.5%) 0.52; IR νmax (DCM)/cm−1 1630 (Ar C = C), 1380 (N = N), 1335 (C = N), 1278 (C-N), 1246 (C-O ester);δH (400 MHz; CDCl3) 8.52 (1H, dd, J 4.2 and 1.6 Hz, H2), 7.93 (1H, dd, J 8.4 and 1.6 Hz, H4), 7.26–7.42 (5H, m, ArH and H3), 7.35 (1H, d, J 2.4 Hz, H7), 6.28 (1H, d, J 2.4 Hz, H5), 6.03 (1H, m, NH), 5.24 (1H, d, J 12.0 Hz, H14), 3.90 (3H, d, J 2.2 Hz, OCH3), 3.63 (1H, m, H9), 2.55 (2H, m, H13), 1.68 (4H, m, H11 and H12), 1.60 (9H, d, J 6.1 Hz, 3 × H17), 1.30 (3H, d, J 6.4 Hz, H10); δC (101 MHz; CDCl3) 159.5, 155.4, 145.0, 144.2 (2C), 137.4, 135.4, 134.7, 134.2, 129.9, 129.4 (2C), 129.1 (2C), 121.8, 96.6, 91.6, 61.3, 58.2, 55.2, 47.8, 34.1, 30.0 (3C), 26.5 and 20.6; LCMS m/z 508 [M + H]+; HPLC purity: 99.7%; tr’ = 17.25 min.
3.3. In Vitro Biological Evaluations
Antiplasmodial activity on the K1 strain, antitrypanosomal activity on T. b. brucei rhodesiense STIB900 and cytotoxicity on the L6 mammalian cell-line were all carried out at the Swiss Tropical and Public Health Institute (STPH), Switzerland as follows:
3.3.1. Activity Against K1 Strain of P. falciparum
In vitro activity against erythrocytic stages of P. falciparum was determined using a 3H-hypoxanthine incorporation assay, using the chloroquine and pyrimethamine-resistant K1 strain that originates from Thailand and the standard drug chloroquine (Sigma C6628) [39,40,41]. Compounds were dissolved in DMSO at 10 mg/mL and added to parasite cultures incubated in RPMI 1640 medium without hypoxanthine, supplemented with HEPES (5.94 g/L), NaHCO3 (2.1 g/L), neomycin (100 U/mL), AlbumaxR (5 g/L) and washed human red cells A+ at 2.5% hematocrit (0.3% parasitemia). Serial drug dilutions of eleven three-fold dilution steps covering a range from 100 to 0.002 μg/mL were prepared. The 96-well plates were incubated in a humidified atmosphere at 37 °C; 4% CO2, 3% O2, 93% N2. After 48 h, 50 μL of 3H-hypoxanthine (= 0.5 μCi) was added to each well of the plate. The test compounds were tested in triplicate ad parasite growth compared to control or blank well. The plates were incubated for a further 24 h under the same conditions. The plates were then harvested with a Betaplate™ cell harvester (Wallac, Zurich, Switzerland), and the red blood cells transferred onto a glass fiber filter then washed with distilled water. The dried filters were inserted into a plastic foil with 10 mL of scintillation fluid and counted in a Betaplate™ liquid scintillation counter (Wallac, Zurich, Switzerland). IC50 values were calculated from sigmoidal inhibition curves by linear regression using Microsoft Excel.
3.3.2. Activity against Trypanosoma brucei rhodesiense STIB900
This stock was isolated in 1982 from a human patient in Tanzania and, after several mouse passages, was cloned and adapted to axenic culture conditions [41,42,43]. Minimum essential medium (50 µL) supplemented with 25 mM HEPES, 1 g/L additional glucose, 1% MEM non-essential amino acids (100×), 0.2 mM 2-mercaptoethanol, 1 mM Na-pyruvate and 15% heat-inactivated horse serum was added to each well of a 96-well microtiter plate. Serial drug dilutions of eleven three-fold dilution steps covering a range from 100 to 0.002 μg/mL were prepared. Then, 4 × 103 bloodstream forms of T. b. rhodesiense STIB 900 in 50 µL was added to each well and the plate incubated at 37 °C under a 5% CO2 atmosphere for 70 h. 10 µL alamarBlue (resazurin, 12.5 mg in 100 mL double-distilled water) was then added to each well and incubation continued for a further 2–4 h. Then the plates were read with a Spectramax Gemini XS microplate fluorometer (Molecular Devices Cooperation, Sunnyvale, CA, USA) using an excitation wavelength of 536 nm and an emission wavelength of 588 nm. The test compounds were tested in triplicate, and their IC50 values were calculated by linear regression from the sigmoidal dose inhibition curves using Softmax Pro software (Molecular Devices Cooperation, Sunnyvale, CA, USA).
3.3.3. In Vitro Cytotoxicity with L-6 Cells
Assays were performed in triplicate in 96-well microtiter plates, each well containing 100 μL of RPMI 1640 medium supplemented with 1% L-glutamine (200 mM) and 10% fetal bovine serum, and 4000 L-6 cells (a primary cell line derived from rat skeletal myoblasts) [41,44,45]. Serial drug dilutions of eleven three-fold dilution steps covering a range from 100 to 0.002 μg/mL were prepared. After 70 h of incubation, the plates were inspected under an inverted microscope to assure the growth of the controls and sterile conditions. 10 μL of alamarBlue was then added to each well, and the plates incubated for another 2 h. Then the plates were read with a Spectramax Gemini XS microplate fluorometer (Molecular Devices Cooperation, Sunnyvale, CA, USA) using an excitation wavelength of 536 nm and an emission wavelength of 588 nm. The IC50 values were calculated by linear regression from the sigmoidal dose inhibition curves using Softmax Pro software (Molecular Devices Cooperation, Sunnyvale, CA, USA).
3.3.4. Aqueous Kinetic Solubility
A 10 mM stock of the compound was diluted in DMSO to give standard solutions at 220 µM, 100 µM and 10 µM in a 96 well plate as described previously [19]. The 10 mM stock was then spiked 1:50 in phosphate-buffered saline pH 7.0 to give a final DMSO concentration of 2%. Both the standard and sample solutions were plated in triplicate. After shaking for 2 h at 25 °C, the solutions were filtered and analyzed on an Agilent 1200 series HPLC with DAD detection. The solubility of the test compounds was determined by comparing the peak area of the sample solutions to that of the standard solutions.
4. Conclusions
In this study, we presented the synthesis of 4-amino-7chloroquinoline tethered β-lactams and primaquine-based tetrazoles, the latter obtained as unresolved 1:1 diastereomeric mixtures. The configuration of the β-lactams was assigned as cis based on the NMR analysis. The in vitro antiplasmodium activity of all the β-lactams was superior to those of the primaquine-based tetrazoles and primaquine reference drug, with compounds 4d–e and 4g being two to three-fold more active than primaquine while 4d was also equipotent to chloroquine. The most active compound in the tetrazole series, 6d, was 2- and 6-fold less active than primaquine and chloroquine, respectively. The antitrypanosomal activity of all the synthesized compounds was moderate, with compounds 4d, 4e–g and 6a–d displaying encouraging antitrypanosomal activities less than 20 µM. Furthermore, three β-lactams and three tetrazoles were assessed for their aqueous kinetic solubility and generally, the β-lactams compounds exhibited superior solubility (>200 µM) compared to those of tetrazoles (<20 µM).
Supplementary Materials
The following are available online. Table S1: Yields and HPLC purity of the synthesized compounds; preparative and analytical HPLC methods, HPLC chromatograms for 1:1 diastereomeric mixtures (Figures S1–S5), 1H and 13C NMR spectra of the synthesized target compounds.
Author Contributions
M.T. synthesized all the target compounds, collected and analyzed the experimental data, and drafted the manuscript. S.L. performed the HPLC and LCMS work and reviewed the manuscript. M.N. performed the kinetic solubility studies and reviewed the manuscript. K.C. conceptualized and provided intellectual leadership of the project, including supervision and also reviewed the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
The UCT Department of Chemistry Equity Development Program scholarship and the South African National Research Foundation (NRF) for financial support (M.T.). MT further acknowledges the NRF Competitive Support for unrated research (CSUR: 116,282) and UKZN for further financial support. The UCT, South African Medical Research Council (MRC), and the South African Research Chairs Initiative of the Department of Science, technology and Innovation administered through the NRF are also gratefully acknowledged (K.C.).
Acknowledgments
We are grateful to Marcel Kaiser from the Swiss Tropical Institute of Health (STPH) for performing the in vitro antiplasmodium and antitrypanosomal evaluation and cytotoxicity studies. The ADME group of the University of Cape Town (UCT) Drug Discovery and Development Center (H3D) is also thanked for their assistance with the kinetic solubility studies and infrastructure for chromatography work.
Conflicts of Interest
The authors declare no conflict of interest.
Data Availability
Sample of compounds reported in this study are available on request from the corresponding author.
References
- Filardy, A.A.; Guimarães-Pinto, K.; Nunes, M.P.; Zukeram, K.; Fliess, L.; Oliveira-Nascimento, D.; Conde, L.; Morrot, A. Human kinetoplastic protozoan infections: Where are we going next? Front. Immunol. 2018, 9, 1493. [Google Scholar] [CrossRef] [PubMed]
- Njoroge, A.; Njuguna, N.M.; Mutai, P.; Ongaror, D.S.; Smith, P.W.; Chibale, K. Recent approaches to chemical discovery and development against malaria and the neglected tropical diseases human African trypanosomiasis and schistosomiasis. Chem. Rev. 2014, 114, 11138–11363. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.P.S.; Barrett, M.P.; Dranoff, G.; Faraday, C.J.; Gimpelewicz, C.R.; Hailu, A.; Jones, C.L.; Kelly, J.M.; Lazdins-Helds, J.K.; Mäser, P.; et al. Drug discovery for kenetoplastic diseases: Future directions. ACS Infect. Dis. 2019, 5, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.C.; Dick, L.R.; Gould, A.; Brand, S.; Tilley, L. The proteasome as a target for protozoan parasites. Expert Opin. Ther. Targets. 2019, 23, 903–914. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, P.G.E. Update on human African trypanosomiasis (Sleeping sickness). J. Neurol. 2019, 266, 2334–2337. [Google Scholar] [CrossRef] [PubMed]
- WHO Report, World Malaria Report 2019. Available online: https://www.who.int/publications-detail/world-malaria-report-2019 (accessed on 2 April 2020).
- WHO Report, Fourth WHO Report on Neglected Tropical Diseases 2017. Available online: https://www.who.int/neglected_diseases/resources/9789241565448/en/ (accessed on 8 April 2020).
- Akazue, P.I.; Ebiloma, G.U.; Ajibola, O.; Isaac, C.; Onyekwelu, K.; Ezeh, C.O.; Eze, A.A. Sustainable elimination (Zero cases) of sleeping sickness: How far are we from achieving this goal? Pathogen 2019, 8, E135. [Google Scholar] [CrossRef]
- Capewell, P.; Atkins, K.; Weir, W.; Jamonneau, V.; Camara, M.; Clucas, C.; Swar, N.-R.K.; Ngoyi, D.M.; Routureau, B.; Garside, P.; et al. Resolving the apparent transmission paradox of African sleeping sickness. PLoS Biol. 2019, 17, e3000105. [Google Scholar] [CrossRef]
- Jacobs, R.T.; Nare, B.; Phillips, M.A. State of the art African trypanosome drug discovery. Curr. Top Med. Chem. 2011, 11, 1255–1274. [Google Scholar] [CrossRef]
- Fairlamb, A.H.; Horn, D. Melarsoprol resistance in African trypanosomiasis. Trends Parasitol. 2018, 34, 481–492. [Google Scholar] [CrossRef]
- Lee, S.M.; Kim, M.S.; Hayat, F.; Shin, D. Recent advances in the discovery of novel antiprotozoal agents. Molecules 2019, 24, e3886. [Google Scholar] [CrossRef]
- Van Voorhis, W.C.; Adams, J.H.; Adelfio, R.; Ahuong, V.; Akabas, M.H.; Alano, P.; Alday, A.; Resto, Y.A.; Alsibaee, A.; Alzualde, A.; et al. Open source drug discovery with malaria box compound collection for neglected diseases and beyond. PLoS Pathog. 2016, 12, 31005763. [Google Scholar] [CrossRef] [PubMed]
- Berniger, M.; Scmidt, I.; Ponte-Sucre, A.; Holzgrabe, U. Novel lead compounds in pre-clinical development against African sleeping sickness. MedChemComm 2017, 8, 1872–1890. [Google Scholar] [CrossRef] [PubMed]
- Ashley, E.A.; Dhorda, M.; Fairhurst, R.M.; Amaratunga, C.; Lim, P.; Suon, S.; Sreng, S.; Anderson, J.M.; Mao, S.; Sam, B.; et al. Spread of artemisinin resistance in Plasmodium falciparum malaria. N. Engl. J. Med. 2014, 371, 411–423. [Google Scholar] [CrossRef] [PubMed]
- Mbengue, A.; Bhattacharjee, S.; Pandharkar, T.; Liu, H.; Estiu, G.; Stahelin, R.V.; Rizk, S.S.; Njimoh, D.L.; Ryan, Y.; Chotivanich, K.; et al. Amolecular mechanism of artemisinin resistance in Plasmodium falciparum malaria. Nature 2015, 520, 683–687. [Google Scholar] [CrossRef] [PubMed]
- Fairhurst, R.M.; Dondorp, A.M. Atermisinin-resistant Plasmodium falciparum malaria. Microbiol. Spectr. 2016, 4. [Google Scholar] [CrossRef]
- Dondorp, A.M.; Yeung, S.; White, L.; Nguon, C.; Day, N.P.; Socheat, D.; von Seidlein, L. Artemisinin-resistance: Current status and scenarios for containment. Nat. Rev. Microbiol. 2010, 8, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Tukulula, M.; Njoroge, M.; Abay, E.T.; Mugumbate, G.C.; Wiesner, L.; Taylor, D.; Gibhard, L.; Norman, J.; Swart, K.J.; Gut, J.; et al. Synthesis and in vitro and in vivo pharmacological evaluation of new 4-aminiquinoline-based compounds. ACS Med. Chem. Lett. 2013, 4, 1198–1202. [Google Scholar]
- Abay, E.T.; van der Westhuizen, J.H.; Swart, K.J.; Gibhard, L.; Tukulula, M.; Chibale, K.; Wiesner, L. The development and validation of an LC-MS/MS method for the determination of a new antimalarial compound (TK900D) in human whole blood and its application to pharmacokinetic studies in mice. Malar. J. 2014, 13, 42. [Google Scholar] [CrossRef]
- Nivsarkar, M.; Thavaselvam, D.; Prasanna, S.; Sharma, M.; Kaushik, M.P. Design, synthesis and biological evaluation of novel bicyclic β-lactams as potential antimalarials. Bioor. Med. Chem. Lett. 2005, 15, 1371–1373. [Google Scholar] [CrossRef]
- D’hooghe, M.; Dekeukeleire, S.; Mollet, K.; Lategan, C.; Smith, P.J.; Chibale, K.; De Kimpe, N. Synthesis of novel 2-alkoxy-3-amino-3-arylpropan-1-ols and 5-alkoxy-4-aryl-1,3-oxazinanes with antimalarial activity. J. Med. Chem. 2009, 52, 4058–4062. [Google Scholar] [CrossRef]
- Singh, P.; Sachedeva, S.; Raj, R.; Kumar, V.; Mahajan, M.P.; Nasser, S.; Vivas, L.; Gut, J.; Rosenthal, P.J.; Feng, T.-S.; et al. Antiplasmodial and cytotoxicity evaluation of 3-functionalized 2-azetedinone derivatives. Bioorg. Med. Chem. Lett. 2011, 21, 4561–4563. [Google Scholar] [CrossRef] [PubMed]
- Raj, R.; Biot, C.; Carrére-Kremer, S.; Kremer, L.; Guérardel, Y.; Gut, J.; Rosenthal, P.J.; Kumar, V. 4-Aminoquinoline-β-lactam conjugates: Synthesis, antimalarial, and antitubercular evaluation. Chem. Biol. Drug Des. 2014, 83, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Singh, P.; Kumar, M.; Gut, J.; Rosenthal, P.J.; Kumar, K.; Kumar, V.; Mahajan, M.P.; Bissetty, K. Synthesis, docking and in vitro antimalarial evaluation of bifunctional hybrids derived from β-lactams and 7-chloroquinoline using click chemistry. Bioorg. Med. Chem. Lett. 2012, 22, 57–61. [Google Scholar] [CrossRef]
- Jarrahpour, A.; Ebrahimi, E.; Sinou, V.; Latour, C.; Brunel, J.M. Diastereselective synthesis of potent antimalarial cis-β-lactam agents through a [2 + 2] cycloaddition of chiral imines with a chiral ketene. Eur. J. Med. Chem. 2014, 87, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Jarrahpour, A.; Aye, M.; Sinou, V.; Latour, C.; Brunel, J.M. Synthesis of some new monocyclic β-lactams as antimalarial agents. J. Iran Chem. 2015, 12, 2083–2092. [Google Scholar] [CrossRef]
- Staudinger, H. Zur Kenntniss der keten. Diphenylketen. Liebigs Ann. Chem. 1907, 356, 51. [Google Scholar] [CrossRef]
- Musonda, C.C.; Gut, J.; Rosenthal, P.J.; Yardley, V.; Carvalho de Souza, R.C.; Chibale, K. Application of multicomponent reactions to antimalarial drug discovery. Part 2: New antiplasmodial and antitrypanosomal 4-aminoquinoline δ- and ϒ-lactams via a ‘catch and release’ protocol. Bioorg. Med. Chem. 2006, 14, 5605–5615. [Google Scholar]
- McCabe, R.E. Primaquine is lethal for intracellular but not for extracellular trypanosome cruzi. J. Parasitol. 1988, 74, 748–753. [Google Scholar] [CrossRef]
- Carvalho, L.; Martínez-Garcia, M.; Pérez-Victoria, L.; Manzano, J.I.; Yardley, V.; Gamarro, F.; Pérez-Victoria, M. The oral antimalarial drug tafenoquine shows activity against trypanosome brucei. Antimicrob. Agents Chemother. 2015, 59, 6151–6160. [Google Scholar] [CrossRef]
- Yardley, V.; Gamarro, F.; Croft, S.L. Antileishmanial and antitrypanosomal activities of the 8-aminoquinoline tafenoquine. Antimicrob. Agents Chemother. 2010, 54, 5356–5358. [Google Scholar] [CrossRef]
- De, D.; Byers, L.D.; Krogstad, D.J. Antimalarial: Synthesis of 4-aminoqquinolines that circumvent resistance in malarial parasite. J. Heterocycl. Chem. 1997, 34, 315–320. [Google Scholar] [CrossRef]
- Jiao, L.; Liang, Y.; Xu, J. Origin of the relative stereoselectivity of the β-lactam formation in the Staudinger reaction. J. Am. Chem. Soc. 2006, 128, 6060. [Google Scholar] [CrossRef] [PubMed]
- Xu, J. Stereoselectivity in the synthesis of 2-azetidinones from ketenes and imines via the Staudinger reaction. ARKIVOC 2009, 9, 21–44. [Google Scholar]
- Tukulula, M.; Little, S.; Gut, J.; Rosenthal, P.J.; Wan, B.; Franzblau, S.G.; Chibale, K. The design, synthesis, in silico ADME profiling, antiplasmodial and antimycobacterial evaluation of the new arylamino quinoline derivatives. Eur. J. Med. Chem. 2012, 57, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Tukulula, M.; Njoroge, M.; Mugumbate, G.C.; Gut, J.; Rosenthal, P.J.; Barteau, S.; Streckfuss, J.; Heidi, O.; Kameni-Tcheudji, J.; Chibale, K. Tetrazole-based deoxyamodiaquines: Synthesis, ADME/PK profiling and pharmacological evaluation as potential antimalarial agents. Bioorg. Med. Chem. 2013, 21, 4904–4913. [Google Scholar] [CrossRef]
- Tukulula, M.; Sharma, R.-J.; Meurillon, M.; Mahajan, A.; Naran, K.; Warner, D.; Huang, J.; Mekonnen, B.; Chibale, K. Synthesis and antiplasmodial and antimycobacterial evaluation of new nitroimidazoleand nitroimizaooxazine derivatives. ACS Med. Chem. 2013, 4, 128–131. [Google Scholar] [CrossRef]
- Desjardins, R.E.; Canfield, C.J.; Haynes, J.D.; Chulay, J.D. Quantitative assessment of antimalarial activity in vitro by a semiautomated microdilution technique. Antimicrob. Agents Chemther. 1979, 16, 710–718. [Google Scholar] [CrossRef]
- Thaithong, S.; Beale, G.H.; Chutmongkonkul, M. Susceptibility of Plasmodium falciparum to five drugs and in vitro study of isolates mainly from Thailand. Trans. R. Soc. Trop. Med. Hyg. 1983, 77, 228–231. [Google Scholar] [CrossRef]
- Hubber, W.; Koella, J.C. A comparison of three methods of estimating EC50 in studies of drug resistance of malaria parasites. Acta Trop. 1993, 55, 257–261. [Google Scholar] [CrossRef]
- Balts, T.; Baltz, D.; Giroud, C.; Crockett, J. Cultivation in a semi-defined medium if animal infective forms of Trypanosoma brucei, T. equiperdum, T. evansi, T. rhodesiense and T. gambiense. EMBO J. 1985, 4, 1273–1277. [Google Scholar]
- Räz, B.; Iten, M.; Grether-Bühler, Y.; Kaminsky, R.; Brun, R. The alma blue assay to determine drug sensitivity of African trypanosomes (T. b. rhodesiense and T. b gambiense) in vitro. Acta Trop. 1997, 68, 139–147. [Google Scholar]
- Page, B.; Page, M.; Noel, C. A new fluorometric assay for cytotoxicity measurement in vitro. Int. J. Oncol. 1993, 3, 473–476. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.A.; Gogal, R.M., Jr.; Walsh, J.E. A new rapid and simple non-radioactive assay to monitor and determine the proliferation of lymphocytes: An alternative to [3H] thymidine incorporation assay. J. Immunol. Methods 1994, 170, 211–224. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).