(Z,Z)-Selanediylbis(2-propenamides): Novel Class of Organoselenium Compounds with High Glutathione Peroxidase-Like Activity. Regio- and Stereoselective Reaction of Sodium Selenide with 3-Trimethylsilyl-2-propynamides
Abstract
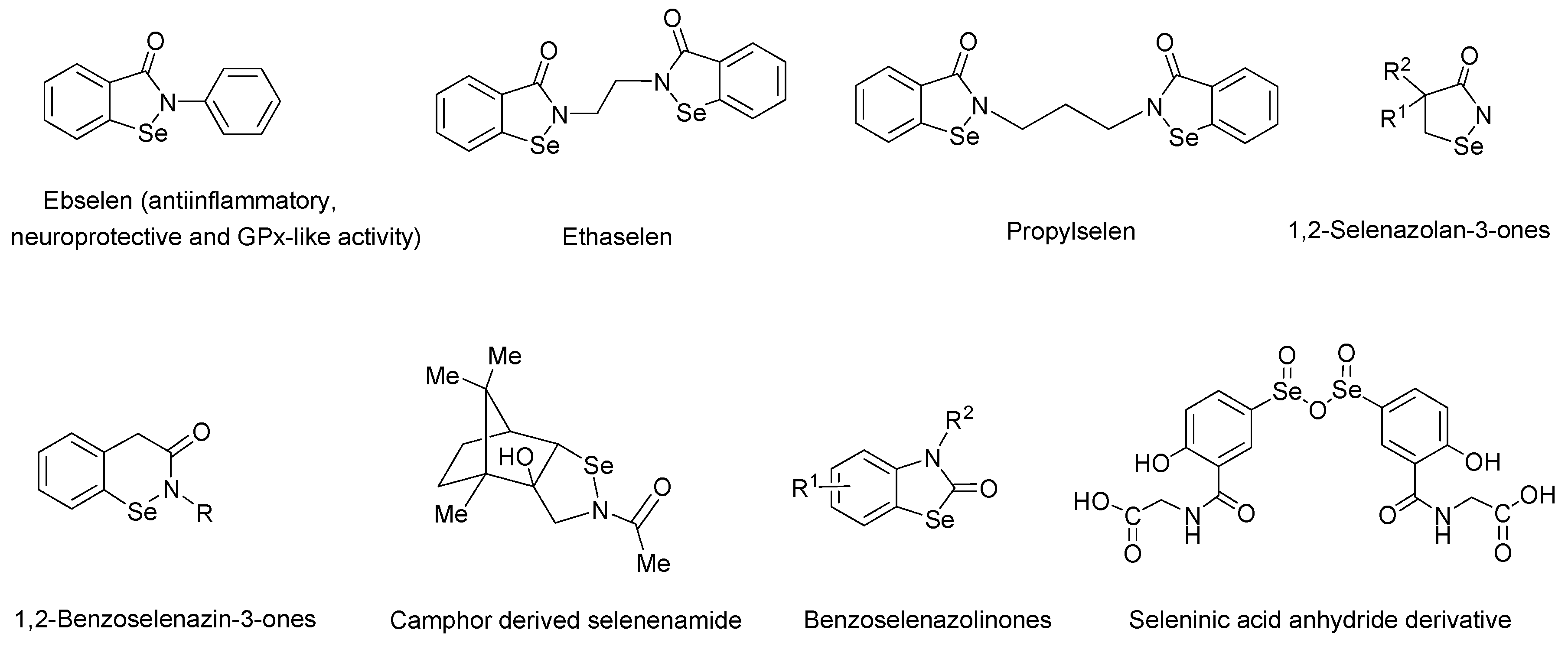
1. Introduction
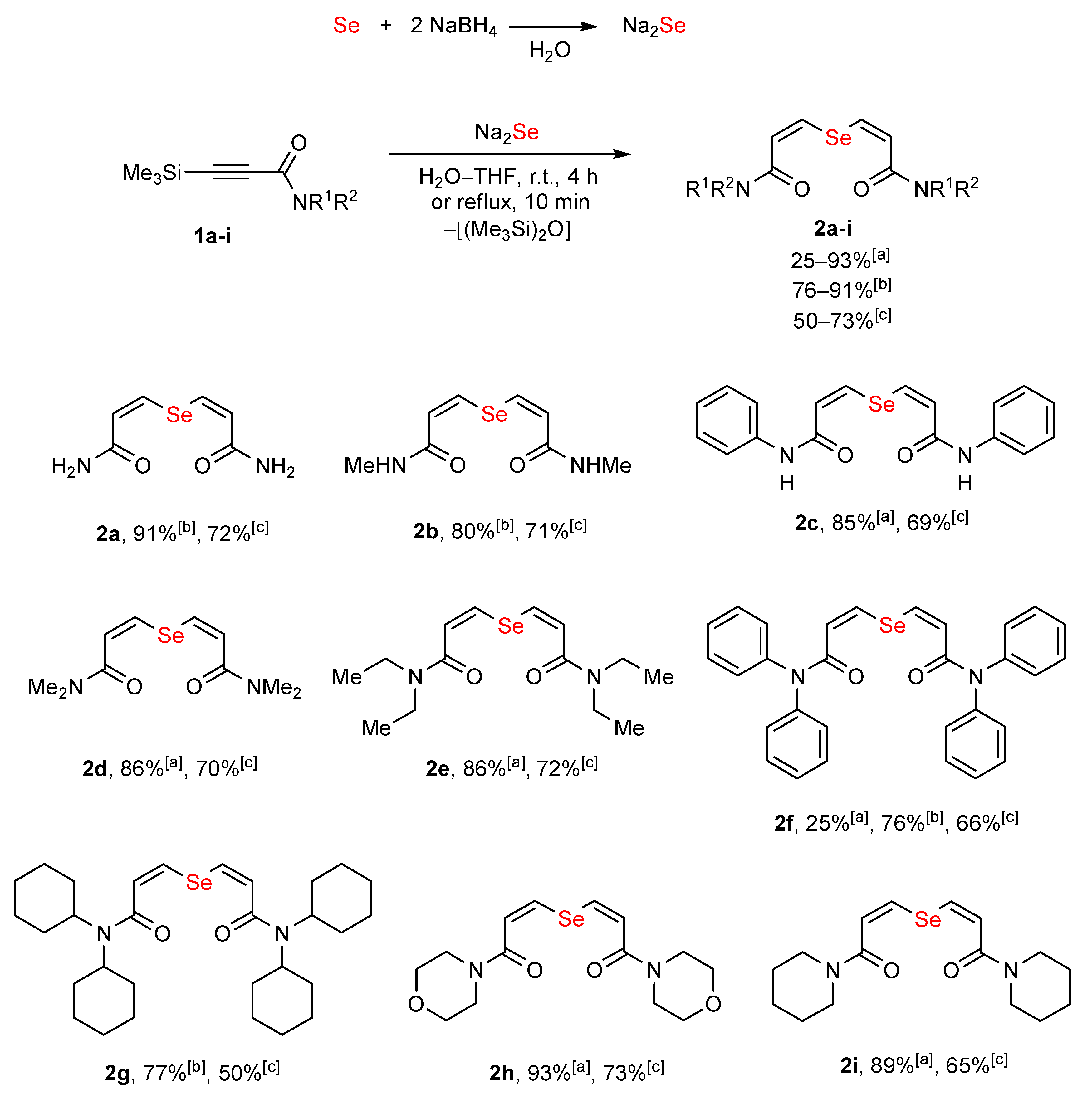
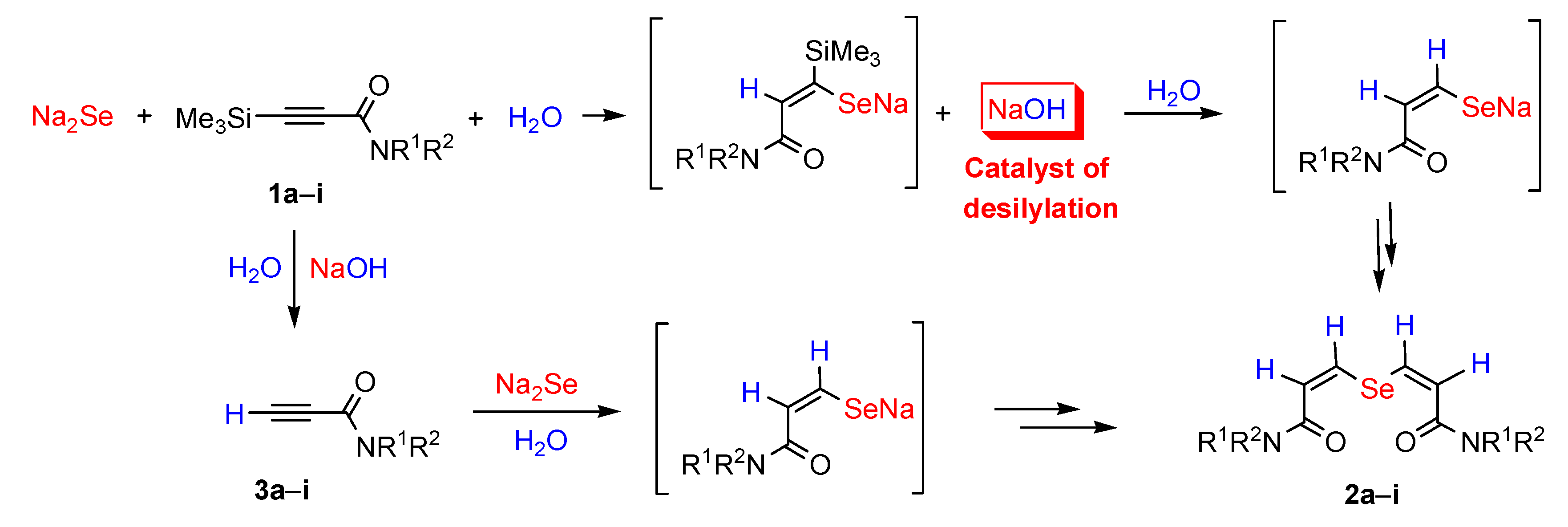
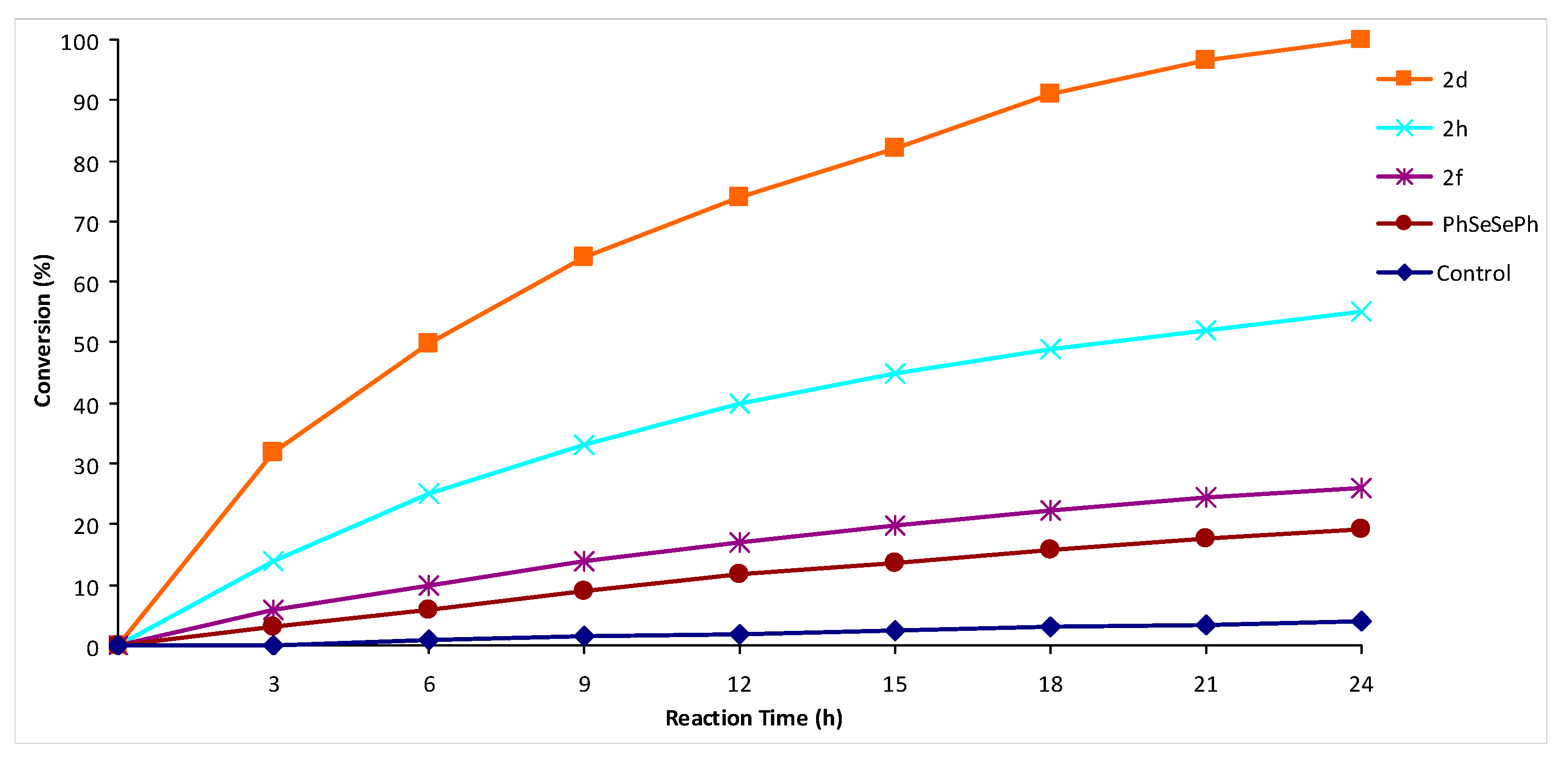
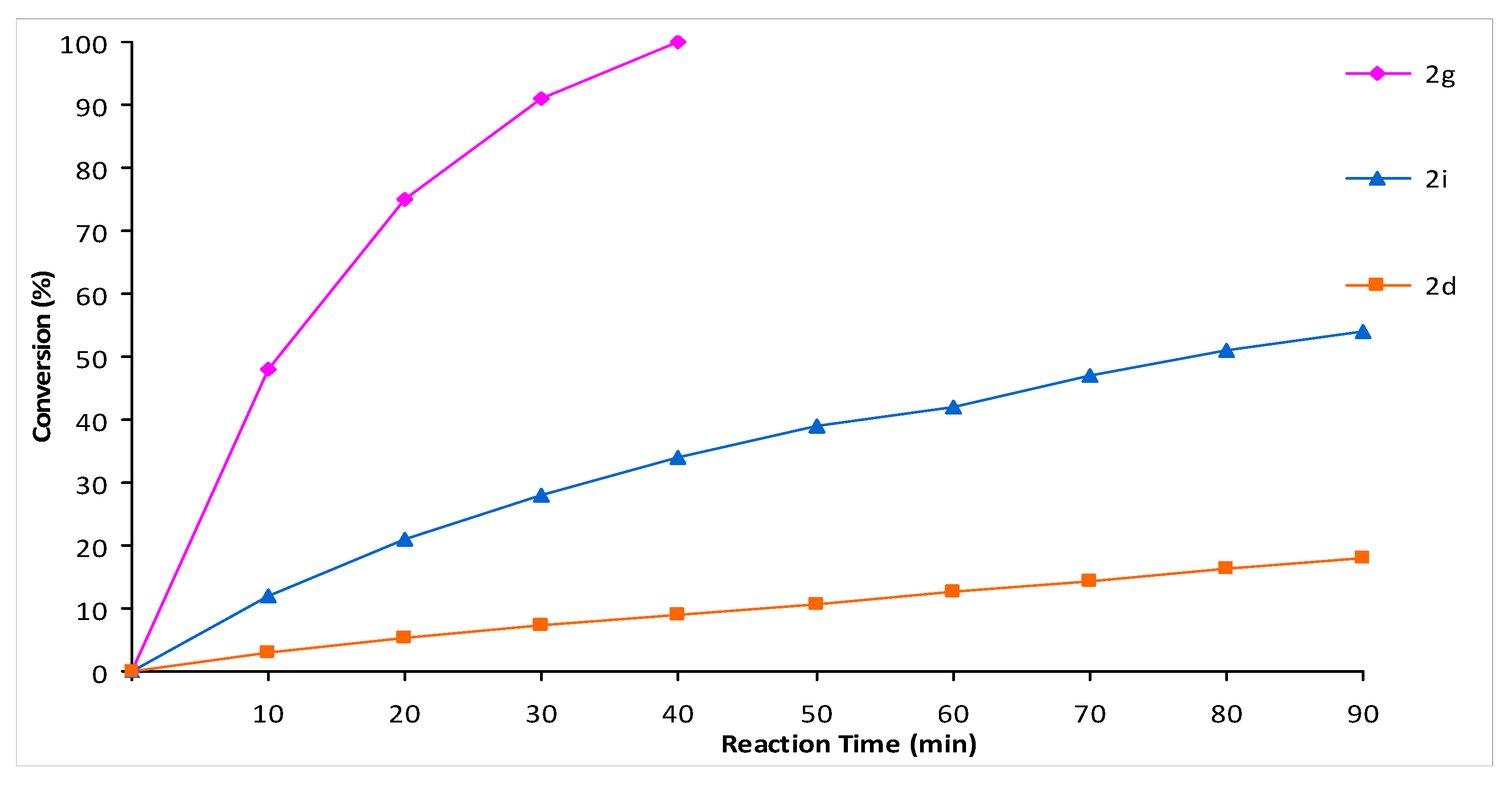
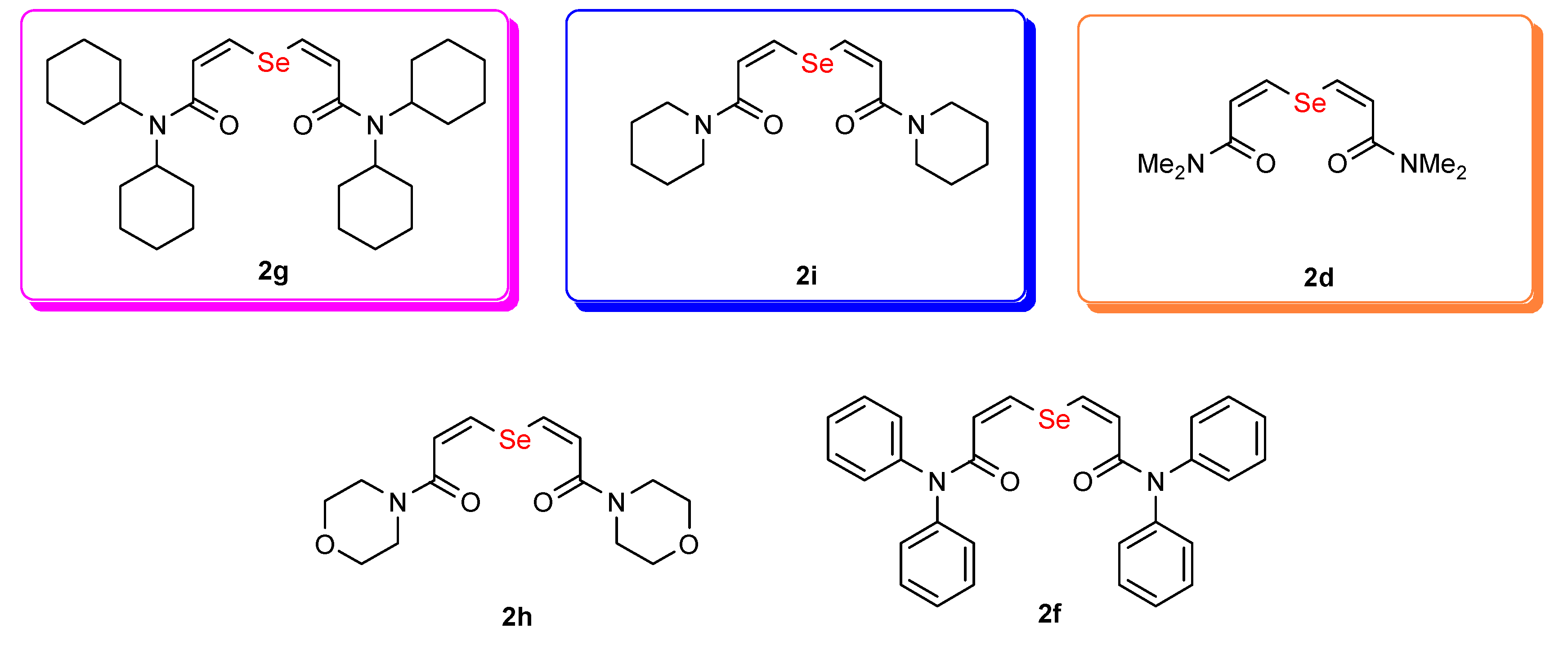
2. Results and Discussion
3. Experimental Section
3.1. General Information
3.2. Method A (Preparation of Compounds 2c–f,h,i)
3.3. Method B (Preparation of Compounds 2a,b,f,g)
3.4. Method C (Preparation of Compounds 2a–i)
3.5. Compounds 2a–i
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Perin, G.; Lenardão, E.J.; Jacob, R.G.; Panatieri, R.B. Synthesis of Vinyl Selenides. Chem. Rev. 2009, 109, 1277–1301. [Google Scholar] [CrossRef] [PubMed]
- Perin, G.; Alves, D.; Jacob, R.G.; Barcellos, A.M.; Soares, L.K.; Lenardão, E.J. Synthesis of Organochalcogen Compounds using Non-Conventional Reaction Media. EJ ChemistrySelect 2016, 2, 205–258. [Google Scholar] [CrossRef]
- Banerjee, B.; Koketsu, M. Recent developments in the synthesis of biologically relevant selenium containing scaffolds. Coord. Chem. Rev. 2017, 339, 104–127. [Google Scholar] [CrossRef]
- Lenardão, E.J.; Cella, R.; Jacob, R.G.; da Silva, T.B.; Perin, G. Synthesis and Reactivity of α-Phenylseleno-β-substituted Styrenes. Preparation of (Z)-Allyl Alcohols, (E)-α-Phenyl-α,β-unsaturated Aldehydes and α-Aryl Acetophenones. J. Braz. Chem. Soc. 2006, 17, 1031–1038. [Google Scholar] [CrossRef]
- Silveira, C.C.; Braga, A.L.; Vieira, A.S.; Zeni, G. Stereoselective Synthesis of Enynes by Nickel-Catalyzed Cross-Coupling of Divinylic Chalcogenides with Alkynes. J. Org. Chem. 2003, 68, 662–665. [Google Scholar] [CrossRef] [PubMed]
- Silveira, C.C.; Mendes, S.R.; Wolf, L. Iron-Catalyzed Coupling Reactions of Vinylic Chalcogenides with Grignard Reagents. J. Braz. Chem. Soc. 2010, 11, 2138–2145. [Google Scholar] [CrossRef]
- Tingoli, M.; Tiecco, M.; Testaferri, L.; Temperini, A. Alkynyl Phenyl Selenides as Convenient Precursors for the Synthesis of Stereodefined Trisubstituted Alkenes. Tetrahedron 1995, 51, 4691–4700. [Google Scholar] [CrossRef]
- Perin, G.; Barcellos, A.M.; Luz, E.Q.; Borges, E.L.; Jacob, R.G.; Lenardão, E.J.; Sancineto, L.; Santi, C. Green Hydroselenation of Aryl Alkynes: Divinyl Selenides as a Precursor of Resveratrol. Molecules 2017, 22, 327. [Google Scholar] [CrossRef]
- Tiecco, M.; Testaferri, L.; Temperini, A.; Bagnoli, L.; Marini, F.; Santi, C. A New Synthesis of α-Phenylseleno γ- and δ-Lactones from Terminal Alkynes. Synlett 2003, 655–668. [Google Scholar] [CrossRef]
- Gonçalves, L.C.C.; Victória, F.N.; Lima, D.B.; Borba, P.M.Y.; Perin, G.; Savegnago, L.; Lenardão, E.J. CuI/glycerol mediated stereoselective synthesis of 1,2-bis-chalcogen alkenes from terminal alkynes: Synthesis of new antioxidants. Tetrahedron Lett. 2014, 55, 5275–5279. [Google Scholar] [CrossRef][Green Version]
- Sartori, G.; Neto, J.S.S.; Pesarico, A.P.; Back, D.F.; Nogueiraa, C.W.; Zeni, G. Bis-vinyl selenides obtained via iron(III) catalyzed addition of PhSeSePh to alkynes: Synthesis and antinociceptive activity. Org. Biomol. Chem. 2013, 11, 1199–1208. [Google Scholar] [CrossRef] [PubMed]
- Bortolatto, C.F.; Wilhelma, E.A.; Roman, S.S.; Nogueira, C.W. (E)-2-Benzylidene-4-phenyl-1,3-diselenole ameliorates signals of renal injury induced by cisplatin in rats. J. Appl. Toxicol. 2014, 34, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Reddy, V.P.; Swapna, K.; Kumar, A.V.; Rao, K.R. Lanthanum-catalyzed stereoselective synthesis of vinyl sulfides and selenides. Tetrahedron Lett. 2010, 51, 293–296. [Google Scholar] [CrossRef]
- Movassagh, B.; Mohammadi, E. Green Trends in Synthesis of Alkenyl and Alkynyl Chalcogenides. Curr. Green Chem. 2016, 3, 18–35. [Google Scholar] [CrossRef]
- Wang, Z.; Mo, H.; Bao, W. Mild, Efficient and Highly Stereoselective Synthesis of (Z)-Vinyl Chalcogenides from Vinyl Bromides Catalyzed by Copper(I) in Ionic Liquids Based on Amino Acids. Synlett 2007, 91–94. [Google Scholar] [CrossRef]
- Mohan, B.; Hwang, S.; Woo, H.; Park, K.H. Transition-metal free synthesis of diaryl vinyl selenides: A simple synthetic approach with high selectivity. Tetrahedron 2014, 70, 2699–2702. [Google Scholar] [CrossRef]
- Lara, R.G.; Borges, E.L.; Lenardão, E.J.; Alves, D.; Jacob, R.G.; Perin, G. Addition of Thiols to Phenylselenoalkynes using KF/Alumina under Solvent-Free Conditions. J. Braz. Chem. Soc. 2010, 21, 2125–2129. [Google Scholar] [CrossRef]
- Tiecco, M.; Testaferri, L.; Temperini, A.; Bagnoli, L.; Marini, F.; Santi, C. A New Synthesis of a-Phenylseleno Esters and Acids from Terminal Alkynes. Synlett 2001, 706–708. [Google Scholar] [CrossRef]
- Orlov, N.V. Metal Catalysis in Thiolation and Selenation Reactions of Alkynes Leading to Chalcogen-Substituted Alkenes and Dienes. ChemistryOpen 2015, 4, 682–697. [Google Scholar] [CrossRef]
- Lenardão, E.J.; Silva, M.S.; Sachini, M.; Lara, R.G.; Jacob, R.G.; Gelson, P. Synthesis of alkenyl selenides and tellurides using PEG-400. Arkivoc 2009, xi, 221–227. [Google Scholar] [CrossRef]
- Lenardão, E.J.; Dutra, L.G.; Saraiva, M.T.; Jacob, R.G.; Perin, G. Hydroselenation of alkynes using NaBH4/BMIMBF4: Easy access to vinyl selenides. Tetrahedron Lett. 2007, 48, 8011–8013. [Google Scholar] [CrossRef]
- Soares, L.K.; Silva, R.B.; Peglow, T.J.; Silva, M.S.; Jacob, R.G.; Alves, D.; Perin, G. Selective Synthesis of Vinyl- or Alkynyl Chalcogenides from Glycerol and their Water-Soluble Derivatives. ChemistrySelect 2016, 1, 2009–2013. [Google Scholar] [CrossRef]
- Potapov, V.A.; Elokhina, V.N.; Larina, L.I.; Yaroshenko, T.I.; Tatarinova, A.A.; Amosova, S.V. Reactions of sodium selenide with ethynyl and bromoethynyl ketones: Stereo- and regioselective synthesis of functionalized divinyl selenides and 1,3-diselenetanes. J. Organomet. Chem. 2009, 694, 3679–3682. [Google Scholar] [CrossRef]
- Knapton, D.J.; Meyer, T.Y. A Palladium-Catalyzed Regio- and Stereoselective Four-Component Coupling Reaction. J. Org. Chem. 2005, 70, 785–796. [Google Scholar] [CrossRef] [PubMed]
- Toyofuku, M.; Fujiwara, S.; Shin-ike, T.; Kuniyasu, H.; Kambe, N. Platinum-Catalyzed Intramolecular Vinylchalcogenation of Alkynes with β-Phenylchalcogeno Conjugated Amides. J. Am. Chem. Soc. 2008, 130, 10504–10505. [Google Scholar] [CrossRef]
- Fujiwara, S.; Toyofuku, M.; Kuniyasu, H.; Kambe, N. Transition-metal-catalyzed cleavage of carbon–selenium bond and addition to alkynes and allenes. Pure Appl. Chem. 2010, 82, 565–575. [Google Scholar] [CrossRef]
- Andreev, M.V.; Potapov, V.A.; Musalov, M.V.; Larina, L.I.; Amosova, S.V. Regio- and stereoselective reaction of sodium benzeneselenolate with 3-(trimethylsilyl)prop-2-ynamides. Russ. Chem. Bull. 2019, 68, 2134–2136. [Google Scholar] [CrossRef]
- Kate, A.S.; George, S.D.; Sonawane, S.; Periyasamy, G. Nucleoside analogue as an anticancer compound. WO Patent 2013144894. Chem. Abstrs. 2013, 159, 557621. [Google Scholar]
- Pacher, T.; Raninger, A.; Lorbeer, E.; Brecker, L.; But, P.P.-H.; Greger, H. Alcoholysis of Naturally Occurring Imides: Misleading Interpretation of Antifungal Activities. J. Nat. Prod. 2010, 73, 1389–1393. [Google Scholar] [CrossRef]
- Moon, J.T.; Ha, S.H.; Lee, S.H.; Kwon, T.H.; Oh, C.R.; Kim, Y.D.; Kim, J.; Choo, D.J.; Lee, J.Y. Total synthesis and biological evaluation of methylgerambullone. Bioorganic Med. Chem. Lett. 2010, 20, 52–55. [Google Scholar] [CrossRef]
- Wang, J.; Xie, L.; Wang, Y.; Wang, X.; Xi, S.; Zeng, T.; Gong, P.; Zhai, X. Design and Synthesis of Novel 4-Phenoxyquinolines Bearing 3-Hydrosulfonylacrylamido or 1H-Imidazole-4-carboxamido Scaffolds as c-Met Kinase Inhibitors. Arch. Pharm. 2017, 350, 1600307. [Google Scholar] [CrossRef]
- Mayer, A.M.S.; Gustafson, K.R. Marine pharmacology in 2001–2: Antitumour and cytotoxic compounds. Eur. J. Cancer 2004, 40, 2676–2704. [Google Scholar] [CrossRef]
- Huang, K.-C.; Chen, Z.; Jiang, Y.; Akare, S.; Kolber-Simonds, D.; Condon, K.; Agoulnik, S.; Tendyke, K.; Shen, Y.; Wu, K.-M.; et al. Apratoxin A Shows Novel Pancreas-Targeting Activity through the Binding of Sec 61. Mol. Cancer Ther. 2016, 15, 1208–1216. [Google Scholar] [CrossRef]
- Suo, R.; Takada, K.; Irie, R.; Watanabe, R.; Suzuki, T.; Ise, Y.; Ohtsuka, S.; Okada, S.; Matsunaga, S. Poecillastrin H, a Chondropsin-Type Macrolide with a Conjugated Pentaene Moiety, from a Characella sp. Marine Sponge. J. Nat. Prod. 2018, 81, 1295–1299. [Google Scholar] [CrossRef]
- Bezerra, D.P.; Ferreira, P.M.P.; Machado, C.M.L.; de Aquino, N.C.; Silveira, E.R.; Chammas, R.; Pessoa, C. Antitumour Efficacy of Piper tuberculatum and Piplartine Based on the Hollow Fiber Assay. Planta Med. 2015, 81, 15–19. [Google Scholar] [CrossRef]
- Elgiushy, H.R.; Hammad, S.F.; Hassan, A.S.; Aboutaleb, N.; Abouzid, K.A.M. Acrylamide Moiety, a Valuable Fragment in Medicinal Chemistry: Insight into Synthetic Methodologies, Chemical Reactivity and Spectrum of Biological Activities of Acrylamide Derivatives. J. Adv. Pharm. Res. 2018, 2, 221–237. [Google Scholar] [CrossRef]
- Lenardao, E.J.; Santi, C.; Sancineto, L. New Frontiers in Organoselenium Compounds; Springer International Publishing AG: Cham, Switzerland, 2018; 189p. [Google Scholar]
- Rappoport, Z. (Ed.) Patai's Chemistry of Functional Groups. Organic Selenium and Tellurium Compounds; John Wiley and Sons: Chichester, UK, 2013; Volume 4, 1678p. [Google Scholar]
- Santi, C. (Ed.) Organoselenium Chemistry: Between Synthesis and Biochemistry; Bentham Science Publishers: Sharjah, UAE, 2014; 563p. [Google Scholar]
- Woollins, J.D.; Laitinen, R.S. (Eds.) Selenium and Tellurium Chemistry. From Small Molecules to Biomolecules and Materials; Springer: Heidelberg, Germany, 2011; 334p. [Google Scholar]
- Azad, G.K.; Tomar, R.S. Ebselen, a promising antioxidant drug: Mechanisms of action and targets of biological pathways. Mol. Biol. Rep. 2014, 41, 4865–4879. [Google Scholar] [CrossRef]
- Back, T.G.; Dyck, B.P. A Novel Camphor-Derived Selenenamide That Acts as a Glutathione Peroxidase Mimetic. J. Am. Chem. Soc. 1997, 119, 2079–2083. [Google Scholar] [CrossRef]
- Ruberte, A.C.; Sanmartin, C.; Aydillo, C.; Sharma, A.K.; Plano, D. Development and Therapeutic Potential of Selenazo Compounds. J. Med. Chem. 2020, 63, 1473–1489. [Google Scholar] [CrossRef]
- Yu, S.-C.; Kuhn, H.; Daniliuc, C.-G.; Ivanov, I.; Jones, P.G.; du Mont, W.-W. 5-Selenization of salicylic acid derivatives yielded isoform-specific 5-lipoxygenase inhibitors. Org. Biomol. Chem. 2010, 8, 828–834. [Google Scholar] [CrossRef]
- Braverman, S.; Cherkinsky, M.; Kalendar, Y.; Jana, R.; Sprecher, M.; Goldberg, I. Synthesis of water-soluble vinyl selenides and their high glutathione peroxidase (GPx)-like antioxidant activity. Synthesis 2014, 46, 119–125. [Google Scholar] [CrossRef]
- Back, T.G.; Moussa, Z. Remarkable Activity of a Novel Cyclic Seleninate Ester as a Glutathione Peroxidase Mimetic and Its Facile in Situ Generation from Allyl 3-Hydroxypropyl. J. Am. Chem. Soc. 2002, 124, 12104–12105. [Google Scholar] [CrossRef]
- Back, T.G.; Moussa, Z. Diselenides and Allyl Selenides as Glutathione Peroxidase Mimetics. Remarkable Activity of Cyclic Seleninates Produced in Situ by the Oxidation of Allyl ω-Hydroxyalkyl Selenides. J. Am. Chem. Soc. 2003, 125, 13455–13460. [Google Scholar] [CrossRef] [PubMed]
- Gusarova, N.K.; Potapov, V.A.; Amosova, S.V.; Trofimov, B.A. Alkylvinyl Selenides from Acetylene, Elemental Selenium and Alkyl Halides. Zhurnal Org. Khimii 1983, 19, 2477–2480. (In Russian) [Google Scholar]
- Gusarova, N.K.; Trofimov, B.A.; Potapov, V.A.; Amosova, S.V.; Sinegovskaya, L.M. Reactions of Elemental Selenium with Acetylenes.1. Identification of Products of Reaction of Elemental Selenium with Acetylene. Zhurnal Org. Khimii 1984, 20, 484–489. (In Russian) [Google Scholar]
- Potapov, V.A.; Gusarova, N.K.; Amosova, S.V.; Kashik, A.S.; Trofimov, B.A. Reactions of Chalcogen with Acetylenes. 2. Reaction of Selenium Metals with Acetylene in the HMPA and DMSO Media. Zhurnal Org. Khimii 1986, 22, 276–281. (In Russian) [Google Scholar]
- Rusakov, Y.Y.; Krivdin, L.B.; Istomina, N.V.; Potapov, V.A.; Amosova, S.V. Divinyl selenide: Conformational study and stereochemical behavior of its 77Se-1H spin-spin coupling constants. Magn. Reson. Chem. 2008, 46, 979–985. [Google Scholar] [CrossRef]
- Potapov, V.A.; Amosova, S.V.; Kashik, A.S. Reactions of selenium and tellurium metals with phenylacetylene in 3-phase catalytical systems. Tetrahedron Lett. 1989, 30, 613–616. [Google Scholar] [CrossRef]
- Musalov, M.V.; Potapov, V.A.; Amosova, S.V. Reaction of diselenium dichloride with acetylene. Russ. J. Org. Chem. 2011, 47, 1115–1116. [Google Scholar] [CrossRef]
- Potapov, V.A.; Musalov, M.V.; Khuriganova, O.I.; Larina, L.I.; Amosova, S.V. Reactions of stereoselective addition of selenium dibromide and monobromide to acetylene. Russ. J. Org. Chem. 2010, 46, 753–754. [Google Scholar] [CrossRef]
- Potapov, V.A.; Khuriganova, O.I.; Musalov, M.V.; Larina, L.I.; Amosova, S.V. Stereospecific synthesis of E,E-bis(2-chlorovinyl)selenide. Russ. J. Gen. Chem. 2010, 80, 541–542. [Google Scholar] [CrossRef]
- Musalov, M.V.; Potapov, V.A.; Musalova, M.V.; Amosova, S.V. Stereoselective synthesis of (E,E)-bis(2-halovinyl) selenides and its derivatives based on selenium halides and acetylene. Tetrahedron 2012, 68, 10567–10572. [Google Scholar] [CrossRef]
- Potapov, V.A.; Ishigeev, R.S.; Amosova, S.V.; Borodina, T.N. Synthesis of a novel family of water-soluble 2H,3H-[1,3]thia- and -selenazolo[3,2-a]pyridin-4-ium heterocycles by annulation reactions. Tetrahedron Lett. 2019, 60, 475–479. [Google Scholar] [CrossRef]
- Medvedeva, A.S. Effect of a Heteroatom on the Reactivity of Silicon and Germanum Acetilenic Alcohols, Ethers, and Carbonyl Compounds. Russ. J. Org. Chem. 1996, 32, 272–287. (In Russian) [Google Scholar]
- Potapov, V.A. Organic diselenides, ditellurides, polyselenides and polytellurides. Synthesis and reactions. In Patai's Chemistry of Functional Groups. Organic Selenium and Tellurium Compounds; Rappoport, Z., Ed.; John Wiley and Sons, Inc.: Chichester, UK, 2013. [Google Scholar] [CrossRef]
- Andreev, M.V.; Safronova, L.P.; Medvedeva, A.S. Highly Efficient Desilylation of 3-Trimethylsilylprop-2-ynamides by the Action of KF–Al2O3. Russ. J. Org. Chem. 2011, 47, 1797–1801. [Google Scholar] [CrossRef]
- Medvedeva, A.S.; Andreev, M.V.; Safronova, L.P. One-Pot Synthesis of 3-(Trimethylsilyl)propynamides. Russ. J. Org. Chem. 2010, 46, 1466–1470. [Google Scholar] [CrossRef]
- Demina, M.M.; Velikanov, A.A.; Medvedeva, A.S.; Larina, L.I.; Voronkov, M.G. Universal method for trimethylsilylation of acetylenic alcohols and glycols. J. Organomet. Chem. 1998, 553, 129–133. [Google Scholar] [CrossRef]
- Medvedeva, A.S.; Novokshonov, V.V.; Demina, M.M.; Voronkov, M.G. An unusual rearrangement of 1-trimethylsiloxy-3-bromomagnesium-2-propyne. J. Organomet. Chem. 1998, 553, 481–482. [Google Scholar] [CrossRef]
- Andreev, M.V.; Medvedeva, A.S.; Larina, L.I.; Demina, M.M. Synthesis of 5-aminoisoxazoles from 3-trimethylsilylprop-2-ynamides. Mendeleev Commun. 2017, 27, 175–177. [Google Scholar] [CrossRef]
- Mareev, A.V.; Andreev, M.V.; Ushakov, I.A. Base-Catalyzed Hydration of Silicon-Containing Activated Alkynes: The Effect of Substituents at the Triple Bond. ChemistrySelect 2020, 5, 10736–10742. [Google Scholar] [CrossRef]
- Andreev, M.V.; Safronova, L.P.; Medvedeva, A.S. Efficient Tandem Synthesis of 3-Alkylaminoprop-2-enamides from 3-trimethylsilylprop-2-ynamide. Russ. J. Org. Chem. 2013, 49, 822–827. [Google Scholar] [CrossRef]


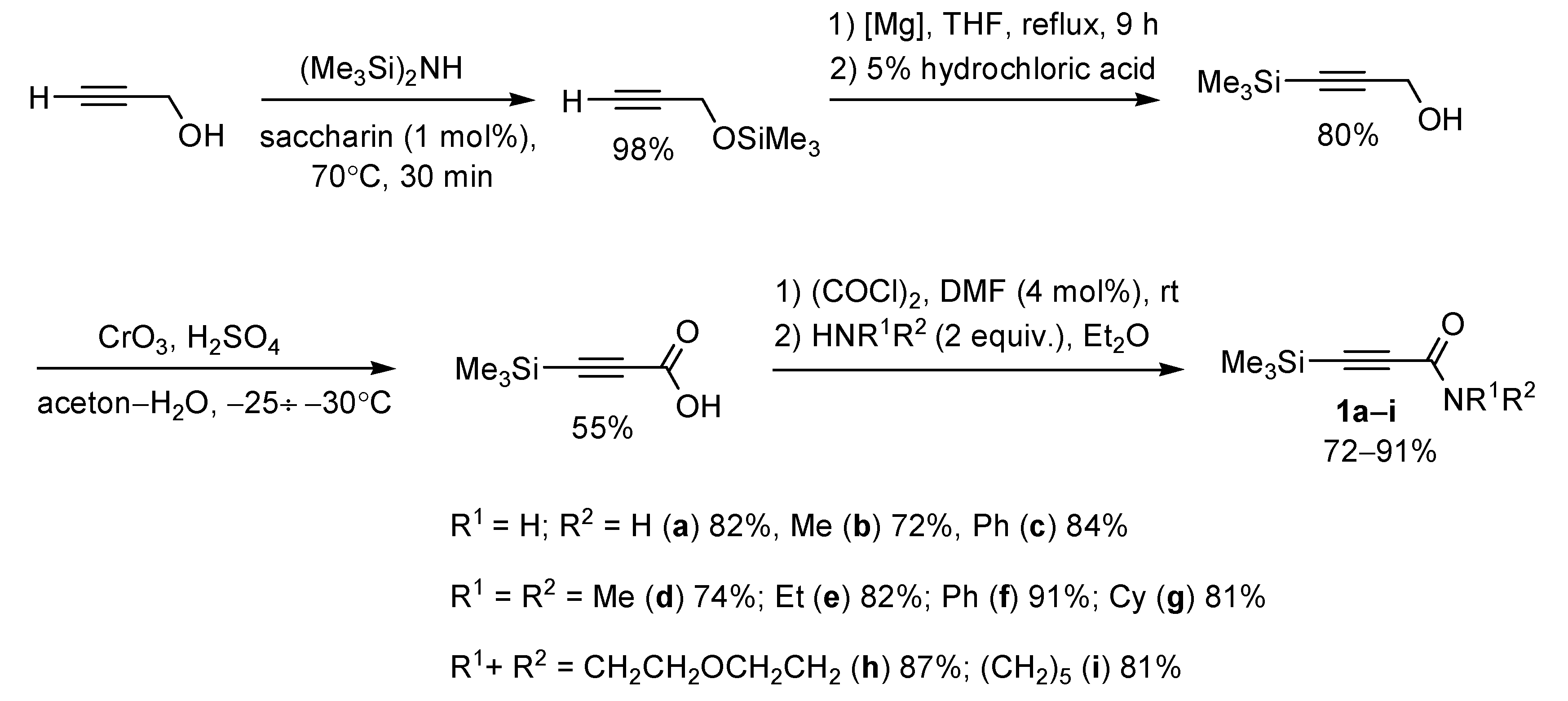
Sample Availability: Samples of the compounds are not available from the authors. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
V. Andreev, M.; Potapov, V.A.; Musalov, M.V.; Amosova, S.V. (Z,Z)-Selanediylbis(2-propenamides): Novel Class of Organoselenium Compounds with High Glutathione Peroxidase-Like Activity. Regio- and Stereoselective Reaction of Sodium Selenide with 3-Trimethylsilyl-2-propynamides. Molecules 2020, 25, 5940. https://doi.org/10.3390/molecules25245940
V. Andreev M, Potapov VA, Musalov MV, Amosova SV. (Z,Z)-Selanediylbis(2-propenamides): Novel Class of Organoselenium Compounds with High Glutathione Peroxidase-Like Activity. Regio- and Stereoselective Reaction of Sodium Selenide with 3-Trimethylsilyl-2-propynamides. Molecules. 2020; 25(24):5940. https://doi.org/10.3390/molecules25245940
Chicago/Turabian StyleV. Andreev, Mikhail, Vladimir A. Potapov, Maxim V. Musalov, and Svetlana V. Amosova. 2020. "(Z,Z)-Selanediylbis(2-propenamides): Novel Class of Organoselenium Compounds with High Glutathione Peroxidase-Like Activity. Regio- and Stereoselective Reaction of Sodium Selenide with 3-Trimethylsilyl-2-propynamides" Molecules 25, no. 24: 5940. https://doi.org/10.3390/molecules25245940
APA StyleV. Andreev, M., Potapov, V. A., Musalov, M. V., & Amosova, S. V. (2020). (Z,Z)-Selanediylbis(2-propenamides): Novel Class of Organoselenium Compounds with High Glutathione Peroxidase-Like Activity. Regio- and Stereoselective Reaction of Sodium Selenide with 3-Trimethylsilyl-2-propynamides. Molecules, 25(24), 5940. https://doi.org/10.3390/molecules25245940







