Synthesis of Novel N-Heterocyclic Compounds Containing 1,2,3-Triazole Ring System via Domino, “Click” and RDA Reactions
Abstract
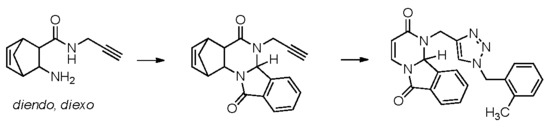
1. Introduction
2. Results
3. Materials and Methods
3.1. General Methods
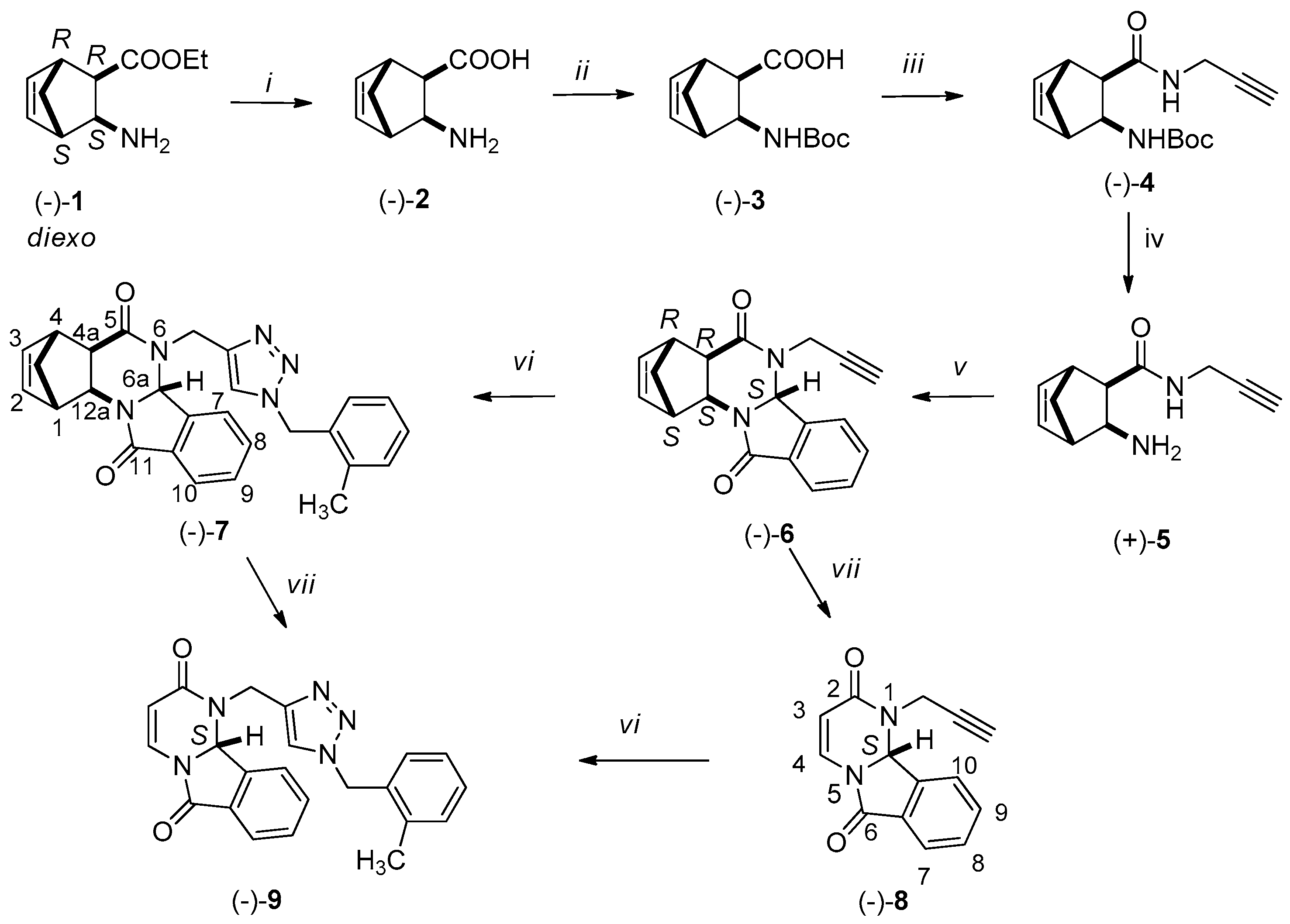
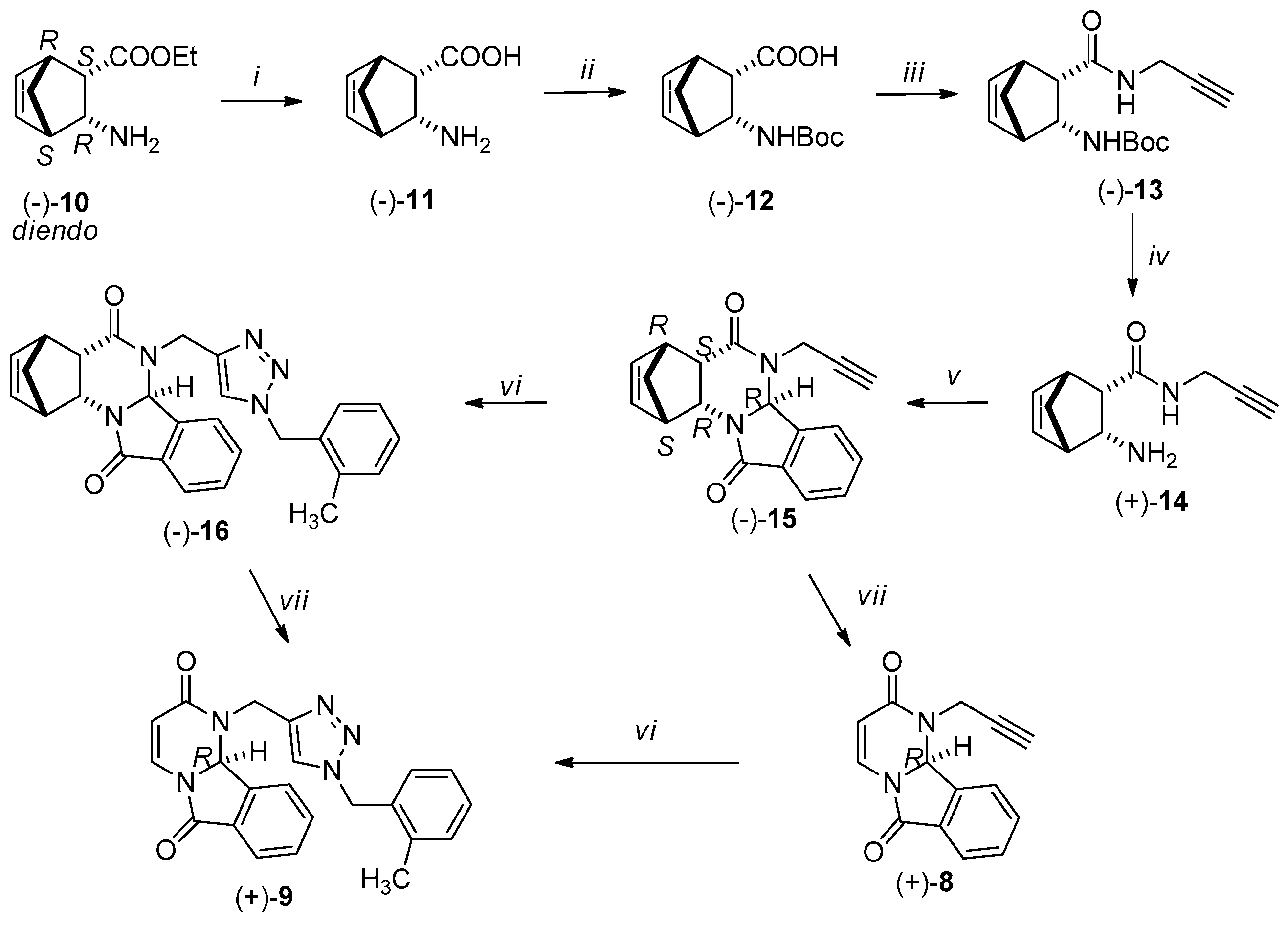
3.2. Synthesis of New Compounds
3.2.1. Synthesis of Amino Acids [(+)-2, (−)-2, (+)-11, (−)-11]
3.2.2. Synthesis of Boc-Protected Amino Acids [(+)-3, (−)-3, (+)-12, (−)-12]
3.2.3. Synthesis of Boc-Protected Propargyl Amides [(+)-4, (−)-4, (+)-13, (−)-13]
3.2.4. Synthesis of Domino Ring Closure Products [(−)-6, (+)-6, (−)-15 and (+)-15]
3.2.5. General Procedure for the Synthesis of (−)-7, (+)-7, (−)-16 and (+)-16 by Click Reaction
3.2.6. RDA Protocols for the Synthesis of Pyrimido[2,1-a]isoindols [(−)-8 (+)-8, (−)-9, (+)-9]
3.2.7. Representative Data for the Racemates (±)-4–(±)-16
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Held, F.E.; Guryev, A.A.; Fröhlich, T.; Hampel, F.; Kahnt, A.; Hutterer, C.; Steingruber, M.; Bahsi, H.; von Bojničić-Kninski, C.; Mattes, D.S. Facile Access to Potent Antiviral Quinazoline Heterocycles with Fluorescence Properties via Merging Metal-Free Domino Reactions. Nat. Commun. 2017, 8, 15071–15080. [Google Scholar] [CrossRef] [PubMed]
- Hutterer, C.; Hamilton, S.; Steingruber, M.; Zeitträger, I.; Bahsi, H.; Thuma, N.; Naing, Z.; Örfi, Z.; Örfi, L.; Socher, E. The Chemical Class of Quinazoline CoMpounds Provides a Core Structure for The Design of Anticytomegaloviral Kinase Inhibitors. Antiviral. Res. 2016, 134, 130–143. [Google Scholar] [CrossRef] [PubMed]
- Alafeefy, A.M.; Kadi, A.A.; Al-Deeb, O.A.; El-Tahir, K.E.; Al-jaber, N.A. Synthesis, Analgesic and Anti-inflammatory Evaluation of Some Novel Quinazoline Derivatives. Eur. J. Med. Chem. 2010, 45, 4947–4952. [Google Scholar] [CrossRef] [PubMed]
- Madapa, S.; Tusi, Z.; Mishra, A.; Srivastava, K.; Pandey, S.; Tripathi, R.; Puri, S.; Batra, S. Search for New Pharmacophores for Antimalarial Activity. Part II: Synthesis and Antimalarial Activity of New 6-ureido-4-anilinoquinazolines. Bioorg. Med. Chem. 2009, 17, 222–234. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jin, L.; Xiang, H.; Wu, J.; Wang, P.; Hu, D.; Xue, W.; Yang, S. Synthesis and Anticancer Activities of 5,6,7-trimethoxy-N-phenyl (ethyl)-4-aminoquinazoline Derivatives. Eur. J. Med. Chem. 2013, 66, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, M.; Zhang, Y.; Heinrich, J.-C.; Haupt, J.; Donakonda, S.; Lennig, P. Thymine derivatives and quinazoline-dione derivatives for the inhibition of HSP27. WO 2016016268 A1, 4 February 2016. [Google Scholar]
- Primeau, J.L.; Garrick, L.M.; Ocain, T.D.; Soll, R.M.; Dollings, P.J. Preparation of pyrimido-cycloalkanes as angiotensin II antagonists and antihyperlipidemics. WO 9308171 A1, 29 April 1993. [Google Scholar]
- Hasegawa, T.; Nakajima, H.; Kubota, D.; Okuma, K. Preparation of pyrimidine derivatives as poly(ADP-ribose) polymerase inhibitors. WO 2000042025 A1, 20 July 2000. [Google Scholar]
- Da Silva, F.d.C.; de Souza, M.C.B.; Frugulhetti, I.I.; Castro, H.C.; Silmara, L.d.O.; de Souza, T.M.L.; Rodrigues, D.Q.; Souza, A.M.; Abreu, P.A.; Passamani, F. Synthesis, HIV-RT Inhibitory Activity and SAR of 1-benzyl-1H-1,2,3-triazole Derivatives of Carbohydrates. Eur. J. Med. Chem. 2009, 44, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Katzung, B.G.; Trevor, A.J. Beta-Lactam and Other Cell Wall- and Membrane-Active Antibiotics. In Basic and Clinical Pharmacology 13E; McGraw-Hill Education: New York, NY, USA, 2014; p. 1145. [Google Scholar]
- Lauria, A.; Delisi, R.; Mingoia, F.; Terenzi, A.; Martorana, A.; Barone, G.; Almerico, A.M. 1,2,3-Triazole in Heterocyclic Compounds, Endowed with Biological Activity, Through 1,3-Dipolar Cycloadditions. Eur. J. Org. Chem. 2014, 2014, 3289–3306. [Google Scholar] [CrossRef]
- Xia, Y.; Qu, F.; Peng, L. Triazole Nucleoside Derivatives Bearing Aryl Functionalities on the Nucleobases Show Antiviral and Anticancer Activity. Mini-Rev. Med. Chem. 2010, 10, 806–821. [Google Scholar] [CrossRef]
- Peterson, L.B.; Blagg, B.S. Click chemistry to probe Hsp90: Synthesis and Evaluation of a Series of Triazole-Containing Novobiocin Analogues. Bioorg. Med. Chem. Lett. 2010, 20, 3957–3960. [Google Scholar] [CrossRef]
- Doiron, J.; Richard, R.; Touré, M.M.; Picot, N.; Richard, R.; Čuperlović-Culf, M.; Robichaud, G.A.; Touaibia, M. Synthesis and Structure–Activity Relationship of 1-and 2-Substituted-1,2,3-Triazole Letrozole-Based Analogues as Aromatase Inhibitors. Eur. J. Med. Chem. 2011, 46, 4010–4024. [Google Scholar] [CrossRef]
- Dheer, D.; Singh, V.; Shankar, R. Medicinal Attributes of 1,2,3-Triazoles: Current Developments. Bioorg. Chem. 2017, 71, 30–54. [Google Scholar] [CrossRef] [PubMed]
- Keivanloo, A.; Bakherad, M.; Lotfi, M. Use of Ligand-Assisted Click Reactions for the Rapid Synthesis of Novel 1,2,3-Triazole Pharmacophore-Based 1,2,4-Triazines and their Benzo-Fused Analogues. Tetrahedron 2017, 73, 5872–5882. [Google Scholar] [CrossRef]
- Maračić, S.; Kraljević, T.G.; Paljetak, H.Č.; Perić, M.; Matijašić, M.; Verbanac, D.; Cetina, M.; Raić-Malić, S. 1,2,3-Triazole Pharmacophore-Based Benzofused Nitrogen/Sulfur Heterocycles with Potential Anti-Moraxella Catarrhalis Activity. Bioorg. Med. Chem. 2015, 23, 7448–7463. [Google Scholar] [CrossRef] [PubMed]
- Ouahrouch, A.; Ighachane, H.; Taourirte, M.; Engels, J.W.; Sedra, M.H.; Lazrek, H.B. Benzimidazole-1,2,3-Triazole Hybrid Molecules: Synthesis and Evaluation for Antibacterial/Antifungal Activity. Arch. Pharm. Chem. Life Sci. 2014, 347, 748–755. [Google Scholar] [CrossRef] [PubMed]
- Massarotti, A.; Aprile, S.; Mercalli, V.; Del Grosso, E.; Grosa, G.; Sorba, G.; Tron, G.C. Are 1,4- and 1,5-Disubstituted 1,2,3-Triazoles Good Pharmacophoric Groups? Chem. Med. Chem. 2014, 9, 2497–2508. [Google Scholar] [CrossRef] [PubMed]
- Kume, M. Synthesis and Structure-Activity Relation-ships of new 7β-[(Z)-2-(2-aminothiazol-4-yl)-2-hydroxyiminoacetamido]-cephalosporins with 1,2,3-Triazole in C-3 Side Chain. J. Antibiot. 1993, 46, 177–192. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Masui, Y.; Goto, Y.; Kitaura, Y.; Mizutani, T.; Matsumura, I.; Sugata, Y.; Ide, Y.; Takayama, M.; Takahashi, H. Practical Large-Scale Synthesis of Cefmatilen, a New Cephalosporin Antibiotic. Org. Process. Res. Dev. 2004, 8, 744–753. [Google Scholar] [CrossRef]
- Chang, K.Y.; Kwon, S.H.; Nam, G.; Seo, J.H.; Kim, S.H.; Choi, K.I.; Kim, J.H.; Ha, D.C. New Cephalosporin Antibiotics with 3-Triazolylpyridiniummethyl Substituents. J. Antibiot. 2001, 54, 460–462. [Google Scholar] [CrossRef]
- Chitasombat, M.N.; Kontoyiannis, D.P. The ‘Cephalosporin Era’of Triazole Therapy: Isavuconazole, a Welcomed Newcomer for the Treatment of Invasive Fungal Infections. Expert Opin. Pharmaco. 2015, 16, 1543–1558. [Google Scholar] [CrossRef]
- Pellissier, H. Stereocontrolled Domino Reactions. Chem. Rev. 2012, 113, 442–524. [Google Scholar] [CrossRef]
- Bharate, J.B.; Vishwakarma, R.A.; Bharate, S.B. Metal-free Domino One-pot Protocols for Quinoline Synthesis. RSC Adv. 2015, 5, 42020–42053. [Google Scholar] [CrossRef]
- Tu, S.J.; Jiang, B. Microwave-Assisted Domino Reaction in Organic Synthesis. In Advances in Induction and Microwave Heating of Mineral and Organic Materials; Grundas, S.A., Ed.; InTech: Xuzhou, China, 2011; pp. 673–696. Available online: https://www.intechopen.com/books/advances-in-induction-and-microwave-heating-of-mineral-and-organic-materials/microwave-assisted-domino-reaction-in-organic-synthesis (accessed on 6 December 2018).
- Zhao, Y.H.; Li, Y.; Guo, T.; Tang, Z.; Deng, K.; Zhao, G. CuI-Catalyzed Domino Reactions for the Synthesis of Benzoxazine-Fused Isoquinolines under Microwave Irradiation. Synth. Commun. 2016, 46, 355–360. [Google Scholar] [CrossRef]
- Tietze, L.F.; Brasche, G.; Gericke, K. Domino Reactions in Organic Synthesis; Weinheim Wiley-VCH: Gottingen, Germany, 2006. [Google Scholar]
- Padwa, A.; Bur, S.K. The Domino Way to Heterocycles. Tetrahedron 2007, 63, 5341–5378. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.F.; Ye, P.; Sprague, K.; Sargent, K.; Yohannes, D.; Baldino, C.M.; Wilson, C.J.; Ng, S.C. Novel One-pot Total Syntheses of Deoxyvasicinone, Mackinazolinone, Isaindigotone, and Their Derivatives Promoted by Microwave Irradiation. Org. Lett. 2005, 7, 3363–3366. [Google Scholar] [CrossRef] [PubMed]
- Abe, T.; Yamada, K. Amination/Cyclization Cascade by Acid-Catalyzed Activation of Indolenine for the One-Pot Synthesis of Phaitanthrin E. Org. Lett. 2016, 18, 6504–6507. [Google Scholar] [CrossRef] [PubMed]
- Abe, T.; Terasaki, M. Synthesis of Phaitanthrin E and Tryptanthrin through Amination/Cyclization Cascade. Helv. Chim. Acta. 2018, 101, e1700284. [Google Scholar] [CrossRef]
- Rolfe, A.; Painter, T.O.; Asad, N.; Hur, M.Y.; Jeon, K.O.; Brzozowski, M.; Klimberg, S.V.; Porubsky, P.; Neuenswander, B.; Lushington, G.H. Triazole-Containing Isothiazolidine 1,1-dioxide Library Synthesis: One-pot, Multi-Component Protocols for Small Molecular Probe Discovery. Am. Chem. Soc. Comb. Sci. 2011, 13, 511–517. [Google Scholar] [CrossRef]
- Lundberg, P.; Hawker, C.J.; Hult, A.; Malkoch, M. Click Assisted One-pot Multi-step Reactions in Polymer Science: Accelerated Synthetic Protocols. Macromol. Rapid Commun. 2008, 29, 998–1015. [Google Scholar] [CrossRef]
- Esmaeili-Marandi, F.; Saeedi, M.; Yavari, I.; Mahdavi, M.; Shafiee, A. Synthesis of Novel Isoindolo [2,1-a] quinazolinedione Derivatives Containing a 1,2,3-Triazole Ring System. Helv. Chim. Acta. 2016, 99, 37–40. [Google Scholar] [CrossRef]
- Totobenazara, J.; Burke, A.J. New Click-chemistry Methods for 1,2,3-Triazoles Synthesis: Recent Advances and Applications. Tetrahedron Lett. 2015, 56, 2853–2859. [Google Scholar] [CrossRef]
- Wang, C.; Ikhlef, D.; Kahlal, S.; Saillard, J.Y.; Astruc, D. Metal-Catalyzed Azide-Alkyne “Click” Reactions: Mechanistic Overview and Recent Trends. Coord. Chem. Rev. 2016, 316, 1–20. [Google Scholar] [CrossRef]
- Singh, M.S.; Chowdhury, S.; Koley, S. Advances of Azide-Alkyne Cycloaddition-Click Chemistry over the Recent Decade. Tetrahedron 2016, 72, 5257–5283. [Google Scholar] [CrossRef]
- Tăbăcaru, A.; Furdui, B.; Ghinea, I.O.; Carac, G.; Dinică, R.M. Recent Advances in Click Chemistry Reactions Mediated by Transition Metal Based Systems. Inorg. Chim. Acta. 2017, 455, 329–349. [Google Scholar] [CrossRef]
- Stájer, G.; Csende, F.; Fülöp, F. The Retro Diels-Alder Reaction as a Valuable Tool for the Synthesis of Heterocycles. Curr. Org. Chem. 2003, 7, 1423–1432. [Google Scholar] [CrossRef]
- Suzuki, K.; Inomata, K.; Endo, Y. Enantiocontrolled Synthesis of Jasmonates via Tandem Retro-Diels− Alder− Ene Reaction Activated by a Silyl Substituent. Org. Lett. 2004, 6, 409–411. [Google Scholar] [CrossRef] [PubMed]
- González-Temprano, I.; Osante, I.; Lete, E.; Sotomayor, N. Enantiodivergent Synthesis of Pyrrolo [2,1-a] Isoquinolines Based on Diastereoselective Parham Cyclization and α-Amidoalkylation Reactions. J. Org. Chem. 2004, 69, 3875–3885. [Google Scholar] [CrossRef] [PubMed]
- Carson, C.A.; Kerr, M.A. Heterocycles from Cyclopropanes: Applications in Natural Product Synthesis. Chem. Soc. Rev. 2009, 38, 3051–3060. [Google Scholar] [CrossRef]
- Yoder, R.A.; Johnston, J.N. A Case Study in Biomimetic Total Synthesis: Polyolefin Carbocyclizations to Terpenes and Steroids. Chem. Rev. 2005, 105, 4730–4756. [Google Scholar] [CrossRef]
- Cheng, Y.; Huang, Z.T.; Wang, M.X. Heterocyclic Enamines: The Versatile Intermediates in the Synthesis of Heterocyclic Compounds and Natural Products. Curr. Org. Chem. 2004, 8, 325–351. [Google Scholar] [CrossRef]
- Palkó, M.; Sohár, P.; Fülöp, F. Synthesis and Transformations of diendo-3-Aminobicyclo[2.2.2]oct-5-ene-2-carboxylic Acid Derivatives. Molecules 2011, 16, 7691–7705. [Google Scholar] [CrossRef]
- Fekete, B.; Palkó, M.; Haukka, M.; Fülöp, F. Synthesis of Pyrrolo[1,2-a]pyrimidine Enantiomers via Domino Ring-Closure followed by Retro Diels-Alder Protocol. Molecules 2017, 22, 613. [Google Scholar] [CrossRef] [PubMed]
- Fekete, B.; Palkó, M.; Mándity, I.; Haukka, M.; Fülöp, F. A Domino Ring-Closure Followed by Retro-Diels–Alder Reaction for the Preparation of Pyrimido[2,1-a]isoindole Enantiomers. Eur. J. Org. Chem. 2016, 3519–3527. [Google Scholar] [CrossRef]
- Fülöp, F.; Miklós, F.; Forró, E. Diexo-3-aminonorbornane-2-carboxylic Acid as Highly Applicable Chiral Source for the Enantioselective Synthesis of Heterocycles. Synlett 2008, 1687–1689. [Google Scholar] [CrossRef]
- Miklós, F.; Tóth, Z.; Hänninen, M.M.; Sillanpää, R.; Forró, E.; Fülöp, F. Retro-Diels–Alder Protocol for the Synthesis of Pyrrolo[1,2-a]pyrimidine and Pyrimido[2,1-a]isoindole Enantiomers. Eur. J. Org. Chem. 2013, 4887–4894. [Google Scholar] [CrossRef]
- Miklós, F.; Bozó, K.; Galla, Z.; Haukka, M.; Fülöp, F. Traceless Chirality Transfer from a Norbornene β-Amino Acid to Pyrimido[2,1-a]isoindole Enantiomers. Tetrahedron: Asymmetry 2017, 28, 1401–1406. [Google Scholar] [CrossRef]
- Nekkaa, I.; Palko, M.; Mandity, I.; Miklos, F.; Fülöp, F. Continuous-Flow retro-Diels–Alder Reaction: A Process Window for Designing Heterocyclic Scaffolds. Eur. J. Org. Chem. 2018, 2018, 4456–4464. [Google Scholar] [CrossRef]
- Miklós, F.; Fülöp, F. “Dry” and “Wet” Green Synthesis of 2,2′-Disubstituted Quinazolinones. Eur. J. Org. Chem. 2010, 2010, 959–965. [Google Scholar] [CrossRef]
- Palkó, M.; Sándor, E.; Sohár, P.; Fülöp, F. Synthesis and Stereostructure of 3-amino-5- and -6-hydroxybicyclo[2.2.1]heptane-2-carboxylic Acid Diastereomers. Monats. Chem. 2005, 136, 2051–2058. [Google Scholar] [CrossRef]
- Stájer, G.; Szabó, E.A.; Fülöp, F.; Bernáth, G.; Sohár, P. Stereochemical Studies. 58. Saturated Heterocycles. 39. Preparation and Steric Structures of dihydro-1,3-oxazines, 1,3-oxazin-2-ones and 1,3-oxazine-2-thiones Fused with Norbornane and Norbornene. J. Heterocycl. Chem. 1983, 20, 1181–1185. [Google Scholar] [CrossRef]
- Stájer, G.; Mód, L.; Szabó, A.E.; Fülöp, F.; Bernáth, G.; Sohár, P. Stereochemical Studies—79 Synthesis and Kinetic Study on the Retrodiene Decomposition of Norbornene-condensed 1,3-oxazin-4-ones. Tetrahedron 1984, 40, 2385–2393. [Google Scholar] [CrossRef]
- Forró, E.; Fülöp, F. Vapour-assisted Enzymatic Hydrolysis of β-Lactams in a Solvent-free System. Tetrahedron: Asymmetry 2008, 19, 1005–1009. [Google Scholar] [CrossRef]
- Lloyd, M.; Lloyd, R.; Keene, P.; Osborne, A. A Concise Synthesis of Single-Enantiomer β-Lactams and β-Amino acids using Rhodococcus Globerulus. J. Chem. Technol. Biotechnol 2007, 82, 1099–1106. [Google Scholar] [CrossRef]
- Canonne, P.; Akssira, M.; Dahdouh, A.; Kasmi, H.; Boumzebra, M. A Convenient Synthesis of Bridged Azatricyclic Anhydrides. Tetrahedron 1993, 49, 1985–1992. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 1–16 are not available from the authors. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palkó, M.; El Haimer, M.; Kormányos, Z.; Fülöp, F. Synthesis of Novel N-Heterocyclic Compounds Containing 1,2,3-Triazole Ring System via Domino, “Click” and RDA Reactions. Molecules 2019, 24, 772. https://doi.org/10.3390/molecules24040772
Palkó M, El Haimer M, Kormányos Z, Fülöp F. Synthesis of Novel N-Heterocyclic Compounds Containing 1,2,3-Triazole Ring System via Domino, “Click” and RDA Reactions. Molecules. 2019; 24(4):772. https://doi.org/10.3390/molecules24040772
Chicago/Turabian StylePalkó, Márta, Mohamed El Haimer, Zsanett Kormányos, and Ferenc Fülöp. 2019. "Synthesis of Novel N-Heterocyclic Compounds Containing 1,2,3-Triazole Ring System via Domino, “Click” and RDA Reactions" Molecules 24, no. 4: 772. https://doi.org/10.3390/molecules24040772
APA StylePalkó, M., El Haimer, M., Kormányos, Z., & Fülöp, F. (2019). Synthesis of Novel N-Heterocyclic Compounds Containing 1,2,3-Triazole Ring System via Domino, “Click” and RDA Reactions. Molecules, 24(4), 772. https://doi.org/10.3390/molecules24040772