Abstract
‘Desymmetrization’ of trans-1,2-diaminocyclohexane by treatment with α,ω-dihalogenated alkylation reagents leads to mono-NH2 derivatives (‘primary-tertiary diamines’). Upon reaction with formaldehyde, these products formed monomeric formaldimines. Subsequently, reactions of the formaldimines with α-hydroxyiminoketones led to the corresponding 2-unsubstituted imidazole N-oxide derivatives, which were used here as new substrates for the in situ generation of chiral imidazol-2-ylidenes. Upon O-selective benzylation, new chiral imidazolium salts were obtained, which were deprotonated by treatment with triethylamine in the presence of elemental sulfur. Under these conditions, the intermediate imidazol-2-ylidenes were trapped by elemental sulfur, yielding the corresponding chiral non-enolizable imidazole-2-thiones in good yields. Analogous reaction sequences, starting with imidazole N-oxides derived from enantiopure primary amines, amino alcohols, and amino acids, leading to the corresponding 3-alkoxyimidazole-2-thiones were also studied.
1. Introduction
Since 1991, after the isolation of the first stable 1,3-di(adamantyl)imidazol-2-ylidene [1], there has been a wealth of both structural studies and practical applications of nucleophilic N-heterocyclic carbenes (NHCs) [2,3]. At the same time, there is continuing demand for catalysts and ligands for asymmetric synthesis [4]. Chiral NHCs are particularly suitable for such applications, and the elaboration of methods for the preparation of their precursors and subsequent applications in asymmetric synthesis are intensively pursued [5,6,7,8]. One efficient method for the preparation of chiral NHCs is the introduction of a chiral fragment attached to the N-atom located within the heterocyclic skeleton (chiral N-substituents).
It is therefore natural that one of the most important motifs in the structure of catalysts and ligands for asymmetric catalysis derives from easily available and inexpensive enantiomerically pure (R,R)-trans-1,2-diaminocylohexane (trans-DACH, Scheme 1) [9,10]. Numerous derivatives of this exceptional diamine have been synthesized via functionalization of only one or both amino groups. In recent years, much attention has been paid to so-called bifunctional catalysts, which are prepared by modifications of the amino groups. Derivatives of type 1 with a cyclic amine motif are promising building blocks for the synthesis of new bifunctional catalysts [11,12] and are important synthons for the preparation of some bioactive compounds [13,14,15,16,17]. An efficient method for the ‘desymmetrization’ of DACH, reported only in recent years, comprises two-fold N,N-alkylation of only one NH2 group using α,ω-dihaloalkylating reagents as shown in Scheme 1 [12].
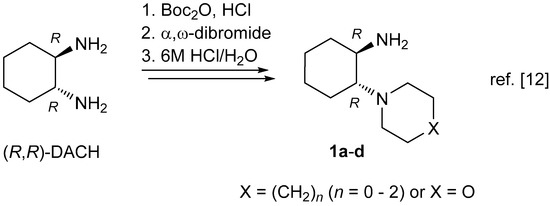
Scheme 1.
trans-1,2-diaminocyclohexane (DACH) and its ‘desymmetrized’ derivatives 1 (Reproduced with permission from [12]).
Along with trans-DACH, another primary amine widely explored in asymmetric synthesis is α-methylbenzylamine (α-MBA), but to date, reports on its application in the synthesis of chiral NHCs are limited [6]. Similarly, chiral β-amino alcohols and amino esters have scarcely been explored. Selected representatives of these two classes of compounds will also be involved in this study.
It is well established that the most efficient and preparatively useful method for the in situ generation of imidazol-2-ylidenes 3 comprises the deprotonation of the corresponding imidazolium salts of type 2 by treatment with a base (Scheme 2) [2,3].
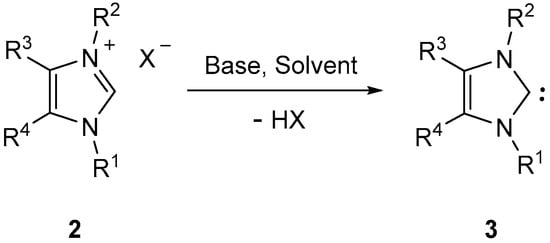
Scheme 2.
Formation of imidazol-2-ylidenes by deprotonation of imidazolium salts.
In our earlier publications, we described an efficient method for the synthesis of enantiomerically pure bis- and mono-imidazole N-oxides derived either from trans-1,2-diaminocyclohexane [18,19], α-methylbenzylamine [20], β-amino alcohols [21], and amino acid esters [22]. In another study, we demonstrated that 2-unsubstituted imidazole N-oxides including some chiral derivatives can be O-alkylated by treatment with alkyl bromides [23]. However, these 1-alkoxyimidazolium salts have never been used for the generation of N-alkoxy-substituted imidazol-2-ylidenes of type 3. In a very recent publication, we reported the synthesis of non-symmetric imidazolium salts prepared in a multistep synthesis starting with adamantyloxyamine [24]. In a test experiment, a symmetric 1,3-di(adamantyloxy)imidazolium salt was converted into the corresponding imidazole-2-thione derivative via an intermediate NHC. Prompted by these results, we decided to synthesize a series of new chiral 1-alkoxyimidazolium salts derived from ‘desymmetrized’ trans-1,2-diaminocylohexane derivatives of type 1, which can subsequently be used as precursors of chiral NHCs. The study was extended by the involvement of α-methylbenzylamine as well as selected amino alcohols and amino acid derivatives.
2. Results and Discussion
In contrast to DACH, which reacts with two equivalents of formaldehyde forming a dimeric product identified as a tetraazaeicosane derivative [25], all ‘primary-tertiary’ diamines 1a–d reacted with formaldehyde yielded the expected formaldimines 4a–d as solid products (Scheme 3). In the solid state they exist as hexahydro-1,3,5-triazines (trimers), which in polar, aprotic solvents undergo almost total dissociation forming the monomeric imines. Similar observations were made for cyclohexylformaldimine [26]. For example, formaldimine 4b dissolved in CDCl3 dissociates with t1/2 of ca. 30 s at 298 K. On the other hand, the same imine observed in the C6D6 solution exists as an equilibrium mixture of trimeric and monomeric forms in a ratio of ca. 2.5:1. The structures of formaldimines 4 were confirmed by 1H and 13C-NMR spectra in CDCl3. For example, in the 1H-NMR spectrum of 4b, the characteristic AB-system of the monomeric form located at 7.13 and 7.28 ppm with J = 18 Hz was attributed to the N=CH2 group. In the 13C-NMR spectrum, the signal of this group appeared at 151.0 ppm, and similar data were found for all formaldimines 4. However, in all samples, the presence of trimeric forms interfered with the integration of signals. The presence of trimers was revealed by signals of the CH2 groups located at 3.80–4.00 ppm. The products were unstable in solution and underwent gradual decomposition over a period of weeks during storage at room temperature. The molecular formulae of the stable, crystalline samples were confirmed by elemental analysis.
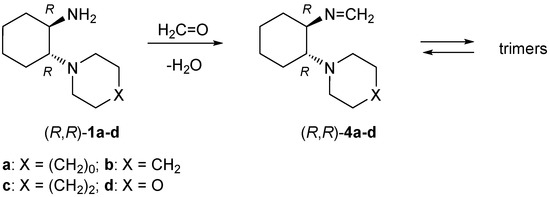
Scheme 3.
Synthesis of monomeric formaldimines 4 from ‘primary–tertiary’ diamines 1.
In the case of 4e derived from (S)-α-methylbenzylamine ((S)-α-MBA), rapid trimerization led to hexahydro-1,3,5-triazine 4′e (Scheme 4) in the course of its synthesis, and after isolation, it could be used for further transformations only in this form [20]. An analogous tendency for trimerization was also observed for 4f–h used in the study.
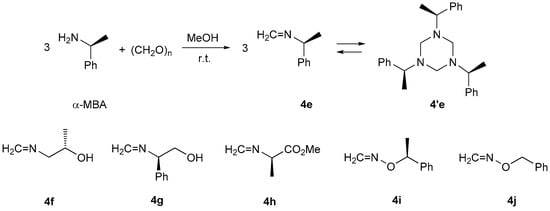
Scheme 4.
Trimerization of formaldimine 4e derived from (S)-α-methylbenzylamine (α-MBA) [20] (Reproduced with permission from [20]) and structures of analogous formaldimines 4f–i.
In contrast, the alkoxyformaldimine 4i derived from (S)-α-methylbenzyloxyamine (α-MBOA) [27] exists in CDCl3 solution in monomeric form, and in that case, no tendency to undergo trimerization was observed even after three days at room temperature. Moreover, a similar behavior was observed in the case of benzyloxyformaldimine (4j) prepared from benzyloxyamine (BOA) [28] and formaldehyde in the course of the present study. Apparently, the presence of an alkoxy moiety reduces the electrophilicity of the =CH2 unit and thereby formation of the trimeric forms is substantially disfavored.
Enantiomerically pure, (R,R)-configured 2-unsubstituted imidazole N-oxides 6 used in this study as the key building blocks were prepared from enantiopure formaldimines 4, derived from the corresponding amines 1, and α-hydroxyiminoketones 5a,b in glacial acetic acid at room temperature [24]. In the case of 4b, along with the (R,R)-configured stereoisomer, the (S,S)-enantiomer was also involved in the study (Scheme 5 and Table 1).
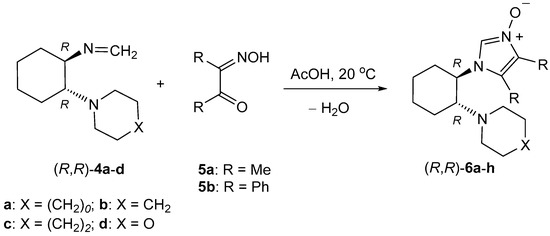

Table 1.
Synthesis of imidazole N-oxides 6 and imidazole-2-thiones 9 from chiral formaldimines 4a–d derived from ‘desymetrized’ trans-1,2-diaminocyclohexanes 1.
Reactions of 4 with α-hydroxyiminoketones 5 leading to imidazole N-oxides 6 were performed at room temperature in glacial acetic acid. After isolation, the products were identified by spectroscopic methods, and the most characteristic signal of HC(2) of the imidazole ring in the 1H-NMR spectra appeared at ca. 8 ppm. Products 6 were alkylated with an equimolar amount of benzyl bromide in CH2Cl2 solution at room temperature. Due to the observed slow decomposition, the (N-benzyloxy)imidazolium salts 7 were used for the next reaction step without further purification. In the case of the azepane derivative 7e, the structure of the crude product was confirmed by 1H-NMR spectroscopy. The characteristic HC(2)-signal was significantly shifted toward the lower field and appeared at 11.35 ppm. In addition, chemoselective O-benzylation was confirmed by the appearance of only one AB-system (5.67 and 5.72 ppm, J = 12.0 Hz) of the OCH2Ph group. Thus, competitive N-benzylation could be ruled out.
The obtained crude imidazolium salts 7 were used as precursors of chiral imidazol-2-ylidenes 8 (Scheme 6). Their intermediacy was proven with known trapping reactions with elemental sulfur [24,29,30] leading to non-enolizable imidazole-2-thiones. The deprotonation of 7 was easily achieved by treatment with triethylamine in pyridine. In the presence of elemental sulfur, the in situ generated NHCs 8 exclusively reacted with the desired imidazole-2-thiones 9 (Scheme 6 and Table 1). Their structures were confirmed by spectroscopic data with the most characteristic signal in the 13C-NMR spectra being the C(2)=S group resonating between 156 and 162 ppm. The obtained imidazole-2-thiones 9a–h were shown to be optically active compounds. The optical rotation for all these products was determined in CHCl3 solutions and no racemization under the reaction conditions is expected.
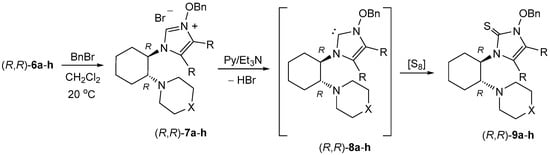
Scheme 6.
Preparation of imidazole-2-thiones 9a–h bearing a chiral substituent via intermediate imidazol-2-ylidenes 8 (see also Table 1).
The inspection of the 1H-NMR spectra of 9 in CDCl3 solution at room temperature evidenced complex signal patterns. It is likely that there are equilibria of different rotamers and/or conformers existing in these solutions. To acquire more information, the 1H-NMR spectra of analytically pure (R,R)-9b were recorded at different temperatures in a 1,1,2,2-tetrachloroethane solution. The spectrum at 294 K showed a set of broadened signals in the region of 4.2–4.8 ppm attributed to the PhCH2O fragment. In addition, two broad signals of H(C1) and HC(2) of the cyclohexane ring were found at 2.8 and 3.1 ppm. In the spectrum measured at 334 K, the benzylic region revealed one broad signal at 4.6 ppm and the signals of two distinct cyclohexane H-atoms were shifted high-field and overlap with the α-H-atoms of the pyrrolidine ring. This observation supports our assumption of the presence of a dynamic equilibrium of different conformers of (R,R)-9b. An analogous explanation is valid for all spectra of 9 bearing two Ph groups at C(4) and C(5) of the imidazole ring.
In extension of the studies performed with ‘desymmetrized’ trans-DACH derivatives, analogous experiments were performed starting with enantiomerically pure functionalized primary amines. Thus, the corresponding formaldimines 4f–h (Scheme 4) were converted into imidazole N-oxides 10a–c by reaction with the α-hydroxyiminoketone 5a under standard conditions (see Experimental Section). In addition, enantiopure 10d, readily available by aminolysis of the corresponding ester with (R)-α-MBA [22] was used. The obtained imidazole N-oxides 10 were used for the preparation of imidazolium salts 11a–d, which upon treatment with triethylamine in pyridine in the presence of elemental sulfur gave the optically active imidazole-2-thiones 13a–d in good yields (Scheme 7). In these reactions, optically active N-butoxyimidazol-2-ylidenes of type 12 were the in situ generated reactive intermediates.
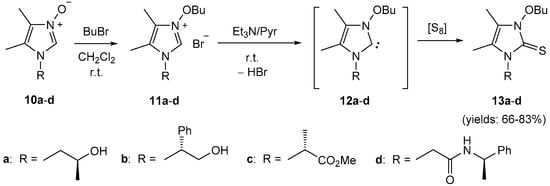
Scheme 7.
Synthesis of optically active 3-butoxyimidazole-2-thiones 13.
In contrast to 9b,d,f, and g derived from (R,R)-trans-DACH, the 1H-NMR spectra of 13 clearly indicated the presence of a single form in the solution. For example, the spectrum of compound 13b showed only one multiplet for the CH2O unit located at 4.34–4.41 ppm, and two characteristic singlets of the Me groups at C(4) and C(5) of the imidazole ring at 1.72 and 2.09 ppm, respectively. In the 13C-NMR spectrum, the signal of the C=S group appeared at 157.4 ppm.
In order to check whether steric hindrance is the reason for the existence of different conformers of 9, four other compounds of that type (i.e., 15a–d derived from (S)-α-methylbenzylamine) were synthesized following the multistep procedure presented in Scheme 6 and Scheme 7. The starting 2-unsubstituted imidazole N-oxides 14a,b are known compounds [20]. Upon treatment with alkylating reagents (benzyl bromide or n-pentyl bromide), they were converted into the corresponding imidazolium salts (Scheme 8). The latter products without purification were treated with triethylamine in the presence of elemental sulfur, yielding the desired 15a–d in good yields (66–81%).
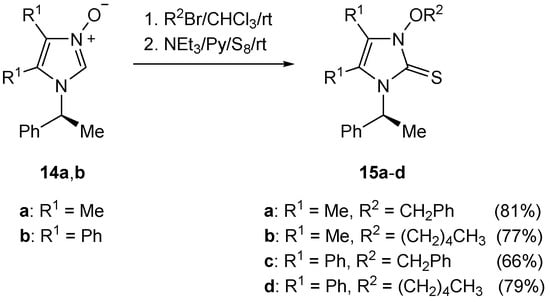
Scheme 8.
3-Alkoxyimidazole-2-thiones 15a–d derived from (S)-α-methylbenzylamine.
In that case, the less bulky (S)-α-methylbenzyl residue was placed at the N(1) atom. The 1H-NMR spectrum of 15a reveals the presence of the expected AB system of the PhCH2O group at 5.45 and 5.58 (J = 10 Hz) ppm. This result emphasizes the importance of the steric demand in the series of compounds 9 derived from bulky trans-1,2-diaminocyclohexane.
3. Conclusions
Enantiomerically pure desymmetrized derivatives of trans-1,2-diaminocyclohexane containing one free NH2 group (i.e., so called ‘primary-tertiary amines’) can be efficiently converted into corresponding formaldimines. The initially obtained formaldimines were transformed into 2-unsubstituted imidazole N-oxides, which upon treatment with benzyl bromide were selectively converted into benzyloxyimidazolium salts. After deprotonation with triethylamine, the latter compounds generated new types of chiral imidazol-2-ylidenes. Their appearance was evidenced by the trapping reaction with elemental sulfur leading to chiral non-enolizable imidazole-2-thiones. The same procedure applied to other optically active functionalized primary amines opens straightforward access for in situ generated nucleophilic carbenes derived from imidazole (imidazol-2-ylidenes) bearing an alkoxy group. This little-known class of alkoxy-substituted optically active NHCs is of interest for potential applications in asymmetric synthesis and in organometallic chemistry for the preparation of transition metal complexes. The multistep synthesis of the optically active imidazole-2-thiones occurs with preservation of the stereochemistry. In addition, many derivatives of chiral 1,2-diamines [13,15,31,32], imidazolium salts [33,34,35] as well as numerous imidazole-2-thiones [36,37] display diverse biological activities. Nevertheless, their alkoxy derivatives are practically unknown. For that reason, the new types of ‘desymmetrized’ derivatives of trans-1,2-diaminocyclohexane and other optically active primary amines reported in this work may also be of interest for medicinal chemistry.
4. Materials and Methods
4.1. General Information
Solvents and chemicals were purchased and used as received without further purification. Products were purified by standard column chromatography on silica gel (230–400 mesh, Merck, Kenilworth, NJ, USA). Unless stated otherwise, yields refer to analytically pure samples. NMR spectra were recorded with a Bruker Avance III 600 MHz instrument (1H-NMR: 600 MHz; 13C-NMR: 151 MHz; Bruker, Billerica, MA, USA). Chemical shifts are reported relative to solvent residual peaks (1H-NMR: δ = 7.26 ppm [CHCl3]; 13C-NMR: δ = 77.0 ppm [CDCl3]). IR spectra were recorded with a FTIR NEXUS spectrometer (as film or KBr pellets) or with a Cary 630 FTIR (Agilent Technologies, Santa Clara, CA, USA) spectrometer, in neat. ESI-MS spectra were performed with a Varian 500-MS LC Ion Trap. High-resolution mass spectrometry (HRMS) measurements were performed using Synapt G2-Si mass spectrometer (Waters, Milford, MA, USA) equipped with an ESI source and quadrupole-Time-of-flight mass analyzer. Elemental analyses were obtained with a Vario EL III (Elementar Analysensysteme GmbH, Langenselbold, Germany) instrument. Optical rotations were determined with an Anton Paar MCP 500 polarimeter (Anton Paar, Graz, Austria) at the temperatures indicated. Melting points were determined in capillaries with a Stuart SMP30 apparatus with automatic temperature monitoring or with a polarizing optical microscope (Opta-Tech, Warszawa, Poland), and are uncorrected.
4.2. Starting Materials
The ‘primary–tertiary’ amines 1 (i.e., compounds (R,R)-1a, (R,R)-1b, (S,S)-1b, (R,R)-1c, and (R,R)-1d) were prepared from the respective, enantiopure diastereomers of trans-1,2-diaminocyclohexane (trans-DACH), according to the literature procedure [12]. α-Hydroxyiminoketones 5 were prepared by nitrozation of ethyl methyl ketone in the case of 7a [38] and by oximation of dibenzoyl in the case of 7b [39] based on the published procedures. α-Methylbenzyloxyamine (α-MBOA) [27] and benzyloxyamine (BOA) [40] were prepared based on modified literature procedures. Formaldimines 4e [20], 4f [21], 4g [21], and 4h [22] were synthetized from corresponding amino compounds and formaldehyde based on the published procedures.
4.3. Synthesis of Formaldimines 4
4.3.1. Reactions of ‘Primary-Tertiary’ Amines 1 with Formaldehyde—General Procedure
A solution of 5 mmol of the respective diamine 1 and 450 mg (15 mmol) of formaldehyde (used as an aqueous solution) in 50 mL of benzene was heated in the Dean-Stark apparatus over 1.5 h. Next, the solvent was evaporated and the crude products were obtained as colorless solids. Analytically pure samples were obtained by recrystallization.
(R,R)-N-[2-(Pyrrolidin-1-yl)cyclohexyl]methanimine ((R,R)-4a): Yield 855 mg (95%). Colorless crystals (diisopropyl ether). M.p. 97–98 °C. = −135.30 (c 0.40, CHCl3). 1H-NMR (CDCl3): δ = 7.39 (d, J = 17.2 Hz, 1H), 7.13 (d, J = 17.2 Hz, 1H), 2.93–2.97 (m, 1H), 2.66–2.71 (m, 1H), 2.52–2.62 (m, 4H), 1.86–1.91 (m, 1H), 1.72–1.77 (m, 1H), 1.60–1.69 (m, 4H), 1.52–1.58 (m, 2H), 1.23–1.30 (m, 3H), 1.12–1.20 (m, 1H) ppm. 13C-NMR (CDCl3): δ = 151.1, 74.4, 63.4, 48.4, 34.0, 24.6, 24.2, 23.9, 23.5 ppm. IR: ν 2920 (vs), 2851 (s), 2801 (s), 1457 (m), 1384 (m), 1369 (m), 1183 (s), 1135 (vs), 997 (vs), 898 (m), 875 (m), 766 (m) cm−1. C11H20N2 (180.29): calcd. C 73.28, H 11.18, N 15.54; found C 73.27, H 11.15, N 15.60.
(R,R)-N-[2-(Piperidin-1-yl)cyclohexyl]methanimine ((R,R)-4b): Yield 756 mg (78%). Colorless crystals (diethyl ether). M.p. 126–128 °C. = −130.92 (c 0.40, CHCl3). 1H-NMR (CDCl3): δ = 7.28 (d, J = 17.2 Hz, 1H), 7.13 (d, J = 17.2 Hz, 1H), 2.93 (td, J = 9.9, 5.2 Hz, 1H), 2.45–2.62 (m, 3H), 2.29–2.37 (m, 2H), 1.8–11.86 (m, 1H), 1.72–1.79 (m, 1H), 1.58–1.69 (m, 3H), 1.44–1.51 (m, 2H), 1.30–1.40 (m, 4H), 1.10–1.25 (m, 3H) ppm. 13C-NMR (CDCl3): δ = 151.0, 72.4, 67.3, 49.6, 34.6, 26.6, 25.6, 25.1, 24.6, 23.6 ppm. IR: ν 2924 (vs), 2849 (s), 1450 (m), 1381 (m), 1360 (m), 1191 (s), 1127 (vs), 1114 (vs), 1004 (vs), 911 (m), 876 (m), 766 (m), 641 (m) cm–1. C12H22N2 (194.32): calcd. C 74.17, H 11.41, N 14.42; found C 74.17, H 11.38, N 14.46.
(S,S)-N-[2-(Piperidin-1-yl)cyclohexyl]methanimine ((S,S)-4b): Yield 775 mg (80%). Colorless crystals (diethyl ether). M.p. 125–127 °C. = +140.97 (c 0.40, CHCl3). 1H-NMR (CDCl3): δ = 7.28 (d, J = 17.2 Hz, 1H), 7.14 (d, J = 17.2 Hz, 1H), 2.94 (td, J = 9.9, 5.3 Hz, 1H), 2.46–2.61 (m, 3H), 2.28–2.36 (m, 2H), 1.82–1.86 (m, 1H), 1.73–1.79 (m, 1H), 1.59–1.70 (m, 3H), 1.44–1.52 (m, 2H), 1.31–1.40 (m, 4H), 1.12–1.26 (m, 3H) ppm. 13C-NMR (CDCl3): δ = 151.1, 72.4, 67.3, 49.6, 34.6, 26.6, 25.6, 25.1, 24.6, 23.6 ppm. IR: ν 2924 (vs), 2849 (s), 1450 (m), 1381 (m), 1360 (m), 1191 (s), 1127 (vs), 1114 (vs), 1004 (vs), 911 (m), 876 (m), 641 (m) cm–1. C12H22N2 (194.32): calcd. C 74.17, H 11.41, N 14.42; found C 73.99, H 11.55, N 14.38.
(R,R)-N-[2-(Azepan-1-yl)cyclohexyl]methanimine ((R,R)-4c): Yield 969 mg (93%). Colorless crystals (diisopropyl ether). M.p. 112–114 °C. = –81.57 (c 0.40, CHCl3). 1H-NMR (CDCl3): δ = 7.32 (d, J = 17.3 Hz, 1H), 7.13 (d, J = 17.3 Hz, 1H), 2.90 (td, J = 10.0, 5.0 Hz, 1H), 2.64–2.68 (m, 2H), 2.59–2.64 (m, 1H), 2.50–2.55 (m, 2H), 1.79–1.82 (m, 1H), 1.71–1.76 (m, 1H), 1.63–1.68 (m, 1H), 1.41–1.62 (m, 10H), 1.15–1.24 (m, 3H) ppm. 13C-NMR (CDCl3): δ = 151.1, 73.7, 68.6, 51.4, 34.5, 30.0, 27.0, 25.6, 25.3, 24.6 ppm. IR: ν 2920 (vs), 2849 (s), 1448 (m), 1355 (m), 1166 (m), 1131 (s), 993 (m), 907 (m), 874 (m) cm−1. C13H24N2 (208.34): calcd. C 74.94, H 11.62, N 13.44; found C 75.10, H 11.73, N 13.61.
(R,R)-N-(2-Morpholinocyclohexyl)methanimine ((R,R)-4d): Yield 1.02 g (98%). Colorless crystals (diisopropyl ether). M.p. 127–129 °C. = −124.52 (c 0.40, CHCl3). 1H-NMR (CDCl3): δ = 7.30 (d, J = 17.1 Hz, 1H), 7.14 (d, J = 17.1 Hz, 1H), 3.59–3.66 (m, 2H), 3.51–3.55 (m, 2H), 2.93 (td, J = 9.8, 5.5 Hz, 1H), 2.50–2.60 (m, 3H), 2.40–2.46 (m, 2H), 1.83–1.88 (m, 1H), 1.75–1.79 (m, 1H), 1.65–1.70 (m, 1H), 1.57–1.63 (m, 2H), 1.16–1.26 (m, 3H) ppm. 13C-NMR (CDCl3): δ = 151.4, 72.5, 67.6, 67.0, 48.9, 34.6, 25.5, 24.6, 23.9 ppm. IR: ν 2920 (s), 2851 (s), 1450 (m), 1258 (m), 1112 (vs), 995 (s), 872 (m) cm–1. C11H20N2O (196.29): calcd. C 67.31, H 10.27, N 14.27; found C 67.07, H 10.39, N 14.23.
4.3.2. Synthesis of Alkoxyformaldimines 4i–j—General Procedure
A solution of 3 mmol of the corresponding primary amine ((S)-MBOA or BOA) and 0.25 mL (ca. 3 mmol) of formaldehyde (as aqueous solution (37%)) in 40 mL benzene was heated in the Dean-Stark apparatus until no formation of water was observed. After cooling to room temperature, the solvent was evaporated and the colorless, oily liquids were used for further reactions without purification.
(S)-(α-Methylbenzyloxy)methanimine (4i): Yield 404 mg (90%). Colorless liquid. = −11.90 (c 0.51, CHCl3). 1H-NMR (CDCl3): δ = 7.38–7.33, 7.30–7.27 (2m, 4H, 1H, Ph), 7.08, 6.43 (2d, 2H, J = 8.5 Hz, =CH2), 5.27 (q, J = 6.7 Hz, 1H), 1.56 (d, J = 6.7 Hz, 3H, Me) ppm. 13C-NMR (CDCl3): δ = 143.0 (t, N=CH2), 137.4, 128.4, 127.6, 126.3 (s, 3d, Ph), 81.1 (d, CH), 21.8 (q, Me) ppm. HRMS (CI+): calcd for C9H13NO [M + H]+ 150.0917; found 150.0919.
(Benzyloxy)methanimine (4j): Yield 360 mg (88%). Colorless liquid (ref. [41], bp 76 °C/15 mmHg). 1H-NMR (CDCl3): δ = 7.40–7.35, 7.34–7.30 (2m, 4H, 1H, Ph), 7.09, 6.47 (2d, 2H, J = 8.2 Hz, =CH2), 5.14 (s, 2H, -CH2-) ppm. 13C-NMR (CDCl3): δ = 137.4 (t, N=CH2), 137.3 (s, Ph), 128.3, 128.1, 127.8 (3d, Ph), 75.8 (t, -CH2-) ppm.
4.4. Synthesis of Imidazole N-Oxides 6—General Procedure
A solution of 1.1 mmol of the respective formaldimine 4 and 1 mmol of α-hydroxyiminoketone 5 in 3 mL of glacial acetic acid was stirred magnetically overnight. The next day, 1 mL of aqueous hydrochloric acid was added and the solution was stirred for 15 min. Then, the resulting solution was evaporated to dryness, the solid residue was dissolved in 20 mL of dichloromethane, and the obtained solution was neutralized with 20 mL of diluted aqueous sodium hydroxide. The organic layer was separated, dried over MgSO4, filtrated, and evaporated. The resulting crude products were washed with two portions (each ca. 10 mL) of diethyl ether, and after separation, the obtained thick oils or amorphous solids were characterized spectroscopically and used for further transformations.
(R,R)-4,5-Dimethyl-1-[2-(pyrrolidin-1-yl)cyclohexyl]-1H-imidazole 3-oxide ((R,R)-6a): Yield 171.0 mg (65%). Colorless, viscous oil. 1H-NMR (CDCl3): δ = 7.88 (s, 1H), 3.73 (td, J = 11.2, 3.5 Hz, 1H), 2.81–2.87 (m, 1H), 2.45–2.49 (m, 2H), 2.37–2.41 (m, 2H), 2.14 (s, 3H), 2.10 (s, 3H), 2.00–2.03 (m, 1H), 1.93–1.97 (m, 1H), 1.80–1.84 (m, 1H), 1.76–1.79 (m, 1H), 1.44–1.60 (m, 4H), 1.14–1.35 (m, 4H) ppm. 13C-NMR (CDCl3): δ = 125.2, 123.0, 120.6, 62.1, 58.7, 48.0, 35.0, 25.2, 24.8, 24.6, 23.6, 8.8, 7.1 ppm. IR: ν 2927 (s), 1655 (m), 1448 (m), 1377 (m), 1332 (vs), 1191 (m), 1142 (m), 883 (s), 702 (s), 579 (s) cm−1. HRMS (ESI+): calcd for C15H26N3O [M + H]+ 264.2076; found 264.2082.
(R,R)-4,5-Diphenyl-1-[2-(pyrrolidin-1-yl)cyclohexyl]-1H-imidazole 3-oxide ((R,R)-6b): Yield 360.0 mg (93%). Yellowish crystals. M.p. 189–191 °C. 1H-NMR (CDCl3): δ = 8.12 (s, 1H), 7.49 (d, J = 7.9 Hz), 7.35–7.39 (m, 3H), 7.18–7.23 (m, 5H), 3.74 (td, J = 11.7, 4.2 Hz, 1H), 2.86 (td, J = 11.1, 3.5 Hz, 1H), 2.32–2.36 (m, 2H), 2.25–2.29 (m, 2H), 2.06–2.11 (m, 1H), 1.89–1.94 (m, 1H), 1.47–1.78 (m, 8H), 1.21–1.29 (m, 1H), 1.08–1.18 (m, 3H) ppm. 13C-NMR (CDCl3): δ = 130.7, 129.6, 129.3, 129.1, 128.9, 128.0, 127.9, 127.8, 127.3, 127.0, 124.2, 62.3, 58.7, 47.6, 35.4, 25.2, 24.6, 24.0, 23.56, 23.51 ppm. IR: ν 2931 (m), 2808 (s), 1448 (m), 1341 (m), 1321 (m), 1224 (m), 881 (m), 754 (s), 710 (s), 689 (vs), 658 cm−1. C25H29N3O·0.5 H2O (387.52 + 9): calcd. C 75.72, H 7.62, N 10.60; found C 75.50, H 7.59, N 10.61.
(R,R)-4,5-Dimethyl-1-[2-(piperidin-1-yl)cyclohexyl]-1H-imidazole 3-oxide ((R,R)-6c): Yield 249.5 mg (90%). Yellowish crystals. M.p. 68–71 °C. 1H-NMR (CDCl3): δ = 7.70 (s, 1H), 3.75 (td, J = 11.5, 3.8 Hz, 1H), 2.47–2.54 (m, 3H), 2.23–2.27 (m, 2H), 2.19 (s, 3H), 2.11 (s, 3H), 1.99–2.02 (m, 2H), 1.78–1.87 (m, 2H), 1.45–1.52 (m, 1H), 1.37–1.42 (m, 2H), 1.24–1.33 (m, 7H) ppm. 13C-NMR (CDCl3): δ = 124.9, 123.4, 120.8, 67.7, 56.6, 50.0, 34.9, 26.5, 25.4, 25.0, 24.7 (2C signals overlap), 8.9, 7.2 ppm. IR: ν 2926 (vs), 2853 (m), 1655 (br.m), 1450 (s), 1377 (m), 1331 (vs), 1103 (m), 881 (m), 702 (s), 579 (s) cm–1. HRMS (ESI+): calcd for C16H28N3O [M + H]+ 278.2232; found 278.2234.
(S,S)-4,5-Dimethyl-1-[2-(piperidin-1-yl)cyclohexyl]-1H-imidazole 3-oxide ((S,S)-6c): Yield: 263.5 g (95%). Yellowish crystals. M.p. 51–54 °C. 1H-NMR (CDCl3): δ = 7.87 (s, 1H), 3.70 (td, J = 11.5, 3.8 Hz, 1H), 2.51–2.56 (m, 1H), 2.41–2.46 (m, 2H), 2.27–2.21 (m, 2H), 2.12 (s, 3H), 2.06 (s, 3H), 1.92–1.96 (m, 2H), 1.71–1.80 (m, 2H), 1.46–1.53 (m, 1H), 1.29–1.35 (m, 2H), 1.18–1.25 (m, 7H) ppm. 13C-NMR (CDCl3): δ = 124.7, 122.8, 120.6, 67.6, 56.3, 49.9, 34.8, 26.3, 25.2, 24.9, 24.51, 24.46, 8.7, 7.1 ppm. IR: ν 2928 (vs), 2855 (m), 1664 (br, m), 1450 (s), 1379 (s), 1332 (vs), 1101 (m), 881 (m), 704 (s), 581 (s) cm−1. HRMS (ESI+): calcd for C16H28N3O [M + H]+ 278.2232; found 278.2238.
(R,R)-4,5-Diphenyl-1-[2-(piperidin-1-yl)cyclohexyl]-1H-imidazole 3-oxide ((R,R)-6d): Yield 261.0 mg (65%). Yellowish crystals. M.p. 192–194 °C. 1H-NMR (CDCl3): δ = 8.00 (s, 1H), 7.57 (d, J = 7.5 Hz, 2H), 7.39–7.45 (m, 3H), 7.23–7.29 (m, 5H), 3.82 (td, J = 11.5, 3.8 Hz, 1H), 2.61 (td, J = 11.3, 3.3 Hz, 1H), 2.25–2.29 (m, 2H), 2.17–2.22 (m, 2H), 1.93–1.97 (m, 1H), 1.78–1.83 (m, 2H), 1.63–1.70 (m, 1H), 1.00–1.44 (m, 10H) ppm. 13C-NMR (CDCl3): δ = 130.6, 129.6, 129.2, 128.9, 128.2, 127.9, 127.7, 127.36, 127.28, 124.1, 68.2, 56.7, 49.7, 35.7, 26.5, 25.3, 25.0, 24.7, 24.0 ppm. IR: ν 2929 (s), 2855 (m), 1444 (m), 1342 (m), 1205 (w), 1101 (w), 1034 (w), 784 (s), 710 (s), 691 (s) cm−1. C26H31N3O (401.54): calcd. C 77.77, H 7.78, N 10.46; found C 77.56, H 7.88, N 10.59.
(S,S)-4,5-Diphenyl-1-[2-(piperidin-1-yl)cyclohexyl]-1H-imidazole 3-oxide ((S,S)-6d): Yield: 333.0 mg (83%) after purification on a short column. Yellowish crystals. M.p. 198–199 °C. 1H-NMR (CDCl3): δ = 8.03 (s, 1H), 7.56 (d, J = 7.5 Hz, 2H), 7.40–7.45 (m, 3H), 7.23–7.29 (m, 5H), 3.81 (td, J = 11.6, 3.8 Hz, 1H), 2.61 (td, J = 11.3, 3.4 Hz, 1H), 2.24–2.28 (m, 2H), 2.17–2.22 (m, 2H), 1.92–1.97 (m, 1H), 1.78–1.83 (m, 2H), 1.68 (qd, J = 12.5, 3.3 Hz, 1H), 1.06–1.44 (m, 10H) ppm. 13C-NMR (CDCl3): δ = 130.5, 129.6, 129.3, 128.95, 128.89, 127.95, 127.91, 127.79, 127.4, 127.1, 68.0, 56.9, 49.6, 35.4, 26.5, 25.3, 24.9, 24.6, 24.0 ppm. IR: ν 2928 (s), 2853 (m), 1444 (m), 1342 (m), 1205 (m), 1103 (m), 1034 (w), 786 (s), 712 (s), 693 (vs) cm−1. HRMS (ESI+): calcd for C26H32N3O [M + H]+ 402.2545; found 402.2546.
(R,R)-1-[2-(Azepan-1-yl)cyclohexyl]-4,5-dimethyl-1H-imidazole 3-oxide ((R,R)-6e): Yield 227.1 g (78 %). Colorless, viscous oil. 1H-NMR (CDCl3): δ = 7.74 (s, 1H), 3.66 (td, J = 11.2, 3.9 Hz, 1H), 2.63–2.67 (m, 1H), 2.53–2.58 (m, 2H), 2.37–2.42 (m, 2H), 2.11 (s, 3H), 2.06 (s, 3H), 1.91–1.96 (m, 2H), 1.71–1.79 (m, 2H), 1.15–1.53 (m, 12H) ppm. 13C-NMR (CDCl3): δ = 124.7, 123.4, 120.1, 67.2, 57.3, 50.4, 35.0, 29.5, 26.5, 25.2, 25.0, 24.7, 8.6, 6.9 ppm. IR: ν 2924 (vs), 2853 (m), 1654 (s), 1448 (s), 1332 (s), 725 (vs), 704 (vs), 581 (m) cm–1. HRMS (ESI+): calcd for C17H30N3O [M + H]+ 292.2389; found 292.2398.
(R,R)-1-[2-(Azepan-1-yl)cyclohexyl]-4,5-diphenyl-1H-imidazole 3-oxide ((R,R)-6f): Yield: 369.6 mg (89%). Yellowish crystals. M.p. 64–68 °C. 1H-NMR (CDCl3): δ = 8.06 (s, 1H), 7.50 (d, J = 7.7 Hz, 2H), 7.37–7.41 (m, 3H), 7.19–7.24 (m, 5H), 3.75 (td, J = 11.4, 3.7 Hz, 1H), 2.71 (td, J = 11.3, 3.5 Hz, 1H), 2.35–2.44 (m, 4H), 2.13–2.17 (m, 1H), 1.88–1.93 (m, 1H), 1.72–1.78 (m, 3H), 1.09–1.64 (m, 11H) ppm. 13C-NMR (CDCl3): δ = 130.5, 129.7, 129.4, 129.0, 128.00, 127.96, 127.5, 127.0, 126.7, 125.9, 125.5, 68.3, 57.9, 50.5, 35.7, 29.7, 26.7, 25.2, 24.9 ppm (one signal of sp3 C atom not observed due to overlap). IR: ν 2926 (s), 2855 (w), 1672 (m), 1444 (m), 1224 (w), 712 (s), 693 (br, s) 635 (m), 508 (br, m), 451 (br, m) cm−1. HRMS (ESI+): calcd for C17H34N3O [M + H]+ 416.2702; found 416.2708.
(R,R)-1-(2-Morpholinocyclohexyl)-4,5-dimethyl-1H-imidazole 3-oxide ((R,R)-6g): Yield: 240.1 mg (86%). Yellowish crystals. M.p. 85–87 °C. 1H-NMR (CDCl3): δ = 9.06 (s, 1H), 3.76–3.82 (m, 1H), 3.58–3.66 (m, 1H), 3.43–3.48 (m, 2H), 3.36–3.40 (m, 2H), 3.10 (td, J = 11.1, 3.1 Hz, 1H), 2.49–2.54 (m, 2H), 2.39–2.43 (m, 2H), 2.15 (s, 3H), 2.09 (s, 3H), 1.95–2.04 (m, 2H), 1.86–1.93 (m, 1H), 1.76–1.82 (m, 2H), 1.42–1.50 (m, 1H), 1.20–1.28 (m, 2H) ppm. 13C-NMR (CDCl3): δ = 126.2, 124.4, 121.0, 67.3, 66.0, 57.2, 48.8, 34.2, 25.3, 24.9, 24.5, 8.9, 7.1 ppm. IR ν 2929 (s), 2855 (m), 1654 (w), 1627 (br, w), 1450 (m), 1332 (s), 1112 (vs), 926 (m), 861 (m), 725 (vs) cm−1. HRMS (ESI+): calcd for C15H26N3O2 [M + H]+ 280.2025; found 280.2026.
(R,R)-1-(2-Morpholinocyclohexyl)-4,5-diphenyl-1H-imidazole 3-oxide ((R,R)-6h): Yield: 266.3 mg (66%). Yellowish crystals. M.p. 152–155 °C. 1H-NMR (CDCl3): δ = 8.08 (s, 1H), 7.54 (d, J = 7.2 Hz, 2H), 7.38–7.43 (m, 3H), 7.24–7.29 (m, 3H), 7.19 (d, J = 6.9 Hz, 2H), 3.84 (td, J = 11.6, 4.0 Hz, 1H), 3.44–3.51 (m, 4H), 2.62 (td, J = 11.3, 3.1 Hz, 1H), 2.22–2.28 (m, 5H), 1.94–1.98 (m, 1H), 1.81–1.86 (m, 2H), 1.69–1.76 (m, 1H), 1.25–1.33 (m, 1H), 1.16–1.24 (m, 1H), 1.08–1.15 (m, 1H) ppm. 13C-NMR (CDCl3): δ = 130.4, 129.7, 129.3, 129.0, 127.99, 127.92, 127.88, 127.4, 127.1, 123.9, 68.1, 67.3, 56.3, 48.6, 35.2, 25.2, 24.8, 24.2 ppm (one signal of an sp2 C atom not observed due to overlap). IR: ν 2926 (s), 2857 (m), 1448 (m), 1341 (m), 1109 (s), 861 (s), 766 (s), 695 (vs), 665 (s), 633 (s) cm−1. C25H29N3O2·0.5 H2O (412.52): calcd. C 72.79, H 7.33, N 10.18; found C 72.68, H 7.25, N 9.90.
4.5. Synthesis of 3-Benzyloxyimidazolium Bromides 7—General Procedure
A solution of 0.5 mmol of crude imidazole N-oxide 6 and 86 mg (0.5 mmol) benzyl bromide in 1 mL of CHCl3 was stirred magnetically overnight at room temperature. The next day, the solvent was evaporated and the obtained crude imidazolium salts were triturated with diethyl ether. The ethereal phase was separated and the non-soluble imidazolium salts 7 were used for the generation of carbenes 8 and their reaction with elemental sulfur without further purification. Whereas di-Me substituted imidazolium salts formed viscous, thick oils, the corresponding di-Ph derivatives were obtained as amorphous solids. The structures of selected imidazolium salts were confirmed by running the 1H-NMR spectra. A representative example of the 1H-NMR spectra registered for 7e is described below and the scanned spectrum is presented in the Supplementary Materials.
(R,R)-1-[2-(Azepan-1-yl)cyclohexyl]-4,5-dimethyl-3-benzyloxy-1H-imidazolium bromide((R,R)-7e): 1H-NMR (CDCl3): δ = 11.09 (s, 1H), 7.51 (d, J = 6.9 Hz, 2H), 7.27–7.37 (m, 3H), 5.70 (d, J= 10.0 Hz, 1H), 5.57 (d, J= 10.0 Hz, 1H), 3.91 (br., 1H), 3.62 (br., 1H), 2.60 (br., 4H), 2.13–2.17 (m, 1H), 2.13 (s, 3H), 2.02–2.06 (m, 1H), 1.96–2.01 (m, 1H), 1.91 (s, 3H), 1.80–1.85 (m, 1H), 1.74–1.79 (m, 1H), 1.55–1.63 (m, 1H), 1.47 (br., 2H), 1.29 (br., 6H), 1.18 (br., 2H) ppm.
4.6. Synthesis of Non-Symmetric Imidazole-2-Thiones 9—General Procedure
The crude imidazolium salt 7 obtained from 0.5 mmol of the corresponding imidazole N-oxide 6 and 86 mg (0.5 mmol) of benzyl bromide according to the general procedure (see above) was dissolved in 1 mL of dry pyridine. Next, 38 mg (1.2 mmol) of elemental sulfur and 122 mg (1.2 mmol) of triethylamine were added to the magnetically stirred, homogenous solution. Stirring at room temperature was continued overnight. Next day, pyridine was removed under reduced pressure and the residual semi-solid material was purified by preparative thin layer chromatography with silica gel. A mixture of dichloromethane and methanol (99:1) was used as an eluent. Imidazole-2-thiones 9 formed a single fraction with Rf ca. 0.3. Solid products were additionally purified by crystallization.
(R,R)-3-Benzyloxy-4,5-dimethyl-1-[2-(pyrrolidin-1-yl)cyclohexyl]-1,3-dihydro-2H-imidazole-2-thione ((R,R)-9a): Yield: 238.8 mg (62%). Colorless, viscous oil, purified by chromatography. = −91.15 (c 0.40, CHCl3). 1H-NMR (CDCl3): δ = 7.32–7.44 (m, 5H), 5.75–5.80 (m, 1H), 5.42 (d, J = 10.0 Hz, 1H), 5.28 (d, J = 10.0 Hz, 1H), 4.57–4.63 (m, 1H), 3.06–3.11 (m, 1H), 2.83–3.02 (m, 3H), 2.36 (s, 3H), 2.26–2.31 (m, 1H), 1.72–2.13 (m, 8H), 1.81 (s, 3H), 1.38–1.51 (m, 3H) ppm. 13C-NMR (CDCl3): δ = 155.1, 133.3, 130.2, 129.5, 128.5, 121.1, 118.4, 78.1, 60.7, 58.4, 52.2, 29.6, 29.4, 24.1, 23.7, 22.6, 9.9, 7.7 ppm. IR ν 2929 (s), 2862 (m), 1377 (br, s), 1401 (br, s), 1321 (vs), 1140 (br, m), 1025 (m),954 (m), 913 (m), 836 (w), 751 (s), 697 (vs), 606 (w), 483 (s) cm−1. HRMS (ESI+): calcd for C22H32N3OS [M + H]+ 386.2266; found 386.2274.
(R,R)-3-Benzyloxy-4,5-diphenyl-1-[2-(pyrrolidin-1-yl)cyclohexyl]-1,3-dihydro-2H-imidazole-2-thione ((R,R)-9b): Yield: 356.8 g (70%). Colorless crystals. M.p. 285–286 °C (from MeOH/CH2Cl2) (decomp.). = −36.65 (c 0.40, CHCl3). 1H-NMR (CDCl3): δ = (signals for both rotamers) 6.96–7.45 (m, 15H), 4.95–5.47 (m, 3H), 3.44 and 3.74 (br., 1H), 2.14–2.70 (m, 4H), 0.80–1.96 (m, 12H) ppm. 13C-NMR (CDCl3): δ = (signals for both rotamers) 157.3, 156.1, 133.2, 132.6, 131.1, 130.6, 129.4, 129.3, 129.0, 128.7, 128.2, 128.0, 127.8, 126.4, 125.2, 124.5, 77.4, 61.7, 58.2, 55.0, 46.4, 33.3, 29.4, 26.0, 25.8, 25.0, 24.8, 23.8, 23.6, 22.1 ppm. IR: ν 2929 (m), 2797 (m), 1425 (m), 1358 (m), 1332 (m), 1306 (br, m), 1187 (w), 1073 (w), 965 (m), 911 (m), 786 (m), 753 (s), 695 (vs), 598 (m), 506 (m) cm−1. HRMS (ESI+): calcd. for C32H36N3OS [M + H]+ 510.2579; found 510.2595. C32H35N3OS (509.70): calcd. C 75.40, H 6.92, N 8.24, S 6.30; found C 75.30, H 6.91, N 8.24, S 6.14.
(R,R)-3-Benzyloxy-4,5-dimethyl-1-[2-(piperidin-1-yl)cyclohexyl]-1,3-dihydro-2H-imidazole-2-thione ((R,R)-9c): Yield: 239.5 mg (60%). Colorless, viscous oil, purified by chromatography. = −16.53 (c 0.40, CHCl3). 1H-NMR (CDCl3): δ = 7.47 (d, J = 7.7 Hz, 2H), 7.32–7.37 (m, 3H), 5.46 (d, J = 10.5 Hz, 1H), 5.32 (d, J = 10.5 Hz, 1H), 5.27 (td, J = 11.6, 3.7 Hz, 1H), 2.89 (td, J = 11.4, 2.8 Hz, 1H), 2.74–2.79 (m, 2H), 2.23–2.27 (m, 2H), 2.08 (s, 3H), 1.94–2.02 (m, 2H), 1.83–1.88 (m, 1H), 1.73–1.78 (m, 1H), 1.70 (s, 3H), 1.59 (qd, J = 12.5, 3.7 Hz, 1H), 1.29–1.52 (m, 8H), 1.17–1.25 (m, 1H) ppm. 13C-NMR (CDCl3): δ = 156.5, 134.2, 130.5, 129.1, 128.4, 119.4, 117.9, 77.6, 65.9, 58.5, 49.4, 32.0, 26.8, 25.9, 25.7, 24.9, 24.6, 10.5, 7.2 ppm. IR: ν 2927 (vs), 2853 (m), 1403 (s), 1377 (s), 1330 (s), 1211 (m), 1105 (m), 963 (m), 911 (m), 749 (vs), 699 (vs), 479 (br.s) cm−1. HRMS (ESI+) calcd. for C23H34N3OS [M + H]+ 400.2423; found: 400.2424.
(S,S)-3-Benzyloxy-4,5-dimethyl-1-[2-(piperidin-1-yl)cyclohexyl]-1,3-dihydro-2H-imidazole-2-thione ((S,S)-9c): Yield: 187.6 mg (47%). Colorless, viscous oil, purified by chromatography. = +26.13 (c 0.40, CHCl3). 1H-NMR (CDCl3): δ = 7.46 (d, J = 7.5 Hz, 2H), 7.32–7.37 (m, 3H), 5.46 (d, J = 10.5 Hz, 1H), 5.31 (d, J = 10.5 Hz, 1H), 5.26 (td, J = 11.9, 4.2 Hz, 1H), 2.89 (td, J = 11.4, 2.8 Hz, 1H), 2.74–2.78 (m, 2H), 2.23–2.27 (m, 2H), 2.07 (s, 3H), 1.94–2.03 (m, 2H), 1.83–1.87 (m, 1H), 1.73–1.77 (m, 1H), 1.68 (s, 3H), 1.58 (qd, J = 12.5, 3.7 Hz, 1H), 1.27–1.51 (m, 8H), 1.17–1.25 (m, 1H) ppm. 13C-NMR (CDCl3): δ = 156.3, 134.2, 130.5, 129.1, 128.4, 119.4, 117.9, 77.6, 65.9, 58.5, 49.4, 32.0, 26.8, 25.8, 25.7, 24.9, 24.5, 10.5, 7.2 ppm. IR: ν 2927 (vs), 2853 (m), 1403 (s), 1377 (s), 1328 (s), 1211 (m), 1105 (m), 963 (m), 911 (w), 749 (vs), 697 (vs), 479 (br, s) cm−1. HRMS (ESI+): calcd for C23H34N3OS [M + H]+ 400.2423; found 400.2426.
(R,R)-3-Benzyloxy-4,5-diphenyl-1-[2-(piperidin-1-yl)cyclohexyl]-1,3-dihydro-2H-imidazole-2-thione ((R,R)-9d): Yield 429.4 mg (82%). Colorless crystals. M.p. 283–285 °C (MeOH/CH2Cl2) (decomp.). = −26.77 (c 0.40, CHCl3). 1H-NMR (CDCl3): δ = (signals for both rotamers in ca. 1:2 ratio) 7.07–7.41 (m, 15H), 5.40 and 5.32 (d, J = 9.4 Hz, 1H), 4.61–4.67 (m) and 5.36 (td, J = 11.8, 3.4 Hz, total: 1H), 4.98 and 5.12 (br. d, J = 9.4 Hz, 1H), 0.77–3.76 (m, 18H) ppm. 13C-NMR (CDCl3): δ = (signals for both rotamers) 157.6, 156.7, 133.2, 133.1, 132.4, 130.6, 130.42, 130.38, 129.5, 129.3, 129.22, 129.15, 128.92, 128.87, 128.84, 128.6, 128.4, 128.3, 128.1, 128.02, 127.97, 127.8, 127.7, 126.9, 126.6, 126.40, 126.31, 124.9, 124.6, 124.3, 77.51, 77.49, 64.4, 59.9, 59.7, 49.1 (br.), 48.8 (br.), 33.2, 29.6, 27.1, 26.8, 26.0, 25.7, 25.2, 24.94, 24.91, 24.6, 23.5 ppm. IR: ν 2924 (s), 2849 (w), 1442 (w), 1397 (s), 1321 (s), 1207 (m), 959 (m), 760 (s), 691 (vs), 596 (m), 568 (m), 475 (w) cm−1. C33H37N3OS (523.73): calcd. C 75.68, H 7.13, N 8.02, S 6.12; found C 75.63, H 7.17, N 8.00. S 6.00.
(S,S)-3-Benzyloxy-4,5-diphenyl-1-[2-(piperidin-1-yl)cyclohexyl]-1,3-dihydro-2H-imidazole-2-thione ((S,S)-9d): Yield 324.7 mg (62%). Viscous oil (after chromatography). = +22.42 (c 0.40, CHCl3). 1H-NMR (CDCl3): δ = (signals for both rotamers in ca. 1:2 ratio) 7.07–7.41 (m, 15H), 5.41 and 5.32 (d, J = 9.3 Hz, 1H), 4.61–4.67 and 5.33–5.38 (m, 1H), 4.98 and 5.12 (br. d, J = 9.3 Hz, 1H), 0.78–3.74 (m, 18H) ppm. 13C-NMR (CDCl3): δ = (signals for both rotamers) 157.6, 156.7, 133.14, 133.06, 132.4, 130.6, 130.43, 130.40, 129.5, 129.4, 129.2, 128.93, 128.89, 128.88, 128.7, 128.5, 128.13, 128.05, 127.8, 127.7, 126.9, 126.7, 126.4, 125.9, 124.9, 124.6, 124.4, 77.53, 77.51, 64.4, 60.0, 59.7, 49.2 (br.), 33.2, 29.6, 27.1, 26.8, 26.0, 25.7, 25.2, 24.9, 24.6, 23.5 ppm. IR: ν 2927 (s), 2853 (m), 1444 (w), 1396 (w), 1321 (m), 1207 (m), 959 (m), 753 (s), 693 (vs), 596 (m), 568 (m), 475 (w) cm−1. HRMS (ESI+): calcd for C33H38N3OS [M + H]+ 524.2736; found 524.2743. C33H37N3OS (523.73): calcd. C 75.68, H 7.13, N 8.02, S 6.12; found C 75.64, H 7.10, N 8.06, S 5.92.
(R,R)-3-Benzyloxy-1-[2-(azepan-1-yl)cyclohexyl]-4,5-dimethyl-1,3-dihydro-2H-imidazole-2-thione ((R,R)-9e): Yield: 173.7 mg (42 %).Viscous oil. = −28.85 (c 0.40, CHCl3). 1H-NMR (CDCl3): δ = 7.46–7.48 (m, 2H), 7.33–7.36 (m, 3H), 5.38 (d, J = 10.4 Hz, 1H), 5.32 (d, J = 10.4 Hz, 1H), 5.21 (td, J = 11.6, 3.4 Hz, 1H), 2.97 (td, J = 11.6, 2.7 Hz, 1H), 2.79–2.84 (m, 2H), 2.40–2.44 (m, 2H), 2.11 (s, 3H), 1.93–2.01 (m, 2H), 1.82–1.86 (m, 1H), 1.73–1.77 (m, 1H), 1.74 (s, 3H), 1.57 (qd, J = 12.4, 3.3 Hz, 1H), 1.31–1.52 (m, 10H) 1.16–1.25 (m, 1H) ppm. 13C-NMR (CDCl3): δ = 156.6, 134.2, 130.3, 129.1, 128.4, 119.2, 118.1, 77.9, 67.1, 59.4, 51.2, 32.1, 30.0, 26.6, 25.8, 25.7, 25.5, 10.6, 7.2 ppm. IR: ν 2922 (vs), 2853 (m), 1403 (s), 1377 (s), 1328 (s), 1170 (w), 1133 (w), 959 (m), 907 (m), 749 (vs), 697 (vs) cm−1. C24H35N3OS (413.62): calcd. C 69.69, H 8.53, N 10.16., S 7.75; found C 69.47, H 8.62, N 10.21, S 7.59.
(R,R)-3-Benzyloxy-1-[2-(azepan-1-yl)cyclohexyl]-4,5-diphenyl-1,3-dihydro-2H-imidazole-2-thione ((R,R)-9f): Yield: 376.4 mg (70%). Colorless crystals. M.p. 264–267 °C (MeOH/CH2Cl2) (decomp.). = −27.17 (c 0.40, CHCl3). 1H-NMR (CDCl3): δ = (signals for both rotamers in ca. 1:2 ratio) 7.05–7.38 (m, 15H), 5.32 and 4.72 (td, J = 11.5, 3.6 Hz, 1H), 5.31 and 5.21 (d, J = 9.3 Hz, 1H), 5.02 (br.) and 5.15 (d, J = 9.3 Hz, 1H), 3.69 (td, J = 11.7, 3.0 Hz, 0.3H), 3.28–3.36 (m, 0.3H), 2.75–2.80 (m, 1H), 2.43–2.47 (m, 0.7H), 2.29–2.32 (m, 0.7H), 1.34–2.21 (m, 15H), 1.16 and 0.98 (qd, J = 12.8, 3.5 Hz, 1H), 1.03–1.11 and 0.73–0.82 (m, 1H) ppm. 13C-NMR (CDCl3): δ = 158.0, 157.0, 133.2, 133.1, 132.5, 130.6, 130.37, 130.3, 129.6, 129.4, 129.2, 129.1, 128.9, 128.6, 128.15, 128.13, 128.10, 128.07, 127.9, 127.8, 127.7, 126.6, 126.4, 126.0, 125.0, 124.7, 77.62, 77.58, 66.1, 61.4, 60.5, 51.4 (br.), 50.5, 33.3, 30.05, 29.97, 29.8, 26.9, 26.6, 26.4, 26.0, 25.8, 25.23, 25.17 ppm. IR: ν 2924 (s), 2853 (m), 1446 (m), 1356 (m), 1332 (m), 1073 (w), 963 (w), 752 (s), 693 (vs) cm−1. C34H39N3OS (537.76): calcd. C 75.94, H 7.31, N 7.81, S 5.96; found C 75.91, H 7.45, N 8.01, S 5.86.
(R,R)-3-Benzyloxy-1-(2-morpholinocyclohexyl)-4,5-dimethyl-1,3-dihydro-2H-imidazole-2-thione ((R,R)-9g): Yield 135 mg (67%). Colorless crystals. M.p. 122–124 °C (after chromatographic separation). = −21.22 (c 0.40, CHCl3). 1H-NMR (CDCl3): δ = 7.43–7.46 (m, 2H), 7.33–7.37 (m, 3H), 5.44 (d, J = 10.4 Hz, 1H), 5.30 (d, J = 10.4 Hz, 1H), 5.27 (td, J = 11.9, 3.7 Hz, 1H), 3.52–3.57 (m, 2H), 3.45–3.50 (m, 2H), 2.92 (dt, J = 11.4, 3.2 Hz, 1H), 2.85–2.90 (m, 2H), 2.30–2.34 (m, 2H), 2.08 (s, 3H), 1.97–2.02 (m, 2H), 1.85–1.90 (m, 1H), 1.75–1.80 (m, 1H), 1.72 (s, 3H), 1.62 (qd, J = 12.4, 3.8 Hz, 1H), 1.42–1.51 (m, 1H), 1.32–1.40 (m, 1H), 1.17–1.27 (m, 1H) ppm. 13C-NMR (CDCl3): δ = 156.5, 134.1, 130.5, 129.3, 128.6, 119.9, 117.7, 77.8, 67.7, 65.4, 58.1, 48.6, 31.9, 25.8, 25.5, 24.5, 10.7, 7.3 ppm. IR: ν 2935 (br, m), 2851 (m) 2816 (w), 1451 (m), 1407 (s), 1334 (s), 1269 (s), 1146 (m), 1108 (vs), 915 (m), 855 (m), 754 (vs), 702 (m) cm−1. C22H31N3O2S (401.56): calcd. C 65.80, H 7.78, N 10.46, S 7.98; found C 65.71, H 7.90, N 10.59, S 7.84.
(R,R)-3-Benzyloxy-1-(2-morpholinocyclohexyl)-4,5-diphenyl-1,3-dihydro-2H-imidazole-2-thione ((R,R)-9h): Yield 213 mg (81%). Colorless crystals. M.p. 279–281 °C (MeOH/CH2Cl2) (decomp.). = −43.58 (c 0.40, CHCl3). 1H-NMR (CDCl3): δ = (signals for both rotamers in ca. 1:1.2 ratio) 7.07–7.43 (m, 15H), 5.40 and 5.33 (d, J = 9.4 Hz, 1H), 5.37 and 4.72 (td, J = 11.8, 3.3 Hz, 1H), 5.09 and 4.92 (d, J = 9.4 Hz, 1H), 3.75 and 3.43 (td, J = 11.7, 3.7 Hz, 1H), 3.44–3.69 (m, 4H), 2.78 and 2.43 (br., 2H), 2.09–2.28 (m, 3H), 1.87–1.93 and 2.01–2.06 (m, 1H), 1.66–1.78 (m, 3H), 1.40–1.49 (m, 1H), 1.20–1.27 and 0.92–1.00 (m, 1H), 1.09–1.17 and 0.81–0.89 (m, 1H) ppm. 13C-NMR (CDCl3): δ = (signals for both rotamers) 157.9, 156.9, 133.13, 133.07, 132.5, 130.6, 130.5, 130.4, 129.6, 129.5, 129.4, 129.3, 129.1, 129.04, 129.02, 128.8, 128.3, 128.0, 127.9, 126.5, 126.34, 126.29, 125.3, 124.5, 77.72, 77.66, 68.0, 67.8, 63.8, 59.8, 59.47, 59.43, 48.3, 33.2, 29.6, 25.93, 25.75, 25.2, 25.0, 24.9, 23.7 ppm. IR: ν 2924 (br m), 2853 (m), 1401 (m), 1360 (w), 1323 (m), 1112 (s), 965 (m), 861 (m), 762 (s), 699 (vs) cm−1. C32H35N3O2S (525.70): calcd. C 73.11, H 6.72, N 7.99; S 6.10; found C 73.12, H 6.76, N 7.93, S 6.03.
4.7. Synthesis of Imidazole N-Oxides 10a–d
A solution of diacetyl monooxime (5a, 354 mg, 3.5 mmol) and the corresponding formaldimine 4 (3.0 mmol) in EtOH (10 mL) was refluxed for 3 h. The solvents were removed in vacuo, and the resulting oil was washed with Et2O (3 × 20 mL). The crude product 10 was either purified by column chromatography on silica gel using AcOEt/MeOH mixtures as the eluent or by recrystallization from the appropriate solvents to give spectroscopically pure imidazole N-oxides isolated as colorless materials. Compounds 10a [21] and 10c [22] were prepared following the analogous method, while imidazole N-oxide 10d was obtained in two steps by condensation of 5a with methyl glycinate-derived formaldimine of type 4 followed by aminolysis of the resulting product with α-MBA as described [22]; the NMR spectra of the obtained samples matches the data reported in the literature.
(R)-1-(2-Hydroxy-1-phenylethyl)-4,5-dimethylimidazole 3-oxide (10b): Yield 474 mg (68%). Off-white solid. M.p. 198–200 °C (CH2Cl2/Et2O) (decomp.). = +49.1 (c 0.22, CHCl3). 1H-NMR (CDCl3): δ = 9.17 (s, 1H, C(2)H), 8.72 (br.s, 1H, OH), 7.01–7.04, 7.27–7.35 (2m, 2H, 3H, Ph), 5.14 (dd, J = 3.8, 10.7 Hz, 1H, CHPh), 4.21 (dd, J = 10.7, 13.4 Hz, 1H, CH2O), 3.95 (dd, J = 3.8, 13.4 Hz, 1H, CH2O), 1.84, 2.10 (2s, 3H each, 2Me) ppm. 13C-NMR (CDCl3): δ = 136.6 (s, Ph), 128.3, 129.0 (2d, 3CH, Ph), 126.9 (d, 2CH, Ph), 126.4 (s, C(4)), 124.9 (d, C(2)), 122.0 (s, C(5)), 63.9 (d, CHPh), 63.6 (t, CH2O), 7.0, 8.9 (2q, 2Me) ppm. IR (neat): ν 3110 (s), 3025–2926 (br, m), 1450 (m), 1405 (m), 1351 (s), 1325 (m), 1208 (m), 1064 (s) cm−1. ESI-MS (m/z): 465.4 (100, [2M + H]+), 233.3 (47, [M + H]+). C13H16N2O2 (232.28): calcd. C, 67.22; H, 6.94; N, 12.06; found: C 67.05, H 6.95, N 12.22.
4.8. Synthesis of Imidazolium Bromides 11a–d
To a solution of imidazole N-oxide 10 (1.0 mmol) in dry CH2Cl2 (1.0 mL) was added an excess of 1-bromobutane (548 mg, 4.0 mmol) and the resulting mixture was stirred until the starting material was fully consumed (TLC monitoring: SiO2, EtOAc/MeOH 7:1). The solvents were removed under reduced pressure to give the corresponding imidazolium bromide 11 quantitatively, which was used for the next step without further purification.
(S)-3-Butoxy-1-(2-hydroxypropyl)-4,5-dimethylimidazolium bromide [23] (11a): Reaction time: 2 d. Pale yellow oil. = +16.0 (c 0.74, CHCl3). 1H-NMR (CDCl3): δ = 10.00 (s, 1H, C(2)H), 4.91 (br.d, J ≈ 5.9 Hz, 1H, OH), 4.47 (td, J = 2.1, 6.5 Hz, 2H, OCH2, Bu), 4.34 (dd, J = 9.1, 14.0 Hz, 1H, NCH2), 4.22 (dd, J = 2.6, 14.0 Hz, 1H, NCH2), 4.16–4.22 (m, 1H, CHCH3), 2.26, 2.27 (2s, 3H each, 2Me), 1.77–1.81 (m, 2H, Bu), 1.47–1.43 (m, 2H, Bu), 1.34 (d, J = 6.3 Hz, 3H, CHCH3), 0.98 (t, J = 7.4 Hz, 3H, CH3, Bu) ppm. 13C-NMR (CDCl3): δ = 131.4 (br.d, C(2)), 123.6, 124.7 (2s, C(4), C(5)), 82.7 (t, OCH2, Bu), 64.8 (d, CHCH3), 53.5 (t, NCH2), 29.6 (t, CH2, Bu), 20.4 (q, CHCH3), 18.7 (t, CH2, Bu), 13.6 (q, CH3, Bu), 7.1, 8.9 (2q, 2Me) ppm. IR (neat): ν3308 (s), 2990–2876 (br, s), 1670 (m), 1634 (m), 1545 (m), 1457 (m), 1377 (m), 1139 (s), 1072 (s), 936 (s) cm−1.
(R)-3-Butoxy-1-(2-hydroxy-1-phenylethyl)-4,5-dimethylimidazolium bromide (11b): Reaction time: 2 d. Yellow solid. M.p. 124–127 °C. = +83.5 (c 1.79, CHCl3). 1H-NMR (CDCl3): δ = 10.50 (s, 1H, C(2)H), 7.15–7.18, 7.32–7.38 (2m, 2H, 3H, Ph), 5.61 (dd, J = 3.8, 10.1 Hz, 1H, CHPh), 5.46 (br.s, 1H, OH), 4.80 (pseudo-q, J ≈ 6.6 Hz, 1H, OCH2, Bu), 4.52–4.59 (m, 2H, CH2OH (1H), OCH2 (1H)), 4.10 (dd, J = 3.8, 13.2 Hz, 1H, CH2OH), 2.06, 2.24 (2s, 3H each, 2Me), 1.79–1.84 (m, 2H, Bu), 1.49–1.55 (m, 2H, Bu), 0.98 (t, J = 7.4 Hz, 3H, CH3, Bu) ppm. 13C-NMR (CDCl3): δ = 134.2 (s, Ph), 131.4 (dbr, C(2)), 126.7, 129.3, 129.5 (3d, 5CH, Ph), 124.3, 125.0 (2s, C(4), C(5)), 83.2 (t, OCH2, Bu), 64.9 (d, CHPh), 62.4 (t, CH2OH), 29.8 (t, CH2, Bu), 18.8 (t, CH2, Bu), 13.7 (q, CH3, Bu), 7.1, 9.1 (2q, 2Me) ppm. IR (neat): ν 3248 (vs), 3027–2876 (br, s), 1636 (m), 1541 (m), 1448 (s), 1359 (m), 1066 (s), 935 (s) cm−1.
(R)-3-Butoxy-4,5-dimethyl-1-[1-(methoxycarbonyl)ethyl]imidazolium bromide [23] (11c): Reaction time: 2 d. Pale yellow oil. = −16.6 (c 0.51, CHCl3). The NMR data in accordance with those reported in [23].
(R)-3-Butoxy-4,5-dimethyl-1-[(N-phenylethyl)acetamido]imidazolium bromide (11d): Reaction time: 4 d. Pale yellow oil. = +51.2 (c 0.52, CHCl3). 1H-NMR (CDCl3): δ = 9.84 (s, 1H, C(2)H), 9.29 (d, J = 7.8 Hz, 1H, NH), 7.14–7.17, 7.23–7.26, 7.39–7.42 (3m, 1H, 2H, 2H, Ph), 5.35, 5.45 (AB system, J = 16.1 Hz, 2H, NCH2), 4.95 (dq, J = 7.1, 7.8 Hz, 1H, CH(Ph)CH3), 4.32 (td, J = 1.4, 6.5 Hz, 2H, OCH2, Bu), 2.14, 2.17 (2s, 3H each, 2Me), 1.71–1.76 (m, 2H, Bu), 1.53 (d, J = 7.1 Hz, 3H, CH(Ph)CH3), 1.42–1.48 (m, 2H, Bu), 0.94 (t, J = 7.4 Hz, 3H, CH3, Bu) ppm. 13C-NMR (CDCl3): δ = 163.6 (s, C=O), 143.6 (s, Ph), 131.4 (br.d, C(2)), 126.3, 126.9, 128.4 (3d, 5CH, Ph), 123.3, 125.9 (2s, C(4), C(5)), 82.6 (t, OCH2, Bu), 50.3 (d, CH(Ph)CH3), 49.7 (t, NCH2), 29.5 (t, CH2, Bu), 22.4 (q, CH(Ph)CH3), 18.6 (t, CH2, Bu), 13.6 (q, CH3, Bu), 7.0, 8.7 (2q, 2Me) ppm. IR (neat): ν 3200 (m), 3032–2872 (br, s), 1679 (vs, C=O), 1547 (s), 1448 (m), 1377 (m), 1250 (m) cm−1.
4.9. Synthesis of Imidazole-2-thiones 13a–d and 15a–d
To a solution of the respective imidazolium bromide (1.0 mmol) in dry pyridine (4.0 mL) was added triethylamine (150 μL) followed by elemental sulfur (33 mg, 1.1 mmol), and the resulting mixture was stirred overnight. The solvents were removed under reduced pressure, and the crude product was purified by standard column chromatography to give imidazole-2-thione of type 13 or 15, respectively.
(S)-3-Butoxy-1-(2-hydroxypropyl)-4,5-dimethylimidazole-2-thione (13a): (chromatographic separation, SiO2, CH2Cl2/EtOAc 5:1); 209 mg (81%). Pale yellow oil. = +1.3 (c 0.77, CHCl3). 1H-NMR (CDCl3): δ = 4.29–4.34 (m, 2H, OCH2, Bu), 4.18–4.23 (m, 1H, CHCH3), 4.03 (dd, J = 8.7, 14.4 Hz, 1H, NCH2), 3.97 (dd, J = 3.2, 14.4 Hz, 1H, NCH2), 3.28 (br.s, 1H, OH), 2.08, 2.12 (2s, 3H each, 2Me), 1.73–1.77 (m, 2H, Bu), 1.46–1.53 (m, 2H, Bu), 1.25 (d, J = 6.3 Hz, 3H, CHCH3), 0.96 (t, J = 7.4 Hz, 3H, CH3, Bu) ppm. 13C-NMR (CDCl3): δ = 156.2 (s, C=S), 118.5, 119.0 (2s, C(4), C(5)), 76.8 (t, OCH2, Bu), 67.3 (d, CHCH3), 51.3 (t, NCH2), 29.8 (t, CH2, Bu), 21.2 (q, CHCH3), 18.9 (t, CH2, Bu), 13.7 (q, CH3, Bu), 7.5, 9.1 (2q, 2Me) ppm. IR (neat): ν 3353 (s), 3040–2874 (br, m), 1433 (m), 1409 (s), 1375 (m), 1347 (m), 1127 (m), 1072 (m), 943 (m) cm−1. ESI-MS (m/z): 281.1 (53, [M + Na]+), 259.1 (100, [M + H]+), 208.1 (42). C12H22N2O2S (258.38): calcd. C 55.78, H 8.58, N 10.84, S 12.41; found: C 55.57, H 8.65, N 10.76, S 12.21.
(R)-3-Butoxy-1-(2-hydroxy-1-phenylethyl)-4,5-dimethylimidazole-2-thione (13b): (chromatographic separation, SiO2, CH2Cl2/EtOAc 5:1); 240 mg (75%). Yellow oil. = −10.8 (c 0.73, CHCl3). 1H-NMR (CDCl3): δ = 7.24–7.30, 7.32–7.35 (2m, 3H, 2H, Ph), 6.26 (m, 1H, CHPh), 4.60–4.65 (m, 1H, CH2OH), 4.34–4.42 (m, 3H, OCH2(Bu) (2H), CH2OH (1H)), 3.09 (br.s, 1H, OH), 2.09 (s, 3H, Me), 1.79–1.84 (m, 2H, Bu), 1.72 (s, 3H, Me), 1.52–1.58 (m, 2H, Bu), 1.00 (t, J = 7.4 Hz, 3H, CH3, Bu) ppm. 13C-NMR (CDCl3): δ = 157.4 (s, C=S), 136.7 (s, Ph), 126.9, 127.8, 128.7 (3d, 5CH, Ph), 118.8, 120.1 (2s, C(4), C(5)), 76.8 (t, OCH2, Bu), 62.8 (t, CH2OH), 60.9 (d, CHPh), 29.9 (t, CH2, Bu), 19.0 (t, CH2, Bu), 13.8 (q, CH3, Bu), 7.4, 9.9 (2q, 2Me) ppm. IR (neat): ν 3347 (s), 3016–2874 (br, s), 1407 (s), 1333 (m), 1156 (m), 1060 (m), 1031 (m) cm–1. ESI-MS (m/z): 343.1 (54, [M + Na]+), 321.2 (100, [M + H]+), 270.1 (45). HRMS (ESI-TOF): calcd for C17H25N2O2S: 321.1637; found: 321.1640.
(R)-3-Butoxy-4,5-dimethyl-1-[1-(methoxycarbonyl)ethyl]imidazole-2-thione (13c): (chromatographic separation, SiO2, CH2Cl2/EtOAc 95:5); 236 mg (83%). Yellow oil. = −45.4 (c 0.20, CHCl3). 1H-NMR (CDCl3): δ = 6.03 (d, J = 7.4 Hz, 1H, CHCH3), 4.31–4.39 (m, 2H, OCH2, Bu), 3.74 (s, 3H, OCH3), 2.02, 2.12 (2s, 3H each, 2Me), 1.75–1.80 (m, 2H, Bu), 1.62 (d, J = 7.4 Hz, 3H, CHCH3), 1.49–1.55 (m, 2H, Bu), 0.98 (t, J = 7.4 Hz, 3H, CH3, Bu) ppm. 13C-NMR (CDCl3): δ = 170.8 (s, C=O), 157.6 (s, C=S), 117.4, 119.8 (2s, C(4), C(5)), 76.7 (t, OCH2, Bu), 53.2 (d, CHCH3), 52.6 (q, OCH3), 29.9 (t, CH2, Bu), 19.0 (t, CH2, Bu), 16.0 (q, CHCH3), 13.8 (q, CH3, Bu), 7.4, 9.8 (2q, 2Me) ppm. IR (neat): ν 2932–2872 (m), 1743 (vs, C=O), 1407 (s), 1344 (m), 1224 (s), 1183 (s), 1109 (m), 1066 (s), 963 (m) cm−1. ESI-MS (m/z): 287.3 (100, [M + H]+). C13H22N2O3S (286.39): calcd. C 54.52, H 7.74, N, 9.78, S 11.19; found: C 54.58, H 7.79, N 9.77, S 11.18.
(R)-3-Butoxy-4,5-dimethyl-1-[(N-phenylethyl)acetamido]imidazole-2-thione (13d): (chromatographic separation, SiO2, CH2Cl2/EtOAc 4:1); 238 mg (66%). Pale orange solid. M.p. 118–119 °C. = +2.7 (c 1.89, CHCl3). 1H-NMR (CDCl3): δ = 7.71 (br.d, J ≈ 8.1 Hz, 1H, NH), 7.20–7.23, 7.25–7.30 (2m, 1H, 4H, Ph), 5.00 (pseudo-p, J ≈ 7.1 Hz, 1H, CH(Ph)CH3), 4.65, 4.70 (AB system, J = 14.9 Hz, 2H, NCH2), 4.35 (dt, J ≈ 6.6, 8.1 Hz, 1H, OCH2, Bu), 4.28 (dt, J ≈ 6.6, 8.1 Hz, 1H, OCH2, Bu), 2.11, 2.13 (2s, 3H each, 2Me), 1.76–1.81 (m, 2H, Bu), 1.50–1.56 (m, 2H, Bu), 1.43 (d, J = 7.0 Hz, 3H, CH(Ph)CH3), 0.99 (t, J = 7.4 Hz, 3H, CH3, Bu) ppm. 13C-NMR (CDCl3): δ = 166.2 (s, C=O), 156.3 (s, C=S), 143.1 (s, Ph), 125.8, 127.0, 128.4 (3d, 5CH, Ph), 118.5, 119.5 (2s, C(4), C(5)), 76.9 (t, OCH2, Bu), 49.3 (d, CH(Ph)CH3), 48.6 (t, NCH2), 29.8 (t, CH2, Bu), 22.5 (q, CH(Ph)CH3), 18.9 (t, CH2, Bu), 13.7 (q, CH3, Bu), 7.4, 9.1 (2q, 2Me) ppm. IR (neat): ν 3282 (m), 2956–2871 (br, m), 1662 (vs, C=O), 1549 (s), 1407 (s), 1372 (m), 1247 (m), 1003 (m) cm−1. ESI-MS (m/z): 384.2 (100, [M + Na]+), 362.2 (21, [M + H]+), 311.1 (48), 241.1 (69). C19H27N3O2S (361.50): calcd. C 63.13, H 7.53, N 11.62, S 8.87; found: C 63.04, H 7.80, N 11.70, S 8.76.
(S)-3-Benzyloxy-4,5-dimethyl-1-(1-phenylethyl)imidazole-2-thione (15a): Yield: 275 mg (81%). Pale yellow crystals. M.p. 102–104 °C (petroleum ether/CH2Cl2). = +110.5 (c 0.32, CHCl3). 1H-NMR (CDCl3): δ = 7.58–7.53 (m, 2H), 7.43–7.40 (m, 3H), 7.37–7.34 (m, 2H), 7.30–7.26 (m, 3H), 6.80 (q, J = 7.3 Hz, 1H), 5.58, 5.45 (AB system, J = 10.0 Hz, 2H, OCH2), 1.83 (d, J = 7.3 Hz, 3H), 1.78, 1.63 (2s, 6H, 2Me) ppm. 13C-NMR (CDCl3): δ = 157.3 (s, C=S), 126.4, 127.4, 128.5, 128.6, 129.3, 130.4 (6d, 10CH), 117.5, 120.7 134.0, 139 (4s), 77.9 (t, OCH2), 53.4 (d, CH), 7.2, 10.2, 17.3 (3q, 3Me) ppm. IR (neat): ν 2971 (m), 2924 (m), 1449 (m), 1403 (s), 1375 (m), 1332 (s), 1299 (m), 1151 (m), 1025 (m), 956 (m), 909 (m), 747 (s), 697 (vs) cm−1. HRMS (ESI-TOF) calcd for C20H23N2OS: 339.1531; found: 339.1535.
(S)-4,5-Dimethyl-3-pentyloxy-1-(1-phenylethyl)imidazole-2-thione (15b): Yield: 245 mg (77%). Pale yellow crystals. M.p. 70–73 °C (petroleum ether/CH2Cl2). = +125.9 (c 0.40, CHCl3). 1H-NMR (CDCl3): δ = 7.28–7.39 (m, 5H), 6.76 (q, J = 7.3 Hz, 1H), 4.43–4.46 (pseudo q, J = 7.2 Hz, 1H, OCH2), 4.37–4.40 (pseudo q, J = 7.2 Hz, 1H, OCH2), 2.09 (s, 3H, Me), 1.83–1.88 (m, 2H, CH2), 1.80 (d, J = 7.3 Hz, 3H), 1.68 (s, 3H, Me), 1.49–1.53 (m, 2H, CH2), 1.41–1.45 (m, 2H, CH2), 0.96 (t, J = 7.3 Hz, 3H, CH3) ppm. 13C-NMR (CDCl3): δ = 157.5 (C=S), 126.5, 127.3, 128.5 (3d, 5CH), 117.8, 119.9, 139.9 (3s), 76.9 (t, OCH2), 53.4 (d, CH), 22.5, 27.7, 27.9 (3t, 3CH2), 7.3, 10.1, 13.9, 16.8 (4q, 4Me) ppm. IR (neat): ν 2954 (m), 2927 (m), 1450 (m), 1410 (s), 1334 (s), 1299 (s), 1157 (m), 1027 (m), 1001 (s), 998 (m), 876 (m), 700 (vs), 687 (s) cm−1. C18H26N2OS (318.48): calcd. C 67.88, H 8.23, N 8.80, S 10.07; found: C 67.61, H 8.28, N 8.70, S 10.06.
(S)-3-Benzyloxy-4,5-diphenyl-1-(1-phenylethyl)imidazole-2-thione (15c): Yield: 305 mg (66%). Pale yellow oil. = +188.5 (c 0.40, CHCl3). 1H-NMR (CDCl3): δ = 7.30–7.32 (m, 2H), 7.20–7.25 (m, 10H), 7.14–7.18 (m, 2H), 7.05–7.12 (m, 6H), 6.75 (br.s, 1H, CH3-CH), 6.67 (br.s, 2H), 5.30, 5.32 (AB system, J = 12.5 Hz, 2H, OCH2), 1.62 (d, J = 6.5 Hz, 3H, CH3-CH) ppm. 13C-NMR (CDCl3): δ = 158.4 (s, C=S), 124.1, 125.9, 126.2, 128.6, 129.8, 132.9 (6s), 126.9, 127.3, 127.9, 128.0, 128.1, 128.2, 128.3, 128.9, 129.1, 129.4, 130.6, 132.0 (12d, 20CH), 77.7 (t, OCH2), 54.6 (d, CH), 17.5 (q, CH3) ppm. IR (neat): ν 3032 (m), 2927 (m), 1496 (m), 1446 (m), 1395 (s), 1325 (m), 1316s, 1215 (m), 1187 (m), 1073 (m), 907 (m), 730 (s), 695 (vs) cm−1. HRMS (ESI-TOF): calcd for C30H27N2O: 463.1844; found: 463.1849.
(S)-3-Pentyloxy-4,5-diphenyl-1-(1-phenylethyl)imidazole-2-thione (15d): Yield: 350 mg (79%). Beige crystals. M.p. 78–81 °C (petroleum ether/CH2Cl2). = +130.1 (c 0.30, CHCl3). 1H-NMR (CDCl3): δ = 7.20–7.25 (m, 9H), 7.06–7.09 (m, 4H), 6.67–6.73 (m, 3H, 2CHarom, CH-CH3), 4.31 (pseudo q, J = 6.8 Hz, 1H, OCH2), 4.14 (pseudo q, J = 6.8 Hz, 1H, OCH2), 1.63–1.66 (m, 2H, CH2), 1.59 (d, J = 6.8 Hz, 3H, CH3-CH), 1.24–1.27 (m, 2H, CH2), 1.19–1.23 (m, 2H, CH2), 0.83 (t, J = 7.3 Hz, 3H, CH3) ppm. 13C-NMR (CDCl3): δ = 158.6 (s, C=S), 126.9, 127.2, 127.9, 128.1, 128.2, 128.3, 128.9, 129.3, 132.0 (9d, 15CH), 124.4, 125.2, 126.0, 140.3 (4s), 76.9 (t, OCH2), 54.7 (d, CH), 22.2, 27.4, 27.8 (3t, 3CH2), 13.9, 17.5 (2q, 2CH3) ppm. IR (neat): ν 2968 (m), 2953 (m), 1448 (m), 1392 (s), 1321 (s), 1187 (m), 1026 (m), 1002 (m), 963 (m), 702 (vs), 695 (vs) cm−1. C28H30N2OS (442.62): C 75.98; H 6.83; N 6.33; S 7.24. Found: C 75.78; H 6.88; N 6.58; S 7.50.
Supplementary Materials
Copies of 1H-NMR and 13C-NMR spectra of all new compounds are available online.
Author Contributions
Conceptualization, G.M. and P.R.S.; Methodology, G.M.; Software, P.J.B. and M.J.; Validation G.M.; P.J.B.; Formal analysis, G.M., P.J.B., and H.H.; Investigation, G.M., M.C, M.J., and K.U.; Resources, G.M. and P.R.S.; Data curation, M.J. and K.U.; Writing—original draft preparation, G.M., H.H., M.J., and K.U.; Writing—review and editing, G.M., H.H., M.J., and P.R.S.; Visualization, M.J.; Supervision, G.M.; Project administration, G.M. and P.R.S.; Funding acquisition, G.M. and P.R.S.
Funding
This research received external funding from the National Science Center (NCN, Cracow, Poland) and the Deutsche Forschungsgemeinschaft (DFG, Bonn, Germany) within the Beethoven-2 program.
Acknowledgments
The authors acknowledge the generous financial support within the Beethoven-2 funding scheme: (a) National Science Centre, Cracow (G. Mlostoń, grant # 2016/23/G/ST5/04115/l) and (b) The Deutsche Forschungsgemeinschaft (DFG, P.R. Schreiner, grant # Schr 597/34-1). Preparation of the analytically pure sample of formaldimine 4i by Dr. Emilia Obijalska and Ms. Karolina Grzybowska (University of Łódź) is acknowledged.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Arduengo, A.J.; Harlow, R.L.; Kline, M. A stable crystalline carbene. J. Am. Chem. Soc. 1991, 113, 361–363. [Google Scholar] [CrossRef]
- Jahnke, M.C.; Hahn, F.E. N-Heterocyclic Carbenes: From Laboratory Curiosities to Efficient Synthetic Tools; The Royal Society of Chemistry: Cambridge, UK, 2011; pp. 1–41. [Google Scholar]
- Hopkinson, M.N.; Richter, C.; Schedler, M.; Glorius, F. An overview of N-heterocyclic carbenes. Nature 2014, 510, 485–496. [Google Scholar] [CrossRef]
- Christmann, M.; Bräse, S. Asymmetric Synthesis-The Essentials; Wiley-VCH: Weinheim, Germany, 2008. [Google Scholar]
- César, V.; Bellemin-Laponnaz, S.; Gade, L.H. Chiral N-heterocyclic carbenes as stereodirectingligands in asymmetric catalysis. Chem. Soc. Rev. 2004, 33, 619–636. [Google Scholar] [CrossRef]
- Benhamou, L.; Chardon, E.; Lavigne, G.; Bellemin-Laponnaz, S.; Cesar, V. Synthetic routes to N-heterocyclic carbene precursors. Chem. Rev. 2011, 111, 2705–2733. [Google Scholar] [CrossRef]
- Flanigan, D.M.; Romanov-Michailidis, F.; White, N.A.; Rovis, T. Organocatalytic reactions enabled by N-heterocyclic carbenes. Chem. Rev. 2015, 115, 9307–9387. [Google Scholar] [CrossRef]
- Janssen-Müller, D.; Schlepphorst, C.; Glorius, F. Privileged chiral N-heterocyclic carbene ligands for asymmetric transition-metal catalysis. Chem. Soc. Rev. 2017, 46, 4845–4854. [Google Scholar] [CrossRef]
- Bennani, Y.L.; Hanessian, S. trans-1,2-Diaminocyclohexane derivatives as chiral reagents, scaffolds, and ligands for catalysis: Application in asymmetric synthesis and molecular recognition. Chem. Rev. 1997, 97, 3161–3195. [Google Scholar] [CrossRef]
- Kizirian, J.-C. Chiral tertiary diamines in asymmetric synthesis. Chem. Rev. 2008, 108, 140–205. [Google Scholar] [CrossRef]
- Nakamura, S.; Hayashi, M.; Kamada, Y.; Sasaki, R.; Hiramatsu, Y.; Shibata, N.; Toru, T. Enantioselective desymmetrization of meso-N-(heteroarenesulfonyl)aziridines with TMSN3 catalyzed by chiral Lewis acids. Tetrahedron Lett. 2010, 51, 3820–3823. [Google Scholar] [CrossRef]
- Dajek, M.; Kowalczyk, R.; Boratyński, P.J. trans-1,2-Diaminocyclohexane-based sulfonamides as effective hydrogen-bonding organocatalysts for asymmetric Michael-hemiacetalization reaction. Catal. Sci. Technol. 2018, 8, 4358–4363. [Google Scholar] [CrossRef]
- De Costa, B.R.; Radesca, L. A practical synthesis, optical resolution and determination of absolute configuration of enantiomerically pure (1S,2R)-(+)- and (1R,2S)-(−)-cis-2-(1-pyrrolidinyl)cyclohexylamines: Important precursors for a new class of sigma-receptor ligands and anticonvulsant drugs. Heterocycles 1990, 31, 1837–1846. [Google Scholar]
- De Costa, B.; George, C.; Rothman, R.B.; Jacobson, A.E.; Rice, K.C. Synthesis and absolute configuration of optically pure enantiomers of Κ-opioid receptor selective agonist. FEBS Lett. 1987, 223, 335–339. [Google Scholar] [CrossRef][Green Version]
- Radesca, L.; Bowen, W.D.; Di Paolo, L.; De Costa, B.R. Synthesis and receptor binding of enantiomeric N-substituted cis-N-[2-(3,4-dichlorophenyl)ethyl]-2-(1-pyrrolidinyl)cyclohexylamines as high-affinity σ receptor ligands. J. Med. Chem. 1991, 34, 3058–3065. [Google Scholar] [CrossRef]
- González-Sabín, J.; Gotor, V.; Rebolledo, F. Chemoenzymatic preparation of optically active trans-cyclohexane-1,2-diamine derivatives: An efficient synthesis of the analgesic U-(–)-50,488. Chem. Eur. J. 2004, 10, 5788–5794. [Google Scholar] [CrossRef]
- Smith, V.C.; Cleghorn, L.A.T.; Woodland, A.; Spinks, D.; Hallyburton, I.; Collie, I.T.; Mok, N.Y.; Norval, S.; Brenk, R.; Fairlamb, A.H.; et al. Optimisation of the anti-Trypanosoma brucei activity of the opioid agonist U50488. Chem. Med. Chem 2011, 6, 1832–1840. [Google Scholar] [CrossRef]
- Mucha, P.; Mlostoń, G.; Jasiński, M.; Linden, A.; Heimgartner, H. A new approach to enantiomerically pure bis-imidazoles derived from trans-1,2-diaminocyclohexane. Tetrahedron: Asymmetry 2008, 19, 1600–1607. [Google Scholar] [CrossRef]
- Mlostoń, G.; Rygielska, D.; Jasiński, M.; Heimgartner, H. Optically active imidazoles derived from enantiomerically pure trans-1,2-diaminocyclohexane. Tetrahedron: Asymmetry 2011, 22, 669–674. [Google Scholar] [CrossRef]
- Mlostoń, G.; Mucha, P.; Urbaniak, K.; Broda, K.; Heimgartner, H. Synthesis of optically active 1-(1-phenylethyl)-1H-imidazoles derived from 1-phenylethylamine. Helv. Chim. Acta 2008, 91, 232–238. [Google Scholar] [CrossRef]
- Jasiński, M.; Mlostoń, G.; Mucha, P.; Linden, A.; Heimgartner, H. Synthesis of new bis-imidazole derivatives. Helv. Chim. Acta 2007, 90, 1765–1780. [Google Scholar] [CrossRef]
- Jasiński, M.; Mlostoń, G.; Linden, A.; Heimgartner, H. Synthesis and selective transformations of 1H-imidazole 3-oxides derived from amino acid esters. Helv. Chim. Acta 2008, 91, 1916–1933. [Google Scholar] [CrossRef]
- Mlostoń, G.; Romański, J.; Jasiński, M.; Heimgartner, H. Exploration of 4,5-dimethyl-1H-imidazole N-oxide derivatives in the synthesis of new achiral and chiral ionic liquids. Tetrahedron: Asymmetry 2009, 20, 1073–1080. [Google Scholar] [CrossRef]
- Mlostoń, G.; Celeda, M.; Urbaniak, K.; Jasiński, M.; Bakhonsky, V.; Schreiner, P.R.; Heimgartner, H. Synthesis and selected transformations of 2-unsubstituted 1-(adamantyloxy)imidazole 3-oxides: Straightforward access to non-symmetric 1,3-dialkoxyimidazolium salts. Beilstein J. Org. Chem. 2019, 15, 497–505. [Google Scholar] [CrossRef]
- Edward, J.T.; Chubb, F.L.; Gilson, D.F.R.; Hynes, R.C.; Sauriol, F.; Wiesenthal, A. Cage peroxides having planar bridgehead nitrogen atoms. Can. J. Chem. 1999, 77, 1057–1065. [Google Scholar] [CrossRef][Green Version]
- Adamczyk-Woźniak, A.; Bujnowski, K.; Sporzyński, A. 1,3,5-Trialkyl-hexahydro-1,3,5-triazines—N-methylenealkylamines equilibria. 1H-NMR studies in solutions. J. Mol. Struct. 2008, 892, 177–181. [Google Scholar] [CrossRef]
- Kleban, I.; Tytsunik, A.V.; Rassukana, Y.V.; Grygorenko, O.O. O-(α-Phenylethyl)hydroxylamine as a ‘chiral ammonia equivalent’: Synthesis and resolution of 5-oxopyrrolidine- and 6-oxopiperidine-3-carboxylic acids. Tetrahedron: Asymmetry 2017, 28, 1817–1822. [Google Scholar] [CrossRef]
- Respondek, J. Reaction product of formaldehyde and O-benzylhydroxylamine: A Schiff base? Z. Naturforsch. 1984, 39b, 1154–1155. [Google Scholar] [CrossRef]
- Funt, L.D.; Tomashenko, O.A.; Khlebnikov, A.F.; Novikov, M.S.; Ivanov, A.Y. Synthesis, transformations of pyrrole- and 1,2,4-triazole-containing ensembles, and generation of pyrrole-substituted triazole NHC. J. Org. Chem. 2016, 81, 11210–11221. [Google Scholar] [CrossRef]
- Jochriem, M.; Kirchler, C.G.; Laus, G.; Wurst, K.; Kopacka, H.; Müller, T.; Schottenberger, H. Synthesis and crystal structure of non-symmetric 1,3-di(alkoxy)imidazolium salts. Z. Naturforsch. B 2017, 72, 617–626. [Google Scholar] [CrossRef]
- Kurteva, V.; Lyapova, M. Synthesis of a series of vicinal diamines with potential biological activity. Cent. Eur. J. Chem. 2004, 2, 686–695. [Google Scholar] [CrossRef]
- Kotti, S.R.S.S.; Timmons, C.; Li, G. Vicinal diamino functionalities as privileged structural elements in biologically active compounds and exploitation of their synthetic chemistry. Chem. Biol. Drug. Des. 2006, 67, 101–114. [Google Scholar] [CrossRef]
- Ocakoglu, K.; Tasli, H.; Limoncu, M.H.; Lambrecht, F.Y. Synthesis and antimicrobial activity of imidazolium salts. Trends Cancer Res. Chemother. 2018. [Google Scholar] [CrossRef]
- Ulucam, G.; Turkyilmaz, M. Synthesis, structural analysis, and biological activities of some imidazolium salts. Bioinorg. Chem. Appl. 2018, 1439810. [Google Scholar] [CrossRef]
- Riduan, S.N.; Zhang, Y. Imidazolium salts and their polymeric materials for biological applications. Chem. Soc. Rev. 2013, 42, 9055–9070. [Google Scholar] [CrossRef]
- Thanigaimalai, P.; Lee, K.-C.; Bang, S.-C.; Lee, J.-H.; Yun, C.-Y.; Roh, E.; Hwang, B.-Y.; Kim, Y.; Jung, S.-H. Inhibitory effect of novel tetrahydropyrimidine-2(1H)-thiones on melanogenesis. Bioorg. Med. Chem. 2010, 18, 1135–1142. [Google Scholar] [CrossRef]
- Savjani, J.K.; Gajjar, A.K. Pharmaceutical importance and synthetic strategies for imidazolidine-2-thione and imidazolie-2-thione derivatives. Pak. J. Biol. Sci. 2011, 14, 1076–1089. [Google Scholar]
- Diels, O.; Jost, H. Über die Darstellung des Diacetyls und ein Polymerisationsproduct desselben. Chem. Ber. 1902, 35, 3290–3299. [Google Scholar] [CrossRef]
- Watson, T.; Taylor, J.; Marks, M.S. The configurations of the benzil-monoximes. J. Chem. Soc. 1930, 2302–2307. [Google Scholar]
- Welch, J.T.; Seper, K.W. Synthesis, regioselective deprotonation, and stereoselective alkylation of fluoroketimines. J. Org. Chem. 1988, 53, 2991–2999. [Google Scholar] [CrossRef]
- Ikeda, K.; Achiwa, K.; Sekiya, M. A convenient synthesis of N-benzyloxy-β-lactams via N-benzyloxyimines. Chem. Pharm. Bull. 1989, 37, 1179–1184. [Google Scholar] [CrossRef][Green Version]
Sample Availability: Samples of formaldimines, imidazole N-oxides and imidazole-2-thiones are available from the authors. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).