Abstract
The current study was chiefly designed to examine the antiproliferative and antioxidant activities of some novel quinazolinone(thione) derivatives 6–14. The present work focused on two main points; firstly, comparing between quinazolinone and quinazolinthione derivatives. Whereas, antiproliferative (against two cell lines namely, HepG2 and MCF-7) and antioxidant (by two methods; ABTS and DPPH) activities of the investigated compounds, the best quinazolinthione derivatives were 6 and 14, which exhibited excellent potencies comparable to quinazolinone derivatives 5 and 9, respectively. Secondly, we compared the activity of four series of Schiff bases which included the quinazolinone moiety (11a–d). In addition, the antiproliferative and antioxidant activities of the compounds with various aryl aldehyde hydrazone derivatives (11a–d) analogs were studied. The compounds exhibited potency that increased with increasing electron donating group in p-position (OH > OMe > Cl) due to extended conjugated systems. Noteworthy, most of antiproliferative and antioxidant activities results for the tested compounds are consistent with the DFT calculations.
1. Introduction
Cancer is the second leading cause of death globally, and the contribution of cancer disease to the overall mortality rate is increasing. Economically, the total annual cost of cancer in 2010 was estimated at approximately US$ 1.16 trillion [1]. So that, more rational design, synthesis, and evaluation of new compounds as anticancer, with higher efficiency is considered as urgent mission in the medicinal chemistry field.
Quinazoline and quinazolinone derivatives are considered as tremendous targets for the medicinal chemists, due to the fact that they are the scaffold of different potent anticancer drugs, such as Gefitinib (trade name Iressa®), Erlotinib (trade name Tarceva®) [2,3,4], Methaqualone [5], Afloqualone (as anticonvulsant activity) [6,7], Chloroqualone (as antitussive), and Diproqualone (as sedative-hypnotic agents) [8] (Figure 1).
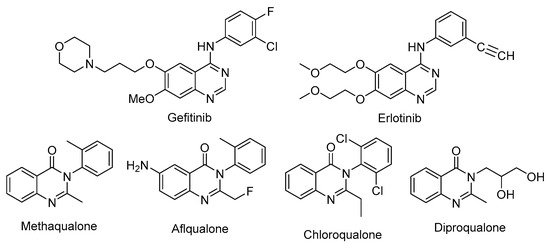
Figure 1.
Some structures of synthetic drugs scaffold quinazoline and quinazolinone derivatives.
In the last decades and until now, various compounds including quinazolinone moiety conspicuously exhibited broad spectrum in numerous pharmacological activities such as anticancer [9,10,11,12,13,14], anticonvulsants [15], antiproliferative [16], anti-inflammatory [17], antihypertensive [18], antifungal [19], antibacterial, antioxidant [20], antimicrobial [21], anti-allergic [22], antimalarial [23], antileishmanial [24], and treatment of Alzheimer’s disease (AD) [25].
Generally, the natural products are considered as one of the most interesting sources of biologically active compounds. Among them, naturally occurring quinazolin-4(3H)-one derivatives, which can be isolated from various plants and microorganisms such as Luotonin A (sources; Peganum nigellastrum) [26], 2-(heptan-3-yl)quinazolinone (sources; Bacillus cereus) [27], Dictyoquinazol A (sources; Dictyophora indusiata) [28], and Echinozolinone (sources; Echinops echinatus) [29] (Figure 2).
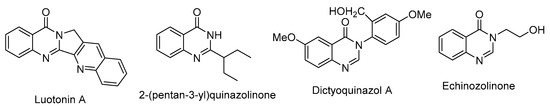
Figure 2.
Some structures of naturally occurring quinazolin-4(3H)-one derivatives.
Quinazolinones have been synthesized by different methodologies [28,30,31,32,33,34,35,36,37], in the present study, the conventional methodology to construct novel qunizolinone compounds has been adopted, followed by the study of the antiproliferative activity, antioxidant activity, and DFT calculations for the synthesized compounds.
2. Results and Discussion
2.1. Chemistry
In this interesting work, curing of anthranilic acid 1 with hexanoyl chloride 2 in dry pyridine afforded the corresponding N-hexanoyl derivative 3 [38], which was cyclized by heating in distilled acetic anhydride to give 2-pentyl-4H-benzo[d][1,3]oxazin-4-one 4 [39,40] (Scheme 1).
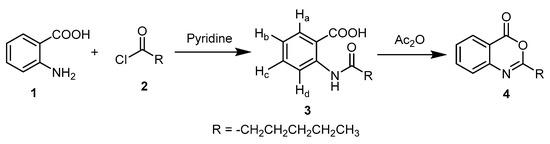
Scheme 1.
The strategy for synthesis of compound 4.
Benzoxazinone derivative 4 was utilized in situ as a precursor to construct new quinazolinone derivatives. For instance, reaction of benzoxazinone derivative 4 with formamide afforded 2-pentylquinazolin-4(3H)-one 5 [41] (Scheme 2). The 1H NMR spectrum of 5 exhibited a singlet peak at 12.13 ppm exchangeable with D2O corresponding to NH proton, two doublet and two triplet peaks in the aromatic region at 8.05–7.42 ppm corresponding to four aromatic protons, and four characteristic peaks upfield at 2.56–0.84 ppm for n-pentyl protons.
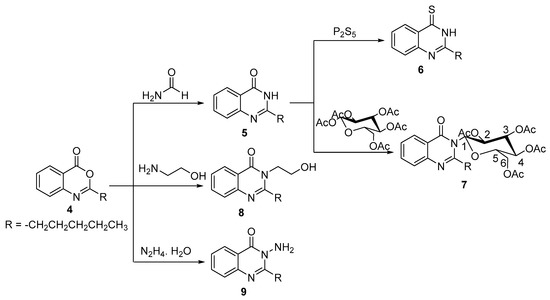
Scheme 2.
Synthetic route to compounds 5–9.
Afterwards, sulfuration of 2-pentylquinazolin-4(3H)-one 5 by utilizing of phosphorus pentasulfide in dry toluene afforded 2-pentylquinazoline-4(3H)-thione 6 (Scheme 2). The formation of compound 6 was unambiguously elaborated by the presence of intense band at 1236 cm−1 corresponding to υc=s and the absence of the stretching band of υc=o in the IR spectrum. On the other hand, the incorporation of β-d-glucose pentaacetate with quinazolinone derivative 5 at the nitrogen atom of the later awarded N-(β-d-glucopyranosyl-2,3,4,6-tetraacetate)-2-pentyl quinazolin-4(3H)-one 7 (Scheme 2), via attacking of the lone pair of nitrogen atom of quinazolinone derivative 5 at the anomeric carbon (C1) of β-d-glucose pentaacetate, followed by ring opening and then ring closure with expulsion of acetate as a leaving group.
The chemical structure of compound 7 was explained by the IR spectrum, whereas it showed a band at 1746 cm−1 compatible with υC=O of the acetate groups and lacked the absorption band for the NH group. Moreover, this structure was also interpreted by the 1H-NMR spectrum which revealed seven signals at 5.92–3.51 ppm and four singlet signals at 1.97–1.91 ppm all of them corresponding to the protons of β-d-glucopyranosyl-2,3,4,6-tetraacetate moiety.
Curing of the benzoxazinone derivative 4 with ethanolamine under reflux for 3 h afforded 3-(2-hydroxyethyl)-2-pentylquinazolin-4(3H)-one 8 as the sole product. The IR spectrum of compound 8 showed a broad band at 3395 cm−1 corresponding to OH functionality. Furthermore, the 1H-NMR spectrum appreciably emerged a triplet peak at 4.95 ppm exchangeable with D2O corresponding to OH proton, triplet, and quartet peaks at 4.11 and 3.65 ppm, respectively, compatible with ethyl protons of 2-hydroxyethyl moiety. As well, the 13C-NMR spectrum exhibited two peaks at 58.8 and 46.1 ppm corresponding to the two carbons of 2-hydroxyethyl moiety.
3-Amino-2-pentylquinazolin-4(3H)-one 9 was commenced by refluxing of compound 4 with hydrazine monohydrate in absolute ethanol for 4 h (Scheme 2). The formation of compound 9 was confirmed by spectroscopic and elemental data. In particular, the 1H-NMR spectrum of compound 9 manifested a singlet signal commutable in D2O at 5.70 ppm corresponding to NH2 protons.
Reaction of 3-amino-2-pentylquinazolin-4(3H)-one 9 with various aldehydes 10a–d gave Schiff bases 11a–d as the sole product in each case (Scheme 3). The 1H-NMR spectra of compounds 11a–d exhibited the appearance of a singlet signal in the region between 8.81–8.69 ppm compatible with methine proton of N=CH group.
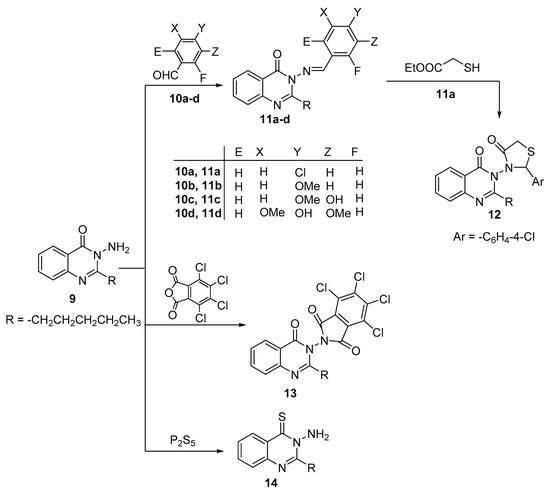
Scheme 3.
Synthetic route to compounds 11–14.
The thiazolidin-4-one moiety 12 was constructed by the reaction of Schiff base 11a with methyl thioglycolate in absolute ethanol including a small amount of piperidine as a catalyst for 3 h (Scheme 3). The prospective structure 12 is in keeping with its spectral and elemental analyses.
Additionally, the nucleophilicity of the amino group of compound 9 was also estimated by fusion of it with 4,5,6,7-tetrachloroisobenzofuran-1,3-dione in oil bath for an hour and that afforded phthalimido derivative 13 in an excellent yield (Scheme 3). The foreseeable structure of compound 13 was elucidated by their spectral data and elemental analysis. Obviously, its IR spectrum showed stretching absorption bands at 1788, and 1746 cm−1 corresponding to the carbonyl groups of phthalimido moiety and at 1707 cm−1 corresponding to carbonyl group of the quinazolinone moiety. The 1H-NMR spectrum exhibited four peaks for four aromatic protons and another four peaks for n-pentyl protons. Furthermore, its 13C-NMR spectrum emerged variant peaks, all of them fit with the proposed structure.
Eventually, the thione derivative 14 was obtained via sulfuration of compound 9 by utilizing phosphorus pentasulfide as the above pervious method (Scheme 3). The structure of 14 was unequivocally explained via the existence of a peak in the 13C NMR spectrum at 182.1 ppm compatible with the carbon of the thione functional group.
2.2. Biological Evaluation
2.2.1. Antiproliferative Screening
Twelve compounds possessing quinazolinone(thione) moieties 5–14 along with compound 3 were screened against two cell lines, namely hepatocellular carcinoma (HepG2) and mammary gland (MCF-7) in vitro by utilizing MTT assay [42,43]. The latter assay is a colorimetric test based on the change of the yellow MTT (3-(4,5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide) to a purple formazan derivative by mitochondrial succinate dehydrogenase in viable cells and the Doxorubicin (DOX) was used as a standard reference.
The results listed in Table 1 and illustrated in Figure 3, demonstrate that compounds 6 and 11d have a very strong efficacy against HePG2 cell line with IC50 values at 5.20 ± 0.5 and 7.63 ± 0.6 μM, respectively. Meanwhile, compounds 6, 11d, and 14 have a very strong efficacy against the MCF-7 cell line with IC50 values at 6.88 ± 0.4, 8.60 ± 0.7, and 10.78 ± 0.9 μM, respectively. Compounds 11b, 11c have a strong efficacy against both cell lines with IC50 values in the range (12.54 ± 1.1–19.68 ± 1.6 μM). For the HePG2 cell line, compounds 5, 9, 11a, and 14 have a moderate efficacy with IC50 values in the range (23.75 ± 1.9–41.92 ± 2.8 μM). Where, for the MCF-7 cell line, compounds 3, 5, 9, and 11a have a moderate efficacy with IC50 in the range (21.98 ± 1.8–47.53 ± 2.9 μM). Ultimately, the remaining compounds in both cases have weak efficacies with IC50 values > 50 μM.

Table 1.
Cytotoxic efficacy of thirteen compounds against hepatocellular carcinoma (HePG2) and mammary gland (MCF-7) cell lines.
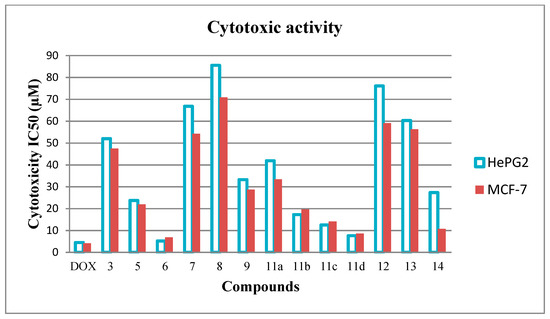
Figure 3.
Cytotoxic efficacy of thirteen compounds against HePG2 and MCF-7 cell lines.
Through our screening of the antiproliferative efficacy of the synthesized compounds, it was determined that the average of relative viability of cells (%) with different concentrations such as 100, 50, 25, 12.5, 6.25, 3.125, and 1.56 μM against two cell lines (HePG2 and MCF-7) as shown in Figure 4 and Figure 5.
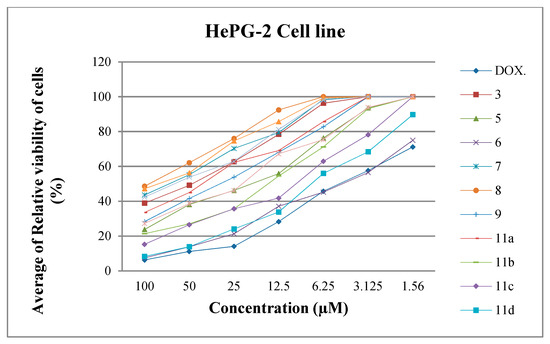
Figure 4.
Average of relative viability of HePG-2 cell line (%) with different concentrations.
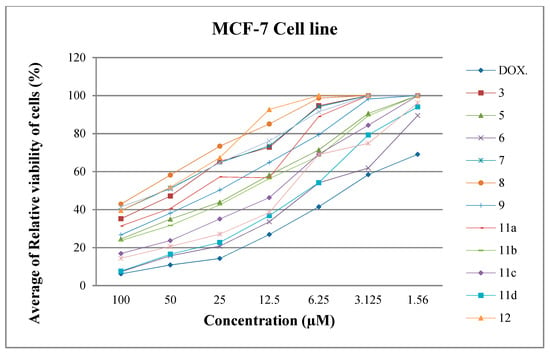
Figure 5.
Average of relative viability of MCF-7 cell line (%) with different concentrations.
Structure Activity Relationship (SAR)
By comparing the antiproliferative efficacy of the thirteen synthesized compounds in this study to their chemical structures, it was concluded that the following structure activity relationship’s (SAR’s) is hypothesized:
1. Conversion of quinazolin-4(3H)-one derivative 5 to quinazolin-4(3H)-thione derivative 6 enhanced the antiproliferative activity against both cell lines from moderate activity to very strong activity.
2. Similarly, conversion of 3-amino-2-pentylquinazolin-4(3H)-one 9 to 3-amino- 2-pentylquinazoline-4(3H)-thione 14 enhanced the antiproliferative activity against both cell lines.
3. Reaction of 9 with various aryl aldehydes afforded hydrazone derivatives (11a–d) analoges with variable potencies according to the following sequence: 3,5-(OMe)2-4-OH-C6H2 11d > 3-OH-4-(OMe)-C6H3 11c > 4-(OMe)-C6H4 11b > 4-Cl-C6H4 11a, whereas, the OH group in p-position is more electron donating group than the OMe group and Cl atom (i.e., the delocalization of n-π electrons decreased in the above sequence).
4. Construction of the thiazolidinone ring in compound 12 decreased the antiproliferative activity comparable with the hydrazone derivative 11a, due to decreasing of the delocalization of n-π electrons after replacement of the C=N group (electron attracting group) by the thiazolidinone ring.
2.2.2. Antioxidant Activity Screening
One of the aims of this work is the screening of all synthesized compounds for antioxidant activity using two different methods, namely ABTS [2,2′-azino-bis(3-ethyl benzothiazoline-6-sulfonic acid)] and DPPH assays. DPPH (2,2-diphenyl-1-picryl-hydrazyl-hydrate) free radical assay based on electron-transfer that produces a violet solution in ethanol. This free radical is stable at ambient temperature and reduced in the presence of an antioxidant molecule, leading to colorless ethanol solution. After investigation of these results as listed in Table 2 and Figure 6, it was realized that, compounds 6 and 11d have promising activity through using ABTS assay. Meanwhile, in the case of using DPPH assay, compounds 6, 11d and 14 have also very high activity. Ascorbic acid was used as a reference through the antioxidant activity screening.

Table 2.
Antioxidant activities of all synthesized compounds by using 2,2′-azino-bis(3-ethyl benzothiazoline-6-sulfonic acid (ABTS) and 2,2-diphenyl-1-picryl-hydrazyl-hydrate (DPPH) methods.
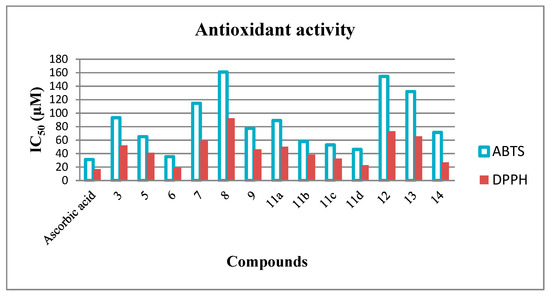
Figure 6.
Antioxidant activities of all synthesized compounds by using ABTS and DPPH methods.
The results depicted in Table 2 and Figure 6 demonstrated that, DPPH assay findings are very approximately related to those of ABTS assay with only one exception, compound 14 has an excellent antioxidant activity against DPPH (IC50 = 26.87 ± 0.23 μM) than that of the ABTS method (IC50 = 71.42 ± 0.52 μM). Noteworthy, all the screened compounds in the case of the DPPH method exhibited IC50 smaller than the corresponding ones of the same compounds in the case of the ABTS method, and it proposed that these compounds are more promising scavengers of the DPPH radical than those of the ABTS radical.
By comparing the antioxidant efficacy of the thirteen synthesized compounds in this study to their chemical structures, it was concluded that the following structure antioxidant activity relationship’s (SAR’s) is hypothesized:
1. The presence of C=S enhanced antioxidant activity than the presence of C=O, as shown in compounds 6 and 14 comparable with compounds 5 and 9, respectively.
2. The hydrazone derivatives (11a–d) analogs have variable potencies according to the following sequence: 3,5-(OMe)2-4-OH-C6H2 11d > 3-OH-4-(OMe)-C6H3 11c > 4-(OMe)-C6H4 11b > 4-Cl-C6H4 11a, whereas, OH group in p-position is a more electron donating group (has more conjugated system) than OMe group and Cl atom.
3. In compound 12, replacement of C=N group by the thiazolidinone ring decreased the antioxidant activity comparable with 11a, because of the lack of the conjugated system.
Previous reports of structurally similar compounds (in quinazoline ring) but with different substituents have demonstrated different results in antiproliferative and antioxidant activities from our results [9,33,44].
2.3. Density Functional Theory
According to the frontier molecular orbital (FMO) theory, the highest occupied molecular orbital (HOMO) acts as an electron-donor and the lowest unoccupied molecular orbital (LUMO) acts as an electron-acceptor [45]. Meanwhile, both play remarkable roles in the electronic studies by using quantum chemical calculations and they are also of significant importance in modern biochemistry and molecular biology [46]. A molecule is considered as a softer and has an excellent chemical reactivity when it has a smaller energy gap. Meanwhile, a molecule is considered to have a higher chemical hardness and assumed to have good stability when it has a larger energy gap [47,48,49,50,51].
The quantum chemical calculations were implemented by the density functional theory (DFT) method by using the Gaussian(R) 09 program at the B3LYP level in conjunction with 6-31G(d,p) basis set and computed parameters are summarized in Table 3.

Table 3.
Quantum chemical parameters of the selected compounds with Density Functional Theory (DFT) at B3LYP/6-31G (d,p) basis set.
By computing and using the energy gap (∆E = ELUMO − EHOMO) and dipole moment values beside another quantum chemical parameters such as ionization energy (I = −EHOMO), electron affinity (A = −ELUMO) [52], chemical hardness (η = (I − A)/2), chemical softness (S = 1/η) [53], and binding energy, we can rationally explicate the relation between the chemical structure and the antiproliferative activity (SAR’s). Whereas, the energy gap of compound 6 (∆E = 3.98 eV) is smaller than that corresponding of compound 5 (∆E = 4.85 eV) and also compound 6 has a higher chemical softness value (S = 0.50 eV−1) than that corresponding of compound 5 (S = 0.41 eV−1). These results are matching with the results of the antiproliferative screening whereas, compound 6 has a higher potency comparable with compound 5 for both cell lines (HepG2 and MCF-7) as shown in Table 1 and Figure 3. Similarly, compound 14 has a smaller energy gap and a higher chemical softness than that corresponding to compound 9 as listed in Table 2. In addition, in vitro compound 14 showed a remarkable higher efficacy comparable with compound 9 as shown in Table 1 and Figure 3. Notably, the dipole moment values of compounds 6 (µ = 3.4641 D) and 14 (µ = 1.852 D) are higher than that of compounds 5 (µ = 3.2867 D) and 9 (µ = 1.764 D), respectively.
On the other hand, compounds 11a–d possess antiproliferative activity in the following order 11d > 11c > 11b > 11a, meanwhile, the energy gaps of these compounds increase in the following order 11a (∆E = 2.99 eV) < 11d (∆E = 3.05 eV) < 11c (∆E = 3.10 eV) < 11b (∆E = 3.13 eV). The lower of the antiproliferative activity of compound 11a may be explained by values of the dipole moment whereas; the dipole moment of compound 11a is smaller than that of compounds 11b–d as shown in Table 3.
The distributions of the HOMO and LUMO orbitals of the selected compounds are computed at the same level of the DFT theory and are provided in Figure 7 and Figure 8. The results manifested that possible reactive sites exist as shown below:
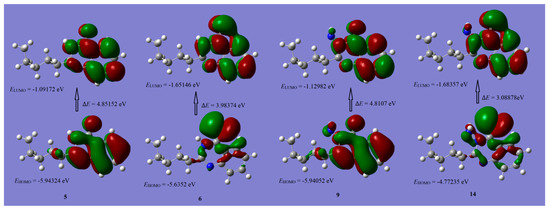
Figure 7.
Schematic representation of HOMO and LUMO coefficient distribution of compounds 5, 6, 9, and 14.
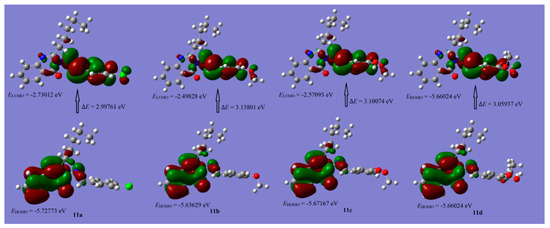
Figure 8.
Schematic representation of highest occupied molecular orbital (HOMO) and loest unoccupied molecular orbital (LUMO) coefficient distribution of compounds 11a–d.
1. The HOMO of compounds 5 and 9 are nearly similar and the distribution of orbitals are mainly situated on the quinazolinone moiety, also, the LUMO of these compounds are situated on the same moiety.
2. The HOMO of compounds 6 and 14 are nearly similar and the distribution of orbitals are mainly situated on C=S, while, the LUMO of these compounds are mainly situated on the quinazolinthione moiety.
3. The HOMO of compounds 11a–d are nearly similar and the distribution of orbitals are mainly situated on the quniazolinone moiety, meanwhile, the LUMO of these compounds are mainly situated on the aryl aldehyde hydrazone system.
3. Materials and Methods
3.1. Chemistry
The melting point is uncorrected and was measured on a Stuart SMP 30 advanced digital electric melting point apparatus (Cole-Parmer, Staffordshire, UK). All reactions were monitored by TLC (Kieselgel 60 F254, Merck, Munchen, Germany) and spots were visualized using UV (254 nm), In the region (400−4000 cm−1), the IR spectrum was measured in the KBr phase by using the Nicolet iS10 FT-IR spectrometer (Shimadzu Corporation, Kyoto, Japan). The 1H-NMR (at 400 MHz) and 13C-NMR (at 100 MHz) spectra were performed at chemical warfare labs, Egypt, with a Varian Gemini spectrometer (Metrohim, California, United States) in DMSO-d6 as a solvent by using tetramethylsilane (TMS) as a reference. Perkin-Elmer 2400 CHN elemental analyzer (Waltham, MA, USA) was used to record CHN elemental analysis at the Faculty of Science, Cairo University, Egypt. The mass spectrum was measured on Shimadzu GC-MS QP1000EX apparatus (Shimadzu Corporation, Kyoto, Japan) at the central analytical lab, Ain Shams University, Cairo, Egypt.
3.1.1. 2-Hexanamidobenzoic Acid 3
Hexanoyl chloride 2 (1.39 mL, 0.01 mol) was added dropwise to anthranilic acid 1 (1.37 g, 0.01 mol) dissolved in dry pyridine (20 mL) at ambient temperature with stirring. The stirring was continued for an hour, and then the resulting emulsion was acidified with cold 10% HCl solution. The white solid which separated was collected by filtration and then recrystallized from benzene to give 3 [38] as white crystals; m.p.: 92–95 °C (Lit. m.p.: 93−95 °C) [38], yield: 92%. IR (KBr, cm−1): 3426−2463 (br) (OH), 3206 (NH), 2959, 2934, 2861 (CHaliph.), 1691, 1637 (C=O). 1H-NMR (400 MHz, DMSO-d6) δ (ppm): 13.51 (br.s, 1H, OH, exchangeable with D2O), 11.09 (s, 1H, NH, exchangeable with D2O), 8.48 (d, 1H, Ar-H, Ha, J = 8.8 Hz), 7.95 (d, 1H, Ar-H, Hd, J = 8.0 Hz), 7.55 (t, 1H, Ar-H, Hc, J = 7.8 Hz, J = 8.0 Hz), 7.11 (t, 1H, Ar-H, Hb, J = 7.4 Hz, J = 7.8 Hz), 2.35 (t, 2H, COCH2, J = 7.2 Hz, J = 7.6 Hz), 1.60 (quintet, 2H, COCH2CH2, J = 7.2 Hz, J = 7.6 Hz), 1.31−1.26 (m, 4H, CH3CH2CH2), 0.85 (t, 3H, CH3, J = 6.8 Hz, J =7.2 Hz), MS m/z (%): 235 (M.+; 29.4). Anal. Calcd. for C13H17NO3 (235.28): C, 66.36; H, 7.28; N, 5.95. Found: C, 66.36; H, 7.28; N, 5.95.
3.1.2. 2-Pentyl-4H-benzo[d][1,3]oxazin-4-one 4
A suspension of 2-hexanamidobenzoic acid 3 (2.35 g, 0.01 mol) in freshly distilled acetic anhydride (10 mL) was heated in water bath for an hour followed by a concentration of the mixture in vacuo and used in situ [39,40].
3.1.3. 2-Pentylquinazolin-4(3H)-one 5
A solution of benzoxazinone 4 (2.17 g, 0.01 mol) in formamide (15 mL) was refluxed for 7 h. After cooling, the reaction mixture was poured onto ice cold water, the obtained solid was filtered off, dried, and recrystallized from petroleum ether 60–80 °C to give 5 [41] as white crystals; m.p.: 142–144 °C (Lit. m.p.: 153–154 °C) [41], yield: 92%. IR (KBr, cm−1): 3171 (NH), 2958, 2928, 2860 (CHaliph.), 1680 (C=O), 1614 (C=N or C=C). 1H-NMR (400 MHz, DMSO-d6) δ (ppm): 12.13 (s, 1H, NH, exchangeable with D2O), 8.05 (d, 1H, Ar-H, Ha, J = 8.0 Hz), 7.74 (t, 1H, Ar-H, Hc, J = 7.6 Hz, J = 7.8 Hz), 7.56 (d, 1H, Ar-H, Hd, J = 8 Hz), 7.42 (t, 1H, Ar-H, Hb, J = 7.6 Hz), 2.56 (t, 2H, N=CCH2, J = 7.6 Hz, J = 8.0 Hz), 1.70 (quintet, 2H, N=CCH2CH2, J = 7.6 Hz, J = 7.2 Hz), 1.30–1.26 (m, 4H, CH3CH2CH2), 0.84 (t, 3H, CH3, J = 6.8 Hz, J = 7.2 Hz). 13C-NMR (100 MHz, DMSO-d6) δ (ppm): 162.2, 157.9, 149.4, 134.7, 127.2, 126.3, 126.1, 121.2, 34.9, 31.1, 26.9, 22.2, 14.2. MS m/z (%): 216 (M.+; 26.3). Anal. Calcd. for C13H16N2O (216.28): C, 72.19; H, 7.46; N, 12.95. Found: C, 72.26; H, 7.49; N, 12.86.
3.1.4. 2-Pentylquinazoline-4(3H)-thione 6
To a solution of quinazolinone 5 (2.16 g, 0.01 mol) in dry toluene (30 mL), P2S5 (2.22 g, 0.01 mol) was added. The reaction mixture was refluxed for 1 h, and then filtered off. The obtained filtrate was evaporated under reduced pressure, the formed solid was collected by filtration, dried, and recrystallized from ethanol to give 6 as light brown crystals; m.p.: 101–103 °C, yield: 76%. IR (KBr, cm−1): 3184, 3141 (NH), 2967, 2935, 2852 (CHaliph.), 1618 (C=N), 1604 (C=C), 1236 (C=S). 1H-NMR (400 MHz, DMSO-d6) δ (ppm): 13.69 (s, 1H, NH, exchangeable with D2O), 8.52 (d, 1H, Ar-H, Hd, J = 8.4 Hz), 7.83 (t, 1H, Ar-H, Hc, J = 7.6 Hz), 7.63 (d, 1H, Ar-H, Ha, J = 8.4 Hz), 7.52 (t, 1H, Ar-H, Hb, J = 7.4 Hz, J = 7.8 Hz), 2.70 (t, 2H, N=CCH2, J = 7.6 Hz, J = 8.0 Hz), 1.72 (quintet, 2H, N=CCH2CH2, J = 7.6 Hz, J = 7.2 Hz), 1.31−1.26 (m, 4H, CH3CH2CH2), 0.85 (t, 3H, CH3, J = 6.8 Hz). MS m/z (%): 232 (M.+; 16.7). Anal. Calcd. for C13H16N2S (232.35): C, 67.20; H, 6.94; N, 12.06; S, 13.80. Found: C, 67.31; H, 7.03; N, 12.01; S, 13.85.
3.1.5. N-(β-d-Glucopyranosyl-2,3,4,6-tetraacetate)-2-pentylquinazolin-4(3H)-one 7
Quinazolinone 5 (2.16 g, 0.01 mol) was refluxed with β-d-glucose pentaacetate (3.90 g, 0.01 mol) in absolute ethanol (50 mL) for 3 h. the solid obtained after slow evaporation of the resulting solution was collected and recrystallized from ethanol to give 7 as white crystals; m.p.: 135–137 °C, yield: 62%. IR (KBr, cm−1): 2955, 2924, 2854 (CHaliph.), 1746 (C=Oester), 1678 (C=Oquinazolinone), 1613 (C=N). 1H-NMR (400 MHz, DMSO-d6) δ (ppm): 8.05 (d, 1H, Ar-H, Ha, J = 8.0 Hz), 7.74 (t, 1H, Ar-H, Hc, J = 8.4 Hz, J = 6.8 Hz), 7.56 (d, 1H, Ar-H, Hd, J = 8.4 Hz), 7.43 (t, 1H, Ar-H, Hb, J = 8.0 Hz, J = 7.2 Hz), 5.92 (d, 1H, C1-H, J = 8.4 Hz), 5.39 (t, 1H, C2-H, J = 9.6 Hz), 4.93 (t, 1H, C3-H, J = 9.6 Hz), 4.90 (t, 1H, C4-H, J = 8.4 Hz, J = 10.0 Hz), 4.14, 412 (d,d, 1H, C6-H, J = 10.4 Hz, J = 5.6 Hz), 3.97 (d, 1H, C6-H, J = 10.4 Hz), 3.51 (m, 1H, C5-H), 2.56 (t, 2H, N=CCH2, J = 8.0 Hz, J = 7.6 Hz), 1.978 (s, 3H, CH3), 1.973 (s, 3H, CH3), 1.961 (s, 3H, CH3), 1.917 (s, 3H, CH3), 1.69 (quintet, 2H, N=CCH2CH2, J = 7.6 Hz, J = 6.8 Hz), 1.31−1.26 (m, 4H, CH3CH2CH2), 0.84 (t, 3H, CH3, J = 6.8 Hz, J = 7.2 Hz). MS m/z (%): 546 (M+; 32.4). Anal. Calcd. for C27H34N2O10 (546.57): C, 59.33; H, 6.27; N, 5.13. Found: C, 59.18; H, 6.21; N, 5.08.
3.1.6. 3-(2-Hydroxyethyl)-2-pentylquinazolin-4(3H)-one 8
A solution of benzoxazinone 4 (2.17 g, 0.01 mol) in ethanolamine (15 mL) was heated under reflux for 3 h. The reaction mixture was poured onto ice cold water, the obtained solid was filtered off, dried, and then recrystallized from ethanol to give 8 as white crystals; m.p.: 84–85 °C, yield: 47%. IR (KBr, cm−1): 3395 (OH), 2953, 2931, 2872 (CHaliph.), 1648 (C=O), 1611 (C=N). 1H-NMR (400 MHz, DMSO-d6) δ (ppm): 8.07 (d, 1H, Ar-H, Ha, J = 8.0 Hz), 7.75 (t, 1H, Ar-H, Hc, J = 7.8 Hz, J = 7.6 Hz), 7.57 (d, 1H, Ar-H, Hd, J = 8.0 Hz), 7.44 (t, 1H, Ar-H, Hb, J = 7.6 Hz, J = 7.2 Hz), 4.95 (t, 1H, OH, exchangeable with D2O, J = 5.6 Hz), 4.11 (t, 2H, CH2CH2OH, J = 5.6 Hz, J = 6.0 Hz), 3.65 (q, 2H, CH2OH, J = 6.0 Hz, J = 5.6 Hz), 2.93 (t, 2H, N=CCH2, J = 7.6 Hz, J = 8.0 Hz), 1.75 (quintet, 2H, N=CCH2CH2, J = 7.6 Hz, J = 7.6 Hz), 1.40−1.32 (m, 4H, CH3CH2CH2), 0.88 (t, 3H, CH3, J = 7.2 Hz). 13C-NMR (100 MHz, DMSO-d6) δ (ppm): 161.7, 158.4, 147.4, 134.6, 127.1, 126.5, 126.4, 120.4, 58.8, 46.1, 34.6, 31.3, 26.3, 22.4, 14.3. MS m/z (%): 260 (M+; 41.2). Anal. Calcd. for C15H20N2O2 (260.34): C, 69.20; H, 7.74; N, 10.76. Found: C, 69.17; H, 7.68; N, 10.81.
3.1.7. 3-Amino-2-pentylquinazolin-4(3H)-one 9
A mixture of benzoxazinone 4 (2.17 g, 0.01 mol) and hydrazine hydrate (1.5 mL) in absolute ethanol (20 mL) was refluxed for 3 h. The mixture was poured onto ice cold water, the formed solid was filtered off, and recrystallized from ethanol to give 9 as buff crystals; m.p.: 58–60 °C, yield: 43%. IR (KBr, cm−1): 3306, 3263 (NH2), 2954, 2931, 2910, 2856 (CHaliph.), 1673 (C=O), 1630 (C=N). 1H NMR (400 MHz, DMSO-d6) δ (ppm): 8.08 (d, 1H, Ar-H, Ha, J = 7.8 Hz), 7.75 (t, 1H, Ar-H, Hc, J = 7.4 Hz, J = 7.8 Hz), 7.59 (d, 1H, Ar-H, Hd, J = 7.6 Hz), 7.45 (t, 1H, Ar-H, Hb, J = 7.2 Hz, J = 7.6 Hz), 5.70 (s, 2H, NH2, exchangeable with D2O), 2.90 (t, 2H, N=CCH2, J = 7.2 Hz, J = 8.0 Hz), 1.74 (quintet, 2H, N=CCH2CH2, J = 7.6 Hz, J = 7.2 Hz), 1.37−1.30 (m, 4H, CH3CH2CH2), 0.87 (t, 3H, CH3, J = 6.4 Hz, J =7.2 Hz). 13C-NMR (400 MHz, DMSO-d6) δ (ppm): 160.9, 158.8, 147.0, 134.3, 127.2, 126.39, 126.31, 120.2, 34.0, 31.4, 26.0, 22.3, 14.3. MS m/z (%): 231 (M+; 41.1). Anal. Calcd. for C13H17N3O (231.30): C, 67.51; H, 7.41; N, 18.17. Found: C, 67.39; H, 7.34; N, 18.24.
3.1.8. General Procedure for Synthesis of 11a–d
A mixture of compound 9 (2.31 g, 0.01 mol) and the appropriate aldehydes 10a–d (0.01 mol) in absolute ethanol (30 mL) was refluxed for 4−6 h. The reaction mixture was evaporated under reduced pressure; the obtained residue was collected and recrystallized from the proper solvent to give the corresponding benzylidene derivatives 11a–d, respectively.
3-((4-Chlorobenzylidene)amino)-2-pentylquinazolin-4(3H)-one 11a
Yellow crystals; m.p.: 176–178 °C (ethanol), yield: 72%. IR (KBr, cm−1): 2943, 2866 (CHaliph.), 1667 (C=O), 1624 (C=N). 1H-NMR (400 MHz, DMSO-d6) δ (ppm): 8.69 (s, 1H, N=CH), 7.95 (d, 1H, Ar-H, Ha, J = 7.8 Hz), 7.88 (d, 2H, Ar-H, HE + HF, J = 8.8 Hz), 7.66 (t, 1H, Ar-H, Hc, J = 8.4 Hz), 7.57 (d, 3H, Ar-H, Hd + HX + HZ), 7.46 (t, 1H, Ar-H, Hb, J = 8.4 Hz), 2.34 (t, 2H, N=CCH2, J = 7.6 Hz), 1.60 (quintet, 2H, N=CCH2CH2, J = 7.2 Hz), 1.31−1.26 (m, 4H, CH3CH2CH2), 0.85 (t, 3H, CH3, J = 6.8 Hz). MS m/z (%): 353 (M+; 4.0). Anal. Calcd. for C20H20ClN3O (353.85): C, 67.89; H, 5.70; Cl, 10.02; N, 11.88. Found: C, 67.78; H, 5.62; Cl, 9.89; N, 11.79.
3-((4-Methoxybenzylidene)amino)-2-pentylquinazolin-4(3H)-one 11b
White crystals; m.p.: 83–84 °C (petroleum ether 60–80 °C), yield: 64%. IR (KBr, cm−1): 2946, 2912, 2882, 2843 (CHaliph.), 1669 (C=O), 1606 (C=N or C=C). 1H-NMR (400 MHz, DMSO-d6) δ (ppm): 8.81 (s, 1H, N=CH), 8.12 (d, 1H, Ar-H, Ha, J = 7.8 Hz), 7.89 (d, 2H, Ar-H, HE + HF, J = 8.8 Hz), 7.80 (t, 1H, Ar-H, Hc, J = 8.2, Hz, J = 7.4 Hz), 7.65 (d, 1H, Ar-H, Hd, J = 8.0 Hz), 7.50 (t, 1H, Ar-H, Hb, J = 7.6, Hz, J = 7.4 Hz), 7.12 (d, 2H, Ar-H, HX + HZ, J = 8.8 Hz), 3.85 (s, 3H, OCH3), 2.80 (t, 2H, N=CCH2, J = 7.6 Hz, J = 8.0 Hz), 1.71 (quintet, 2H, N=CCH2CH2, J = 7.6 Hz, J = 7.2 Hz), 1.33−1.26 (m, 4H, CH3CH2CH2), 0.81 (t, 3H, CH3, J = 7.2 Hz). MS m/z 7(%): 349 (M+; 11.). Anal. Calcd. for C21H23N3O2 (349.43): C, 72.18; H, 6.63; N, 12.03. Found: C, 72.29; H, 6.69; N, 11.88.
3-((3-Hydroxy-4-methoxybenzylidene)amino)-2-pentylquinazolin-4(3H)-one 11c
White crystals; m.p.: 150–152 °C (ethanol), yield: 57%. IR (KBr, cm−1): 3277 (OH), 2956, 2927, 2892, 2863, 2845 (CHaliph.), 1678 (C=O), 1603 (C=N or C=C). 1H-NMR (400 MHz, DMSO-d6) δ (ppm): 9.49 (s, 1H, OH, exchangeable with D2O), 8.70 (s, 1H, N=CH), 8.12 (d, 1H, Ar-H, Ha, J = 8.0 Hz), 7.79 (t, 1H, Ar-H, Hc, J = 8.0 Hz, J =8.4 Hz), 7.65 (d, 1H, Ar-H, Hd, J = 7.6 Hz), 7.49 (t, 1H, Ar-H, Hb, J = 8.0 Hz, J = 7.2 Hz), 7.42 (d, 1H, HF, Jm = 2 Hz), 7.30, 7.28 (d,d, 1H, Ar-H, HE, Jo = 8.4 Hz, Jm = 2 Hz), 7.07 (d, 1H, Ar-H, HX, J = 8.4 Hz), 3.85 (s, 3H, OCH3), 2.78 (t, 2H, N=CCH2, J = 7.6 Hz), 1.71 (quintet, 2H, N=CCH2CH2, J = 7.6 Hz, J = 7.2 Hz), 1.31−1.28 (m, 4H, CH3CH2CH2), 0.81 (t, 3H, CH3, J = 6.8 Hz, J = 7.2 Hz). MS m/z (%): 365 (M+; 23.4). Anal. Calcd. for C21H23N3O3 (365.43): C, 69.02; H, 6.34; N, 11.50. Found: C, 68.88; H, 6.28; N, 11.62.
3-((4-Hydroxy-3,5-dimethoxybenzylidene)amino)-2-pentylquinazolin-4(3H)-one 11d
White crystals; m.p.: 148–150 °C (benzene), yield: 61%. IR (KBr, cm−1): 3408 (OH), 2952, 2911, 2844 (CHaliph.), 1668 (C=O), 1591 (C=N or C=C). 1H-NMR (400 MHz, DMSO-d6) δ (ppm): 9.36 (br.s, 1H, OH, exchangeable with D2O), 8.72 (s, 1H, N=CH), 8.12 (d, 1H, Ar-H, Ha, J = 8.2 Hz), 7.79 (t, 1H, Ar-H, Hc, J = 8.0 Hz, J = 7.4 Hz), 7.65 (d, 1H, Ar-H, Hd, J = 7.6 Hz), 7.50 (t, 1H, Ar-H, Hb, J = 8.0 Hz, J = 7.2 Hz), 7.23 (s, 2H, HE + HF), 3.82 (s, 6H, 2OCH3), 2.81 (t, 2H, N=CCH2, J = 7.6 Hz, J = 8.0 Hz), 1.72 (quintet, 2H, N=CCH2CH2, J = 7.6 Hz, J = 7.2 Hz), 1.36−1.26 (m, 4H, CH3CH2CH2), 0.81 (t, 3H, CH3, J = 6.8 Hz, J =7.2 Hz). 13C-NMR (100 MHz, DMSO-d6) δ (ppm): 169.8, 158.0, 156.5, 148.6 (2), 146.7, 140.8, 134.6, 127.4, 127.0, 126.7, 122.8, 121.3, 106.8 (2), 56.5 (2), 34.5, 31.3, 26.0, 22.2, 14.2. MS m/z (%): 395 (M+; 62.1), Anal. Calcd. for C22H25N3O4 (395.46): C, 66.82; H, 6.37; N, 10.63. Found: C, 66.95; H, 6.41; N, 10.58.
3.1.9. 2-(4-Chlorophenyl)-3-(4-oxo-2-pentylquinazolin-3(4H)-yl)thiazolidin-4-one 12
A mixture of compound 11a (3.53 g, 0.01 mol) and methyl thioglycolate (0.89 mL, 0.01 mol) in absolute ethanol (30 mL) containing piperidine (0.5 mL) was refluxed for 3 h. The obtained solid after evaporation of the solvent was collected and recrystallized from petroleum ether 60–80 °C to give 12 as pale yellow crystals; m.p.: 78–80 °C, yield: 47%. IR (KBr, cm−1): 2951, 2925, 2868 (CHaliph.), 1736 (C=Othiazolidinone), 1671 (C=Oquinazolinone), 1608 (C=N or C=C). 1H-NMR (100 MHz, DMSO-d6) δ (ppm): 8.13 (d, 1H, Ar-H, Ha, J = 8.2 Hz), 7.96 (d, 2H, Ar-H, HE + HF, J = 8.0 Hz), 7.80 (t, 1H, Ar-H, Hc, J = 7.6 Hz), 7.65 (d, 1H, Ar-H, Hd, J = 8.0 Hz), 7.64 (d, 2H, Ar-H, HX + HZ, J = 8.4 Hz), 7.51 (t, 1H, Ar-H, Hb, J = 7.6 Hz, J = 7.8 Hz), 5.70 (s, 1H, SCH), 3.75, 3.67 (d,d, 2H, CH2(thiazolidinone), J = 23.6 Hz, J = 23.2 Hz), 2.81 (t, 2H, N=CCH2, J = 8.0 Hz, J = 7.6 Hz), 1.71 (quintet, 2H, N=CCH2CH2, J = 7.6 Hz, J = 8.0 Hz), 1.35−1.17 (m, 4H, CH3CH2CH2), 0.80 (t, 3H, CH3, J = 7.2 Hz). MS m/z (%): 427 (M+; 11.8). Anal. Calcd. for C22H22ClN3O2S (427.95): C, 61.75; H, 5.18; Cl, 8.28; N, 9.82; S, 7.49. Found: C, 61.66; H, 5.12; Cl, 8.31; N, 9.75; S, 7.55.
3.1.10. 4,5,6,7-Tetrachloro-2-(4-oxo-2-pentylquinazolin-3(4H)-yl)isoindoline-1,3-dione 13
Compound 9 (2.31 g, 0.01 mol) was fused with 4,5,6,7-tetrachloroisobenzofuran-1,3-dione (2.85 g, 0.01 mol) in oil bath for an hour. The resulting solid was recrystallized from ethanol to give 13 as orange crystals; m.p.: 178–180 °C, yield: 86%. IR (KBr, cm−1): 2943, 2856 (CHaliph.), 1788, 1746 (C=Oimide), 1705 (C=Oquinazolinone), 1606 (C=N or C=C). 1H-NMR (400 MHz, DMSO-d6) δ (ppm): 8.08 (d, 1H, Ar-H, Ha, J = 8 Hz), 7.94 (t, 1H, Ar-H, Hc, J = 8.0 Hz, J = 7.2 Hz), 7.76 (d, 1H, Ar-H, Hd, J = 8.0 Hz), 7.60 (t, 1H, Ar-H, Hb, J = 7.6 Hz), 2.73 (t, 2H, N=CCH2, J = 6.8 Hz, J = 8.0 Hz), 1.70−1.60 (m, 2H, N=CCH2CH2), 1.29−1.27 (m, 4H, CH3CH2CH2), 0.81 (t, 3H, CH3, J = 6.8 Hz, J =7.2 Hz). 13C-NMR (100 MHz, DMSO-d6) δ (ppm): 161.5, 161.3, 157.9, 157.7, 147.4, 146.7, 140.0, 138.8, 136.6 (2), 128.2 (2), 128.0, 127.1, 126.8, 119.8, 32.6, 30.9, 26.0, 22.2, 14.2. MS m/z (%): 231 (M+; 41.1). Anal. Calcd. for C21H15Cl4N3O3 (499.17): C, 50.53; H, 3.03; Cl, 28.41; N, 8.42. Found: C, 50.61; H, 3.09; Cl, 28.37; N, 8.53.
3.1.11. 3-Amino-2-pentylquinazoline-4(3H)-thione 14
A mixture of compound 9 (2.31 g, 0.01 mol) and P2S5 (2.22 g, 0.01 mol) in dry toluene (15 mL) was heated under reflux for 4 h. The mixture was filtered off, the filtrate was evaporated under reduced pressure, the obtained solid was collected, dried, and recrystallized from ethanol to give 14 as yellow crystals; m.p.: 57–59 °C, yield: 53%. IR (KBr, cm−1): 3240, 3200 (NH2), 2925, 2855 (CHaliph.), 1591 (C=N or C=C), 1238 (C=S). 1H-NMR (400 MHz, DMSO-d6) δ (ppm): 8.51 (d, 1H, Ar-H, Ha, J = 8.2 Hz), 7.82 (t, 1H, Ar-H, Hc, J = 7.6 Hz, J = 7.8 Hz), 7.69 (d, 1H, Ar-H, Hd, J = 7.6 Hz), 7.57 (t, 1H, Ar-H, Hb, J = 7.6 Hz), 7.04 (s, 2H, NH2, exchangeable with D2O), 3.06 (t, 2H, N=CCH2, J = 7.2 Hz, J = 8.0 Hz), 1.81 (quintet, 2H, N=CCH2CH2, J = 7.6 Hz, J = 7.2 Hz), 1.42−1.32 (m, 4H, CH3CH2CH2), 0.88 (t, 3H, CH3, J = 6.8 Hz, J =7.2 Hz). 13C NMR (100 MHz, DMSO-d6) δ (ppm): 182.1, 155.5, 142.0, 134.5, 130.6, 128.1 (2), 127.4, 34.4, 31.3, 25.5, 22.4, 14.3. MS m/z (%): 247 (M+; 74.3). Anal. Calcd. for C13H17N3S (247.36): C, 63.12; H, 6.93; N, 16.99; S, 12.96. Found: C, 63.19; H, 6.96; N, 17.11; S, 12.82.
3.2. Cytotoxicity and Antiproliferative Evaluation
MTT Assay
The implement of MTT methodology for the antiproliferative screening of quinazolinone derivatives 5–14 along with compound 3 against two cell lines, namely, hepatocellular carcinoma (HepG2) and mammary gland (MCF-7) were obtained from ATCC through the Holding company for biological products and vaccines (VACSERA), Cairo, Egypt. The reference anticancer drug used was Doxorubicin. The MTT assay was carried out at the pharmacology department, Faculty of pharmacy, Mansoura University, Egypt according to the reported literatures [42,43,54]. The cells were cultured in a RPMI-1640 medium with 10% fetal bovine serum, followed by the addition of antibiotics (100 units/mL penicillin and 100 µg/mL streptomycin) at 37 °C in a 5% CO2 incubator. The cells were seeded in a 96-well plate at a density of (1.0 × 104 cells/well) at 37 °C for 48 h under 5% CO2. Treatment of cells with different concentrations of compounds such as 100, 50, 25, 12.5, 6.25, 3.125, and 1.56 μM was carried out and placed in the incubator for 24 h. Then, 20 µL of MTT solution at 5 mg/mL was added and incubated for 4 h. DMSO (100 µL) was added into each well to dissolve the purple formazan formed. At 570 nm absorbance the colorimetric assay was measured and recorded by using a plate reader (BioTek EL ×800 Microplate Reader, BioTek Instruments, Inc, Winooski, VT, USA).
Calculation of the relative cell viability (%) = (A of treated samples /A of untreated sample) × 100.
3.3. Antioxidant Assay
3.3.1. Antioxidant Activity Screening Assay
ABTS Method
By the bleaching of ABTS derived radical cations, the detections of antioxidant activities were estimated. The radical cation was prepared by the reaction of ABTS [2,2′-azino-bis(3-ethyl benzothiazoline-6-sulfonic acid)] (60 µL) with MnO2 (3 mL, 25 mg/mL) in a phosphate buffer solution (10 µM, pH 7, 5 mL). The solution was shaken for 3 min, centrifuged, filtered, and recorded at λmax 734 nm the absorbance A(control) of the resulting ABTS radical solution (green-blue). Upon the addition of the tested sample solution (20 µl) with different concentrations of compounds such as 200, 100, 50, 25, and 12.5 μM in spectroscopic grade MeOH/buffer (1:1 v/v) to the ABTS solution, the absorbance A(test) was measured. The decreasing in the absorbance is expressed as % inhibition which was calculated according to the following equation [55]:
where; the reference and standard antioxidant compound in this test is the ascorbic acid solution (20 µL, 2 mM) and the blank sample was performed by the solvent without ABTS.
% Inhibition = [A(control) − A(test)/A(control)] × 100
DPPH Method
According to the methodology described by Brand-Williams et al. [56], the measurement of the DPPH radical scavenging activity was implemented. The samples with different concentrations of compounds such as 200, 100, 50, 25, and 12.5 μM were allowed to react with the stable DPPH radical in ethanol solution. Whereas, the reaction mixture consisted of sample (0.5 mL), absolute ethanol (3 mL), and DPPH radical solution (0.3 mL) 0.5 mM in ethanol. DPPH is reduced when it reacts with an antioxidant compound, which can donate hydrogen. The changes in color (from deep violet to light yellow) were recorded [absorbance (Abs)] at λmax 517 nm after 100 min of reaction using a UV-Vis spectrophotometer (Schimadzu Co., Tokyo, Japan). The blank solution was prepared by mixing ethanol (3.3 mL) and the sample (0.5 mL). Meanwhile, the mixture of ethanol (3.5 mL) and DPPH radical solution (0.3 mL) serve as a positive control.
The scavenging activity percentage (AA %) was determined according to Mensor et al. [57]:
AA % = 100 − [(Abs(sample) − Abs(blankl)/Abs(control)) × 100]
3.4. Computational Procedures
All theoretical calculations and results of the studied compounds were implemented by utilizing Gaussian(R) 09 D.01 [58] (Semichem Inc., Shawnee Mission, KS, USA) by applying the DFT operation with the hybrid functional B3LYP level [59,60] in conjunction with the 6−31G(d,p) basis set. The visualization of these results was achieved using GaussView 6.0.16 software (Semichem Inc., Shawnee Mission, KS, USA).
4. Conclusions
In conclusion, this work focused on the study of the antiproliferative and antioxidant activities in vitro in addition to the theoretical calculation of the DFT theory of some novel quinazolinone(thione) derivatives 6–14. Two main points were the principal targets; firstly, by comparing the activities of quinazolinone and quinazolinthione derivatives. Secondly, comparing the activities of four series of Schiff bases, that have quinazolinone moiety. The results of this study imply that the quinazolinthione derivatives 6 and 14 have promising potent antiproliferative activity comparable with quinazolinone derivatives 5 and 9, respectively. According to the DFT study, compounds 6 and 14 have a smaller energy gap and a higher chemical softness than that of compounds 5 and 9, respectively. Additionally, screening of various aryl aldehyde hydrazone derivatives (11a–d) analogs exhibited that the potency increased with increasing the electron donating group in p-position due to increasing of the conjugated system, and that was supported by the DFT study.
On the other hand, compounds 6 and 11d showed promising antioxidant activity using ABTS assay. While in the DPPH assay, compounds 6, 11d, and 14 have showed potent activities comparable to the ascorbic acid which was used as a reference drug. Noteworthy, the results of both antiproliferative and antioxidant activities for each compound individually are nearly the same.
Author Contributions
The listed authors contributed to this work as described in the following: A.A.E.-S., M.F.I., and A.E.-G.E.A. designed the research idea, implemented the synthesis and characterization of novel compounds, and contributed to the data interpretation. A.E.-G.E.A. and A.M.N. contributed to discuss the results, writing the original draft manuscript, and revisions. All authors read and approved the final manuscript.
Funding
The authors are grateful to the Deanship of Scientific Research, King Saud University for funding this work through research group project “RGP-172”.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Stewart, B.W.; Wild, C.P. (Eds.) World Cancer Report; International Agency for Research on Cancer: Lyon, France, 2014. [Google Scholar]
- Spanò, V.; Montalbano, A.; Carbone, A.; Parrino, B.; Diana, P.; Cirrincione, G.; Castagliuolo, I.; Brun, P.; Issinger, O.G.; Tisi, S.; et al. Synthesis of a new class of pyrrolo[3,4-h]quinazolines with antimitotic activity. Eur. J. Med. Chem. 2014, 74, 340–357. [Google Scholar] [CrossRef] [PubMed]
- Sordella, R.; Bell, D.W.; Haber, D.A.; Settleman, J. Gefitinib-sensitizing EGFR mutations in lung cancer activate anti-apoptotic pathways. Science 2004, 305, 1163–1167. [Google Scholar] [CrossRef] [PubMed]
- Raymond, E.; Faivre, S.; Armand, J.P. Epidermal growth factor receptor tyrosine kinase as a target for anticancer therapy. Drugs 2000, 60, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Ko, H.C.; Wang, Y.H.; Liou, K.T.; Chen, C.M.; Chen, C.H.; Wang, W.Y.; Chang, S.; Hou, Y.C.; Chen, K.T.; Chen, C.F.; et al. Antiinflammatory effects and mechanisms of the ethanol extract of Evodia rutaecarpa and its bioactive components on neutrophils and microglial cells. Eur. J. Pharmacol. 2007, 555, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Keller, T.L.; Zocco, D.; Sundrud, M.S.; Hendrick, M.; Edenius, M.; Yum, J.; Kim, Y.J.; Lee, H.K.; Cortese, J.F.; Wirth, D.F.; et al. Halofuginone and other febrifugine derivatives inhibit prolyl-tRNA synthetase. Nat. Chem. Biol. 2012, 8, 311–317. [Google Scholar] [CrossRef]
- El-Azab, A.S.; Eltahir, K.E. Synthesis and anticonvulsant evaluation of some new 2,3,8-trisubstituted-4(3H)-quinazoline derivatives. Bioorg. Med. Chem. Lett. 2012, 22, 327–333. [Google Scholar] [CrossRef]
- Shcherbakova, I.; Balandrin, M.F.; Fox, J.; Ghatak, A.; Heaton, W.L.; Conklin, R.L. 3H-Quinazolin-4-ones as a new calcilytic template for the potential treatment of osteoporosis. Bioorg. Med. Chem. Lett. 2005, 15, 1557–1560. [Google Scholar] [CrossRef]
- Reddy, A.G.; Babu, V.H.; Rao, Y.J.P. A review on quinazolines as anticancer agents. J. Chem. Pharm.Sci. 2017, 10, 1492–1504. [Google Scholar]
- Mohamed, M.A.; Ayyad, R.R.; Shawer, T.Z.; Abdel-Aziz, A.A.-M.; El-Azab, A.S. Synthesis and antitumor evaluation of trimethoxyanilides based on 4(3H)-quinazolinone scaffolds. Eur. J. Med. Chem. 2016, 112, 106–113. [Google Scholar] [CrossRef]
- Chinigo, G.M.; Paige, M.; Grindrod, S.; Hamel, E.; Dakshanamurthy, S.; Chruszcz, M.; Minor, W.; Brown, M.L. Asymmetric synthesis of 2,3-dihydro-2-arylquinazolin-4-ones: methodology and application to a potent fluorescent tubulin inhibitor with anticancer activity. J. Med. Chem. 2008, 51, 4620–4631. [Google Scholar] [CrossRef]
- Kubo, K.; Shimizu, T.; Ohyama, S.; Murooka, H.; Iwai, A.; Nakamura, K.; Hasegawa, K.; Kobayashi, Y.; Takahashi, N.; Takahashi, K.; et al. Novel potent orally active selective VEGFR-2 tyrosine kinase inhibitors: synthesis, structure-activity relationships, and antitumor activities of N-phenyl-N’-{4-(4-quinolyloxy)phenyl}ureas. J. Med. Chem. 2005, 48, 1359–1366. [Google Scholar] [CrossRef] [PubMed]
- Hour, M.-J.; Huang, L.-J.; Kuo, S.-C.; Xia, Y.; Bastow, K.; Nakanishi, Y.; Hamel, E.; Lee, K.-H. 6-Alkylamino- and 2,3-Dihydro-3‘-methoxy-2-phenyl-4-quinazolinones and related compounds: Their synthesis, cytotoxicity, and inhibition of tubulin polymerization. J. Med. Chem. 2000, 43, 4479–4487. [Google Scholar] [CrossRef] [PubMed]
- Takase, Y.; Saeki, T.; Watanabe, N.; Adachi, H.; Souda, S.; Saito, I. Cyclic GMP Phosphodiesterase inhibitors. 2. requirement of 6-substitution of quinazoline derivatives for potent and selective inhibitory activity. J. Med. Chem. 1994, 37, 2106−2111. [Google Scholar] [CrossRef] [PubMed]
- Khodarahmi, G.; Jafari, E.; Hakimelahi, G.; Abedi, D.; Rahmani, K.M.; Hassanzadeh, F. Synthesis of some new quinazolinone derivatives and evaluation of their antimicrobial activities. Iran, J. Pharm. Res. 2012, 11, 789–797. [Google Scholar]
- Venkatesh, R.; Kasaboina, S.; Jain, N.; Janardhan, S.; Holagunda, U.D.; Nagarapu, L. Design and synthesis of novel sulphamide tethered quinazolinone hybrids as potential antitumor agents. J. Mol. Struct. 2019, 1181, 403–411. [Google Scholar] [CrossRef]
- Patel, M.B.; Kumar, S.P.; Valand, N.N.; Jasrai, Y.T.; Menon, S.K. Synthesis and biological evaluation of cationic fullerene quinazolinone conjugates and their binding mode with modeled Mycobacterium tuberculosis hypoxanthine-guanine phosphoribosyltransferase enzyme. J. Mol. Model. 2013, 19, 3201–3217. [Google Scholar] [CrossRef]
- Mhaske, S.B.; Argade, N.P. the chemistry of recently isolated naturally occurring quinazolinone alkaloids. Tetrahedron 2006, 62, 9787–9826. [Google Scholar] [CrossRef]
- Song, F.; Ren, B.; Yu, K.; Chen, C.; Guo, H.; Yang, N.; Gao, H.; Liu, X.; Liu, M.; Tong, Y.; et al. Quinazolin-4-one coupled with pyrrolidin-2-iminium alkaloids from marine-derived fungus Penicillium aurantiogriseum. Mar Drugs. 2012, 10, 1297–1306. [Google Scholar] [CrossRef]
- Obafemi, C.A.; Fadare, O.A.; Jasinski, J.P.; Millikan, S.P.; Obuotor, E.M.; Iwalewa, E.O.; Famuyiwa, S.O.; Sanusi, K.; Yilmaz, Y.; Ceylan, Ü. Microwave-assisted synthesis, structural characterization, DFT studies, antibacterial and antioxidant activity of 2-methyl-4-oxo-1,2,3,4- tetrahydroquinazoline-2-carboxylic acid. J. Mol. Struct. 2018, 1155, 610–622. [Google Scholar] [CrossRef]
- Patel, N.B.; Patel, V.N. New 2,3-disubstituted quinazolin-4 (3H)-ones as antimicrobial agents. Ind. J. Heterocycl. Chem. 2007, 16, 247–250. [Google Scholar]
- Peet, N.P.; Baugh, L.E.; Sunder, S.; Lewis, J.E.; Matthews, E.H.; Olberding, E.L.; Shah, D.N. 3-(1H-Tetrazol-5-yl)-4(3H)-quinazolinone sodium salt (MDL 427): A new antiallergic agent. J. Med. Chem. 1986, 29, 2403–2409. [Google Scholar] [CrossRef] [PubMed]
- Patil, A.; Barge, M.; Rashinkar, G.; Salunkhe, R. Aqueous hydrotrope: An efficient and reusable medium for a green one-pot, diversity-oriented synthesis of quinazolinone derivatives. Mol. Divers. 2015, 19, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Khattab, S.N.; Haiba, N.S.; Asal, A.M.; Bekhit, A.A.; Guemei, A.A.; Amer, A.; El-Faham, A. Study of antileishmanial activity of 2-aminobenzoyl amino acid hydrazides and their quinazoline derivatives. Bioorg. Med. Chem. Lett. 2017, 27, 918–921. [Google Scholar] [CrossRef] [PubMed]
- Haghighijoo, Z.; Firuzi, O.; Hemmateenejad, B.; Emami, S.; Edraki, N.; Miri, R. Synthesis and biological evaluation of quinazolinone-based hydrazones with potential use in Alzheimer’s disease. Bioorg. Chem. 2017, 74, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Mhaske, S.B.; Argade, N.P. Regioselective quinazolinone-directed ortho lithiation of quinazolinoylquinoline: practical synthesis of naturally occurring human DNA topoisomerase I poison luotonin a and luotonins B and E. J. Organomet. Chem. 2004, 69, 4563–4566. [Google Scholar] [CrossRef]
- Houck, D.R.; Ondeyka, J.; Zink, D.L.; Inamine, E.; Goetz, M.A.; Hensens, O.D. On the biosynthesis of asperlicin and the directed biosynthesis of analogs in Aspergillus alliaceus. J. Antibiot. (Tokyo) 1988, 41, 882–891. [Google Scholar] [CrossRef]
- He, L.; Li, H.; Chen, J.; Wu, X. Recent advances in 4(3H)-quinazolinone syntheses. RSC Adv. 2014, 4, 12065–12077. [Google Scholar] [CrossRef]
- Chaudhuri, P.K. Echinozolinone, an alkaloid from Echinops echinatus. Phytochemistry 1987, 26, 587–589. [Google Scholar] [CrossRef]
- Ramanathan, M.; Hsu, M.T.; Liu, S.T. Preparation of 4(3H)-quinazolinones from aryldiazonium salt, nitriles and 2-aminobenzoate via a cascade annulation. Tetrahedron 2019, 75, 791–796. [Google Scholar] [CrossRef]
- Sales, Z.S.; Mani, N.S.; Allison, B.D. The synthesis of 2-amino-4(3H)-quinazolinones and related heterocycles via a mild electrocyclization of aryl guanidines. Tetrahedron Lett. 2018, 59, 1623–1626. [Google Scholar] [CrossRef]
- Ismail, M.F.; El-sayed, G.A. Dodecanoyl isothiocyanate and N’-(2-cyanoacetyl)dodecane- hydrazide as precursors for the synthesis of different heterocyclic compounds with interesting antioxidant and antitumor activity. Synth. Commun. 2018, 48, 892–905. [Google Scholar] [CrossRef]
- Ajani, O.O.; Audu, O.Y.; Aderohunmu, D.V.; Owolabi, F.E.; Olomieja, A.O. Review Article Undeniable Pharmacological Potentials of Quinazoline Motifs in Therapeutic Medicine. Am. J. Drug Discov. Dev. 2017, 7, 1–24. [Google Scholar] [CrossRef]
- Abbas, S.Y.; El-Bayouki, K.A.M.; Basyouni, W.M. Utilization of isatoic anhydride in the syntheses of various types of quinazoline and quinazolinone derivatives. Synth. Commun. 2016, 46, 993–1035. [Google Scholar] [CrossRef]
- Maiden, T.M.M.; Harrity, J.P.A. Recent developments in transition metal catalysis for quinazolinone synthesis. Org. Biomol. Chem. 2016, 14, 8014–8025. [Google Scholar] [CrossRef]
- Rohokale, R.S.; Kshirsagar, U.A. Advanced Synthetic Strategies for Constructing Quinazolinone Scaffolds. Synthesis 2016, 48, 1253–1268. [Google Scholar] [CrossRef]
- Youssef, Y.M.; El-Sayed, A.A.; Azab, M.E. Utility of Benzoxazin-4-one and 3-amino- quinazolin-4-one Derivatives as Precursors for Construction of Potent Insecticidal Heterocycles. J. Heterocyclic Chem. 2019. [Google Scholar] [CrossRef]
- Park, W.J.; Ma, E. Inhibition of PCAF histone acetyltransferase and cytotoxic effect of N-acylanthranilic acids. Pharmacal. Res. 2012, 35, 1379–1386. [Google Scholar] [CrossRef]
- Li, W.; Wu, X.-F. Palladium-catalyzed carbonylative synthesis of benzoxazinones from N-(o-Bromoaryl)amides using paraformaldehyde as the carbonyl source. J. Org. Chem. 2014, 79, 10410–10416. [Google Scholar] [CrossRef]
- Mahindroo, N.; Ahmed, Z.; Bhagat, A.; Bedi, K.L.; Khajuria, R.K.; Kapoor, V.K.; Dhar, K.L. Synthesis and structure-activity relationships of vasicine analogues as bronchodilatory agents. Med. Chem. Res. 2006, 14, 347–368. [Google Scholar] [CrossRef]
- Zhang, W.; Meng, C.; Liu, Y.; Tang, Y.; Li, F. Auto-tandem catalysis with ruthenium: From o-aminobenzamides and allylic alcohols to quinazolinones via redox isomerization/acceptorless dehydrogenation. Adv. Synth. Catal. 2018, 360, 3751–3759. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Denizot, F.; Lang, R. Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J. Immunol. Methods 1986, 89, 271–277. [Google Scholar] [CrossRef]
- He, D.; Wang, M.; Zhao, S.; Shu, Y.; Zeng, H.; Xiao, C.; Lu, C.; Liu, Y. Pharmaceutical prospects of naturally occurring quinazolinone and its derivatives. Fitoterapia 2017, 119, 136–149. [Google Scholar] [CrossRef] [PubMed]
- Karunakaran, V.; Balachandran, V. FT-IR, FT-Raman spectra, NBO, HOMO-LUMO and thermodynamic functions of 4-chloro-3-nitrobenzaldehyde based on ab initio HF and DFT calculations. Spectrochim. Acta A 2012, 98, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Ayyamperumal, N.; Balachandran, V.; Thangavel, K. Molecular structure, vibrational spectra, first hyperpolarizability and HOMO-LUMO analysis of p-acetylbenzonitrile using quantum chemical calculation. J. Mol. Struct. 2013, 1038, 134–144. [Google Scholar] [CrossRef]
- Prashanth, J.; Ramesh, G.; Naik, J.L.; Ojha, J.K.; Reddy, B.V. Molecular geometry, NBO analysis, Hyperpolarizability and HOMO-LUMO energies of 2-azido-1-phenylethanone using Quantum chemical calculations. Mater. Today Proc. 2016, 3, 3761–3769. [Google Scholar] [CrossRef]
- Al-Omary, F.A.M.; Mary, Y.S.; Panicker, C.Y.; El-Emam, A.A.; Al-Swaidan, I.A.; Al-Saadi, A.A.; Alsenoy, C.V. Spectroscopic investigations, NBO, HOMO-LUMO, NLO analysis molecular docking of 5-(adamantan-1-yl)-3anilinomethyl-2,3-dihydro-1,3,4-oxadiazole-2-thione, a potential bioactive agent. J. Mol. Struct. 2015, 1096, 1–14. [Google Scholar] [CrossRef]
- Koparir, M.; Orek, C.; Koparir, P.; Sarac, K. Synthesis, experimental, theoretical characterization and biological activities of4-ethyl-5-(2-hydroxyphenyl)-2H-1,2,4-triazole- 3(4H)-thione. Spectrochim. Acta, Part A 2013, 105, 522–531. [Google Scholar] [CrossRef]
- Fleming, I. Frontier Orbitals and Organic Chemical Reactions; John Wiley & Sons: New York, NY, USA, 1976. [Google Scholar]
- Şahin, Z.S.; Kantar, G.K.; Şaşmaz, S.; Büyükgüngör, O. Synthesis, molecular structure, spectroscopic analysis, thermodynamic parameters and molecular modeling studies of (2-methoxyphenyl)oxalate. J. Mol. Struct. 2015, 1087, 104–112. [Google Scholar] [CrossRef]
- Koopmans, T. Über die Zuordnung von Wellenfunktionen und Eigenwerten zu den Einzelnen Elektronen Eines Atoms. Physica 1934, 1, 104–113. [Google Scholar] [CrossRef]
- Pearson, R.G. Absolute electronegativity and hardness correlated with molecular orbital theory. Proc. Natl. Acad. Sci. USA. 1986, 83, 8440–8441. [Google Scholar] [CrossRef] [PubMed]
- Ismail, M.F.; El-sayed, A.A. Synthesis and in-vitro antioxidant and antitumor evaluation of novel pyrazole-based heterocycles. J. Iran. Chem. Soc. 2019, 16, 921–937. [Google Scholar] [CrossRef]
- Lissi, E.A.; Modak, B.; Torres, R.; Escobar, J.; Urzua, A. Total antioxidant potential of resinous exudates from Heliotropium species, and a comparison of the ABTS and DPPH methods. Free Radic. Res. 1999, 30, 471. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. use of a free radical method to evaluate antioxidant activity. Lebensm. Wiss. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Mensor, L.L.; Menezes, F.S.; Leitao, G.G.; Reis, A.S.; dos Santos, T.C.; Coube, C.S.; Leitão, S.G. Screening of Brazilian plant extracts for antioxidant activity by the use of DPPH free radical method. Phytother. Res. 2001, 15, 127–130. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision D.01; Gaussian Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).