Erythroxylum in Focus: An Interdisciplinary Review of an Overlooked Genus
Abstract
:1. Introduction
2. History and Evolution of the Erythroxylum Genus
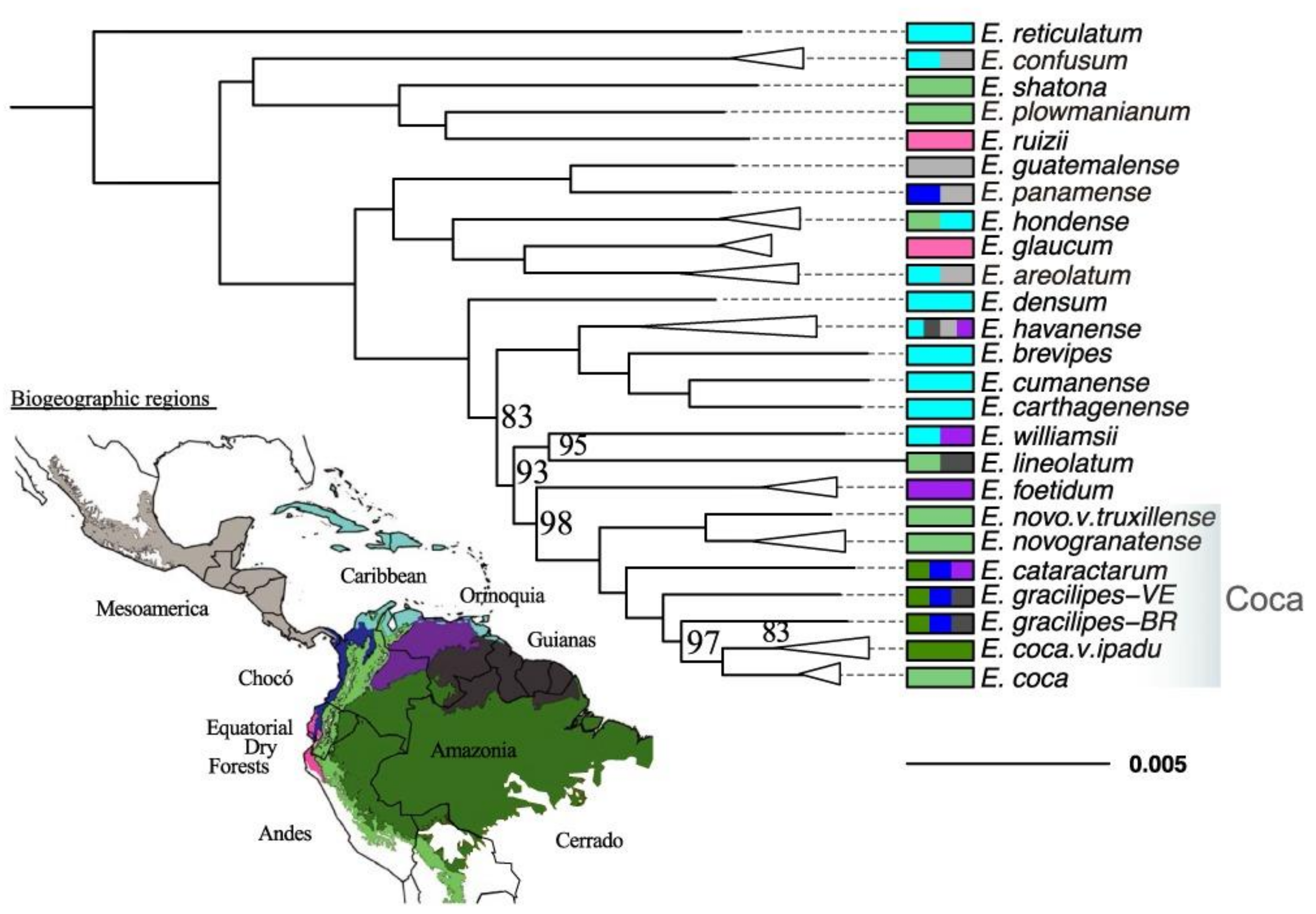
2.1. The Phylogeny of Erythroxylum and Related Genera
2.2. Domestication of the Coca Plant
2.3. Omic Studies on Erythroxylum to Shed Light on Evolution and Functional Background
3. An Overview of Bioprospection and Pharmacological Research in the Erythroxylum Genus
3.1. Erythroxylum vacciniifolium Mart.
3.2. Erythroxylum ovalifolium Peyr.
3.3. Erythroxylum pervillei Baill.
3.4. Erythroxylum macrocarpum 0.E. Schulz
3.5. Erythroxylum caatingae Plowman
3.6. Erythroxylum Suberosum A.St.-Hill., A.Juss and Cambess
3.7. Erythroxylum laurifolium Lam.
4. Erythroxylum coca and E. novogranatense: Coca’s Productive Uses
4.1. Context of Coca’s Uses
4.2. Whole Coca versus Isolated Cocaine
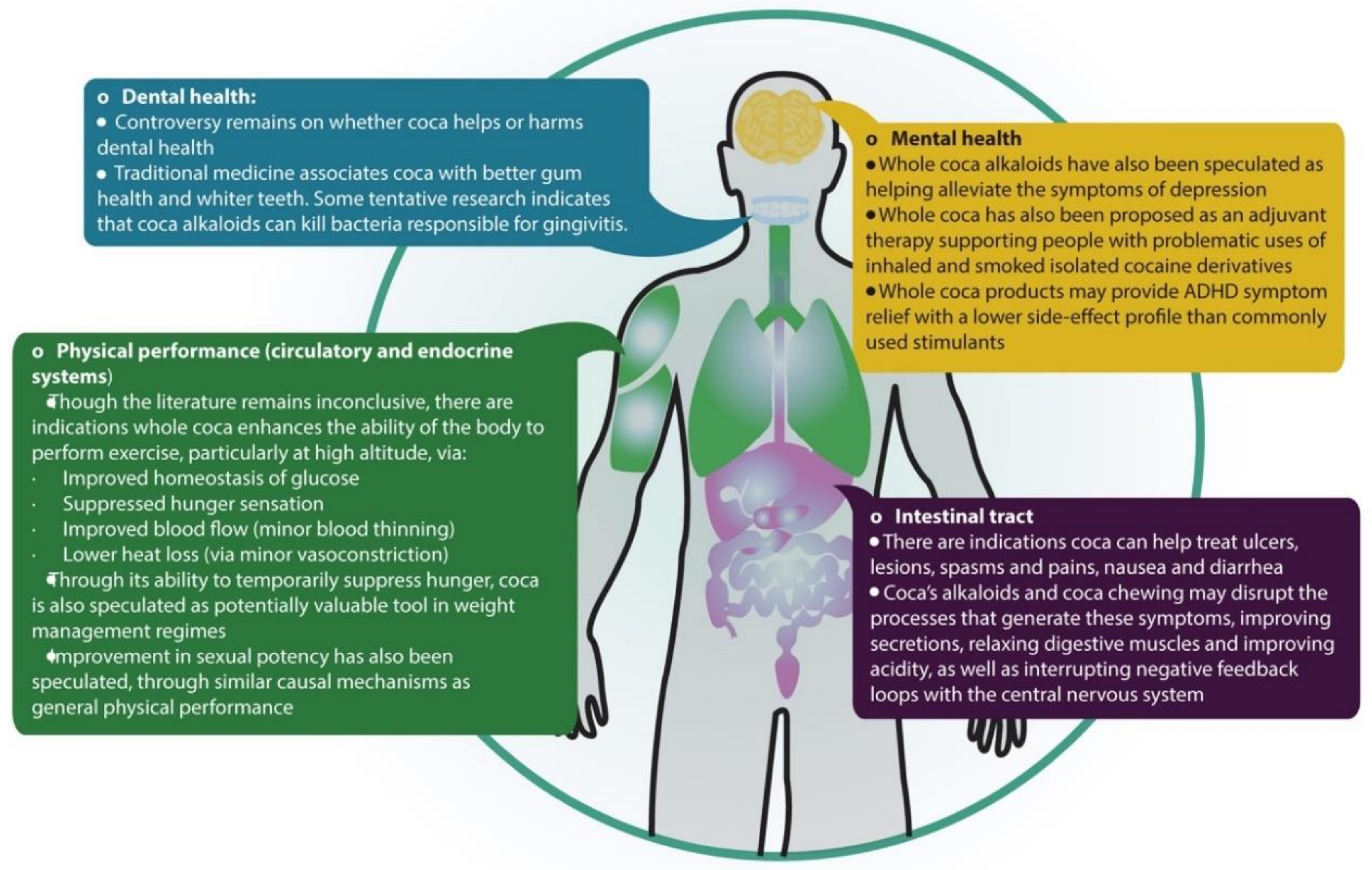
4.3. Potential Uses in Contemporary Medicine
4.3.1. Coca and Physical Performance: Metabolic and Cardiovascular Effects
4.3.2. Coca and Digestive and Oral Health
4.3.3. Sexual Impotence
4.3.4. Mental Health and Problematic Drug Use
4.4. Potential Uses in Nutrition
4.5. Potential Uses in Agriculture
4.6. Legality and Development of the Coca Industry in the Andean Region and Beyond
5. Inclusive and Equitable Research and Commercialization of Erythroxylum Species
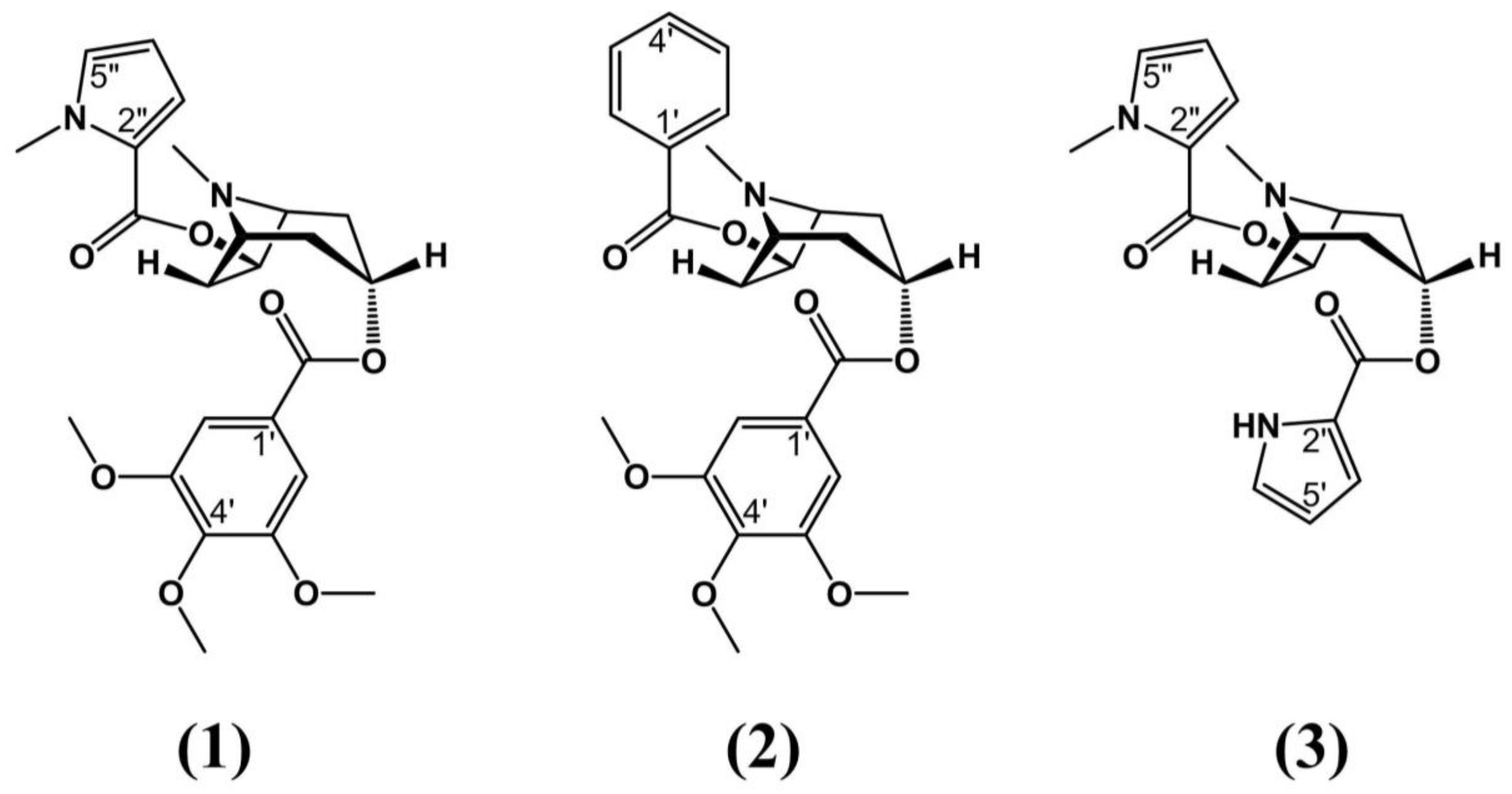
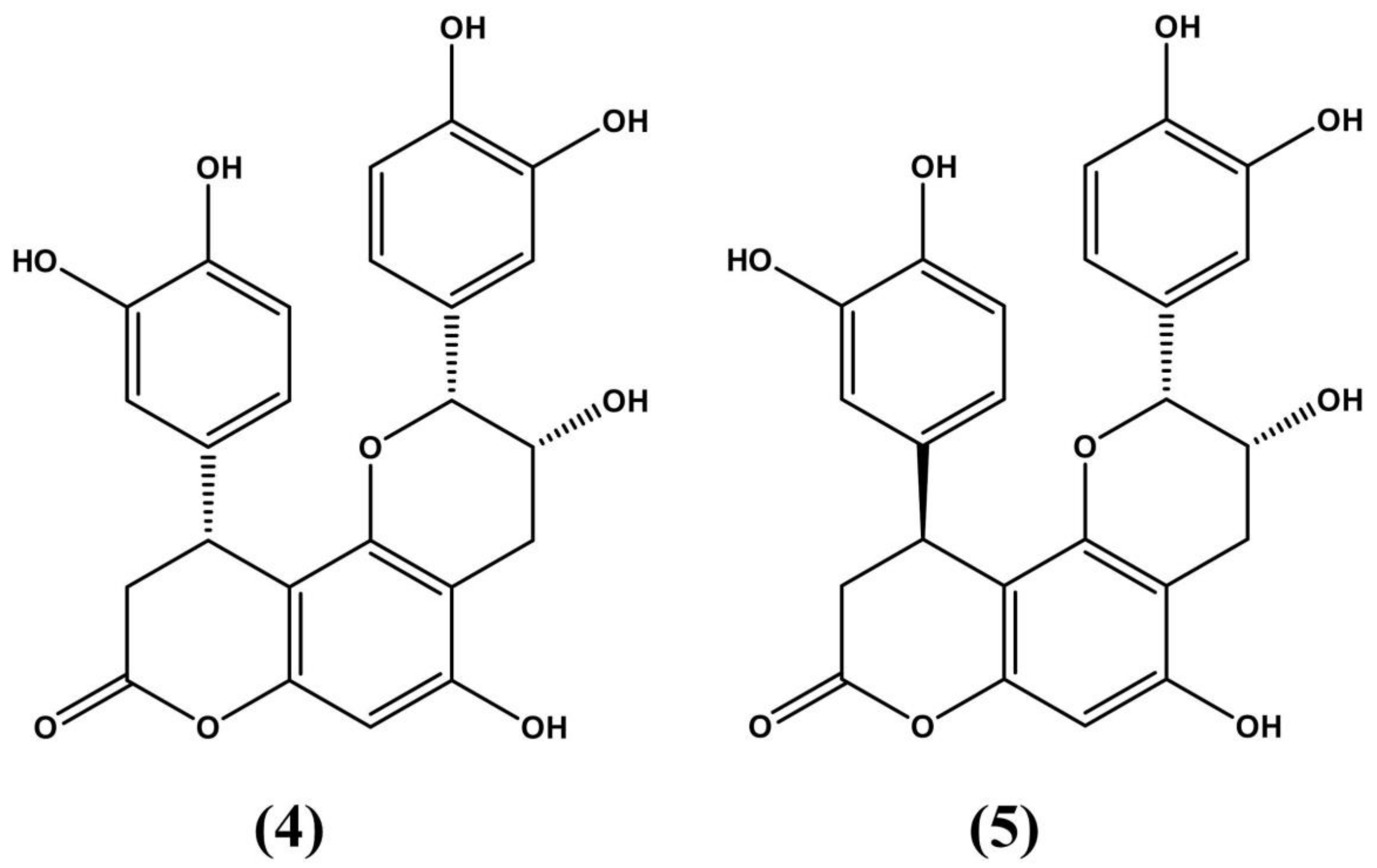
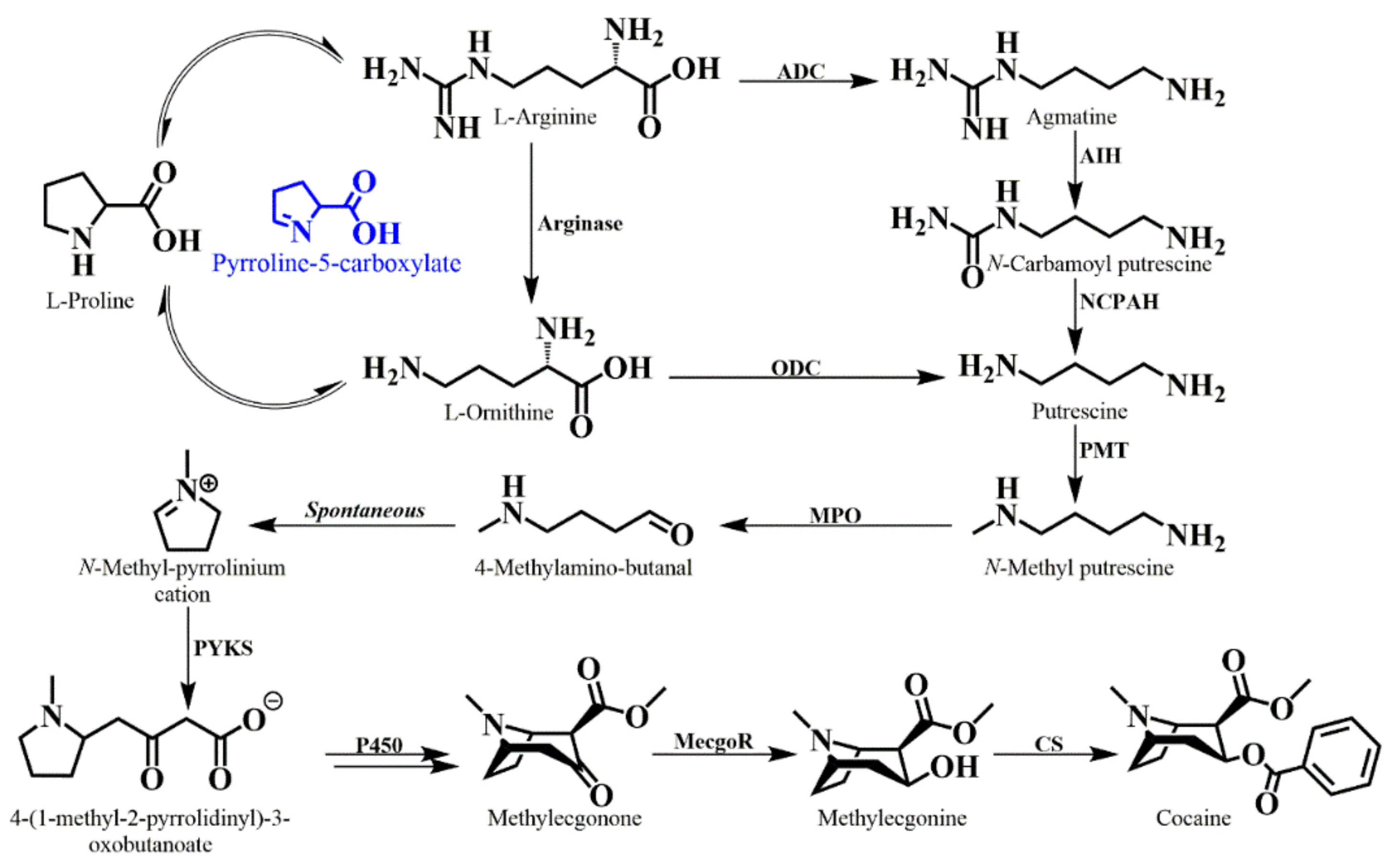
6. Tropane Alkaloid Biosynthesis in E. coca
7. Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mishra, B.B.; Tiwari, V.K. Natural Products: An Evolving Role In Future Drug Discovery. Eur. J. Med. Chem. 2011, 46, 4769–4807. [Google Scholar] [CrossRef] [PubMed]
- Veeresham, C. Natural Products Derived From Plants As A Source Of Drugs. J. Adv Pharm. Technol. Res. 2012, 3, 200–201. [Google Scholar] [CrossRef] [PubMed]
- Davalos, E. New Answers To An Old Problem: Social Investment And Coca Crops In Colombia. Int. J. Drug Policy. 2016, 31, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Metaal, P. Coca in Debate: The Contradiction and Conflict Between the UN Drug Conventions and the Real World. In Prohibition, Religious Freedom, and Human Rights: Regulating Traditional Drug Use; Labate, B.C., Cavnar, C., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; ISBN 978-3-642-40957-8. [Google Scholar]
- Grisaffi, T. Social Control in Bolivia: A Humane Alternative to the Forced Eradication of Coca Crops. In Drug Policies and the Politics of Drugs in the Americas; Springer: New York, NY, USA, 2016; pp. 149–166. [Google Scholar]
- Plowman, T.; Hensold, N. Names, Types, And Distribution Of Neotropical Species Of Erythroxylum (Erythroxylaceae). Brittonia 2004, 56, 1–53. [Google Scholar] [CrossRef]
- Plowman, T. Coca Chewing And The Botanical Origins Of Coca (Erythroxylum spp.). In Proceedings of the Coca and Cocaine: Effects on People and Policy in Latin America; Stark, J., Pacini, D., Franquemont, C., Eds.; Cultural Survival, Inc. and LASP (Cornell University): Cambridge, MA, USA, 1986; Volume 30, p. 235. [Google Scholar]
- Dillehay, T.D.; Rossen, J.; Ugent, D.; Karathanasis, A.; Vásquez, V.; Netherly, P.J. Early Holocene Coca Chewing In Northern Peru. Antiquity 2010, 84, 939–953. [Google Scholar] [CrossRef]
- Plowman, T. The Ethnobotany Of Coca (Erythroxylum spp., Erythroxylaceae). Repos. Inst. CEDRO 1984. [Google Scholar]
- Ministerio de Gobierno de Bolivia - Consejo Nacional de Lucha contra el Tráfico Ilícito de Drogas (CONALTID). Estudio integral de la demanda de la hoja de coca en Bolivia. In Proceedings of the Quincuagésimo Quinto Periodo Ordinario de Sesiones. Symposium conducted at the meeting of Comisión Interamericana para el Control del Abuso de Drogas (CICAD), Washington, DC, USA, 28 May 2014; Washington, DC, USA. [Google Scholar]
- Instituto Nacional de Estadística e Informática (INEI). Análisis de los Resultados de la Encuesta Hogares Sobre Demanda de la Hoja de Coca 2013; INEI: Lima, Peru, 2015. [Google Scholar]
- Garcia-Yi, J. Social Control And As Supply-Side Harm Reduction Strategies. The Case Of An Indigenous Community In Peru. Rev. Iberoam. Estud. Desarro. Iberoam. J. Dev. Stud. 2014, 3, 58–82. [Google Scholar] [CrossRef]
- Johnson, E.L. Alkaloid Content In Erythroxylum Coca Tissue During Reproductive Development. Phytochemistry 1996, 42, 35–38. [Google Scholar] [CrossRef]
- Plowman, T.; Rivier, L. Cocaine and Cinnamoylcocaine Content of Erythroxylum Species. Ann. Bot. 1983, 51, 641–659. [Google Scholar] [CrossRef]
- Acock, M.C.; Lydon, J.; Johnson, E.; Collins, R. Effects Of Temperature And Light Levels On Leaf Yield And Cocaine Content In Two Erythroxylum Species. Ann. Bot. 1996, 78, 49–53. [Google Scholar] [CrossRef]
- Henman, A. Mamacoca (Un Studio Complete De La Coca); Juan Gutenborg: Lima, Peru, 2005. [Google Scholar]
- Schultes, R. Coca In The Northwest Amazon. In Botanical Museum Leaflets; Harvard University Herbaria: Cambridge, MA, USA, 1980; Volume 28, pp. 47–60. [Google Scholar]
- Burchard, R. Chewing C: A New Perspective. Cannabis Cult. 1975, 463–484. [Google Scholar]
- Allen, C.J. To Be Quechua: The Symbolism Of Coca Chewing In Highland Peru. Am. Ethnol. 1981, 8, 157–171. [Google Scholar] [CrossRef]
- Echeverri, J.A.; Pereira, E. Amazônica. In O uso ritual das plantas de poder; Labate, B., Goulart, S., Eds.; Mercado de Letras/FAPESP: Campinas, Brasil, 2005; pp. 117–185. [Google Scholar]
- Allen, C.J. The Hold Life Has: Coca And Cultural Identity In An. Andean Community; Smithsonian Institution: Lima, Peru, 2002; ISBN 1-58834-359-6. [Google Scholar]
- Weil, A.T. The Therapeutic Value Of Coca In Contemporary Medicine. J. Ethnopharmacol. 1981, 3, 367–376. [Google Scholar] [CrossRef]
- The Angiosperm Phylogeny Group* An Update Of The Angiosperm Phylogeny Group Classification For The Orders And Families Of Flowering Plants: APG II. Bot. J. Linn. Soc. 2003, 141, 399–436. [CrossRef]
- Matthews, M.L.; Endress, P.K. Comparative Floral Structure And Systematics In Rhizophoraceae, Erythroxylaceae And The Potentially Related Ctenolophonaceae, Linaceae, Irvingiaceae And Caryocaraceae (Malpighiales). Bot. J. Linn. Soc. 2011, 166, 331–416. [Google Scholar] [CrossRef]
- Dahlgren, R.M.T. Rhizophoraceae and Anisophylleaceae: Summary Statement, Relationships. Ann. Mo. Bot. Gard. 1988, 75, 1259–1277. [Google Scholar] [CrossRef]
- Schwarzbach, A.E.; Ricklefs, R.E. Systematic Affinities Of Rhizophoraceae And Anisophylleaceae, And Intergeneric Relationships Within Rhizophoraceae, Based On Chloroplast DNA, Nuclear Ribosomal DNA, And Morphology. Am. J. Bot. 2000, 87, 547–564. [Google Scholar] [CrossRef]
- Islam, M. Tracing The Evolutionary History Of Coca (Erythroxylum). Ph.D. Thesis, University of Colorado, Boulder, CO, USA, 2011. [Google Scholar]
- White, D.M.; Islam, M.B.; Mason-Gamer, R.J. Phylogenetic Inference In Section Archerythroxylum Informs Taxonomy, Biogeography, And The Domestication Of Coca (Erythroxylum Species). Am. J. Bot. 2019, 106, 154–165. [Google Scholar] [CrossRef]
- Schulz, O.E. Erythroxylaceae; Engelmann: Wiesloch, Germany, 1907; Volume 29. [Google Scholar]
- Jara Muñoz, O.A. El Complejo Erythroxylum macrophyllum (Erythroxylaceae): Delimitación Taxonómica Y Posición Filogenética. Universidad Nacional de Colombia: Bogota, Colombia, 2011. [Google Scholar]
- Loiola, M.I.B. Revisão Taxonômica De Erythroxylum, P. Browne Sect. Rhabdophyllum, O.E. Schulz (Erythroxylaceae Kunth); Herbarium Senckenbergianum: Frankfurt am Main, Germany, 2006. [Google Scholar]
- Rury, P.M. Systematic Anatomy Of Erythroxylum, P. Browne: Practical And Evolutionary Implications For The Cultivated Cocas. J. Ethnopharmacol. 1981, 3, 229–263. [Google Scholar] [CrossRef]
- Bohm, B.A.; Ganders, F.R.; Plowman, T. Biosystematics and Evolution of Cultivated Coca (Erythroxylaceae). Syst. Bot. 1982, 7, 121–133. [Google Scholar] [CrossRef]
- Darwin, C. The Different Forms Of Flowers On Plants Of The Same Species; John Murray: London, UK, 1877. [Google Scholar]
- Ganders, F.R. Heterostyly in Erythroxylum coca (Erythroxylaceae). Bot. J. Linn. Soc. 1979, 78, 11–20. [Google Scholar] [CrossRef]
- Ruiz-zapata, T.; Kalin-Arroyo, M. Plant Reproductive Ecology of a Secondary Deciduous Tropical Forest in. Biotropica 1978, 10, 221–230. [Google Scholar] [CrossRef]
- Kalin-Arroyo, M.; Cabrera, E. Preliminary Self Incompatibility Tests For Some Tropical Cloud Forest Species In Venezuela. Incompat. Newsl. 1977, 8. [Google Scholar]
- Cuevas, E.; Molina-Freaner, F.; Eguiarte, L.E.; Domínguez, C.A. Patterns Of Male Sterility Within And Among Populations Of The Distylous Shrub Erythroxylum havanense (Erythroxylaceae). Plant. Ecol. 2005, 176, 165–172. [Google Scholar] [CrossRef]
- Dominguez, C.A.; Avila-Sakar, G.; Vázquez-Santana, S.; Márquez-GuzmáN, J. Morph-Biased Male Sterility In The Tropical Distylous Shrub Erythroxylum havanense (Erythroxylaceae). Am. J. Bot. 1997, 84, 626–632. [Google Scholar] [CrossRef] [PubMed]
- Rosas, F.; Domínguez, C.A. Male Sterility, Fitness Gain Curves And The Evolution Of Gender Specialization From Distyly In Erythroxylum havanense. J. Evol. Biol. 2009, 22, 50–59. [Google Scholar] [CrossRef]
- Berry, P.E.; Tober, H.; Gomez, J. Agamospermy And The Loss Of Distyly In Erythroxylum undulatum (Erythroxylaceae) From Northern Venezuela. Am. J. Bot. 1991, 78, 595–600. [Google Scholar] [CrossRef]
- Emche, S.D.; Zhang, D.; Islam, M.B.; Bailey, B.A.; Meinhardt, L.W. AFLP Phylogeny of 36 Erythroxylum Species. Trop. Plant. Biol. 2011, 4, 126–133. [Google Scholar] [CrossRef]
- Johnson, E.L.; Zhang, D.; Emche, S.D. Inter- and Intra-specific Variation among Five Erythroxylum Taxa Assessed by AFLP. Ann. Bot. 2005, 95, 601–608. [Google Scholar] [CrossRef]
- Plowman, T. The Identity Of Amazonian And Trujillo Coca. Bot. Mus. Leafl. Harv. Univ. 1979, 27, 45–68. [Google Scholar]
- Plowman, T. The Identification Of Coca (Erythroxylum Species): 1860–1910. Bot. J. Linn. Soc. 1982, 84, 329–353. [Google Scholar] [CrossRef]
- Johnson, E.L.; Schmidt, W.F.; Emche, S.D.; Mossoba, M.M.; Musser, S.M. Kaempferol (Rhamnosyl) Glucoside, A New Flavonol From Erythroxylum coca var. ipadu. Biochem. Syst. Ecol. 2003, 31, 59–67. [Google Scholar] [CrossRef]
- Johnson, E.L.; Emche, S.D. Variation of Alkaloid Content in Erythroxylum coca Leaves from Leaf Bud to Leaf Drop. Ann. Bot. 1994, 73, 645–650. [Google Scholar] [CrossRef]
- Johnson, E.L.; Schmidt, W.F.; Norman, H.A. Leaf Flavonoids as Chemotaxonomic Markers for Two Erythroxylum Taxa. Z. Für Naturforschung C 1997, 52, 577–585. [Google Scholar] [CrossRef]
- Johnson, E.L.; Schmidt, W.F.; Norman, H.A. Flavonoids As Markers For Erythroxylum Taxa: E. coca var. ipadu and E. novogranatense var. truxillense. Biochem. Syst. Ecol. 1998, 26, 743–759. [Google Scholar] [CrossRef]
- Docimo, T.; Reichelt, M.; Schneider, B.; Kai, M.; Kunert, G.; Gershenzon, J.; D’Auria, J.C. The First Step In The Biosynthesis Of Cocaine In Erythroxylum coca: The Characterization Of Arginine And Ornithine Decarboxylases. Plant. Mol. Biol. 2012, 78, 599–615. [Google Scholar] [CrossRef]
- Schmidt, G.W.; Jirschitzka, J.; Porta, T.; Reichelt, M.; Luck, K.; Torre, J.C.P.; Dolke, F.; Varesio, E.; Hopfgartner, G.; Gershenzon, J.; et al. The Last Step in Cocaine Biosynthesis Is Catalyzed by a BAHD Acyltransferase. Plant. Physiol. 2015, 167, 89–101. [Google Scholar] [CrossRef]
- Davey, J.W.; Blaxter, M.L. RADseq: Next-Generation Population Genetics. Brief. Funct. Genomics 2010, 9, 416–423. [Google Scholar] [CrossRef]
- Davey, J.W.; Hohenlohe, P.A.; Etter, P.D.; Boone, J.Q.; Catchen, J.M.; Blaxter, M.L. Genome-Wide Genetic Marker Discovery And Genotyping Using Next-Generation Sequencing. Nat. Rev. Genet. 2011, 12, 499–510. [Google Scholar] [CrossRef]
- Andrews, K.R.; Luikart, G. Recent Novel Approaches For Population Genomics Data Analysis. Mol. Ecol. 2014, 23, 1661–1667. [Google Scholar] [CrossRef]
- Van Bakel, H.; Stout, J.M.; Cote, A.G.; Tallon, C.M.; Sharpe, A.G.; Hughes, T.R.; Page, J.E. The Draft Genome And Transcriptome Of Cannabis sativa. Genome Biol. 2011, 12, R102. [Google Scholar] [CrossRef] [PubMed]
- Laverty, K.U.; Stout, J.M.; Sullivan, M.J.; Shah, H.; Gill, N.; Holbrook, L.; Deikus, G.; Sebra, R.; Hughes, T.R.; Page, J.E.; et al. A Physical And Genetic Map Of Cannabis sativa Identifies Extensive Rearrangements At The THC/CBD acid Synthase Loci. Genome Res. 2019, 29, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Zanolari, B.; Guilet, D.; Marston, A.; Queiroz, E.F.; de, Q.; Paulo, M.; Hostettmann, K. Tropane Alkaloids From The Bark Of Erythroxylum vacciniifolium. J. Nat. Prod. 2003, 66, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Graf, E.; Lude, W. Alkaloide aus Erythroxylum vacciniifolium Martius, 2. Mitt. Strukturaufklärung von Catuabin, A., B und C. Arch. Pharm. 1978, 311, 139–152. [Google Scholar] [CrossRef] [PubMed]
- Manabe, H.; Sakagami, H.; Ishizone, H.; Kusano, H.; Fujimaki, M.; Wada, C.; Komatsu, N.; Nakashima, H.; Murakami, T.; Yamamoto, N. Effects Of Catuaba Extracts On Microbial and HIV Infection. Vivo Athens Greece 1992, 6, 161–165. [Google Scholar]
- Satoh, M.; Satoh, Y.; Fujimoto, Y. Cytotoxic Constituents From Erythroxylum catuaba Isolation And Cytotoxic Activities Of Cinchonain. Nat. Med. 2000, 54, 97–100. [Google Scholar]
- Coriolano de Oliveira, E.; Alves Soares Cruz, R.; de Mello Amorim, N.; Guerra Santos, M.; Carlos Simas Pereira Junior, L.; Flores Sanchez, E.; Pinho Fernandes, C.; Garrett, R.; Machado Rocha, L.; Lopes Fuly, A. Protective Effect Of The Plant Extracts Of Erythroxylum Sp. Against Toxic Effects Induced By The Venom Of Lachesis Muta Snake. Molecules 2016, 21, 1350. [Google Scholar] [CrossRef]
- Chin, Y.-W.; Jones, W.P.; Waybright, T.J.; McCloud, T.G.; Rasoanaivo, P.; Cragg, G.M.; Cassady, J.M.; Kinghorn, A.D. Tropane Aromatic Ester Alkaloids From A Large-Scale Re-Collection Of Erythroxylum pervillei Stem Bark Obtained In Madagascar. J. Nat. Prod. 2006, 69, 414–417. [Google Scholar] [CrossRef]
- Aguiar, J.S.; Araújo, R.O.; do Desterro Rodrigues, M.; Sena, K.X.; Batista, A.M.; Guerra, M.M.; Oliveira, S.L.; Tavares, J.F.; Silva, M.S.; Nascimento, S.C. Antimicrobial, Antiproliferative And Proapoptotic Activities Of Extract, Fractions And Isolated Compounds From The Stem Of Erythroxylum caatingae Plowman. Int. J. Mol. Sci. 2012, 13, 4124–4140. [Google Scholar] [CrossRef]
- Silva, G.L.; Cui, B.; Chávez, D.; You, M.; Chai, H.-B.; Rasoanaivo, P.; Lynn, S.M.; O’Neill, M.J.; Lewis, J.A.; Besterman, J.M. Modulation of the Multidrug-Resistance Phenotype by New Tropane Alkaloid Aromatic Esters from Erythroxylum pervillei. J. Nat. Prod. 2001, 64, 1514–1520. [Google Scholar] [CrossRef]
- Mahomoodally, M.F.; Gurib-Fakim, A.; Subratty, A.H. Antimicrobial Activities And Phytochemical Profiles Of Endemic Medicinal Plants Of Mauritius. Pharm. Biol. 2005, 43, 237–242. [Google Scholar] [CrossRef]
- Mahomoodally, M.F.; Fakim, A.-G.; Subratty, A.H. Effects Of Erythroxylum macrocarpum (Erythroxylaceae), An Endemic Medicinal Plant Of Mauritius, On The Transport Of Monosaccharide, Amino Acid And Fluid Across Rat Everted Intestinal Sacs in vitro. J. Cell Mol. Biol. 2005, 4, 93–98. [Google Scholar]
- Al-said, M.S.; Evans, W.C.; Grout, R.J. Alkaloids of Erythroxylum macrocarpum and E. sideroxyloides. Phytochemistry 1986, 25, 851–853. [Google Scholar] [CrossRef]
- De Oliveira, S.L.; Tavares, J.F.; Branco, M.V.S.C.; Lucena, H.F.S.; Barbosa-Filho, J.M.; de Agra, M.F.; do Nascimento, S.C.; dos Aguiar, J.S.; da Silva, T.G.; de Simone, C.A.; et al. Tropane Alkaloids from Erythroxylum caatingae Plowman. Chem. Biodivers. 2011, 8, 155–165. [Google Scholar] [CrossRef]
- Ribeiro, G.; de Amorim, L.L.; Guimarães, S.S. Antioxidant Activity and Phytochemical Screening of Extracts of Erythroxylum suberosum A. St.-Hil (Erythroxylaceae). Res. J. Phytochem. 2015, 9, 68–78. [Google Scholar]
- De Barros, I.M.C.; Leite, B.H.; Leite, C.F.; Fagg, C.W.; Gomes, S.M.; Resck, I.S.; Fonseca-Bazzo, Y.M.; Magalhães, P.O.; Silveira, D. Chemical Composition And Antioxidant Activity Of Extracts From Erythroxylum suberosum A. St. Hil. leaves. J. Appl. Pharm. Sci. 2017, 7, 088–094. [Google Scholar]
- Macedo, T.B.; Elias, S.T.; Torres, H.M.; Yamamoto-Silva, F.P.; Silveira, D.; Magalhães, P.O.; Lofrano-Porto, A.; Guerra, E.N.; Silva, M.A.G. Cytotoxic Effect Of Erythroxylum suberosum combined With Radiotherapy In Head And Neck Cancer Cell Lines. Braz. Dent. J. 2016, 27, 108–112. [Google Scholar] [CrossRef]
- Picot, C.; Subratty, A.H.; Mahomoodally, M.F. Inhibitory Potential Of Five Traditionally Used Native Antidiabetic Medicinal Plants On A-Amylase, A-Glucosidase, Glucose Entrapment, And Amylolysis Kinetics in vitro. Adv. Pharmacol. Sci. 2014, 2014. [Google Scholar]
- Hansen, K.; Adsersen, A.; Smitt, U.W.; Nyman, U.; Christensen, S.B.; Schwartner, C.; Wagner, H. Angiotensin Converting Enzyme (ACE) Inhibitory Flavonoids From Erythroxylum laurifolium. Phytomedicine 1996, 2, 313–317. [Google Scholar] [CrossRef]
- Lohezic, F.; Amoros, M.; Boustie, J.; Girre, L. In-vitro Antiherpetic Activity of Erythroxylon laurifolium (Erythroxylaceae). Pharm. Pharmacol. Commun. 1999, 5, 249–253. [Google Scholar] [CrossRef]
- Jelager, L.; Gurib-Fakim, A.; Adsersen, A. Antibacterial And Antifungal Activity Of Medicinal Plants Of Mauritius. Pharm. Biol. 1998, 36, 153–161. [Google Scholar] [CrossRef]
- Gootenberg, P. Andean Cocaine: The Making of a Global Drug; Univ of North Carolina Press: Chapel Hill, NC, USA, 2008; ISBN 978-0-8078-3229-5. [Google Scholar]
- Muro, A.; Aguirre, P.; Parra, D.; Piza, M. Usos, Impactos y Derechos: Posibilidades, Políticas y Jurídicas para la Investigación de la Hoja de Coca en Colombia; Elementa: Bogota, DC, Colombia, 2018. [Google Scholar]
- Troyano, D.L.; Restrepo, D. Coca Industrialization: A Path to Innovation, Development and Peace in Colombia; Open Society Foundations: New York, NY, USA, 2018; ISBN 978-1-940983-80-6. [Google Scholar]
- Biondich, A.S.; Joslin, J.D. Coca: The History And Medical Significance Of An Ancient Andean Tradition. Emerg. Med. Int. 2016. [Google Scholar] [CrossRef] [PubMed]
- Rivier, L. Analysis Of Alkaloids In Leaves Of Cultivated Erythroxylum And Characterization Of Alkaline Substances Used During Coca Chewing. J. Ethnopharmacol. 1981, 3, 313–335. [Google Scholar] [CrossRef]
- Jenkins, A.J.; Llosa, T.; Montoya, I.; Cone, E.J. Identification And Quantitation Of Alkaloids In Coca Tea. Forensic Sci. Int. 1996, 77, 179–189. [Google Scholar] [CrossRef]
- Novák, M.; Salemink, C.A.; Khan, I. Biological activity Of The Alkaloids Of Erythroxylum coca and Erythroxylum novogranatense. J. Ethnopharmacol. 1984, 10, 261–274. [Google Scholar] [CrossRef]
- Rubio, N.C.; Hastedt, M.; Gonzalez, J.; Pragst, F. Possibilities For Discrimination Between Chewing Of Coca Leaves And Abuse Of Cocaine By Hair Analysis Including Hygrine, Cuscohygrine, Cinnamoylcocaine And Cocaine Metabolite/Cocaine Ratios. Int. J. Legal Med. 2015, 129, 69–84. [Google Scholar] [CrossRef]
- Nersesyan, A.; Kundi, M.; Krupitza, G.; Barcelos, G.; Mišík, M.; Wultsch, G.; Carrion, J.; Carrion-Carrera, G.; Knasmueller, S. Induction Of Nuclear Anomalies In Exfoliated Buccal Cells Of Coca Chewers: Results Of A Field Study. Arch. Toxicol. 2013, 87, 529–534. [Google Scholar] [CrossRef]
- WHO/UNICRI Cocaine Project. Available online: https://www.brucekalexander.com/articles-speeches/cocaine/188-whounicri-cocaine-project (accessed on 30 August 2019).
- WHO: “Six Horsemen ride out”. Available online: https://www.tni.org/es/node/17310 (accessed on 29 August 2019).
- Galarza, M.; Peñaloza, R.; Echalar, L.; Aguilar, M.; Souvain, M.; Spielvogel, H. Efectos Del Acullico De Coca En La Prueba De Tolerancia A La Glucosa. Biofarbo 1997, 57, 57–60. [Google Scholar]
- Brutsaert, T.; Milotich, M.; Frisancho, A.R.; Spielvogel, H. Coca Chewing Among High Altitude Natives: Work And Muscular Efficiencies Of Nonhabitual Chewers. Am. J. Hum. Biol. 1995, 7, 607–616. [Google Scholar] [CrossRef]
- Favier, R.; Caceres, E.; Guillon, L.; Sempore, B.; Sauvain, M.; Koubi, H.; Spielvogel, H. Coca Chewing For Exercise: Hormonal And Metabolic Responses Of Nonhabitual Chewers. J. Appl. Physiol. 1996, 81, 1901–1907. [Google Scholar] [CrossRef]
- Favier, R.; Caceres, E.; Sempore, B.; Cottet-Emard, J.M.; Gauquelin, G.; Gharib, C.; Spielvogel, H. Fluid Regulatory Hormone Response To Exercise After Coca-Induced Body Fluid Shifts. J. Appl. Physiol. 1997, 83, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Spielvogel, H.; Caceres, E.; Koubi, H.; Sempore, B.; Sauvain, M.; Favier, R. Effects Of Coca Chewing On Metabolic And Hormonal Changes During Graded Incremental Exercise To Maximum. J. Appl. Physiol. 1996, 80, 643–649. [Google Scholar] [CrossRef] [PubMed]
- Hurtado Sánchez, C.A.; Cartagena Triveño, D.; Erostegui Revilla, C.P. Evaluación De La Respuesta Glucemica Post-Ingesta De La Hoja De Coca (Erythroxylum Coca) En Personas Sin Antecedente Patológico Metabólico. Rev. Científica Cienc. Médica 2013, 16, 20–24. [Google Scholar]
- Casikar, V.; Mujica, E.; Mongelli, M.; Aliaga, J.; Lopez, N.; Smith, C.; Bartholomew, F. Does Chewing Coca Leaves Influence Physiology At High Altitude? Indian, J. Clin. Biochem. 2010, 25, 311–314. [Google Scholar] [CrossRef]
- Hanna, J.M. Responses Of Quechua Indians To Coca Ingestion During Cold Exposure. Am. J. Phys. Anthropol. 1971, 34, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Little, M.A. Effects Of Alcohol And Coca On Foot Temperature Responses Of Highland Peruvians During A Localized Cold Exposure. Am. J. Phys. Anthropol. 1970, 32, 233–242. [Google Scholar] [CrossRef]
- Rodríguez, A.; Guillon, L.; de Chavez, M. Uso De La Hoja De Coca Y Hematología; Instituto Boliviano de Biologia de Altura: La Paz, Bolivia, 1997. [Google Scholar]
- Fuchs, A.; Burchard, R.E.; Curtain, C.C.; De Azeredo, P.R.; Frisancho, A.R.; Gagliano, J.A.; Katz, S.H.; Little, M.A.; Mazess, R.B.; Picón-Reátegui, E.; et al. Coca Chewing and High-Altitude Stress: Possible Effects of Coca Alkaloids on Erythropoiesis. Curr. Anthropol. 1978, 19, 277–291. [Google Scholar] [CrossRef]
- Biondich, A.S.; Joslin, J.D. Coca: High Altitude Remedy Of The Ancient Incas. Wilderness Environ. Med. 2015, 26, 567–571. [Google Scholar] [CrossRef]
- Montesinos, F. Metabolism Of Cocaine. Bull. Narc. 1965, 17, 11–17. [Google Scholar]
- Langsjoen, O.M. Dental Effects Of Diet And Coca-Leaf Chewing On Two Prehistoric Cultures Of Northern Chile. Am. J. Phys. Anthropol. 1996, 101, 475–489. [Google Scholar] [CrossRef]
- Ramos, A.W. Actividad Antibacteriana Del Extracto De Erythroxylum coca sobre Porphyromonas Gingivalis, estudio in vitro. Undergraduate Thesis, Universidad Nacional Mayor de San Marcos, Lima, Peru, 2012. [Google Scholar]
- Seki, K.; Nishi, Y. Coca, un Biobanco: Investigación Científica Sobre Alimentación, Curación y Regeneración, 1st ed.; t’ika & teko: La Paz, Bolivia, 2012; ISBN 978-99954-824-8-0. [Google Scholar]
- Mantegazza, P. Sulle virtù igieniche e medicinali della coca e sugli alimenti nervosi in generale. Soc. Per Gli Annali Delle Scienze E Dell’Industria 1859. [Google Scholar]
- Carter, W.E.; Mamani, M. Coca en Bolivia; Librería Editorial “Juventud”: La Paz, Bolivia, 1986. [Google Scholar]
- Sharma, A.; Couture, J. A Review Of The Pathophysiology, Etiology, And Treatment Of Attention-Deficit Hyperactivity Disorder (ADHD). Ann. Pharmacother. 2014, 48, 209–225. [Google Scholar] [CrossRef] [PubMed]
- Storebø, O.J.; Ramstad, E.; Krogh, H.B.; Nilausen, T.D.; Skoog, M.; Holmskov, M.; Rosendal, S.; Groth, C.; Magnusson, F.L.; Moreira-Maia, C.R. Methylphenidate For Children And Adolescents With Attention Deficit Hyperactivity Disorder (ADHD). Cochrane Database Syst. Rev. 2015. [Google Scholar] [CrossRef] [PubMed]
- Gumucio, J.H. Cocaine, the Legend About Coca and Cocaine; Accion Andina, ICORI: La Paz, Bolivia, 1995. [Google Scholar]
- Collazos, C.; Urquieta, R.; Alvistur, E. Nutrición Y Coqueo. Rev. Viernes Méd. 1965, 16, 36–44. [Google Scholar]
- Duke, J.A.; Aulik, D.; Plowman, T. Nutritional Value Of Coca. Bot. Mus. Leafl. Harv. Univ. 1975, 24, 113–119. [Google Scholar]
- Olivier, J.; Symington, E.A.; Jonker, C.Z.; Rampedi, I.T.; van Eeden, T.S. Comparison Of The Mineral Composition Of Leaves And Infusions Of Traditional And Herbal Teas. South. Afr. J. Sci. 2012, 108, 01–07. [Google Scholar] [CrossRef]
- Penny, M.E.; Zavaleta, A.; Lemay, M.; Liria, M.R.; Huaylinas, M.L.; Alminger, M.; McChesney, J.; Alcaraz, F.; Reddy, M.B. Can Coca Leaves Contribute to Improving the Nutritional Status of the Andean Population? Food Nutr. Bull. 2009, 30, 205–216. [Google Scholar] [CrossRef]
- Hallberg, L.; Hulthén, L. Prediction Of Dietary Iron Absorption: An Algorithm For Calculating Absorption And Bioavailability Of Dietary Iron. Am. J. Clin. Nutr. 2000, 71, 1147–1160. [Google Scholar] [CrossRef]
- Del Anaya, M.S.; Troyano, D.L. Guía: Producción Tecnificada De Abonos Orgánicos, Sólidos Y Líquidos A Partir De La Hoja De Coca Para Fertilización De Cultivos Transitorios; Servicio Nacional de Aprendizaje: La Paz, Bolivia, 2017. [Google Scholar]
- Cordero, T.; TeÓfila, A. Evaluación nutricional de la proteína de la hoja de Coca (Erythroxylum coca Lamarck var. coca). Undergrad. Thesis, Chem. Dep. UNMSM, Lima, Peru, 2002. [Google Scholar]
- Nathanson, J.A.; Hunnicutt, E.J.; Kantham, L.; Scavone, C. Cocaine As A Naturally Occurring Insecticide. Proc. Natl. Acad. Sci. 1993, 90, 9645–9648. [Google Scholar] [CrossRef]
- Blanco, E.C.; González, J.C.M. El Uso De La Hoja De Coca Como Manifestación Cultural Inmaterial. Revistas 2014, 6, 11. [Google Scholar]
- Aguilar, A.; Chulver, P. Análisis Para La Factibilidad De Exportación De La Hoja De Coca; Fundación Acción Semilla: La Paz, Bolivia, 2019. [Google Scholar]
- Martínez, T.; Castro, E. ¿Es Eficaz La Erradicacion Forzosa De Cultivos De Coca? Centro de Estudios sobre Seguridad y Drogas: Bogota, DC, Colombia, 2018. [Google Scholar]
- Glave, M.; Rosemberg, C. La Comercialización De Hoja De Coca En El Perú; Análisis Del Mercado Formal (Grade): Lima, Peru, 2005. [Google Scholar]
- United Nations Office on Drugs and Crime (UNODC) Colombia. Monitoreo de Territorios Afectados por Cultivos Ilícitos 2018; UNODC: Bogota, DC, Colombia, 2019. [Google Scholar]
- United Nations Office on Drugs and Crime (UNODC) Perú. Monitoreo de Cultivos de Coca, 2013; UNODC: Lima, Peru, 2014. [Google Scholar]
- United Nations Office on Drugs and Crime (UNODC) Estado Plurinacional de Bolivia. Monitoreo de Cultivos de Coca 2018; UNODC: La Paz, Bolivia, 2019. [Google Scholar]
- Channing, G. May Transnational Crime and the Developing World; Global Financial Integrity: Washington, DC, USA, 2017. [Google Scholar]
- Ruiz, M. Las Relaciones entre las Herramientas de la Propiedad Intelectual, los Conocimientos Tradicionales y Recursos Genéticos, en el Contexto de la Aplicación del Protocolo de Nagoya: Alcances y Aproximaciones; Deutsche Gesellschaft für Internationale Zusammenarbeit (GIZ) GmbH: San Salvador, El Salvador, 2016. [Google Scholar]
- Niemann, A. Ueber eine neue organische Base in den Cocablättern. Arch. Pharm. 1860, 153, 291–308. [Google Scholar] [CrossRef]
- Humphrey, A.J.; O’Hagan, D. Tropane alkaloid biosynthesis. A century old problem unresolved. Nat. Prod. Rep. 2001, 18, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Jirschitzka, J.; Mattern, D.J.; Gershenzon, J.; D’Auria, J.C. Learning From Nature: New Approaches To The Metabolic Engineering Of Plant Defense Pathways. Curr. Opin. Biotechnol. 2013, 24, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, T.; Yukimune, Y.; Yamada, Y. Putrescine and putrescine N-methyltransferase In The Biosynthesis Of Tropane Alkaloids In Cultured Roots Of Hyoscyamus albus.2. Incorporation of labeled precursors. Planta 1989, 178, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Leete, E. Stereospecific Incorporation Of Ornithine Into Tropine Moiety Of Hyoscyamine. J. Am. Chem. Soc. 1962, 84, 55–57. [Google Scholar] [CrossRef]
- Leete, E. Biosynthesis Of Hyoscyamine: Proof That Ornithine-2-C14 Yields Tropine Labelled at C-1. Tetrahedron Lett. 1964, 1619–1622. [Google Scholar] [CrossRef]
- Leete, E. Biosynthesis Of The Pyrrolidine Rings Of Cocaine And Cuscohygrine From [5-14C]-Labeled Ornithine Via A Symmetrical Intermediate. J. Am. Chem. Soc. 1982, 104, 1403–1408. [Google Scholar] [CrossRef]
- Liebisch, H.W.; Ramin, H.; Schoffin, I.; Schütte, H.R. Zur Biosynthese der Tropanalkaloide. Z. Naturforschung Part. B-Chem. Biochem. Biophys. Biol. Verwandten Geb. 1965, 20, 1183–1185. [Google Scholar]
- Alcazar, R.; Altabella, T.; Marco, F.; Bortolotti, C.; Reymond, M.; Koncz, C.; Carrasco, P.; Tiburcio, A.F. Polyamines: Molecules With Regulatory Functions In Plant Abiotic Stress Tolerance. Planta 2010, 231, 1237–1249. [Google Scholar] [CrossRef]
- Hoye, T.R.; Bjorklund, J.A.; Koltun, D.O.; Renner, M.K. Methylputrescine Oxidation During Cocaine Biosynthesis: Study Of Prochiral Methylene Hydrogen Discrimination Using The Remote Isotope Method. Org. Lett. 2000, 2, 3–5. [Google Scholar] [CrossRef]
- Wigle, I.D.; Mestichelli, L.J.J.; Spenser, I.D. 2H NMR-spectroscopy as A Probe Of The Stereochemistry Of Biosynthetic Reactions - The Biosynthesis Of Nicotine. J. Chem. Soc. Chem. Commun. 1982, 662–664. [Google Scholar] [CrossRef]
- Hibi, N.; Higashiguchi, S.; Hashimoto, T.; Yamada, Y. Gene Expression In Tobacco Low-Nicotine Mutants. Plant. Cell 1994, 6, 723–735. [Google Scholar] [PubMed]
- Kai, G.; Zhang, Y.; Chen, J.; Li, L.; Yan, X.; Zhang, R.; Liao, P.; Lu, X.; Wang, W.; Zhou, G. Molecular Characterization And Expression Analysis Of Two Distinct Putrescine N-Methyltransferases From Roots Of Anisodus acutangulus. Physiol. Plant. 2009, 135, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Teuber, M.; Azemi, M.E.; Namjoyan, F.; Meier, A.C.; Wodak, A.; Brandt, W.; Drager, B. Putrescine N-methyltransferases--A Structure-Function Analysis. Plant. Mol. Biol. 2007, 63, 787–801. [Google Scholar] [CrossRef]
- Leete, E. Spermidine: An Indirect Precursor Of The Pyrrolidine Rings Of Nicotine And Nornicotine in Nicotiana glutinosa. Phytochemistry 1985, 24, 957–960. [Google Scholar] [CrossRef]
- Leete, E. Recent Developments In The Biosynthesis Of The Tropane Alkaloids. Planta Med. 1990, 56, 339–352. [Google Scholar] [CrossRef]
- Leete, E.; Kim, S.H.; Rana, J. Chemistry Of The Tropane Alkaloids And Related-Compounds.38. The Incorporation Of [2-13C,14C,15N]-1-Methyl-D1-Pyrrolinium Chloride Into Cuscohygrine in Erythroxylum coca. Phytochemistry 1988, 27, 401–406. [Google Scholar] [CrossRef]
- Abraham, T.W.; Leete, E. New Intermediate In The Biosynthesis Of The Tropane Alkaloids in Datura innoxia. J. Am. Chem. Soc. 1995, 117, 8100–8105. [Google Scholar] [CrossRef]
- Kaczkowski, J.; Schütte, H.R.; Mothes, K. Die Rolle des Acetats in der Biosynthese der Tropanalkaloide. Biochim. Biophys. Acta 1961, 46, 588–594. [Google Scholar] [CrossRef]
- Liebisch, H.W.; Peisker, K.; Radwan, A.S.; Schütte, H.R. Zur Biosynthese der Tropanalkaloide.XI. Die Bildung der C3-Brücke des Tropins. Z. Pflanzenphysiol. 1972, 67, 1–9. [Google Scholar] [CrossRef]
- Robins, R.J.; Abraham, T.W.; Parr, A.J.; Eagles, J.; Walton, N.J. The Biosynthesis Of Tropane Alkaloids In Datura stramonium: The Identity Of The Intermediates Between N-Methylpyrrolinium Salt And Tropinone. J. Am. Chem. Soc. 1997, 119, 10929–10934. [Google Scholar] [CrossRef]
- Leete, E.; Bjorklund, J.A.; Couladis, M.M.; Kim, S.H. Late Intermediates In The Biosynthesis Of Cocaine: 4-(1-Methyl-2-Pyrrolidinyl)-3-Oxobutanoate And Methylecgonine. J. Am. Chem. Soc. 1991, 113, 9286–9292. [Google Scholar] [CrossRef]
- Heim, W.G.; Sykes, K.A.; Hildreth, S.B.; Sun, J.; Lu, R.H.; Jelesko, J.G. Cloning And Characterization Of A Nicotiana tabacum Methylputrescine Oxidase Transcript. Phytochemistry 2007, 68, 454–463. [Google Scholar] [CrossRef] [PubMed]
- Katoh, A.; Shoji, T.; Hashimoto, T. Molecular Cloning Of N-Methylputrescine Oxidase From Tobacco. Plant. Cell Physiol. 2007, 48, 550–554. [Google Scholar] [CrossRef]
- Bedewitz, M.A.; Jones, A.D.; D’Auria, J.C.; Barry, C.S. Tropinone Synthesis Via An Atypical Polyketide Synthase And P450-Mediated Cyclization. Nat. Commun. 2018, 9. [Google Scholar] [CrossRef]
- Novák, M.; Salemink, C.A. The Essential Oil of Erythroxylum coca. Planta Med. 1987, 53, 113. [Google Scholar] [CrossRef]
- Jirschitzka, J.; Schmidt, G.W.; Reichelt, M.; Schneider, B.; Gershenzon, J.; D’Auria, J.C. Plant Tropane Alkaloid Biosynthesis Evolved Independently In The Solanaceae and Erythroxylaceae. Proc. Natl. Acad. Sci. 2012, 109, 10304–10309. [Google Scholar] [CrossRef]
- Casale, J.F.; Moore, J.M. Lesser Alkaloids Of Cocaine-Bearing Plants.2. 3-Oxo-Substituted Tropane Esters: Detection And Mass Spectral Characterization Of Minor Alkaloids Found In South American Erythroxylum coca var coca. J. Chromatogr. A 1996, 749, 173–180. [Google Scholar] [CrossRef]
- Casale, J.F.; Moore, J.M. Lesser Alkaloids Of Cocaine-Bearing Plants.3. 2-Carbomethoxy-3-Oxo Substituted Tropane Esters: Detection And Gas Chromatographic Mass Spectrometric Characterization Of New Minor Alkaloids Found In South American Erythroxylum coca var coca. J. Chromatogr. A 1996, 756, 185–192. [Google Scholar] [CrossRef]
- Casale, J.F.; Toske, S.G.; Colley, V.L. Alkaloid Content Of The Seeds From Erythroxylum coca var. coca. J. Forensic Sci. 2005, 50, 1402–1406. [Google Scholar]
- Kim, N.; Estrada, O.; Chavez, B.; Stewart, C.; D’Auria, J.C. Tropane and Granatane Alkaloid Biosynthesis: A Systematic Analysis. Molecules 2016, 21. [Google Scholar] [CrossRef] [PubMed]
- Torre, J.C.P.; Schmidt, G.W.; Paetz, C.; Reichelt, M.; Schneider, B.; Gershenzon, J.; D’Auria, J.C. The Biosynthesis Of Hydroxycinnamoyl Quinate Esters And Their Role In The Storage Of Cocaine In Erythroxylum coca. Phytochemistry 2013, 91, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Efferth, T. Biotechnology Applications of Plant Callus Cultures. Engineering 2019, 5, 50–59. [Google Scholar] [CrossRef]
- Docimo, T.; Davis, A.J.; Luck, K.; Fellenberg, C.; Reichelt, M.; Phillips, M.; Gershenzon, J.; D’Auria, J.C. Influence Of Medium And Elicitors On The Production Of Cocaine, Amino Acids And Phytohormones By Erythroxylum coca calli. Plant. Cell Tissue Organ. Cult. 2015, 120, 1061–1075. [Google Scholar] [CrossRef]
- Ping, Y.; Li, X.D.; Xu, B.F.; Wei, W.; Wei, W.P.; Kai, G.Y.; Zhou, Z.H.; Xiao, Y.L. Building Microbial Hosts for Heterologous Production of N-Methylpyrrolinium. Acs Synth. Biol. 2019, 8, 257–263. [Google Scholar] [CrossRef]
- Srinivasan, P.; Smolke, C.D. Engineering A Microbial Biosynthesis Platform For De Novo Production Of Tropane Alkaloids. Nat. Commun. 2019, 10. [Google Scholar] [CrossRef]
- Ping, Y.; Li, X.D.; You, W.J.; Li, G.Q.; Yang, M.Q.; Wei, W.P.; Zhou, Z.H.; Xiao, Y.L. De Novo Production of the Plant-Derived Tropine and Pseudotropine in Yeast. Acs Synth. Biol. 2019, 8, 1257–1262. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
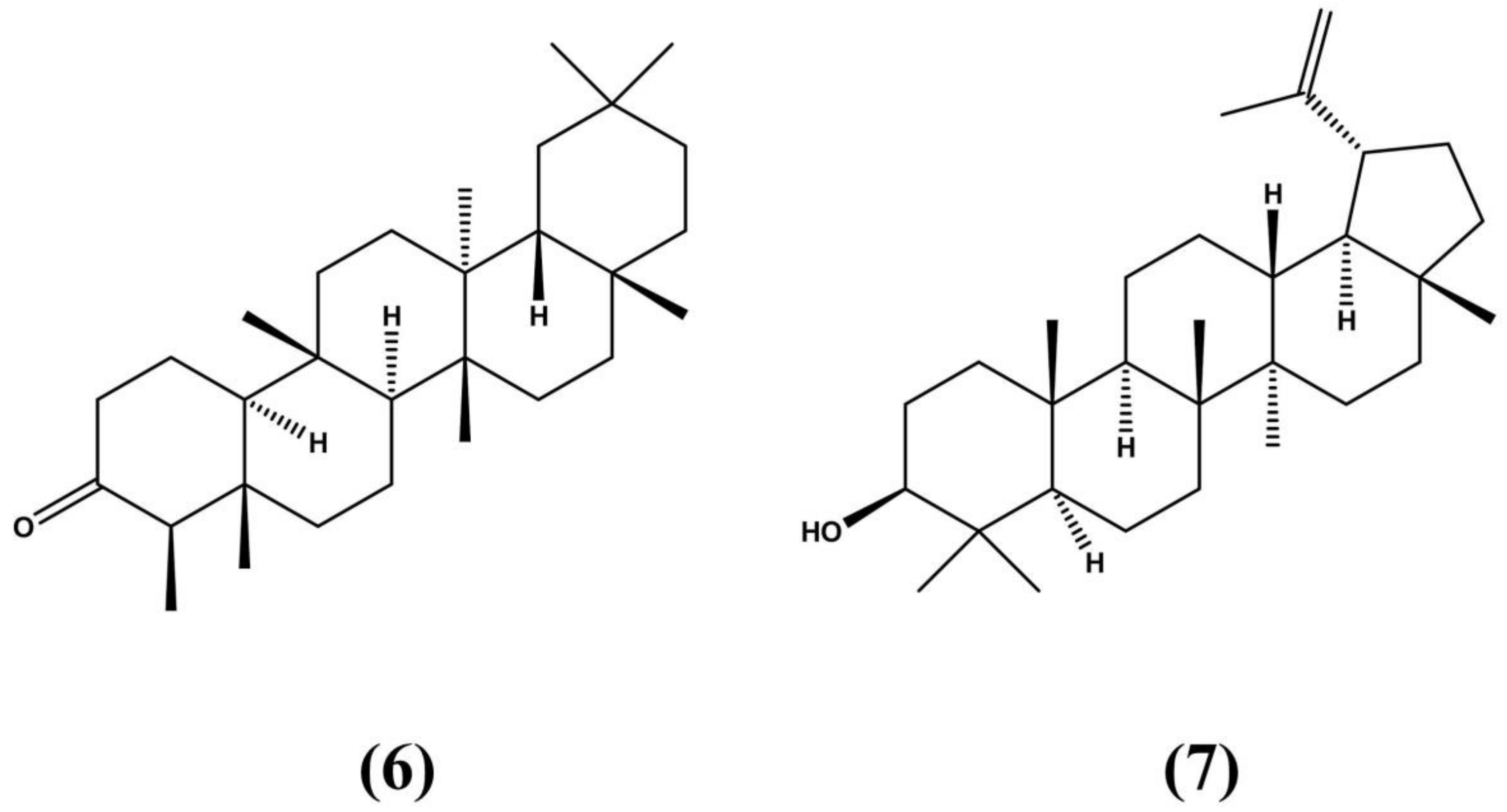
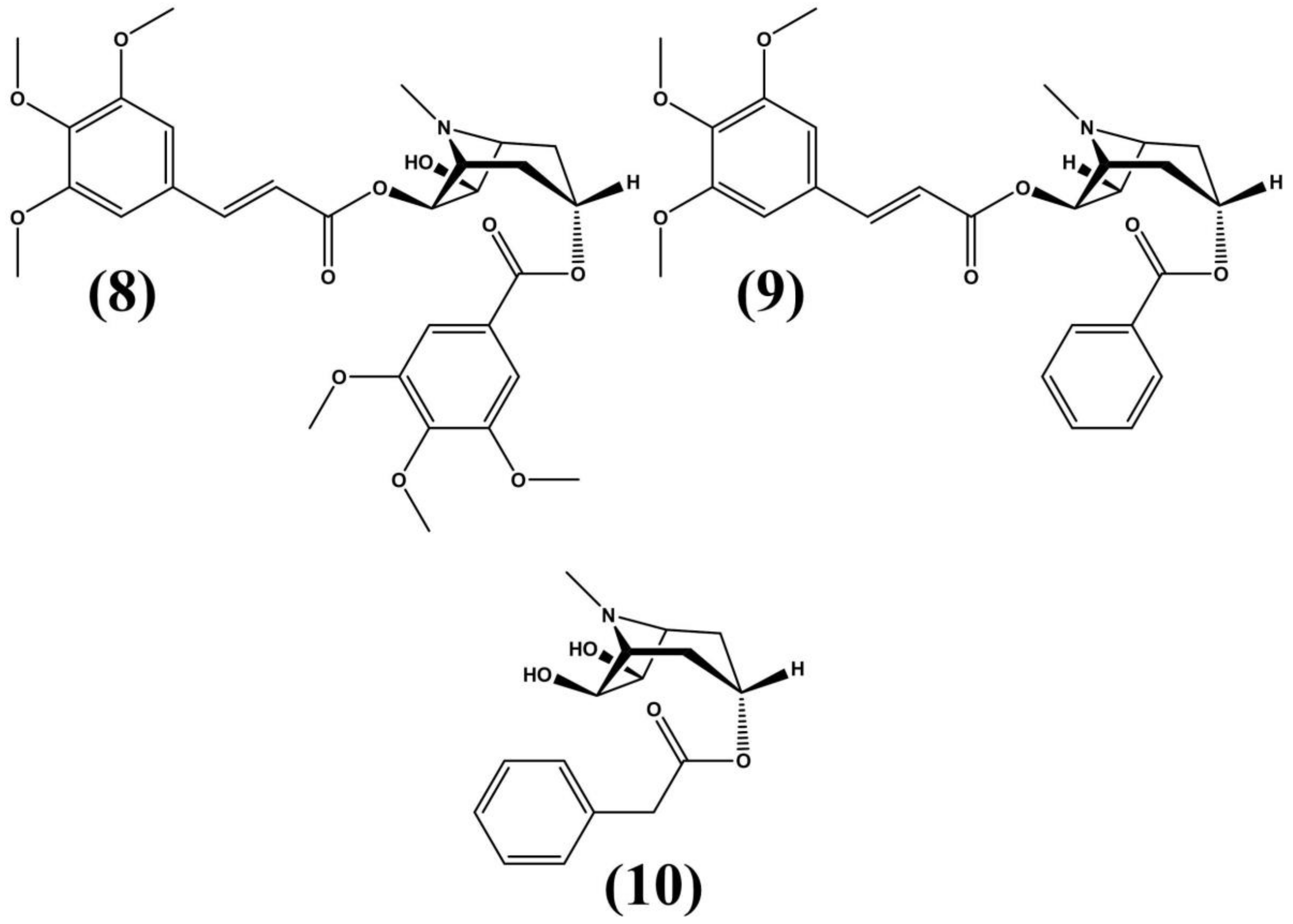
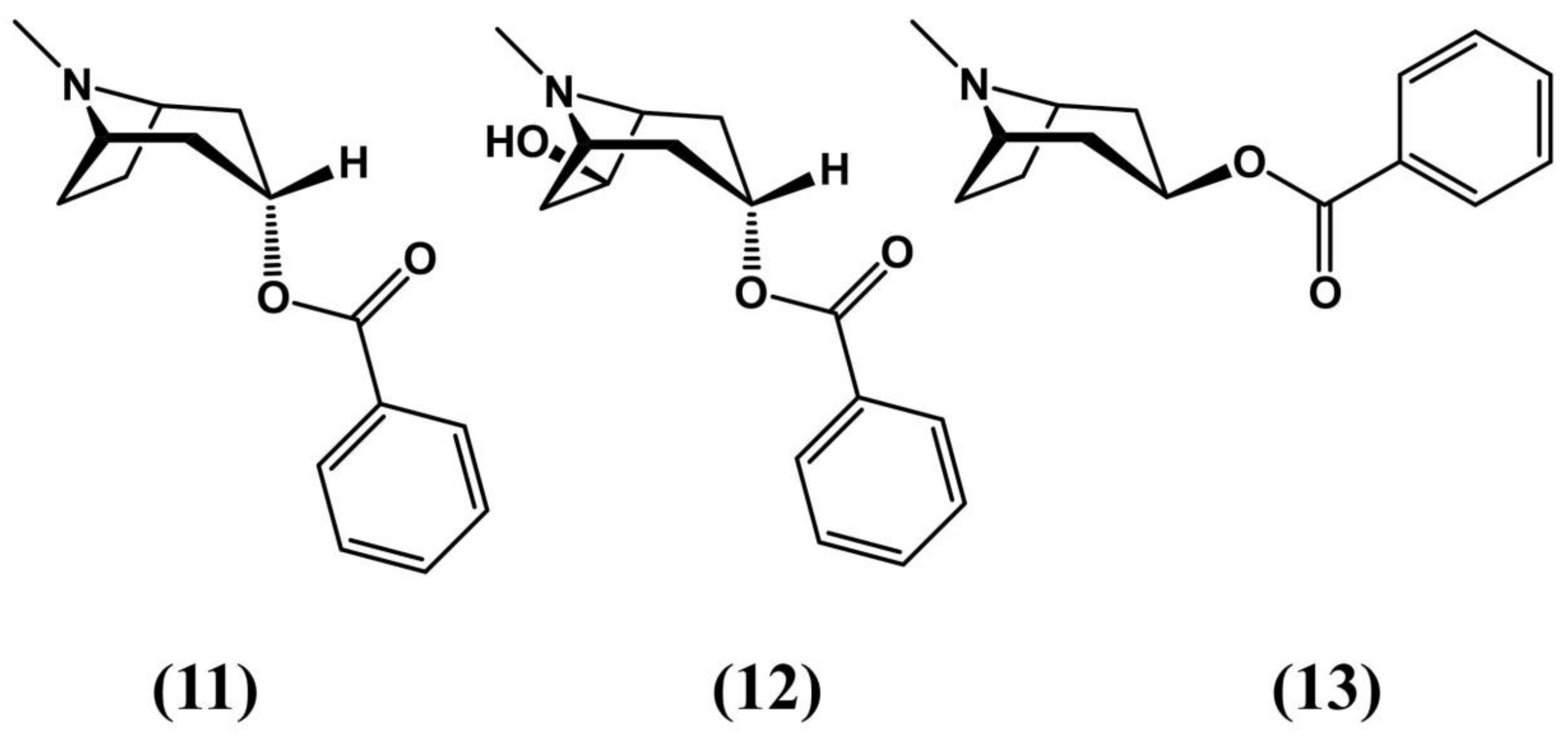
| Species | Distribution | Type of Study | Bioactive Properties | Extract Source | Active Compounds | References |
|---|---|---|---|---|---|---|
| Erythroxylum vacciniifolium Mart. | Brazilian northeast, Atlantic Forest | Pre-clinical testing: Lymphotropic virus type I (HTLV-1) positive MT-4 cells and mice | - Aphrodisiac - Tonic - Antimicrobial - Anticancer | Stem bark | C-3 α ester; C-3 3,4,5 trimethoxybenzoic acid; pyrrole-2-carboxylic acid; cinchonains 1a and 1b | Zanolari et al. (2003) Graf et al. (1978) Manabe et al. (1992) Satoh et al. (2000) |
| Erythroxylum ovalifolium Peyr. | Restinga (sandbanks) in the state of Rio de Janeiro (Brazil) | Pre-clinical testing: Swiss mice | - Neutralize toxicity of snake venom - Treat edemas and hemorrhages - Anti-fungal | Stem bark | Friedelin and Lupeol | Coriolano de Olivero et al. (2016) |
| Erythroxylum pervillei Baill. | Endemic to Madagascar | Testing: Human ovarian adenocarcinoma (SKVLB) cells and multidrug-resistance oral epidermoid carcinoma (KB-V1) cells | - Anticancer - Treat abdominal pain | Stem bark and roots | - Previlleine A, G and H - Aromtic sters | Chin et al. (2006) Silva et al. (2001) |
| Erythroxylum macrocarpum O.E. Schulz | Endemic to Mauritius | Pre-clinical testing: Swis albino rats | - Antibacterial - Diuretic | Leaves and twigs | - Tannins - Flavonoids - Tropan-3α-ol - tropan-3β-ol - 6β-diol | Mahomoodally et al. (2005) Al-said et al. (1986) |
| Erythroxylum caatingae Plowman | Dry forest in northeastern Brazil known as Caatinga | Pre-clinical testing: Swiss mice and human cancer cells from leukemia (K562), lung (NCI-H292) and larynx (Hep-2) | - Anticancer - Antimicrobial | Stem | - 6β-Benzoyloxy-3α-(3,4,5-trimethoxybenzoyloxy) | Aguiar et al. (2012) |
| Erythroxylum suberosum A.St.-Hill., A.Juss & Cambess. | Savannahs in Brazil, Bolivia, Paraguay, Venezuela and the Guyanas. | Testing: Human cancer cells of oral squamous carcinoma (SCC-9), hypopharynx squamous carcinoma (FaDu) and human keratinocyte (HaCaT) | - Antidiarrhea - Astringent - Antirheumatoid - Anesthetic - Antioxidant | Leaves | - Coumarins - Flavonoids - Isoquercitrin - Catechin | Riberio et al. (2015) Barros et al. (2017) Macedo et al. (2016) |
| Erythroxylum laurifolium Lam. | Endemic to Mauritius | Testing: Kidney epithelial cells (VERO) | - Anti-diabetic - Anti-hypertension - Effects against Herpes I virus | Leaves | - Afzelin - Quercitrin - Tannins - Flavonoids | Picot et al. (2014) Hansen et al. (1996) Lohezic et al. (1999) Jelager et al. (1998) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Restrepo, D.A.; Saenz, E.; Jara-Muñoz, O.A.; Calixto-Botía, I.F.; Rodríguez-Suárez, S.; Zuleta, P.; Chavez, B.G.; Sanchez, J.A.; D’Auria, J.C. Erythroxylum in Focus: An Interdisciplinary Review of an Overlooked Genus. Molecules 2019, 24, 3788. https://doi.org/10.3390/molecules24203788
Restrepo DA, Saenz E, Jara-Muñoz OA, Calixto-Botía IF, Rodríguez-Suárez S, Zuleta P, Chavez BG, Sanchez JA, D’Auria JC. Erythroxylum in Focus: An Interdisciplinary Review of an Overlooked Genus. Molecules. 2019; 24(20):3788. https://doi.org/10.3390/molecules24203788
Chicago/Turabian StyleRestrepo, David A., Ernesto Saenz, Orlando Adolfo Jara-Muñoz, Iván F. Calixto-Botía, Sioly Rodríguez-Suárez, Pablo Zuleta, Benjamin G. Chavez, Juan A. Sanchez, and John C. D’Auria. 2019. "Erythroxylum in Focus: An Interdisciplinary Review of an Overlooked Genus" Molecules 24, no. 20: 3788. https://doi.org/10.3390/molecules24203788
APA StyleRestrepo, D. A., Saenz, E., Jara-Muñoz, O. A., Calixto-Botía, I. F., Rodríguez-Suárez, S., Zuleta, P., Chavez, B. G., Sanchez, J. A., & D’Auria, J. C. (2019). Erythroxylum in Focus: An Interdisciplinary Review of an Overlooked Genus. Molecules, 24(20), 3788. https://doi.org/10.3390/molecules24203788