Abstract
Electrochemical anodized titanium dioxide (TiO2) nanotubes are of immense significance as electrochemical energy storage devices owing to their fast electron transfer by reducing the diffusion path and paving way to fabricating binder-free and carbon-free electrodes. Besides these advantages, when nitrogen is doped into its lattice, doubles its electrochemical activity due to enhanced charge transfer induced by oxygen vacancy. Herein, we synthesized nitrogen-doped TiO2 (N-TiO2) and studied its electrochemical performances in supercapacitor and as anode for a lithium-ion battery (LIB). Nitrogen doping into TiO2 was confirmed by Raman spectroscopy and X-ray photoelectron spectroscopy (XPS) techniques. The electrochemical performance of N-TiO2 nanotubes was outstanding with a specific capacitance of 835 µF cm−2 at 100 mV s−1 scan rate as a supercapacitor electrode, and it delivered an areal discharge capacity of 975 µA h cm−2 as an anode material for LIB which is far superior to bare TiO2 nanotubes (505 µF cm−2 and 86 µA h cm−2, respectively). This tailor-made nitrogen-doped nanostructured electrode offers great promise as next-generation energy storage electrode material.
1. Introduction
Lithium-ion batteries (LIBs) and supercapacitors are the best known electrochemical energy storage (EES) devices for their high energy density (kW h kg−1) and power density (kW h−1), respectively. LIBs have found applications in our day-to-day electronic devices while supercapacitor-based trams and buses are being tested in a few countries [1]. The existing research progress on the materials chemistry of LIBs and supercapacitors are in focus to replace the fossil fuel-based internal combustion (IC) engine with plug-in/hybrid electric vehicles. Another important prospect is to store and provide electricity when it is needed inorder to minimize the transmission loss and maximize power utilizations. LIBs possess low self-discharge, high gravimetric and volumetric density (W h L−1), while a supercapacitor exhibits high charge-discharge rate, power density, and long cyclic performance of over 10,000 cycles [1,2,3,4,5].
TiO2 is considered as an alternative anode material that could potentially substitute commercialized graphite. Some of its merits are: higher Li insertion potential (~1.7 V vs. Li+/Li0), prohibiting lithium plating/dendrite growth (proven safety concern), fast lithium insertion/extraction, low volume change, being environmentally friendly, chemically stable, and having a low cost [6,7,8]. Additionally, it offers theoretical capacity of 332 mA h g−1 [9], but due to its poor electronic conductivity (~1 × 10−12 S cm−1 to 1 × 10−7 S cm−1) and low Li-ion diffusion (~1 × 10−15 cm2 s−1 to 1 × 10−9 cm2 s−1), the ability of TiO2 has affected the lithium-ion storage capacity, which possibly limits its practical use. Therefore, there is a need to develop nanostructures of TiO2 with short diffusion length for electronic and Li+ transport, increasing the contact area between electrode and electrolyte, and better accommodation of the strain during Li insertion/extraction [10,11]. TiO2 nanotubes achieved the above properties, especially, when directly grown from Ti metal foil by the electrochemical anodization technique and, furthermore, displayed an additional advantage such as additive free electrode [12]. However, recent research is driven to tailor the properties of TiO2 nanotubes to improve the electronic conductivity and ionic diffusion by making composites with carbon materials [13], metal oxides [14], and doping with nitrogen [15] to increase its electrochemical activity.
Depending upon experimental conditions, nitrogen doping in TiO2 (N-TiO2) phases leads to different forms such as nitrogen substitution to oxygen atoms, or interstitial NO2-, or surface adsorbed N2, etc [16]. Some studies found that ammonia-treated TiO2 exhibits the occurrence of interstitial atoms and it form N-Ti-O [17]. Therefore, nitrogen substitution in TiO2 causes a reduction and as a result Ti3+ and oxygen vacancy are formed. This formation creates additional electrons in the structure and leads to the increase of electronic conductivity [18]. Recently, more studies were carried out on nitrogen-doped 1-D TiO2 nanostructures in wide range of potential applications especially in energy storages devices. Hyungku Han et al. [19], reported the performance of LIB with nitrated TiO2 hollow nanofibers, which had been prepared by the electrospinning method and demonstrated an excellent improvement in the rate capability and exhibited discharge capacity of 85 mA h g−1, which was nearly twice as that of 45 mA h g−1 (bare TiO2). Hydrothermally prepared N-TiO2 nanotubes/graphene composites presented the discharge capacity of 369 mA h g−1 at 0.1 Ag−1 as better performance in LIBs [20]. N-TiO2-B nanowires have been studied for LIB as anode materials and they exhibit enhanced electrochemical performance of 153 mA h g−1 at 20 C with a 76% capacity retention even after 1000 cycles, which make them potential candidate in a high-power lithium battery [21]. However, it is valuable that TiO2 nanotubes prepared by electrochemical anodization technique have in particular shown additional advantages over other synthesis techniques, particularly, in energy storage devices. These include: (i) titanium itself acts as a current collector and, therefore, minimizes the resistance between the active materials and the current collector; and (ii) enables binder free and conducting agents free active materials. Due to these advantages, anodized TiO2 nanotubes can be effectively used as a Li-ion battery anode material.
As the nitrogen-doped anodized TiO2 exhibits superior electrochemical performance, this work attempts to report on the comparative performances of rate capability, specific capacitance, cyclic stability, and specific capacity of TiO2 nanotubes and N-TiO2 nanotubes for both LIB and supercapacitor applications.
2. Results and Discussions
2.1. Structural Analysis
2.1.1. X-ray Diffraction (XRD)
The XRD patterns of TiO2 nanotubes annealed at 450 °C for 3 h in (a) air and (b) NH3 atmosphere, respectively, are presented in Figure 1. The diffraction peaks corresponding to highly crystalline TiO2 anatase phase along with Ti metal peaks were observed for TiO2 nanotubes (JCPDS data file no: 89-4921) with no sign of rutile phase, which is in good agreement with reported literature [22]. However, TiO2 nanotubes treated in NH3 atmosphere, that is, the N-TiO2 nanotubes displayed a dominant anatase phase with some significant difference in diffraction patterns compared to TiO2 nanotubes that included: (i) decreased intensity; (ii) peak broadening, and (iii) peaks shifting, which is clearly shown inset in Figure 1. In the N-TiO2 nanotubes, above mentioned change in XRD could be attributed to the TiO2 nanotubes treated in NH3 atmosphere resulting in nitrogen substitution into TiO2 that induces structural changes in the lattice that prompted peak shifting and broadening and decreased intensity when compared to the TiO2 nanotubes [23].
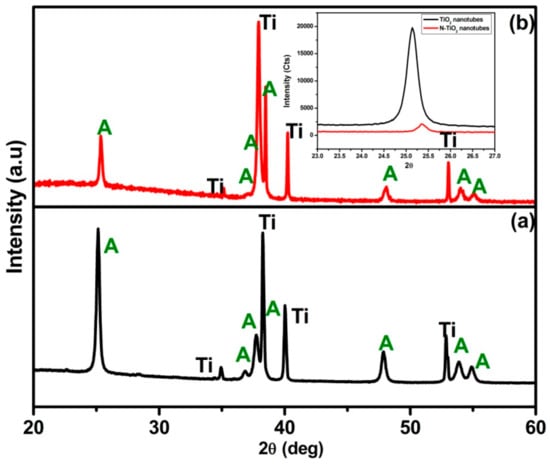
Figure 1.
X-ray diffraction (XRD) pattern of (a) TiO2 nanotubes and (b) N-TiO2 nanotubes. Inset contains magnified view between 23–27° (2θ Position).
2.1.2. Raman Spectroscopy
Furthermore, in order to distinguish, the TiO2 nanotubes and N-TiO2 nanotubes, structural analysis was carried out using Raman spectroscopy as shown in Figure 2. According to the reported data [24], TiO2 anatase phase would predominantly display a characteristic line of six fundamental modes that includes A1g (519 cm−1), B1g (399 cm−1 and 519 cm−1) and Eg (144 cm−1, 197 cm−1, and 639 cm−1). Herein, TiO2 nanotubes spectra showed the presence of active modes peaks at 144.3 cm−1, 395.8 cm−1, 515.7 cm−1 and 636 cm−1, which directly confirms the pure anatase phase and no other peaks of rutile phase. In N-TiO2 nanotubes, the strongest Eg mode at 150 cm−1 was clearly visible and could be ascribed to the external vibration of the anatase phase. When compared to TiO2 nanotubes, the N-TiO2 nanotubes spectra exhibit weak intensity, and shifting toward high frequency, which clearly confirms the nitrogen substitution into the TiO2 lattice, which is in good agreement with the reported literatures [25].
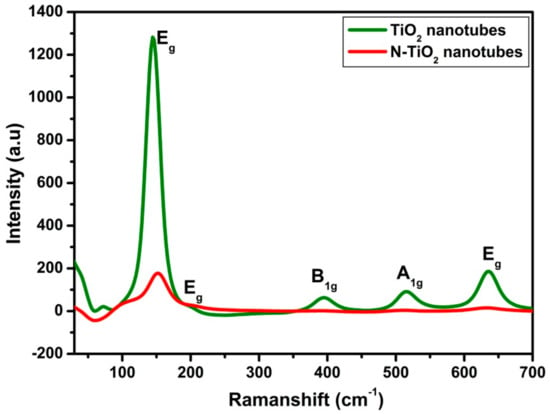
Figure 2.
Raman spectra of TiO2 nanotubes and N-TiO2 nanotubes.
2.2. Morphological and Compositional Analysis: Field Emission Scanning Electron Microscopy (FESEM) and Energy Dispersive Spectroscopy (EDS)
Topographical views and cross-sectional views of TiO2 nanotubes and N-TiO2 nanotubes were carried out by FESEM as shown in Figure 3. It is clearly observed that both TiO2 nanotubes treated in air and NH3 atmosphere have displayed homogenous nanotube morphology. Since, both TiO2 nanotubes were synthesized at the same anodization conditions, but annealed in different atmospheres, they do not show any noticeable change in pore diameter. Figure 3d shows the presence of nitrogen in the N-TiO2 nanotubes as confirmed by EDS elemental analysis.
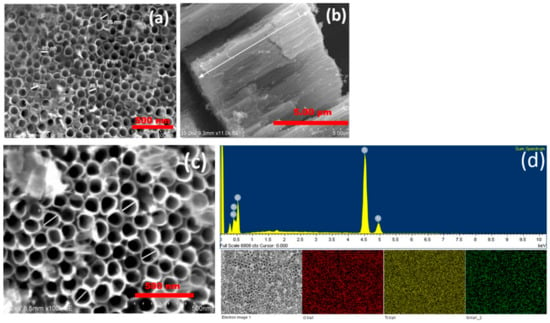
Figure 3.
Field emission scanning electron microscopy (FESEM) micrograph of (a) top view-TiO2 nanotubes, (b) cross sectional view-TiO2 nanotubes, (c) top–view-N-TiO2 nanotubes and (d) energy dispersive spectroscopy (EDS) of N-TiO2 nanotubes.
2.3. Chemical Analysis: X-ray Photoelectron Spectroscopy (XPS)
To investigate the chemical changes that occur during different annealing atmosphere of TiO2 nanotubes, XPS measurements were carried out for TiO2 nanotubes and N-TiO2 nanotubes and the survey scan spectra are shown in Figure 4. The spectra show the presence of elements of Ti, O, and N with trace amounts of carbon in respective samples.
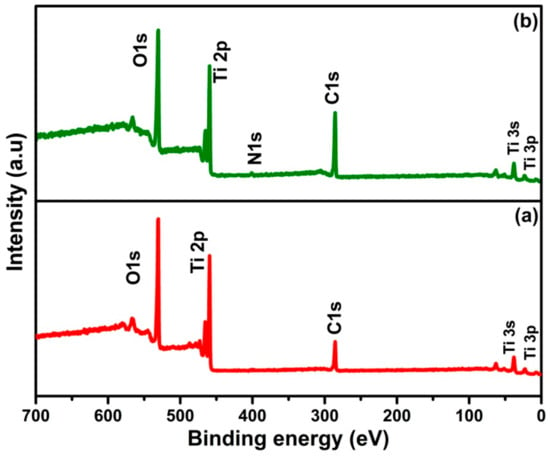
Figure 4.
X-ray photoelectron spectroscopy (XPS) survey scan spectra of (a) TiO2 nanotubes and (b) N-TiO2 nanotubes.
To investigate the nitrogen doping effect in TiO2 nanotubes further, narrow scans of N 1s, Ti 2p, O 1s spectra of N-TiO2 nanotubes were measured as shown in Figure 5. The observed core-level N 1s spectra have shown broad range spectrum from 394 eV to 404 eV and it can be interpreted that the N 1s spectra for nitrogen substitutions or interstitial doping, as it still should be a complex process and subject to debate as stated by Asahi et al. [26], and these may be caused by different synthesis procedures adopted by different groups. For spectra, fitting was applied and three peaks of binding energy nearly were exhibited at 396.1 eV, 402.2 eV, and 399.8 eV, which are well in agreement with reported literature [27]. From the narrow scan spectra of Ti 2p of N-TiO2 nanotubes (Figure 5b), the peaks at 464.1 eV and 458.3 eV correspond to the 2p1/2 and 2p3/2, respectively, which clearly indicates the incorporation of nitrogen in TiO2 nanotubes [27]. It has been assessed that peaks corresponding to 396.1 eV binding energy were attributed to the substitution of nitrogen to replace lattice oxygen atoms and formation of oxy-nitrides (O–Ti–N) [28] and peaks at binding energy 399.8 eV and 402.2 eV were corresponding to the interstitial doping of nitrogen atoms, and to form bond with oxygen atoms (Ti–O–N), which is well agreement with report literature [29]. This is further confirmed from O 1s narrow scan spectra of TiO2 and N-TiO2 nanotubes as shown in Figure 5c. While comparing two spectra of O 1s, there are some additional peaks grown in N-TiO2 nanotubes at 531.7 eV, and this is due to the interstitial doping of nitrogen into the TiO2 lattice. Therefore, the above results confirm the doping of nitrogen atoms into surface of TiO2 nanotubes.
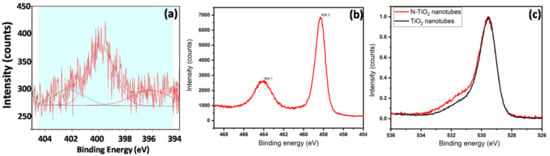
Figure 5.
Narrow scan XPS spectra of (a) N 1s, (b) Ti 2p and (c) O 1s of N-TiO2 nanotubes.
2.4. Supercapacitor Application
2.4.1. Cyclic Voltammetry (CV)
The electrochemical performances of TiO2 nanotubes and N-TiO2 nanotubes as electrode materials for supercapacitor application were carried out by an identical two-electrode system using swagelok cells in an aqueous solution of 1 M KOH. Figure 6 shows the CV curves of TiO2 nanotubes and N-TiO2 nanotube samples at a scan rate of 100, 200, and 500 mV s−1 in the potential window of 0 to 0.6 V. Both CV curves present the typical rectangular shape, which resembles the electrochemical double-layer capacitor (EDLC) as reported in our previous work [30]. Clearly, current density continues to increase as the scan rate increases without any change in curve shape indicating the good rate capability of both samples. From these CV curves, the specific capacitance of both electro-active materials were calculated and found to be 505 µF cm−2 for TiO2 nanotubes and 835 µF cm−2 for N-TiO2 nanotubes at a scanning rate of 100 mV s−1. Table 1 displays the current density, and specific capacitance as a function of different scanning rates for the TiO2 nanotubes- and N-TiO2 nanotubes.
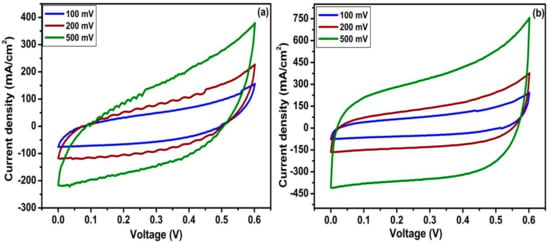
Figure 6.
Cyclic voltammetric (CV) curves of (a) TiO2 nanotubes and (b) N-TiO2 nanotubes.

Table 1.
Displays the current density, and specific capacitance as a function of different scan rates.
To further study the electrochemical performance of TiO2 nanotubes and N-TiO2 nanotubes for supercapacitor applications, galvanostatic charge/discharge measurement were taken at a different current density. Figure 7 shows the first charge/discharge curves of TiO2 nanotubes and N-TiO2 nanotubes samples at a current density of 80, 160, 240 and 320 µA cm−2, which are linear and symmetrical indicating good electrochemical capability and ensuring the electrochemical double layer capacitor behavior [31]. The specific capacitance of the electrode was estimated from the galvanostatic discharge curves according to the following equation:
where I represents charge/discharge current (A), Δt is the charge/discharge time (s), ΔV represents the potential window (V), and A represents the electrode area (cm2). The specific capacitances of two samples recorded at different current densities have been summarized in Table 2. The results obtained reveal the difference between TiO2 nanotubes and N-TiO2 nanotubes samples. At current density of 160 µA cm−2, the TiO2 nanotubes sample delivered a specific capacitance of 1508 µF cm−2 while N-TiO2 nanotubes exhibited overwhelming 3121 µF cm−2, which is double the specific capacitance of the former. Therefore, from the aforementioned electrochemical studies, the specific capacitance doubled from N-TiO2 nanotubes and could be attributed to its improved electronic conductivity, which facilitates the transport of charge carriers.
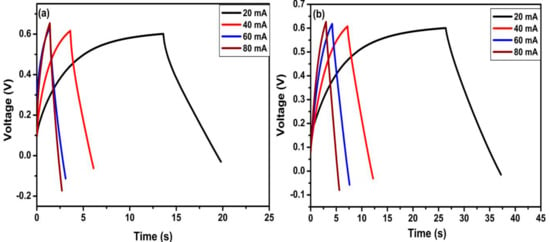
Figure 7.
Galvanostatic first charge/discharge curves of TiO2 nanotubes and N-TiO2 nanotubes.

Table 2.
Comparative electrochemical performance of undoped TiO2 - and N-doped TiO2 nanotubes.
2.4.2. Electrochemical Impedance Spectroscopy
Electrochemical impedance spectroscopy measurements were performed for the TiO2 nanotubes and N-TiO2 nanotubes at a frequency range from 1 Hz to 1 MHz and its Nyquist plot is shown in Figure 8. From the spectra, it can be seen that both samples could not show any semicircle in the high-frequency region, which indicates good capacitive electrodes. Compared to TiO2 nanotubes, low frequency region of N-TiO2 nanotubes exhibits clear vertical line, which is due to the ion’s diffusion in the electrolyte to the electrode interface that results in the better performance of the supercapacitor electrode.
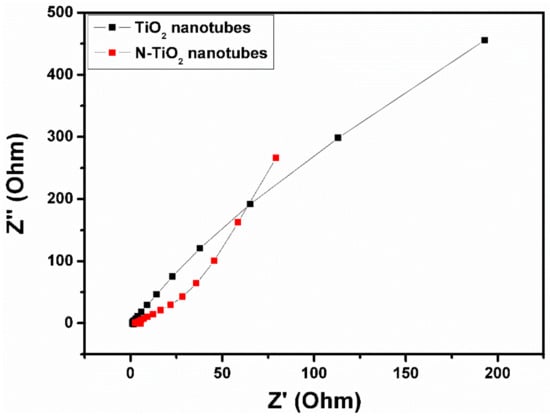
Figure 8.
Electrochemical impedance spectra of TiO2 nanotubes and N-TiO2 nanotubes.
Therefore, enhanced capacitive performance of N-TiO2 nanotubes could be ascribed to the improved conductivity of the electrode [32].
2.5. Lithium-Ion Battery Application
To investigate the electrochemical performance of TiO2 nanotubes and N-TiO2 nanotubes as anode materials for LIB, we have initially recorded cyclic voltammetry for 5 cycles as shown in Figure 9a–d, which were tested under the same conditions and found a significant difference in lithium insertion/extraction during the discharge/charge process between the two samples. CV curves of both samples that were tested at 0.1 mV s−1 scan rate, displayed cathodic and anodic peaks, which are associated with Li+ intercalation and de-intercalation into TiO2 nanotubes. The overall cell reaction for the Li insertion/extraction into TiO2 nanotubes can be written as follows:
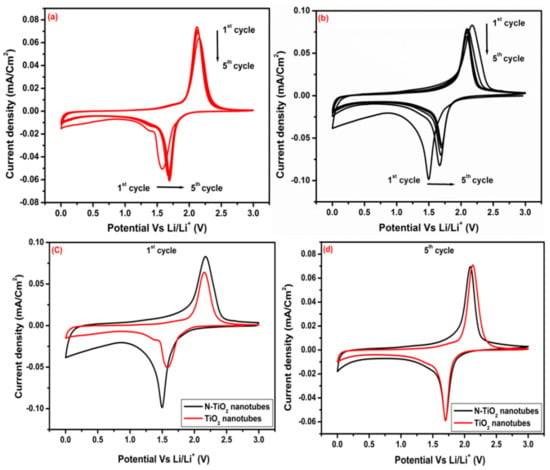
Figure 9.
Cyclic voltammetry of (a) TiO2 nanotubes and (b) N-TiO2 nanotubes for 5 cycles, respectively, comparing CV of both samples (c) 1st cycle and (d) 5th cycle.
For the first cycle of TiO2- and N-TiO2 nanotubes samples, cathodic /anodic peaks are located at 1.58/2.15 V and 1.49/2.17 versus Li0/Li+, respectively, which were attributed to the lithium insertion/extraction from the anatase phase of TiO2 and agree well with the peak’s position from reported literature [33]. Here, lithium insertion into TiO2 anatase phase is a two-phase process of Li poor (Li0.01TiO2) and Li rich (Li0.6TiO2). Table 3 shows the peak position of cathodic, anodic, potential difference of TiO2 nanotubes and N-TiO2 nanotubes samples. In detailed analysis of CV curves, some clear information has been notified regarding lithiation and delithiation of Li+ ions into the TiO2 lattice. During 1st cycle, cathodic peaks potential is lower in N-TiO2 nanotubes sample compared to TiO2 nanotubes, with increasing cycles, the peak position in N-TiO2 nanotubes shifted slightly towards higher potential probably due to the activation process for the Li+ lithiation in the first cycle, which is in agreement with some of the reported literature [34]. The potential separation between anodic and cathodic peak for N-TiO2 nanotubes (0.39 V) is smaller than TiO2 nanotubes samples (0.437 V) in the 5th cycle. This reduction in potential difference suggested that N-TiO2 nanotubes display high reversibility and faster Li+ diffusion [7]. Figure 9c,d, compares the CV curves of the 1st and 5th cycle of TiO2 nanotubes and N-TiO2 nanotubes samples. It is clear that in the 1st cycle, sweeping area and current density of N-TiO2 nanotubes is more than that of TiO2 nanotubes, which indicates higher lithium ions storage capabilities and high electrochemical activity. But in the 5th cycle, almost both samples exhibit similar type of behavior and show good reversible capability.

Table 3.
The peak position of cathodic, anodic, potential difference of TiO2 nanotubes and N-TiO2 nanotubes electrodes.
Figure 10 shows the charge/discharge curves in the 1st, 2nd, 50th, 100th, and 200th cycles for the TiO2 nanotubes’ and N-TiO2 nanotubes’ electrodes between 0 V to 3 V at a current density of 20 µA cm−2. Both samples exhibit voltage plateaus that occur at 1.75 V and 1.9 V, which were due to the insertion and extraction of Li+ from TiO2 structures. The initial discharge capacities of TiO2 nanotubes and N-TiO2 nanotubes for the 1st cycles were 86 µA h cm−2 and 975 µA h cm−2, respectively, which indicated that N-TiO2 nanotubes exhibit superior performance than the TiO2 nanotubes. These can be attributed to accommodation of more Li+ in N-TiO2 nanotubes due to its increased electronic and ionic conductivity.
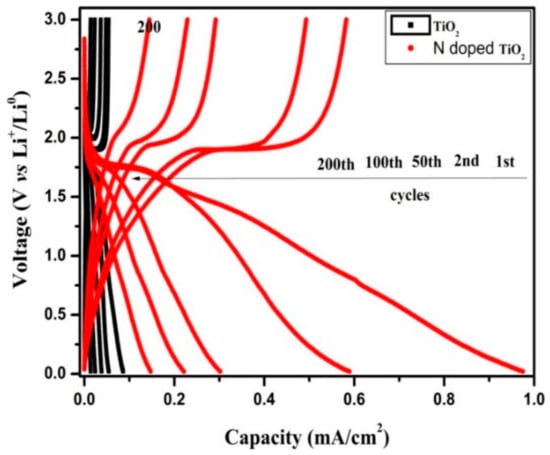
Figure 10.
Galvanostatic charge/discharges curves of TiO2 nanotubes and N-TiO2 nanotubes.
Figure 11 shows the specific capacities as a function of cycle number plot to understand the long cyclic stability of TiO2 nanotubes and N-TiO2 nanotubes samples. It is seen that initial reversible discharge capacity of N-TiO2 nanotubes and TiO2 nanotubes samples are 975 µA h cm−2 and 86 µA h cm−2, respectively, and with further cycling of 200 cycles, the specific capacity comes down to 145 µA h cm−2 and 13 µA h cm−2 for N doped TiO2 nanotubes and TiO2 nanotubes samples, respectively, which is a higher areal capacity compared to other reported TiO2 nanotubes based anode materials. For both samples, coulombic efficiency of more than 98% has been achieved even after 200 cycles and as a result it indicates that the nitrogen-doped TiO2 nanotubes exhibit superior cyclic performance that can be attributed to the fast Li+ diffusion and increased electronic conductivity.
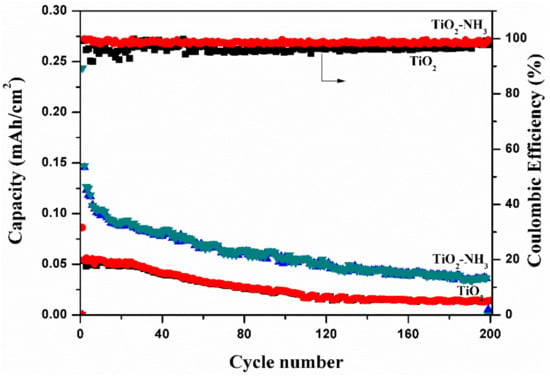
Figure 11.
Cyclic performances of TiO2 nanotubes and N-TiO2 nanotubes.
As, N-TiO2 nanotubes outperformed the TiO2 nanotubes with the highest ever reported areal capacity, we intended to test its rate capability at various current densities from 5 to 500 mA cm−2 so as to check its feasibility for high-power applications, which is shown in Figure 12. For these measurements, a fresh cell was made and, therefore, in an initial current density of 5 mA cm−2 the capacity (1.3 mA h cm−2) fell rapidly due to untreated electrochemical process [35]. For N-TiO2 nanotubes, it exhibited reversible capacity of 145.6, 81.6, 57.2, 44.0, 35.2, 31.6 and 28.4 µA h cm−2 at a current density of 10, 20, 50, 100, 200, 300, and 500 mA cm−2, respectively. For each current density, the capacities were recorded for 20 cycles and it displayed constant capacity except in lower current density. This reveals that discharge capacity of the electrode decreases along with the increase of current densities and this may be attributed to the insulating character of the N-TiO2 nanotubes sample. When it reverted back to current density of 50 and 10 mA cm−2, it displayed a reversible capacity of 58 and 101.2 µA h cm−2, respectively, even after 200 cycles. As a result, the N-TiO2 nanotubes sample exhibits superior cyclic performances, which makes it a suitable negative material for LIB.
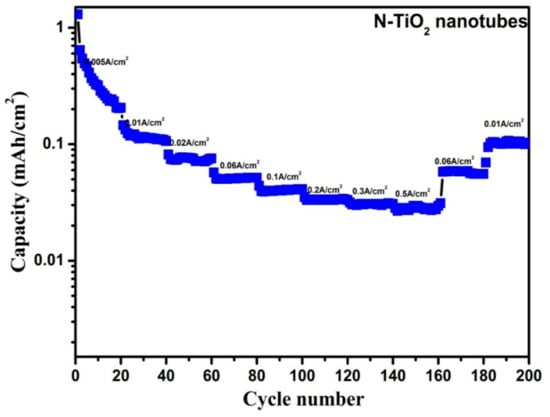
Figure 12.
Rate capability performances of N-TiO2 nanotubes.
3. Materials and Methods
All chemicals of analytical grade were used without any further purification. This section includes the synthesis of TiO2 nanotubes and nitrogen doped by cracking ammonia gas. The as-obtained TiO2 nanotubes were physically and electrochemically characterized for LIBs and supercapacitor applications.
3.1. Synthesis of TiO2 Nanotubes and N-Doped TiO2 (N-TiO2) Nanotubes
In a typical experimental procedure, TiO2 nanotubes were synthesized as reported in our previous work [30], here, the electrolyte consists of 70% ethylene glycol, 30% glycerol, 2% distilled water (DD) water containing 0.3 M ammonium fluoride. For anodization, Ti foils were polished as described in our previous work [30] and to get mirror polish of Ti metal, diamond paste of 3 µm and 0.5 µm were used. Anodization process was carried out at an anodizing voltage of 50 V for 4 h using titanium foil 2 cm × 1 cm as working electrode and platinum foil as counter/reference electrode. As-obtained anodized TiO2 nanotubes was washed and air dried at room temperature. To obtain TiO2 nanotubes and N-TiO2 nanotubes, the samples were further subjected to thermal annealing at 450 °C for 3 h in air and ammonia atmosphere, respectively.
3.2. Material Characterizations
All samples were characterized for phase purity and morphology using various sophisticated analytical techniques. Phase purity was analyzed using the X-ray diffraction (XRD) technique with X’PERT PRO PANalytical equipment operated at 1°/min scan rate and 0.02° step size while Raman spectroscopy (Nanophoton Raman-11, Japan) measured at a wavelength of 532 nm line of Nd-YAG laser. Morphology of particle distribution and its elemental compositional were visualized using field emission scanning electron microscopy (FESEM Hitachi, Japan, Model No.: SU6600) operated at 5 kV and 10 µA coupled with energy dispersive spectroscopy (EDS). The chemical composition and N 1s, O1 s and Ti 2p spectra were determined by using an X-ray photoelectron spectroscopy (XPS) instrument (Omicron nanotechnology) with monochromatized Al Kα X–rays (energy: 1486.6 eV) at 300 W.
3.3. Electrochemical Characterization
N-TiO2 nanotubes were tested for supercapacitor and as an anode material for LIB applications. For a comparative purpose, non-doped TiO2 samples were subjected to above applications at the same operating conditions.
3.3.1. Supercapacitor
Electrochemical supercapacitor characterizations were carried out using the AUTOLAB workstation (PGSTAT-12). Two-electrode system was employed for electrochemical measurement using swagelok-type cells. Both the working and counter electrodes were of the same active material separated by Whatman filter paper in an electrolytic solution of 1 M KOH. Cyclic voltammetric (CV) curves were obtained between the potential ranges of 0 and +0.6 V at different scanning rates (100, 200 and 500 mV s−1). Electrochemical impedance spectroscopy (EIS) measurement was carried out by applying a voltage of 5 mV in the frequency range between 1 Hz and 10 MHz.
3.3.2. Li-Ion Battery Anode
As the active materials (TiO2 nanotubes and N-TiO2 nanotubes) were contained directly over the current collector (Ti plate), the electrodes were used as it is without binder and conducting agents. All electrodes were tested as the LIB anode in a typical CR 2032-type coin cell that was fabricated in an argon containing a MBraun glove box maintained with <1 ppm O2 and <1 ppm H2O. The electrode of dimensions (0.5 cm × 0.5 cm) containing N-TiO2 were used as working electrode while Li foil as counter/reference electrode, separated by Celgard, the separator soaked in 1 M LiPF6 (1:1 (v/v) EC/DEC) as electrolyte. The fabricated cells were subjected to testing at constant current density of 20 µA cm−2 unless otherwise mentioned in a precision battery system (Landt CT2001A, New York, NY, USA).
4. Conclusions
In summary, we have employed N-TiO2 nanotubes for enhancing the electrochemical properties of a supercapacitor and LIB. From XPS spectra, peak at binding energy of 399.8 eV ensured that nitrogen was substituted in the TiO2 lattice from the N 1s core level spectra. N-TiO2 nanotubes as a supercapacitor electrode exhibited a specific capacitance of 835 µF cm−2 at a scan rate of 100 mV s−1, which is far superior to TiO2 nanotubes (505 µF cm−2). Similarly, areal discharge capacities of 975 µA h cm−2 and 86 µA h cm−2 for N-TiO2 nanotubes and TiO2 nanotubes, respectively, were obtained as anode material for LIB. Cyclic stability and rate capability studies of N-TiO2 nanotubes exhibits enhanced performance compared to TiO2 nanotubes. As a result, the N-TiO2 nanotubes sample exhibits better performance, which provides suitable active materials for supercapacitor and Li ion battery applications.
Author Contributions
T.A. prepared samples and carried out for characterization such as structural, morphological, chemical analysis, and article drafting; C.M.S. carried out LIB testing and article writing; R.K. carried out supercapacitor testing; B.S. and S.K. have participated in discussions of the results and article writing.
Funding
The authors acknowledge the UGC, India, for funding this project. S.Z.K. acknowledges financial support from the M-ERA, net project 272806 by the Research Council of Norway.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Alireza, K.; Li, Z. Battery, Ultracapacitor, Fuel Cell, and Hybrid Energy Storage Systems for Electric, Hybrid Electric, Fuel Cell, and Plug-In Hybrid Electric Vehicles: State of the Art. IEEE Trans. Veh. Technol. 2010, 59, 2806–2814. [Google Scholar]
- Bruce, P.G.; Scrosati, B.; Tarascon, J.M. Nanomaterials for Rechargeable Lithium Batteries. Angew. Chem. Int. Ed. 2008, 47, 2930–2946. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.K.; Muralidharan, P.; Lee, H.-W.; Ruffo, R.Y.; Yang, C.K.; Chan, H.; Peng, R.A.; Huggins, Y. Spinel LiMn2O4 Nanorods as Lithium Ion Battery Cathodes. Nano Lett. 2008, 8, 3948–3952. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Zhang, L.; Zhang, J. A review of electrode materials for electrochemical supercapacitors. Chem. Soc. Rev. 2012, 41, 797–828. [Google Scholar] [CrossRef] [PubMed]
- Chockla, A.M.; Harris, J.T.; Akhavan, V.A.; Bogart, T.D.; Holmberg, V.C.; Steinhagen, C.; Mullins, C.B.; Stevenson, K.J.; Korgel, B.A. Silicon Nanowire Fabric as a Lithium Ion Battery Electrode Material. J. Amer. Chem. Soc. 2011, 133, 20914–20921. [Google Scholar] [CrossRef]
- Wei, Z.; Liu, Z.; Jiang, R.; Bian, C.; Huang, T.; Yu, A. TiO2 nanotube array film prepared by anodization as anode material for lithium ion batteries. J. Solid State Electrochem. 2010, 14, 1045–1050. [Google Scholar]
- Wang, Y.; Liu, S.; Huang, K.; Fang, D.; Zhuang, S. Electrochemical properties of freestanding TiO2 nanotube membranes annealed in Ar for lithium anode material. J. Solid State Electrochem. 2012, 16, 723–729. [Google Scholar]
- Mancini, M.; Nobili, F.; Tossici, R.; Wohlfahrt-Mehrens, M.; Marassi, R. High performance, environmentally friendly and low cost anodes for lithium-ion battery based on TiO2 anatase and water soluble binder carboxymethyl cellulose. J. Power Sources 2011, 196, 9665–9671. [Google Scholar] [CrossRef]
- Yang, Z.; Choi, D.; Kerisit, S.; Rosso, K.M.; Wang, D.; Zhang, J.; Graff, G.; Liu, J. Nanostructures and lithium electrochemical reactivity of lithium titanites and titanium oxides: A review. J. Power Sources 2009, 192, 588–598. [Google Scholar] [CrossRef]
- Pei, Z.; Zhu, M.; Huang, Y.; Huang, Y.; Xue, Q.; Geng, H.; Zhi, C. Dramatically improved energy conversion and storage efficiencies by simultaneously enhancing charge transfer and creating active sites in MnOx/TiO2 nanotube composite electrodes. Nano Energy 2016, 20, 254–263. [Google Scholar] [CrossRef]
- Liu, Z.; Li, H.; Zhu, M.; Huang, Yan.; Tang, Z.; Pei, Z.; Wang, Z.; Shi, Z.; Liu, J.; Huang, Y.; et al. Towards wearable electronic devices: A quasi-solid-state aqueous lithium-ion battery with outstanding stability, flexibility, safety and breathability. Nano Energy 2018, 44, 164–173. [Google Scholar]
- Pan, D.; Huang, H.; Wang, X.; Wang, L.; Liao, H.; Li, Z.; Wu, M. C-axis preferentially oriented and fully activated TiO2 nanotube arrays for lithium ion batteries and supercapacitors. J. Mater. Chem. A 2014, 2, 11454–11464. [Google Scholar] [CrossRef]
- Bresser, D.; Oschmann, B.; Muhammad, N.; Tahir, F.M.; Ingo, L.; Wolfgang, T.; Rudolf, Z.; Stefano, P. Carbon-Coated Anatase TiO2 Nanotubes for Li- and Na-Ion Anodes. J. Electrochem. Soc. 2015, 162, A3013–3020. [Google Scholar] [CrossRef]
- Madian, M.; Giebeler, L.; Klose, M.; Jaumann, T.; Uhlemann, M.; Gebert, A.; Oswald, S.; Ismail, N.; Eychmüller, A.; Eckert, J. Self-Organized TiO2/CoO Nanotubes as Potential Anode Materials for Lithium Ion Batteries. ACS Sustain. Chem. Eng. 2015, 3, 909–919. [Google Scholar] [CrossRef]
- Liu, D.; Xiao, P.; Zhang, Y.; Betzaida, B.; Garcia, Q.; Zhang, F.; Guo, Q.; Champion, R.; Cao, G. TiO2 Nanotube Arrays Annealed in N2 for Efficient Lithium-Ion Intercalation. J. Phys. Chem. C 2008, 112, 11175–11180. [Google Scholar] [CrossRef]
- Palgrave, R.G.; Payne, D.G.; Egdell, R.G. Nitrogen diffusion in doped TiO2 (110) single crystals: A combined XPS and SIMS study. J. Mater. Chem. 2009, 19, 8418–8425. [Google Scholar] [CrossRef]
- Chen, Y.; Cao, X.; Lin, B.; Gao, B. Origin of the visible-light photoactivity of NH3-treated TiO2: Effect of nitrogen doping and oxygen vacancies. Appl. Surf. Sci. 2013, 264, 845–852. [Google Scholar] [CrossRef]
- Lee, K.; Mazare, A.; Schmuki, P. One-Dimensional Titanium Dioxide Nanomaterials: Nanotubes. Chem. Rev. 2014, 114, 9385–9454. [Google Scholar] [CrossRef]
- Han, H.; Song, T.; Bae, J.-Y.; Nazar, L.F.; Kim, H.; Paik, U. Nitridated TiO2 hollow nanofibers as an anode material for high power lithium ion batteries. Energy Environ. Sci. 2011, 4, 4532–4536. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Z.; Lv, X.-J. N-doped TiO2 nanotubes/N-doped graphene nanosheets composites as high performance anode materials in lithium-ion battery. J. Mater. Chem. A 2014, 2, 15473–15479. [Google Scholar] [CrossRef]
- Zhang, Y.; Fu, Q.; Xu, Q.; Yan, X.; Zhang, R.; Guo, Z.; Du, F.; Wei, Y.; Zhang, D.; Chen, G. Improved electrochemical performance of nitrogen doped TiO2-B nanowires as anode materials for Li-ion batteries. Nanoscale 2015, 7, 12215–12224. [Google Scholar] [CrossRef]
- Pan, X.; Xu, Y.-J. Defect-Mediated Growth of Noble-Metal (Ag, Pt, and Pd) Nanoparticles on TiO2 with Oxygen Vacancies for Photocatalytic Redox Reactions under Visible Light. J. Phys. Chem. C 2013, 117, 17996–18005. [Google Scholar] [CrossRef]
- Sun, H.; Bai, Y.; Jin, W.; Xu, N. Visible-light-driven TiO2 catalysts doped with low-concentration nitrogen species. Solar Energy Mater. Solar Cells 2008, 92, 76–83. [Google Scholar] [CrossRef]
- Yanagisawa, K.; Ovenstone, J. Crystallization of Anatase from Amorphous Titania Using the Hydrothermal Technique: Effects of Starting Material and Temperature. J. Phys. Chem. B 1999, 103, 7781–7787. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, W.; Zhang, Y.; Liu, S. An Efficient Two-Step Technique for Nitrogen-Doped Titanium Dioxide Synthesizing: Visible-Light-Induced Photodecomposition of Methylene Blue. J. Phys. Chem. C 2007, 111, 1010–1014. [Google Scholar] [CrossRef]
- Asahi, R.; Morikawa, T.; Irie, H.; Ohwak, T. Nitrogen-Doped Titanium Dioxide as Visible-Light-Sensitive Photocatalyst: Designs, Developments, and Prospects. Chem. Rev. 2014, 114, 9824–9852. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Burda, C. Photoelectron Spectroscopic Investigation of Nitrogen-Doped Titania Nanoparticles. J. Phys. Chem. B 2004, 108, 15446–15449. [Google Scholar] [CrossRef]
- Sathish, M.; Viswanathan, B.; Viswanath, R.P.; Gopinath, C.S. Synthesis, Characterization, Electronic Structure, and Photocatalytic Activity of Nitrogen-Doped TiO2. Nanocatalyst. Chem. Mater. 2005, 17, 6349–6353. [Google Scholar] [CrossRef]
- Emerson, C.; Kohlrausch, M.; Zapata, J.M.; Renato, V.; Gonçalves, S.K.; de O. Vaz, M.; Sérgio, J.D.; Marcos, R.T.; Santos, J.L. Polymorphic phase study on nitrogen-doped TiO2 nanoparticles: Effect on oxygen site occupancy dye sensitized solar cells efficiency and hydrogen production. RSC Adv. 2015, 5, 101276–101286. [Google Scholar]
- Tamilselvan, A.; Balakumar, S. Anatase TiO2 nanotube by electrochemical anodization method: Effect of tubes dimension on the supercapacitor application. Ionics 2016, 22, 99–105. [Google Scholar] [CrossRef]
- Ratha, S.; Rout, C.S. Supercapacitor Electrodes Based on Layered Tungsten Disulfide-Reduced Graphene Oxide Hybrids Synthesized by a Facile Hydrothermal Method. ACS Appl. Mater. Interfaces 2013, 5, 11427–11433. [Google Scholar] [CrossRef]
- Salari, M.; Aboutalebi, S.H.; Konstantinov, K.; Liu, H.K. A highly ordered titania nanotube array as a supercapacitor electrode. Phys. Chem. Chem. Phys. 2011, 13, 5038–5041. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.G.; Lee, Y.-G.; Song, S.-W.; Kim, K.M. Lithium-ion battery anode properties of TiO2 nanotubes prepared by the hydrothermal synthesis of mixed (anatase and rutile) particle. Electrochim. Acta 2010, 55, 5975–5983. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, Y.; Xiao, P.; Garcia, B.B.; Zhang, Q.; Zhou, X.; Jeong, Y.-H.; Cao, G. TiO2 nanotube arrays annealed in CO exhibiting high performance for lithium ion intercalation. Electrochim. Acta 2009, 54, 6816–6820. [Google Scholar] [CrossRef]
- Zakharova, G.S.; Jähne A Popa, C.; Täschner, Ch.; Gemming, Th.; Leonhardt, A.; Büchner, B.; Klingeler, R. Anatase Nanotubes as an Electrode Material for Lithium-Ion Batteries. J. Phys. Chem. C 2012, 116, 8714–8720. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available with author-T.A. and B.S. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).