Abstract
Novel 3D-configurated stereoisomers cis-cup-tris[C60>(DPAF-C9)] and trans-chair-tris[C60>(DPAF-C9)] were designed and synthesized in good yields. The former, with three C60> cages per molecule facing at the same side of the geometrical molecular cup-shape, was proposed to provide excellent binding interaction forces at the gold surface of core-shell γ-FeOx@AuNP nanoparticles and to direct the subsequent formation of a fullerene cage array (defined as fullerosome). Upon photoactivation of the Au-layer and cis-cup-tris[C60>(DPAF-C9)] itself, the degree of photoinduced intramolecular e−-transfer from DPAF to a C60> moiety was found to be largely enhanced by the accumulated plasmonic resonance energy at the near-field surface. Distribution of resulting negative charges along the outer (C60>)-derived fullerosome shell layer of the trilayered NPs was correlated with the detected photoswitchable dielectric amplification phenomena using white LED light at 1.0 GHz.
1. Introduction
Electronic charge-polarization of organic substances under the externally applied field is the basic phenomenon leading to dielectric characteristics. It can be correlated with electrons shifting within polarizable molecules or the dielectric medium showing electronic polarizability. Certain polymers including poly(vinylidene fluoride) (PVDF, having asymmetrical electron-withdrawing fluorine) [1,2], polyarylene ether nitrile (PEN, having polar cyano groups) with conducting polyaniline [3,4] and azobenzene dyes (having large dipole moments of polar push-pull donor-acceptor terminal groups) [5] may exhibit such behavior; however, with a low dielectric constant. Advantages of organic dielectrics over inorganic ones, such as barium calcium titanate [6], boron nitride on graphenes [7], and titanium dioxide [8], lay on their facile chemical modification and easy processing, intrinsic excellent mechanical performance, recyclability, and multiple components blending. Example of the latter case was given by hybrid preparation of PEN on core-shell nanoparticles [9]. These dielectric materials and polymers may exhibit potential in applications of capacitor-type charge and energy storage [10,11,12] and organic photovoltaics [13]. Recently, we found [14,15,16] that the dielectric constant of a medium can be amplified to more than 200–350% by a photoswitching technique using the construction of multi-layered core-shell nanoparticles. They consist of two main photoresponsive components, such as organic electron (e−)-polarizable nanostructure of C60(>DPAF-C9) (1-C9, Figure 1) and inorganic plasmonic gold nanocrystal hybrids in a form of core and shell layered nanomaterials. The degree variation of dielectric amplification depends on the core-shell composition and the distribution of polarized charges.
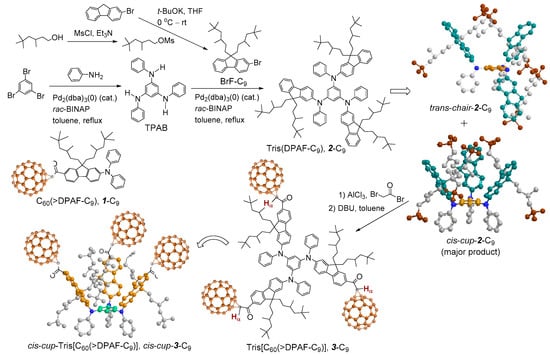
Figure 1.
Synthetic route to the preparation of cis-cup-tris[C60(>DPAF-C9)] (cis-cup-3-C9) from the isolated precursor cis-cup-tris(DPAF-C9) (cis-cup-2-C9) with reaction reagents provided.
The compound 1-C9 analogous nanostructure is an electronically polarizable push-pull donor-acceptor type conjugative compound having a C60 cage as the acceptor moiety and a DPAF-C9 [9,9-di(3,5,5-trimethylhexyl)-2-diphenylaminofluorene] chromophore as the light harvesting donor antenna moiety. It is a part of the general composition of C60(>light-harvesting antenna)x exhibiting ultrafast inter- and intramolecular photoinduced electron-transfer processes going from the electron-donating diphenylaminofluorene moiety to the electron-accepting C60 cage moiety [17,18,19,20]. The phenomena resulted in the formation of a positively charged (DPAF)+·-C9 and a negatively charged (C60>)−· cage moieties, respectively. These two charge states form the foundation of our observed photoswitchable dielectric property enhancements [14,15,16]. Recently, we further extended the study to design and synthesize highly restricted 3D-stereoisomers based on inter-connected three DPAF-C9 chromophore units giving a structure of tris(DPAF-C9) or 2-C9 (Figure 1). The structural modification allowed us to investigate the stereo-configuration-dependence of organic fluorophore on the enhancement of their photophysical properties, including photoluminescence (PL) and electroluminescence (EL) emission in solid-state thin-films [21]. The stereochemical modification was based on the construction of 3D-geometrically branched chromophores having sterically hindered alkyl side-chains that resulted in the physical separation of each DPAF-C9 moiety of 2-C9 from each other. It prevents and minimizes the tendency of planar organic π-conjugated fluorophore molecules, such as DPAF, to undergo aromatic-aromatic stacking, overlapping, and aggregation, via intermolecular hydrophobic-hydrophobic interactions, in solid state thin-films. The packing action triggers concentration-dependent self-quenching effects at excited states, resulted in the reduction of photophysical properties at the condensed phase. In the case of tris(DPAF-C9), attachment of large bulky groups at the vicinity of planar molecular benzene core region can restrict and reduce the degree of freedom in intramolecular DPAF-C9 rotation via steric hindrance, and thus the tendency of intermolecular aggregation. During the synthesis of tris(DPAF-C9), two stereoisomeric forms were isolated, namely, trans- and cis-tris(DPAF-C9) defined as trans-chair-2-C9 and cis-cup-2-C9 [22], respectively, as shown in Figure 1. We selected the latter cup-form to undergo further attachment of a C60> cage as the end group of each DPAF-C9 arm that resulted in a nanostructure of tris[60]fullerenylated cis-tris(diphenylaminofluorene) as cis-cup-tris[C60(>DPAF-C9)] or cis-cup-3-C9 for the photoswitchable dielectric property amplification study. This design of new stereo-isomeric structures may be beneficial for uses as positive charge carriers in enhancing photoinduced dielectric characteristics [23] and as the precursor building blocks in the synthesis of several C60- and C70-based ultrafast photoresponsive nanomaterials in correlation to our recent studies [17,18,19,20]. Therefore, we applied cis-cup-3-C9 as a photoswitchable dielectric charge-polarizer in the fabrication of microwave-responsive plasmonic trilayered core-shell nanoparticles.
2. Results and Discussion
An electromagnetic (EM) wave consists of both components of perpendicular electric-fields and magnetic-fields. Most organic photoresponsive materials are non-magnetic. Therefore, our main design consideration of organic microwave-responsive materials in tuning a material’s refractive index was based on the electric susceptibility, in terms of the degree of dielectric polarization in response to photoactivation instead of an applied electric field. In general, for disordered amorphous charge-transfer complex solids, the charge transfer events will not lead to significant alternation of the dielectric property of solids. Our approaches were to achieve the ferroelectric-like characteristics by firstly demonstrating ultrafast photoinduced intramolecular electron-transfer rate going from DPAF donor moiety to the C60> acceptor moiety of C60(>DPAF-C9) (1-C9) within <130 femtoseconds [17,18,19,20], resembling nearly spontaneous photoinduced charge-polarization action. Secondly, we then fabricated the C60> cage assembly into an aligned partial bilayered fullerosome membrane structure [24]. This type of fullerosome consists of a C60-C60 cage aligned array into a nano-layer to host the polarized negative charges at the inner membrane area and all DPAF donor-antennae moieties facing outside surface of the fullerosome to host the polarized positive charges. The self-assemble action was induced by strong hydrophobic-hydrophobic (C60>)-(C60>) interaction forces among fullerene cages. The resulting layered, polarized charges distribution affords a close resemblance to the characteristics of dielectrics, also as nano-capacitor-like assemblies, upon LED irradiation [14,15,16].
In the material’s design, we replaced 1-C9 by cis-cup-tris[C60(>DPAF-C9)] (cis-cup-3-C9, Figure 1) as the outer shell donor-acceptor nanoconjugates on the construction of core-shell nanoparticles. It was derived from a C3-symmetrical 1,3,5-triaminobenzene ring as the central core for connecting three fused 2-diphenylaminofluorene moieties, forming a new class of 3D-stereomeric tris(fluorenylphenylamino)-benzene as tris(DPAF-C9) 2-C9. These 3D-stereomers faces high torsional stress at the central benzene core region that forces all fluorene chromophore groups located at the outer-edge area to be oriented in a non-coplanar 3D-configuration [22]. Two stereoisomeric forms are possible, namely, cis-cup-2-C9 and trans-chair-2-C9 (Figure 1). They can be separated by thin-layer chromatographic technique with the former as the major product. Based on the density functional theory (DFT) calculation with the geometries optimized at the B97-D3/SVP level of theory [21], the cis-cup-form having three bis(3′,5′,5′-trimethylhexyl)fluorene moieties on 1,3,5-tris(phenylamino)benzene was found to be essentially C3 symmetric with a higher formation tendency than the corresponding trans-chair-form. It is owing to the influence by strong dispersion interactions within the alkyl chains that are enhanceable in the presence of more polar media. Upon a 180°-rotation of one of three amino groups, the structure changes to produce a conformational isomer in a trans-chair-form which is less cup-like than the cis-cup-form. In principle, subsequent attachment of three C60> cages on cis-cup-2-C9 should lead to cis-cup-3-C9 having all C60> moieties facing outward from the central benzene core at the same side with each other. This will facilitate the cup-attachment on the gold layer of core-shell nanoparticles induced by the strong C60-Au binding force [25,26] and, thus, enhance the distribution of polarized negative charges at gold surface bound monolayered fullerosome region, as the positive contribution to the photoswitchable relative dielectric properties.
2.1. Synthesis of Electron-Polarizable Tris[C60(>DPAF-C9)] Stereoisomer cis-cup-3-C9
Synthetic procedure for the preparation of tris(DPAF-C9) with the detailed spectroscopic characterization was reported previously by us [21,22]. Geometrically conformational isomer cis-cup-2-C9 was clearly isolated from the trans-chair-stereoisomer and confirmed by the use of high performance liquid chromatography (HPLC, µPorasil™ 125 Å, 10 µm, 35 × 300 mm, mobile phase: hexane-EtOAc (9.0:1.0, v/v), flow rate: 1.0 mL/min, detector: UV at λ 350 nm) in an analytical scale. For the scale-up separation, column chromatography (CC) using the same solvent system did not provide satisfactory results. However, the application of thin-layer chromatography (TLC) technique using the solvent eluent in slightly lower polarity (hexane-ethylacetate/9.5:0.5, v/v) than those of HPLC or the CC formed an overlap band of the stereoisomers cis-cup-2-C9 and trans-chair-2-C9. By cutting the corresponding fluorescent TLC band of tris(DPAF-C9) into two portions and performing several repeated TLC procedures, we were able to significantly increase the degree of product purity sufficiently enough to allow our confirmation of their entity by 1H NMR spectrum [21,22]. It indicated a higher Rf value for cis-cup-2-C9 than that of trans-chair-2-C9. With a C3 symmetry in structure, the former displayed a differentiable singlet peak for three central benzene protons (cis-Hb) at δ 6.55 (Figure 2(Aa)), while the latter with a less symmetry resulted in two proton peaks (trans-Hb) at δ 6.48 (1H) and 6.55 (2H).
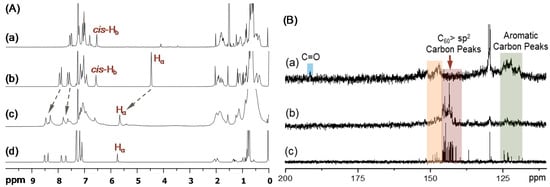
Figure 2.
(A) 1H NMR spectra (CDCl3) of (a) cis-cup-tris(DPAF-C9) (cis-cup-2-C9), (b) cis-cup-tris(BrDPAF-C9), (c) cis-cup-tris[C60(>DPAF-C9)] (cis-cup-3-C9), and (d) C60(>DPAF-C9) as a reference for comparison. (B) 13C NMR spectra of (a) cis-cup-tris(BrDPAF-C9), (b) cis-cup-tris[C60(>DPAF-C9)], and (c) C60(>DPAF-C9) (1-C9), showing in (b) the main group of fullerenyl sp2 carbon peaks at δ140–147 that indicated the attachment of C60> cages on 2-C9, as compared with those of (Bc).
Prior to the attachment of three C60> cages on cis-cup-2-C9, it was functionalized by the Friedel–Crafts acylation at C7 position of diphenylaminofluorene moiety with α-bromoacetyl bromide in the presence of aluminum chloride (6.0 eq.) in 1,2-dichloroethane at 0 °C to ambient temperature overnight to afford the corresponding α-bromoacetylfluorene derivative, as the intermediate step of reactions. It resulted in yellow semi-solids in 53% yield of cis-N1,N3,N5-tris(7-α-bromoacetyl-9,9-di(3′,5′,5′-trimethyl-1′-hexyl)fluoren-2-yl)-1″,3″,5″-tris(phenylamino)benzene as cis-cup-tris(BrDPAF-C9). It was purified by either column or TLC chromatography (silica gel, hexane–toluene, 3:2, Rf = 0.3 on TLC). The functional structure of cis-cup-tris(BrDPAF-C9) was verified by both infrared (FT-IR) and 1H NMR spectra with the former showing a strong carbonyl (-C=O) stretching absorption band centered at 1675 cm−1 indicating this functional group being linked on a phenyl moiety since a clear band absorption shift from 1725 cm−1, normally detectable for an alkyl carbonyl group, was evident. In the case of 1H NMR spectrum, tris(Br-DPAF-C9) displayed characteristic new peak signals of two methylene protons (Hα) next to the carbonyl group of the α-bromoacetyl moiety at δ 4.46 (Figure 2(Ab)). Subsequence attachment of a C60> cage to each of three DPAF moieties was made via a cylopropylacetyl bridge by the treatment of three α-bromoacetylfluorene moieties of cis-cup-tris(BrDPAF-C9) with C60 in toluene in the presence of DBU at ambient temperature for 8.0 h. An excessive amount of C60 (4.5 eq.) was applied to avoid the formation of C60-bisadducts or trisadducts during the cyclopropanation reaction. The product of cis-cup-tris[C60(>DPAF-C9)] stereoisomer as cis-cup-3-C9 was confirmed by the evidence of changing solubility characteristics matching those of C60 and a slight shift of cyclopropyl keto group absorption in IR spectrum to υmax 1627 cm−1, which was assigned to the carbonyl stretching band. It was also accompanied by two typical fullerenyl cage signals at υmax 575 (w) and 529 (s) cm−1. These two bands are characteristic absorptions used to provide evidence of (C60>)-related monoadduct with relative intensity ratio differentiable from those of starting C60 itself. They will disappear from the spectrum once becoming a (C60>)-bisadduct. Therefore, this IR technique was applied often in the [60]fullerenyl product structure verification. In addition, disappearance of α-proton peaks of the α-bromoacetyl group at δ 4.46 (Figure 2(Ab)) with the appearance of new peaks centered at δ 5.67 (Figure 2(Ac)) can be indicative of successful formation of the cyclopropanyl keto-bridge between a C60> cage and the fluorene moiety, using previously reported C60(>DPAF-C9) characterization (Figure 2(Ad)) as the reference [17]. The latter peak δ 5.67 was assigned to the chemical shift of α-proton (Hα) of 3-C9 indicated in Figure 1. Its large down-fielded shift from the normal chemical shift value of a alkyl acetyl proton at ~δ 2.1–2.6 is due to the influence of strong [60] fullerenyl current in the close vicinity. Accordingly, the detection of δ 5.67 peak can also be served as the verification of short covalent-bonding between C60> and DPAF moieties. The half-height width of Hα peak in Figure 2(Ac) is slightly wider than that of Figure 2(Ab) that revealed less symmetrical environment among three Hα protons of cis-cup-3-C9. Similarly, by using 13C NMR spectrum of C60(>DPAF-C9) (Figure 2(Bc)) as the reference for comparison, we were able to detect calculated 29 fullerenyl sp2 carbon peaks corresponding to the C60> moiety (C2 symmetry) of cis-cup-tris[C60(>DPAF-C9)] as a matching multi-peak band in the range of δ 140–147 (Figure 2(Bb)). The reduced spectrum resolution is due to the increasing molecular complexity along with the reduced solubility of cis-cup-3-C9.
2.2. Synthesis of cis-cup-Tris[C60(>DPAF-C9)]-Encapsulated Core-Shell Nanoparticles
Synthetic procedures for the preparation of core-shell γ-FeOx@AuNP nanoparticles (4-NPs) in an average particle diameter of ~20 nm were reported by us recently [14,15]. A slightly modified method was applied in this study to afford similar results based on the verification by transmission electron microscopy (TEM) micrographs and microanalyses of energy dispersive x-ray spectra (EDS). Subsequent encapsulation of γ-FeOx@AuNPs by cis-cup-tris[C60(>DPAF-C9)] leading to the formation of trilayered core-shell NPs (Figure 3) was performed by mixing these two components in a predefined ratio in solvent under ultrasonication. The initial binding force of cis-cup-3-C9 to γ-FeOx@AuNP was governed by strong binding interaction forces of C60> cages to the gold surface [26,27] that should result in the partial replacement of 1-octanethiol capping molecules on the gold surface via alkylthiol ligand-C60> exchange induced by sonochemical energy to invert cis-cup-3-C9 molecules under ultrasonication. The exchange should form a monolayer of cis-cup-tris[C60(>DPAF-C9)] with the orientation of all three C60> cages per nanomolecule in a cup-capping manner facing on the surface of Au layer with interlinked three DPAF-C9 moieties facing outward. Attachment of additional cis-cup-3-C9 nanomolecules will follow the strong hydrophobic-hydrophobic interactions among (C60>)-(C60>) cages to form a partial trilayered fullerosome membrane resembling that of reported fullerenyl nanovesicle [24]. Since all encapsulated magnetic nanoparticles were physically removed from the container solution by the assistance of an external permanent magnet and washed repeatedly by ethanol and diethyl ether, we were able to ensure the products being free from residual non-binding cis-cup-3-C9 molecules at the NP surface. We assigned the resulting trilayered core-shell nanostructures of (γ-FeOx@AuNP)@{cis-cup-tris[C60(>DPAF-C9)]}n as 5-NPs.
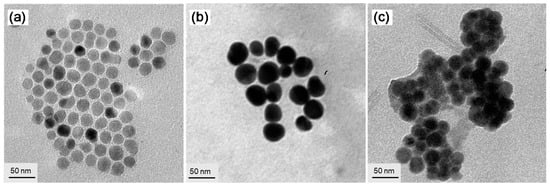
Figure 3.
TEM micrograph images of (a) γ-FeOx NPs, (b) γ-FeOx@AuNP (4-NPs), and (c) (γ-FeOx@AuNP)@{cis-cup-tris[C60(>DPAF-C9)]}n (5-NPs), showing evolution of particle morphology changes and the soft organic material encapsulation on dark nanoparticles on the latter micrograph.
TEM micrographs of Figure 3 showed the morphology and topography of NPs that indicated a roughly homogeneous narrow size distribution of the parent γ-FeOx NPs (Figure 3a) at a diameter of ~20 nm, on average. Subsequent deposition of solid gold nanocrystals on the surface of γ-FeOx NPs to a structure of core-shell γ-FeOx@AuNP (4-NP) was controlled to a shell layer thickness of 6.0–8.0 nm by the calculated quantity of HAuCl4 applied. This thickness was measured by the comparison of average particle size between Figure 3a,b, where the latter displayed much higher contrast of the Au layer shell. With the encapsulation of 4-NPs by cis-cup-3-C9-derived fullerosome (array of C60> cages) as the outer shell layer, the morphology of soft organic materials in lighter contrast covering all hard nanoparticles can be observed that allowed us to measure an average thickness of roughly 10 nm (Figure 3c), matching well with the weight ratio of 4-NPs and cis-cup-3-C9 applied in the core-shell nanoparticle fabrication.
2.3. Physical Properties of cis-cup-Tris[C60(>DPAF-C9)] and Its Encapsulated Core-Shell Nanoparticles
Photophysical properties of cis-cup-tris[C60(>DPAF-C9)] are dominated by two components, namely, C60> cages as the electron (e−)-acceptors and light-harvesting DPAF antenna units as electron (e−)-donors. Both components are photoresponsive at a different wavelength range. For example, optical absorption of C60> cages occurs mainly the band centered at 335 nm (1.76 × 105 L mol−1 cm−1) [17], whereas the band centered at 402 nm (4.19 × 104 L mol−1 cm−1) is attributed to the absorption of DPAF moieties, as shown in the UV–vis spectra of Figure 4c. Characteristics of the latter band were compared with that of cis-cup-2-C9 (Figure 4a), cis-cup-tris(BrDPAF-C9) (Figure 4b), and C60(>DPAF-C9) (1-C9, Figure 4d) showing a clear bathochromic shift of the 353-nm band of the former to 395 nm (1.10 × 105 L mol−1 cm−1), which is matching roughly with the 404-nm band of 1-C9 and the 402-nm band of cis-cup-3-C9 for the peak assignment. This assignment was also consistent with the observation of 2.9-folds higher in the absorption extinction coefficient (ε) value for cis-cup-tris(BrDPAF-C9), having three DPAF moieties per molecule, as comparing with that of 1-C9 with one DPAF moiety per molecule. Upon the attachment of three C60> cages, optical absorptions of fullerene moieties of cis-cup-3-C9 became dominating in the spectrum with a much higher ε value for the 335-nm band (Figure 4c). It was accompanied by a weak characteristic (forbidden) steady state absorption band of the C60> moiety appearing at 692 nm (the insert of Figure 4c) that provided further confirmation of a fullerene conjugate structure.
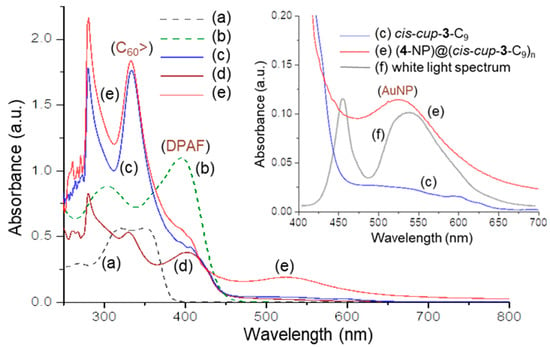
Figure 4.
UV-vis spectra of (a) cis-cup-tris(DPAF-C9) (cis-cup-2-C9), (b) cis-cup-tris(BrDPAF-C9), (c) cis-cup-tris[C60(>DPAF-C9)] (cis-cup-3-C9), (d) C60(>DPAF-C9) (1-C9), (e) (4-NP)@(cis-cup-3-C9)n (5-NPs), and (f) white light emission spectrum, where (a) and (b) were taken in EtOAc and (c), (d), and (e) were taken in toluene. The concentration of all samples is 1.0 × 10−5 M.
Interestingly, distinctly 3.3-folds lower in the ε value of the 402 nm peak in Figure 4c than that of 1-C9, making it as a shoulder band to the main C60> absorption, was interpreted by a phenomenon likely caused by the specific molecular 3D-conformation of cis-cup-tris[C60(>DPAF-C9)]. Owing to good solubility and compatibility of fullerenes to toluene solvent used, the orientation of three C60> cages was likely to face outward into the solvent phase while DPAF moieties were hidden inside the partially aggregated cluster nanoparticle. The hypothesis was also verified by observation of the distinguishable solubility decrease of cis-cup-3-C9 in CHCl3; however, increase in toluene and CS2 matches well with the solubility characteristics of C60.
Our study of photoswitchable dielectric amplification phenomena is based on photoexcited plasmonic resonance energy transfer to induce the intramolecular charge polarization of cis-cup-tris[C60(>DPAF-C9)] forming the corresponding dielectric ion-radical components (C60>)−· and DPAF+·-C9. In this event, C60> served as an electron (e−)-acceptor, whereas light-harvesting DPAF antenna worked as an electron (e−)-donor. Accordingly, we first investigated the characteristics of redox potentials of both C60> and DPAF moieties in the 3D-configurated nanostructure. Measurements of the cyclic voltammetry (CV) were carried out on the sample of cis-cup-3-C9 in a mixture of toluene and (n-butyl)4N+-PF6− electrolyte, using Pt as both working and counter electrodes and Ag/AgCl as the reference electrode. To provide a clear data interpretation, CV characteristics of C60 (>DPAF-C9) were applied as the reference under variation of cyclic oxidation and reduction voltages vs. Ag/Ag+ from −2.0 to 1.5 V. It displayed one reversible oxidation (1Eox of 0.95 V)-reduction (1Ered of 1.07 V) cycle wave at positive voltages and at least two reversible reduction (1Ered of −0.43 V and 2Ered of −0.88 V)-oxidation (1Eox of −0.62 V and 2Eox of −1.10 V) cycle waves at negative voltages (Figure 5(Ac)). These potential values were compared by those of a set of separated molecular components using a C60> monoadduct and cis-cup-tris(DPAF-C9) as models for confirmation, showing reversible 1Ered/1Eox of −0.40/−0.71 V, 2Ered/2Eox of −0.82/−1.14 V, and 3Ered/3Eox of −1.33/−1.62 V vs. Ag/Ag+ for the C60> monoadduct (Figure 5(Aa)) and one reversible cyclic wave of 1Eox/1Ered as 0.93/1.03 V for cis-cup-tris(DPAF-C9) (Figure 5(Ab)). Once the compound 1-C9 was molecularly tripled to cis-cup-3-C9, the cyclic redox wave corresponding to DPAF moieties at positive voltages fully disappeared, as shown in Figure 5(Ad). However, two clearly reversible 1Ered/1Eox (−0.23/−0.55 V) and 2Ered/2Eox (−0.75/−0.99 V) with one less obvious cyclic wave of 3Ered/3Eox (−0.97/−1.15 V) vs. Ag/Ag+ corresponding to the redox characteristics of C60> moieties of cis-cup-3-C9 were observed. This revealed the occurrence of a partial one-electron oxidation process of e−-donor DPAF-C9 moiety in the structure of cis-cup-3-C9 in the presence of three C60> cages. This inhibited the further electron-oxidation event in a clear manner. Since C60> is capable of undergoing at least three reversible reduction/oxidation waves (Figure 5(Aa)), partial e−-transfer from DPAF may not give significant influence on its CV diagram, except the first and second reduction potentials being modified from −0.43 to −0.23 and −0.88 to −0.75 V vs. Ag/Ag+, respectively.
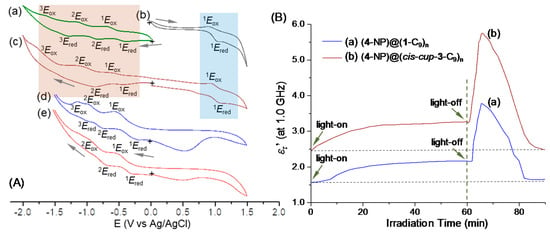
Figure 5.
(A) Cyclic voltammograms (CV) of (a) C60>, (b) cis-cup-tris(DPAF-C9), (c) C60 (>DPAF-C9) (1-C9), (d) cis-cup-tris[C60(>DPAF-C9)] (with no light), and (e) cis-cup-tris[C60(>DPAF-C9)] (after white LED light irradiation for 30 min) at different voltages vs Ag/Ag+ in a solution concentration of 1.0 × 10−3 M in CH3CN-CH2Cl2, containing (n-butyl)4N+-PF6− electrolyte (0.2 M), using Pt as working and counter electrodes and Ag/AgCl as the reference electrode at a scan rate of 10 mV/s. (B) Irradiation time-dependent relative dielectric constant (εr′) amplification of trilayered core-shell nanoparticles of (a) (γ-FeOx@AuNP)@[C60(>DPAF-C9)]n (6-NPs) and (b) (γ-FeOx@AuNP)@{cis-cup-tris[C60(>DPAF-C9)]}n (5-NPs) at the frequency of 1.0 GHz. White LED light irradiation period was 60 min.
Most importantly, we were able to apply the white LED light irradiation (30 min) concurrent with the CV measurement to obtain the insight of redox characteristics of cis-cup-3-C9 under similar photoexcitation conditions used in the dielectric property measurements of the same compound in a form of core-shell nanoparticles, as described below. As a result, Figure 5(Ae) displayed a similar cyclic wave profiles as those of Figure 5(Ad) showing two clearly reversible waves with 1Ered/1Eox (−0.32/−0.55 V) and 2Ered/2Eox (−0.70/−0.99 V) vs. Ag/Ag+ that was attributed to the redox characteristics of C60> moieties. No clear oxidation waves of DPAF moieties were detected that was indicative of an effective intramolecular e−-transfer from DPAF-C9 to C60> upon light irradiation to produce three ion-radical pairs per molecule as cis-cup-tris[C60−·(>DPAF-C9)+·]. At this polarized charge-form, DPAF moieties were not able to undergo further electron-oxidation process, consistent with the observed data. Furthermore, these CV results may be correlated with or indicative of similar charge-polarization phenomena to occur in the solid state of (cis-cup-3-C9)-encapsulated core-shell 5-NPs.
The reflectivity of a subject is controlled by the refractive index of the subject surface medium that is a function of the product of permittivity (εr) in a complex form and permeability (µ). Both are relevant material parameters in response to incident electromagnetic waves. The former complex permittivity can be presented by the equation εr* = εr′ − iεr″, where εr′ is the real part as the relative dielectric constant and εr″ is the imaginary part as the loss factor. The former parameter εr′ is dependent on charge-polarization of the material at the wavelength of measurements. In the experimental data collection, the complex relative electric permittivity (and) values were measured in terms of complex reflection coefficient of electromagnetic waves in proportion to a complex scattering parameter, defined as S11. The scattering parameter S11 was measured by a high-performance coaxial probe (Agilent 85070E) [27]. It was then converted to complex relative electric permittivity (εr*) values using the Nicolson-Ross algorithm [28]. From this measurement and calculation, the value of εr′ was derived.
Surface plasmon resonance (SPR) energy phenomena has been demonstrated and applied in many technological areas including the use of it as an alternative means to increase either light absorption or scattering in a thin film to enhance solar cells efficiency [29,30]. In principle, SPR energy occurs as the result of collective oscillation of surface electrons in gold nanoparticles that is induced by the interaction with the incident light. It leads to polarization with the formation of polaritons leading to the influence of absorption cross-section enhancement and light emitting [31]. In our study, we applied core-shell nanoparticles to investigated the possibility of tuning the SPR energy band to match with absorptions of dielectric materials for the induced intramolecular electron polarization that led to the modulation of dielectric constant and related physical property of the material layer on core-shell nanoparticles of 5-NPs. The approach coincides with our recent results of ultrafast photoinduced intramolecular e−-transfer phenomena in femtoseconds [19,20]. Therefore, we found that a near-field effect within a few nanometers was crucial to enhance SPR energy accumulation during the energy-transfer event between plasmonic AuNPs and C60-(antenna)x core-shell layers by direct contacts. Accordingly, we assembled a multiple layered structure of NPs having an inner magnetic γ-FeOx@AuNP core encapsulated by an outer cis-cup-tris[C60(>DPAF-C9)] shell, as described above. The NP fabrication was coupled by the control of the layer thickness of cis-cup-3-C9 in terms of the quantity weight ratio to that of γ-FeOx@AuNP (4-NPs).
We first evaluated the degree difference of dielectric property amplification between 1-C9 (giving 6-NPs) and cis-cup-3-C9 (giving 5-NPs) using the same plasmonic core-shell γ-FeOx@AuNP core. As a result, Figure 5B showed irradiation time-dependent relative dielectric constant (εr′) curves of 5-NPs and 6-NPs taken at the microwave frequency of 1.0 GHz during and after a 60-min white LED light illumination (corresponding to a total light fluence of 12.8 J/cm2). Apparently, microwave EM wave showed a higher sensitivity to the NP surface materials for us to note the detected initial dielectric constant value at the time zero min being comparable in value to that of organic 1-C9 (εr′0min = 1.55) and cis-cup-3-C9 (εr′0min = 2.48) even though the inner shell layer of AuNPs and the core γ-FeOx NPs having a much higher εr′0min (=9.0) value. A slightly higher εr′0min for the latter is interesting since higher dielectric constant organics may be advantageous in energy devices. In all experiments, white light illumination led to a slight rise in temperature to 35–45 °C in the first 15 min. Slight increase of chamber temperatures away from 25 °C resulted in a small increase of the εr′ value in a similar degree among these two samples. The εr′ value was then remaining relatively steady during the rest of irradiation period up to 60 min. Upon turning-off the light at the end of 60-min irradiation, we observed a large sharp raise of the εr′ value for both samples while the chamber temperature dropped quickly back to 25 °C. Sharp increase of the permittivity at the light-off stage was found to reach a peak maximum (εr′max) value of 5.75 for 5-NPs (Figure 5(Bb)) and 3.75 for 6-NPs (Figure 5(Ba)). They can be accounted by a ratio of εr′max/εr′0min in a 2.35- and 2.42-fold increase for 5-NPs and 6-NPS, respectively. Similar calculations based on the ratio of εr′max/εr′60min (the εr′ value at time 60-min) were found to be 1.76- and 1.74-fold increase in the εr′ value for 5-NPs (εr′60min = 3.26, Figure 5(Bb)) and 6-NPs (εr′60min = 2.16, Figure 5(Ba)), respectively. Apparently, fabricated core-shell structural configurations were capable of inducing permittivity amplification at a RF-frequency of 1.0 GHz.
We also investigated the weight ratio (cis-cup-3-C9/γ-FeOx@AuNP)-dependent dielectric property of trilayered (γ-FeOx@AuNP)@{cis-cup-tris[C60(>DPAF-C9)]}n (5-NPs) for the comparison at the frequency of 1.0 GHz. Similar sharp increase of the permittivity at the light-off stage was found for all samples to reach a peak maximum (εr′max) value of 5.46 (Figure 6(Aa)), 6.60 (Figure 6(Ab)), 7.03 (Figure 6(Ac)), and 8.39 (Figure 6(Ad)) for the weight ratio of 1:2, 1:1, 2:1, and 3:1, respectively. They can be accounted by a corresponding ratio of εr′max/εr′0min in a 2.80- (εr′0min = 1.95), 2.75- (εr′0min = 2.40), 2.42- (εr′0min = 2.90), and 1.90-fold (εr′0min = 4.42) increase, respectively. Interestingly, a higher quantity of cis-cup-3-C9 applied led to a progressively increase of the initial dielectric εr′0min value up to 4.42 higher than that of many organic materials and polymers. This higher εr′0min value resulted in a lower degree of amplification εr′max/εr′0min. However, a high dielectric εr′max value of 8.39 (Figure 6(Ad)) achieved in organic-inorganic hybrid nanomaterials is significant for potential applications. Finally, similar calculations based on the ratio of εr′max/εr′60min were found to be 1.82- (εr′60min = 3.0, Figure 6(Aa)), 1.80- (εr′60min = 3.67, Figure 6(Ab)), 1.67- (εr′60min = 4.21, Figure 6(Ac)), and 1.42-fold (εr′60min = 5.91, Figure 6(Ad)) increase, respectively. Relative dielectric constant ratios of either εr′max/εr′0min or εr′max/εr′60min as the εr′ value shown at each peak maximum vs that at either 0 min or 60 min, respectively, in Figure 6B indicated a progressive increase of the degree of dielectric amplification upon the quantity increase of cis-cup-3-C9 up to a weight ratio of cis-cup-3-C9/γ-FeOx@AuNP as 2.0/1.0.
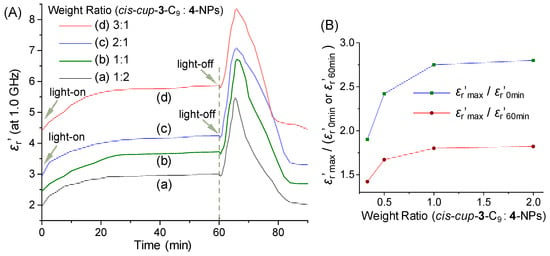
Figure 6.
(A) Irradiation time-dependent relative dielectric constant (εr′) amplification of trilayered core-shell nanoparticles of (γ-FeOx@AuNP)@{cis-cup-tris[C60(>DPAF-C9)]}n (5-NPs) with a different weight ratio of cis-cup-3-C9 vs γ-FeOx@AuNP (4-NPs) at the frequency of 1.0 GHz with a white LED light irradiation period of 60 min. (B) Relative dielectric constant (εr′) ratios of the values at peak maximum vs that at either 0 min or 60 min, showing a progressive increase of the degree of dielectric amplification.
3. Experimental Section
3.1. Chemicals and Reagents
Reagents of aluminum chloride (AlCl3), tris(dibenzylideneacetone)dipalladium(0) [Pd2(dba)3(0)], α-bromoacetyl bromide, rac-2,2′-bis(diphenylphosphino)-1,1′-binaphthyl (BINAP), sodium t-butoxide, and 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) were purchased from Aldrich Chemicals (St. Louis, MO, USA) and used without further purification. A C60 sample with a purity of 99.0% was purchased from Term USA, Inc. (Fort Bragg, CA, USA) The anhydrous grade solvent of THF was refluxed over sodium and benzophenone overnight and distilled under reduced pressure (10−1 mmHg). Sodium sulfate was used as the drying agent. The precursors 1,3,5-tris(N-phenylamino)benzene (TPAB) and 2-bromo-9,9-bis(3′,5′,5′-trimethyl-1′-hexyl)fluorene (BrF-C9) were synthesized according to our previous procedures [22].
3.2. Instruments for Spectroscopic Measurements
Infrared spectra were recorded as KBr pellets on a Thermo Nicolet AVATAR 370 FTIR spectrometer (Thermo Scientific Nicolet, Waltham, MA, USA). UV-vis spectra were recorded on a PerkinElmer Lambda 750 UV spectrometer (PerkinElmer, Shelton, CT, USA). 1H NMR and 13C NMR spectra were recorded on Bruker & Spectrospin Avance 500 (Bruker, Billerica, MA, USA). The high performance liquid chromatography (HPLC) was performed using the µPorasilTM 125 Å Column (10 µm, 35 × 300 mm, Waters, Milford, MA, USA) with a mixture of hexane-EtOAc as the mobile phase and the flow rate of 1.0 mL/min. The eluted compounds were detected by their UV absorption at λ 350 nm.
Cyclic voltammetry (CV) was record on EG&G Princeton Applied Research 263A Potentiostat/Galvanostat (Ametek Inc., Berwyn, PA, USA) using Pt metal as the working electrode, Ag/AgCl as the reference electrode, and Pt wire as the counter electrode at a scan rate of 10 mV/s. The solution for CV measurements was prepared in a concentration of 1.0 × 10−3 M in CH3CN–CH2Cl2, containing the electrolyte Bu4N+-PF6− (0.2 M). Transmission electron microscopy (TEM) measurements were carried out on Philips EM400T Transmission Electron Microscope (FEI Co., Hillsboro, OR, USA). In the sample preparation, a carbon-Copper film grid in a 200-mesh size was used as the supporting plate for directly coating of a nanoparticle sample in a solution of 1.0 × 10−6 M on the grid. All solvents were removed and dried by the freeze-dry technique to prevent the solvent-removal induced particle aggregation.
3.3. Synthesis of N1,N3,N5-Tris(9,9-di(3′,5′,5′-trimethyl-1′-hexyl)fluoren-2-yl)-1″,3″,5″-tris(phenylamino)benzene, tris(DPAF-C9) as 2-C9
Synthetic procedure of tris(DPAF-C9) was followed by and slightly modified from the recently reported methods [22]. A typical procedure was given as follows. A mixture of BrF-C9 (10.0 g, 20.3 mmol, excess), TPAB (1.16 g, 3.30 mmol), Pd2(dba)3(0) (0.023 g, 0.25 mol%), BINAP (0.046 g, 0.75 mol%), and sodium t-butoxide (1.94 g, 20.3 mmol) taken in anhydrous toluene (75 mL) was heated to refluxing temperature under nitrogen for a period of 72 h. After being cooled to room temperature, the reaction mixture was washed with water for three times and dried over sodium sulfate. A crude brown colored paste was obtained after evaporating the solvent. It was subjected to column chromatography purification using silica gel as the stationary phase and hexane-ethylacetate (9:1) as the eluent. The product of tris(DPAF-C9) was collected at Rf = 0.8 as light yellow solids in 83% yield (4.35 g).
Since the HPLC indicated a mixture of tris(DPAF-C9) stereoisomers, further separation and isolation were carried out by thin-layer chromatography (TLC), using silica gel as the stationary phase and hexane-ethylacetate (9.5:0.5, v/v) as the eluent. A mid-broad band at Rf 0.55−0.7 was identified as the tris(DPAF-C9) products that can be cut evenly into two parts at Rf 0.55–0.63 (as the bottom cut) and Rf 0.63–0.7 (as the top cut). Based on their corresponding 1H NMR spectra, the top cut was confirmed to be the pure cis-cup-tris(DPAF-C9) stereoisomer and the bottom cut was consisting of a mixture of trans-chair-tris(DPAF-C9) and cis-cup-tris(DPAF-C9) stereoisomers in the ratio of roughly 2:3. This fraction was subjected to further TLC separation and elution two more times using hexane-ethylacetate (9.7:0.3, v/v) as the eluent for the first run and the similar mixture (9.8:0.2, v/v) for the second run to obtain cis-cup-tris(DPAF-C9) in improved purity and the pure form of trans-chair-tris(DPAF-C9) fraction.
Spectroscopic data of cis-cup-tris(DPAF-C9): FT-IR (KBr) υmax 3064 (w, aromatic C-H stretching), 3038 (w), 3011 (w), 2956 (vs, aliphatic C-H stretching), 2908 (m), 2865 (s), 1596 (m), 1584 (s, C=C), 1495 (s, anti-symmetric deformations of CH3 groups and scissor vibrations of CH2 groups), 1468 (m), 1450 (s), 1363 (m, symmetric deformations of CH3 groups), 1296 (m, asymmetric stretching vibrations of C-N-C), 1246 (m, asymmetric stretching vibrations of C-N-C), 1215 (w), 1176 (w), 1157 (w), 1030 (w), 933 (w), 826 (w), 754 (m), 736 (s, C-H out-of-plan deformation), 710 (m, C-H out-of-plan deformation), 694 (m), and 508 (w) cm−1; UV-vis (EtOAc, 1.0 × 10−5 M) λmax (ε) 323 (5.62 × 104 L mol−1 cm−1) and 353 nm (5.65 × 104 L mol−1 cm−1); 1H NMR (500 MHz, CDCl3) δ 7.58 (s, 3H, br), 7.52 (d, 3H), 7.32–7.21 (m, 9H), 7.09–7.01 (m, 18H), 6.82 (t, 3H), 6.55 (s, 3H), 1.92–1.67 (m, 12H), 1.10 (m, 6H), and 0.97–0.39 (m, 96H); 13C NMR (500 MHz, CDCl3) δ 151.7, 150.0, 149.2, 147.7, 146.5, 141.2, 136.8, 129.2, 126.6, 126.6, 124.0, 122.9, 121.7, 120.0, 119.3, 115.6, 54.5, 51.0, 50.6, 50.6, 38.3, 38.1, 37.4, 37.6, 33.1, 32.7, 31.1, 30.2, 29.6, 29.4, 27.2, 22.9, and 22.4; MALDI–TOF MS calcd for C117H153N3, m/z 1600.21; found, m/z 1601.26 (MH+), 1602.30, 1603.29, 1260.95, 1092.81, 752.23 and 508.79 (PhN+H + fluorene-C9).
Spectroscopic data of trans-chair-tris(DPAF-C9): FT-IR (KBr) υmax 3062 (w, aromatic C-H stretching), 3037 (w), 3012 (w), 2955 (vs, aliphatic C-H stretching), 2925 (vs., aliphatic C-H stretching), 2855 (s), 1630 (vs), 1588 (s, C=C), 1495 (s, anti-symmetric deformations of CH3 groups and scissor vibrations of CH2 groups), 1450 (s), 1362 (m, symmetric deformations of CH3 groups), 1294 (m, asymmetric stretching vibrations of C-N-C), 1250 (m, asymmetric stretching vibrations of C-N-C), 1218 (w), 1158 (w), 1035 (w), 803 (w), 763 (m), 736 (m, C-H out-of-plan deformation), 709 (w, C-H out-of-plan deformation), 691 (m), and 510 (w) cm−1; UV-vis (EtOAc, 1.0 × 10−5 M) λmax (ε) 322 (5.50 × 104) and 353 nm (5.68 × 104 L mol−1 cm−1); 1H NMR (500 MHz, CDCl3) δ 7.58 (s, 3H, br), 7.51 (d, 3H), 7.34–7.20 (m, 9H), 7.09–7.01 (m, 18H), 6.84 (m, 3H), 6.55 (m, 2H), 6.48 (m, 1H), 2.02–1.67 (m, 12H), 1.11 (m, 6H), and 0.97–0.39 (m, 96H); 13C NMR [similar to those of cis-cup-tris(DPAF-C9)]; MALDI−TOF MS calcd for C117H153N3, m/z 1600.21; found, m/z 1601.37 (MH+), 1602.36, 1603.35, 1604.40, 1260.00, 1093.86, and 508.73 (PhN+H + fluorene-C9).
3.4. Synthesis of N1,N3,N5-Tris(7-a-bromoacetyl-9,9-di(3′,5′,5′-trimethyl-1′-hexyl)fluoren-2-yl)-1″,3″,5″-tris(phenylamino)benzene, cis-cup-Tris(BrDPAF-C9)
To a suspension of AlCl3 (1.0 g, 7.5 mmol) in 1,2-dichloroethane (40 mL) at 0 °C was added cis-cup-tris(DPAF-C9) (0.7 g, 0.44 mmol) with vigorously stirring. The compound of α-bromoacetyl bromide (1.0 g, 5.0 mmol) was then slowly added over 10 min while maintaining the temperature between 0–10 °C. The mixture was warmed to ambient temperature and stirred overnight. The reaction was quenched by slow addition of water (50 mL) while maintaining the temperature below 45 °C. The organic layer was washed sequentially with dil. HCl (1.0 N, 50 mL) and water (50 mL × 2), and dried over sodium sulfate and then concentrated in vacuo to give the crude product as viscous yellow semi-solids. It was purified by column chromatography (silica gel) using hexane–EtOAc (9:1, v/v) as eluent to afford tris(Br-DPAF-C9) in 53% yield (0.46 g). Spectroscopic data: FT-IR (KBr) υmax 3066 (w), 3035 (w), 2954 (s), 2907 (m), 2864 (m), 1733 (w), 1675 (s), 1594 (s), 1584 (s), 1492 (m), 1464 (m), 1429 (m), 1393 (w), 1364 (w), 1343 (w), 1288 (s), 1262 (m), 1205 (w), 1180 (m), 1107 (w), 1022 (w), 819 (m), 748 (m), 713 (m), and 696 (m) cm−1; UV-vis (EtOAc, 1.0 × 10−5 M) λmax (ε) 304 (8.88 × 104 L mol−1 cm−1) and 395 nm (1.10 × 105 L mol−1 cm−1); 1H NMR (500 MHz, CDCl3) δ 7.94 (m, 3H), 7.88 (m, 3H), 7.63 (m, 3H), 7.56 (m, 3H), 7.22−6.92 (m, 21H), 6.56 (m, 3H), 4.46 (m, 6H), 2.11−1.67 (m, 12H), 1.10 (m, 6H), 0.99–0.26 (m, 96H).
3.5. Synthesis of N1,N3,N5-Tris(7-(1,2-dihydro-1,2-methanofullerene[60]-61-carbonyl)-9,9-di(3′,5′,5′-trimethyl-1′-hexyl)fluoren-2-yl)-1″,3″,5″-tris(phenylamino)benzene, cis-cup-Tris[C60>(DPAF-C9)] as cis-cup-3-C9
A homogeneous solution of C60 (0.70 g, 0.97 mmol)) in anhydrous toluene (700 mL) was prepared by ultrasonication for a period of 1.0 h that was then stirred overnight under nitrogen. To this was added by cis-cup-tris(BrDPAF-C9) (0.40 g, 0.20 mmol) and 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU, 0.21 g, 1.38 mmol) sequentially and stirred at room temperature for a period of 8.0 h. At the end of stirring, the reaction mixture was concentrated to a 10%-volume. Methanol (100 mL) was added to effect precipitation of the crude product, which was isolated by centrifugation. Further purification by column chromatography (silica gel) using a solvent mixture of hexane–toluene (3:2) as the eluent afforded cis-cup-tris[C60>(DPAF-C9)] as brown solids in 68% yield (0.52 g). Spectroscopic data: FT-IR (KBr) υmax 2951 (m), 2923 (m), 2861 (w), 1681 (m), 1627 (s), 1594 (s), 1585 (s), 1490 (m), 1462 (m), 1429 (w), 1361 (w), 1317 (m), 1292 (m), 1247 (m), 1213 (m), 1184 (m), 1093 (w), 1035 (w), 908 (m), 819 (m), 732 (s), 698 (m), 575 (w), and 529 (s) cm−1; UV-vis (toluene, 1.0 × 10−5 M) λmax (ε) 335 nm (1.76 × 105 L mol−1 cm−1) and 402 nm (shoulder band, 4.19 × 104 L mol−1 cm−1); 1H NMR (500 MHz, CDCl3) δ 8.46 (m, 3H), 8.30 (m, 3H), 7.81 (m, 3H), 7.63 (m, 3H), 7.31−6.70 (m, 21H), 6.60 (m, 3H), 5.67 (m, 3H), 1.92−1.67 (m, 12H), 1.14 (m, 6H), 0.70 (m, 96H); 13C NMR (500 MHz, CDCl3) δ 148.67, 148.56, 148.46, 147.89, 147.70, 147.18, 145.96, 145.82, 145.72, 145.68, 145.58, 145.47, 145.24, 145.13, 145.05, 144.73, 144.60, 144.48, 144.35, 144.28, 144.18, 144.14, 144.03, 143.94, 143.87, 143.72, 143.50, 143.41, 143.20, 142.92, 142.65, 142.50, 141.64, 141.35, 139.92, 137.07, 134.75, 134.03, 133.86, 129.61, 129.47, 124.34, 124.13, 123.53, 119.78, 116.61, 84.81, 72.88, 55.60, 51.55, 51.32, 51.02, 50.89, 45.07, 38.33, 38.02, 37.85, 37.67, 33.33, 31.31, 30.37, 29.93, 27.66, and 22.99.
3.6. Preparation of cis-cup-Tris[C60>(DPAF-C9)]-Encapsulated γ-FeOx@AuNP Yielding Trilayered Core-Shell Nanoparticles, (γ-FeOx@AuNP)@{cis-cup-tris[C60(>DPAF-C9)]}n (5-NPs)
Nanoparticles of γ-FeOx and the corresponding plasmonic metallic gold-coated γ-FeOx@AuNP nanoparticles (4-NPs) were synthesized according to a slightly modified procedure reported previously [14,15]. A large scale preparation was carried out for the subsequent fabrication procedure using the same batch of materials to give consistency of γ-FeOx@AuNP characteristics in terms of the particle size and the Au-layer thickness. Spectroscopy data of 4-NPs: FT-IR (KBr) νmax 3027 (w), 2956 (w), 2923 (m), 2856 (m), 1631 (s), 1492 (m), 1461 (s), 1378 (m), 1328 (w), 1263 (w), 1166 (w), 1128 (w), 1076 (w), 1037 (w), 804 (m), 728 (m), and 588 (s) cm−1.
Encapsulation of cis-cup-tris[C60>(DPAF-C9)] on nanoparticles of γ-FeOx@AuNP was carried out by the method as follows. A mixture of γ-FeOx@AuNPs (100 mg) and cis-cup-3-C9 in a predefined weight ratio amount were dissolved in toluene (30 mL) with stirring for 30 min and then ultrasonicated for an additional >30 min until a clear solution being obtained showing a homogenized nanoparticles dispersion. The solution was concentrated via rotary evaporation to less than 3.0 mL in volume to increase the molecular contact of cis-cup-3-C9 to 4-NPs. It was then diluted by toluene to a volume of 30 mL with stirring and subsequent sonication for 10 min to dissolve cis-cup-3-C9 fully. All encapsulated magnetic nanoparticles were physically removed from the container solution by the assistance of an external permanent magnet. The separated nanoparticles were washed repeatedly by ethanol and ether, followed by drying in vacuo to afford dark brown solids of 5-NPs. Spectroscopy data: FT-IR (KBr) νmax 3089 (w), 3064 (w), 3031 (w), 2952 (m, aromatic C-H stretching), 2927 (s, aliphatic C-H stretching), 2860 (m), 1679 (m, -C=O), 1636 (m), 1587 (s, -C=C-), 1492 (m), 1463 (m), 1430 (m), 1363 (w), 1291 (m, asymmetric stretching vibrations of C-N-C), 1247 (w), 1214 (m), 1187 (m), 1126 (w), 1091 (w), 1035 (m), 904 (m), 815 (w), 730 (m, C-H out-of-plan deformation), 695 (m), 576 (m), and 526 (s, C60>) cm−1.
3.7. Measurements of Dielectric Properties and Permittivity as Relative Dielectric Constant (εr′)
Microwave-responsive dielectric property measurements were carried out by the use of an Agilent Network Analyzer (Agilent Technologies, Inc., Santa Clara, CA, USA) equipped with an open-ended Agilent 85070E dielectric probe kit in a detection range of 200 MHz to 50 GHz. Prior to each measurement, the system base-line calibration was conducted by using open-ended, close-ended, and attenuated calibrators to ensure the minimization of signal instability that was induced by the cable connection and system drift errors. In the data collection, a complex scattering parameter, defined as S11, was measured and converted to relative complex electric dielectric constant values using Agilent 85071E Materials Measurement Software. This complex form of dielectric constant (ε*) consists of both a real (εr′) and an imaginary (εr″) part. The former term represents the value of dielectric constant and the latter is defined as the loss factor.
All permittivity measurements were performed in a custom-built chamber that was conducted under a circumferentially uniform illumination environment. The uniformness was achieved by the installation of a reflective half-circular aluminum plate at the back-wall side surrounding the testing tube which was located at the center of the chamber. The light source used in this measurement included a collimated white LED light with an output power of 2.0 W (Prizmatix, Southfield, MI, USA). The light beam was allowed to pass through a small window opening at the front side of the chamber for the sample irradiation. Some reflected light beams were designed to refocus from the back-side aluminum mirror plate back to the sample-containing tube. We installed four small fans with two at the top and one at each side-wall of the chamber to control and prevent the temperature increase inside the chamber during the experiment. The illumination period was fixed at 60 min. Poly(dimethylsiloxane) (PDMS, 1.0 g) semi-solid was applied as a polymer matrix host which is capable of forming a paste-like material sample in mixing with a predefined quantity of nanoparticles. In a typical preparation, a mixture of PDMS and core-shell nanoparticle materials (100 mg) were blended by dissolving both components in ethyl acetate (20 mL) in a testing tube under sonication. It was followed by solvent evaporation to yield paste-like semi-solid materials filling in the tube.
4. Conclusions
We designed and synthesized novel 3D-configurated stereoisomers cis-cup-tris[C60>(DPAF-C9)] and trans-chair-tris[C60>(DPAF-C9)] in good yields. Efficient chromatographic separation was carried out to obtain both stereoisomers in a pure form with their corresponding nanostructure verified by spectroscopic techniques. The former with three C60> cages per molecule facing at the same side of the geometrical molecular cup-shape was proposed to provide excellent binding interaction forces at the gold surface of core-shell γ-FeOx@AuNP nanoparticles to direct the subsequent formation of a fullerene cage array (defined as fullerosome). Upon photoactivation of the Au-layer and cis-cup-tris[C60>(DPAF-C9)] itself, the level of photoinduced intramolecular e−-transfer from DPAF to C60> moieties was found to be largely enhanced by the accumulated plasmonic resonance energy at the near-field surface. Distribution of resulting negative charges along the outer (C60>)-derived fullerosome shell layer of the trilayered NPs was correlated with the detected photoswitchable dielectric amplification changes using white LED light at 1.0 GHz. This class of new materials exhibits potential in microwave applications as its photoswitching responsive wavelength range is being extended to cover WiFi-bands over 2.4/3.6/5.0 GHz.
Author Contributions
All authors contribute a significant effort on this work. H.Y. and M.W. carried out the main synthetic works, spectroscopic characterization, data analysis, and permittivity measurements; T.Y. setup the permittivity and reflectivity measurements and carried out dielectric properties conversion; T.Y., L.-S.T. and L.Y.C. participated in the discussion and experimental studies and contributed to a part of manuscript writing; All authors read and approved the final manuscript.
Funding
The authors at UML thank the financial support of Air Force Office of Scientific Research (AFOSR) under the grant number FA9550-14-1-0153.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Sharma, M.; Singh, M.P.; Srivastava, C.; Madras, G.; Bose, S. Poly(vinylidene fluoride)-based flexible and lightweight materials for attenuating microwave radiations. ACS Appl. Mater. Interfaces 2014, 6, 21151–21160. [Google Scholar] [CrossRef] [PubMed]
- Gadinski, M.R.; Chanthad, C.; Han, K.; Dong, L.; Wang, Q. Synthesis of poly(vinylidene fluoride-co-bromotrifluoroethylene) and effects of molecular defects on microstructure and dielectric properties. Polym. Chem. 2014, 5, 5957–5966. [Google Scholar] [CrossRef]
- Wei, R.; Li, K.; Ma, J.; Zhang, H.; Liu, X. Improving dielectric properties of polyarylene ether nitrile with conducting polyaniline. J. Mater. Sci. Mater. Electron. 2016, 27, 9565–9571. [Google Scholar] [CrossRef]
- You, Y.; Du, X.; Mao, H.; Tang, X.; Wei, R.; Liu, X. Synergistic enhancement of mechanical, crystalline and dielectric properties of polyarylene ether nitrile-based nanocomposites by unidirectional hot stretching–quenching. Polym. Int. 2017, 66, 1151–1158. [Google Scholar] [CrossRef]
- Lei, D.; Runt, J.; Safari, A.; Newnham, R.E. Dielectric properties of azo dye-poly(methyl methacrylate) mixtures. Macromolules 1987, 20, 1797–1801. [Google Scholar] [CrossRef]
- Puli, V.S.; Pradhan, D.K.; Riggs, B.C.; Chrisey, D.B.; Katiyar, R.S. Investigations on structure, ferroelectric, piezoelectric and energy storage properties of barium calcium titanate (BCT) ceramics. J. Alloys Compd. 2014, 584, 369–373. [Google Scholar] [CrossRef]
- Kim, K.K.; Hsu, A.; Jia, X.; Kim, S.M.; Shi, Y.; Dresselhaus, M.; Palacios, T.; Kong, J. Synthesis and characterization of hexagonal boron nitride film as a dielectric layer for graphene devices. ACS Nano 2012, 6, 8583–8590. [Google Scholar] [CrossRef] [PubMed]
- Wypych, A.; Bobowska, I.; Tracz, M.; Opasinska, A.; Kadlubowski, S.; Krzywania-Kaliszewska, A.; Grobelny, J.; Wojciechowski, P. Dielectric properties and characterisation of titanium dioxide obtained by different chemistry methods. J. Nanomater. 2014, 2014, 124814. [Google Scholar] [CrossRef]
- You, Y.; Han, W.; Tu, L.; Wang, Y.; Wei, R.; Liu, X. Doublelayer core/shell-structured nanoparticles in polyarylene ether nitrile-based nanocomposites as flexible dielectric materials. RSC Adv. 2017, 7, 29306–29311. [Google Scholar] [CrossRef]
- Chu, B.; Zhou, X.; Ren, K.; Neese, B.; Lin, M.; Wang, Q.; Bauer, F.; Zhang, Q.M. A dielectric polymer with high electric energy density and fast discharge speed. Science 2006, 313, 334–336. [Google Scholar] [CrossRef] [PubMed]
- Khanchaitit, P.; Han, K.; Gadinski, M.R.; Li, Q.; Wang, Q. Ferroelectric polymer networks with high energy density and improved discharged efficiency for dielectric energy storage. Nat. Commun. 2013, 4, 2845. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Yao, F.-Z.; Liu, Y.; Zhang, G.; Wang, H.; Wang, Q. High-temperature dielectric materials for electrical energy storage. Annu. Rev. Mater. Res. 2018, 48, 219–243. [Google Scholar] [CrossRef]
- Brebels, J.; Manca, J.V.; Lutsen, L.; Vanderzande, D.; Maes, W. High dielectric constant conjugated materials for organic photovoltaics. J. Mater. Chem. A 2017, 5, 24037–24050. [Google Scholar] [CrossRef]
- Wang, M.; Yu, T.; Tan, L.-S.; Urbas, A.; Chiang, L.Y. Tunability of rf-responses by plasmonic dielectric amplification using branched e−-polarizable C60-adducts on magnetic nanoparticles. J. Phys. Chem. C 2016, 120, 17711–17721. [Google Scholar] [CrossRef]
- Wang, M.; Su, C.; Yu, T.; Tan, L.-S.; Hu, B.; Urbas, A.; Chiang, L.Y. Novel photoswitchable dielectric properties on nanomaterials of electronic core-shell γ-FeOx@Au@fullerosomes for GHz frequency applications. Nanoscale 2016, 8, 6589–6599. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Yu, T.; Tan, L.-S.; Urbas, A.; Chiang, L.Y. Enhancement of photoswitchable dielectric property by conducting electron donors on plasmonic core-shell gold-fluorenyl C60 nanoparticles. J. Phys. Chem. C 2018, 122, 12512–12523. [Google Scholar] [CrossRef]
- Padmawar, P.A.; Canteenwala, T.; Tan, L.-S.; Chiang, L.Y. Synthesis and characterization of photoresponsive diphenylaminofluorene chromophore adducts of [60]fullerene. J. Mater. Chem. 2006, 16, 1366–1378. [Google Scholar] [CrossRef]
- Jeon, S.; Wang, M.; Ji, W.; Tan, L.-S.; Cooper, T.; Chiang, L.Y. Broadband two-photon absorption characteristics of highly photostable fluorenyl-dicyanoethylenylated [60]fullerene dyads. Molecules 2016, 21, 647. [Google Scholar] [CrossRef] [PubMed]
- Padmawar, P.A.; Rogers, J.O.; He, G.S.; Chiang, L.Y.; Canteenwala, T.; Tan, L.-S.; Zheng, Q.; Lu, C.; Slagle, J.E.; Danilov, E.; et al. Large cross-section enhancement and intramolecular energy transfer upon multiphoton absorption of hindered diphenylaminofluorene-C60 dyads and triads. Chem. Mater. 2006, 18, 4065–4074. [Google Scholar] [CrossRef]
- Luo, H.; Fujitsuka, M.; Araki, Y.; Ito, O.; Padmawar, P.; Chiang, L.Y. Inter- and intramolecular photoinduced electron-transfer processes between C60 and diphenylaminofluorene in solutions. J. Phys. Chem. B 2003, 107, 9312–9318. [Google Scholar] [CrossRef]
- Lee, Y.-T.; Wang, M.; Kokubo, K.; Kang, N.-G.; Wolf, L.; Tan, L.-S.; Chen, C.-T.; Chiang, L. New 3D-stereoconfigurated cis-tris(fluorenylphenylamino)-benzene with large steric hindrance to minimize π–π stacking in thin-film devices. Dyes Pigm. 2018, 149, 377–386. [Google Scholar] [CrossRef]
- Kang, N.-G.; Kokubo, K.; Jeon, S.; Wang, M.; Lee, C.-L.; Canteenwala, T.; Tan, L.-S.; Chiang, L. Synthesis and photoluminescent properties of geometrically hindered cis-tris(diphenyl-aminofluorene) as precursors to light-emitting devices. Molecules 2015, 20, 4635–4654. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Jeon, S.; Su, C.; Yu, T.; Tan, L.-S.; Chiang, L.Y. Synthesis of photoswitchable magnetic Au–fullerosome hybrid nanomaterials for permittivity enhancement applications. Molecules 2015, 20, 14746–14760. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Hauck, T.; El-Khouly, M.E.; Padmawar, P.A.; Canteenwala, T.; Pritzker, K.; Ito, O.; Chiang, L.Y. Self-assembled photoresponsive amphiphilic diphenylaminofluorene-C60 conjugate vesicles in aqueous solution. Langmuir 2005, 21, 3267–3272. [Google Scholar] [CrossRef] [PubMed]
- Kuzume, A.; Herrero, E.; Feliu, J.M.; Nichols, R.J.; Schiffrin, D.J. Fullerene monolayers adsorbed on high index gold single crystal surfaces. Phys. Chem. Chem. Phys. 2004, 6, 619–625. [Google Scholar] [CrossRef]
- Islam, M.T.; Molugu, S.K.; Cooke, P.H.; Noveron, J.C. Fullerene stabilized gold nanoparticles. New J. Chem. 2015, 39, 5923. [Google Scholar] [CrossRef]
- Agilent Technologies. Basics of Measuring the Dielectric Properties of Materials; Agilent Technical Note 5989-2589EN; Agilent Technologies: Santa Clara, CA, USA, 2006; pp. 17–18. [Google Scholar]
- Nicolson, A.M.; Ross, G.F. Measurement of the intrinsic properties of materials by time-domain techniques. IEEE Trans. Instrum. Meas. 1970, 19, 377–382. [Google Scholar] [CrossRef]
- Pillai, S.; Green, M.A. Plasmonics for photovoltaic applications. Sol. Energy Mater. Sol. Cells 2010, 94, 1481–1486. [Google Scholar] [CrossRef]
- Losurdo, M.; Giangregorio, M.M.; Bianco, G.V.; Sacchetti, A.; Capezzuto, P.; Bruno, G. Enhanced absorption in Au nanoparticles/a-Si:H/c-Si heterojunction solar cells exploiting Au surface plasmon resonance. Sol. Energy Mater. Sol. Cells 2009, 93, 1749–1754. [Google Scholar] [CrossRef]
- Coronado, E.A.; Ezequiel, R.E.; Stefani, F.D. Optical properties of metallic nanoparticles: Manipulation light, heat and forces at the nanoscale. Nanoscale 2011, 3, 4042–4059. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of all synthetic compounds including cis-cup-tris(BrDPAF-C9) and cis-cup-tris[C60>(DPAF-C9)] are available from the authors.. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).