Chemical Composition and Antioxidant Characteristic of Traditional and Industrial Zhenjiang Aromatic Vinegars during the Aging Process
Abstract
1. Introduction
2. Results and Discussion
2.1. Proximate Composition of Traditional Zhenjiang Aromatic Vinegar (TZAV) and Industrial Zhenjiang Aromatic Vinegar (IZAV)
2.2. Organic Acid Contents in TZAV and IZAV
2.3. Total Phenolic, Flavonoid Contents and Antioxidant Activities in TZAV and IZAV
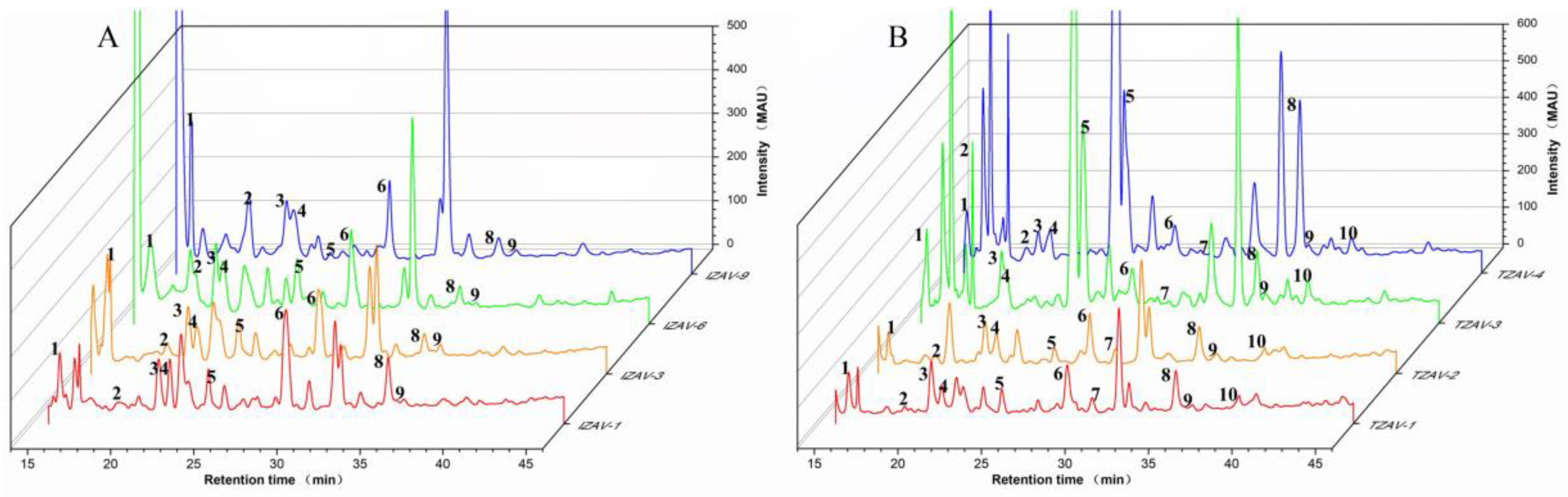
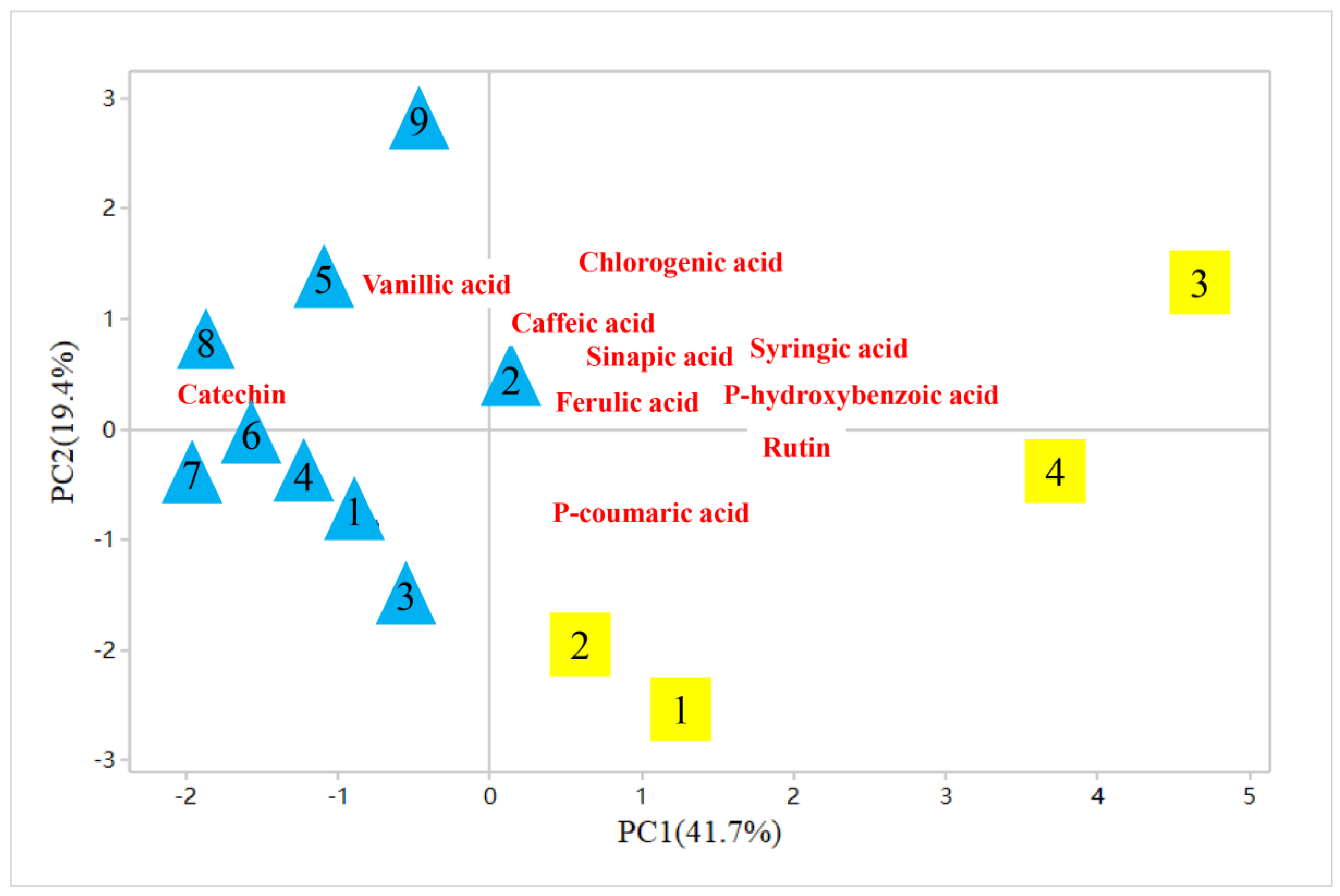
2.4. Phenolic Compounds and Their Contributions to Total Antioxidant Activity (TAA) in TZAV and IZAV
3. Materials and Methods
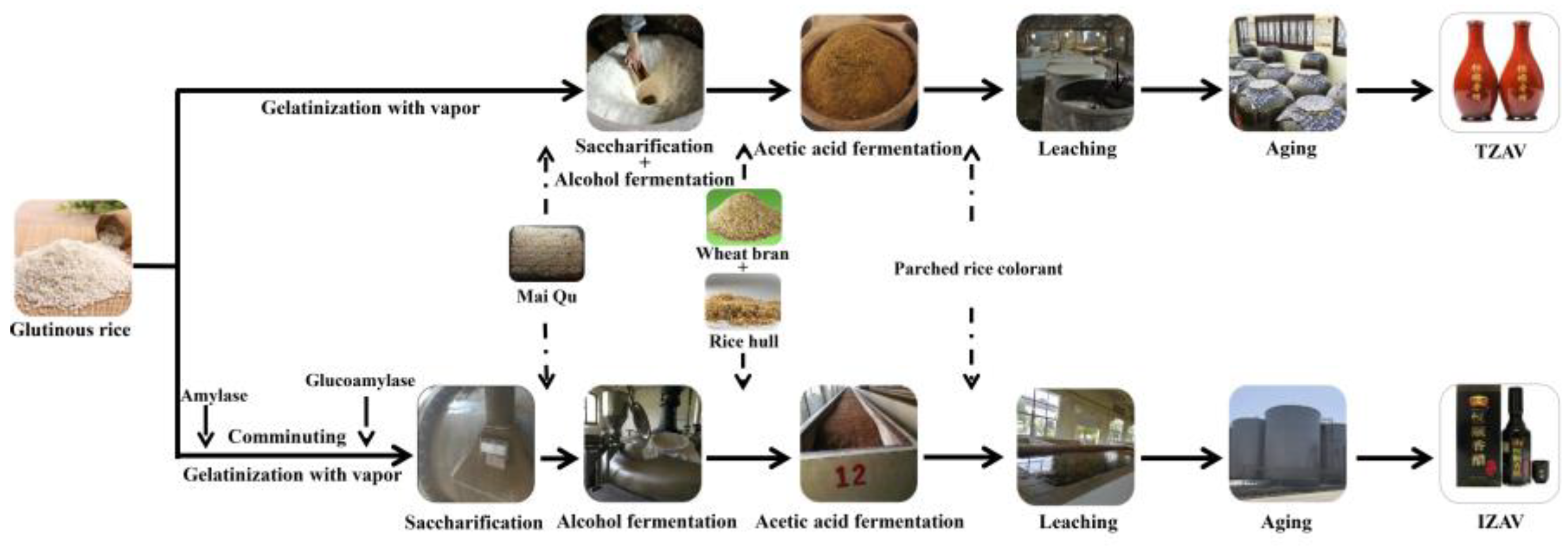
3.1. Samples and Reagents
3.2. Proximate Compositions
3.3. Analysis of Organic Acids
3.4. Measurement of Total Phenolic and Flavonoid Contents
3.5. Determination of TAA
3.6. Identification of Phenolic Compositions
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ABTS | 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid |
| ANOVA | analysis of variance |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| FRAP | ferric reducing antioxidant power |
| GAE | gallic acids equivalents |
| HPLC | high performance liquid chromatography |
| IZAV | industrial Zhenjiang aromatic vinegar |
| LOD | limits of detection |
| LOQ | limits of detection quantitation |
| PC | principal components |
| PCA | principal component analysis |
| RE | rutin equivalents |
| TAA | total antioxidant activity |
| TEAC | Trolox equivalent antioxidant capacity |
| TFC | total flavonoid content |
| TPC | total phenolic content |
| TEAC | Trolox equivalent antioxidant capacity |
| TZAV | traditional Zhenjiang aromatic vinegar |
| ZAV | Zhenjiang aromatic vinegar |
References
- Mas, A.; Torija, M.J.; García-Parrilla, M.C.; Troncoso, A.M. Acetic acid bacteria and the production and quality of wine vinegar. Sci. World J. 2014, 394671. [Google Scholar] [CrossRef] [PubMed]
- Tesfaye, W.; Morales, M.L.; García-Parrilla, M.C.; Troncoso, A.M. Wine vinegar: Technology, authenticity and quality evaluation. Trends Food Sci. Technol. 2002, 13, 12–21. [Google Scholar] [CrossRef]
- Liu, D.; Zhu, Y.; Beeftink, R.; Ooijkaas, L.; Rinzema, A.; Chen, J.; Tramper, J. Chinese vinegar and its solid-state fermentation process. Food Res. Int. 2004, 20, 407–424. [Google Scholar] [CrossRef]
- Nie, Z.; Zheng, Y.; Wang, M.; Han, Y.; Wang, Y.; Luo, J.; Niu, D. Exploring microbial succession and diversity during solid-state fermentation of Tianjin duliu mature vinegar. Bioresour. Technol. 2013, 148, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Tao, W.; Ao, Z. Antioxidant activity of vinegar melanoidins. Food Chem. 2007, 102, 841–849. [Google Scholar] [CrossRef]
- Xu, W.; Xu, Q.; Chen, J.; Lu, Z.; Xia, R.; Li, G.; Xu, Z.; Ma, Y. Ligustrazine formation in Zhenjiang aromatic vinegar: Changes during fermentation and storing process. J. Sci. Food Agric. 2011, 91, 1612–1617. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Gan, J.; Yu, Y.; Zhu, S.; Yin, L.; Cheng, Y. Effect of laboratory-scale decoction on the antioxidative activity of Zhenjiang aromatic vinegar: The contribution of melanoidins. J. Funct. Foods 2016, 21, 75–86. [Google Scholar] [CrossRef]
- Zhou, Z.; Liu, S.; Kong, X.; Ji, Z.; Han, X.; Wu, J.; Mao, J. Elucidation of the aroma compositions of Zhenjiang aromatic vinegar using comprehensive two dimensional gas chromatography coupled to time-of-flight mass spectrometry and gas chromatography-olfactometry. J. Chromatogr. A 2017, 1487, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Budak, N.H.; Aykin, E.; Seydim, A.C.; Greene, A.K.; Guzel-Seydim, Z.B. Functional properties of vinegar. J. Food Sci. 2014, 79, 757–764. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Chen, T.; Giudici, P.; Chen, F. Vinegar functions on health: Constituents, sources, and formation mechanisms. Compr. Rev. Food. Sci. Food Saf. 2016, 15, 1124–1138. [Google Scholar] [CrossRef]
- Dávalos, A.; Bartolomé, B.; Gómez-Cordovés, C. Antioxidant properties of commercial grape juices and vinegars. Food Chem. 2005, 93, 325–330. [Google Scholar] [CrossRef]
- Verzelloni, E.; Tagliazucchi, D.; Conte, A. Changes in major antioxidant compounds during aging of traditional balsamic vinegar. J. Food Biochem. 2010, 34, 152–171. [Google Scholar] [CrossRef]
- Pazuch, C.M.; Siepmann, F.B.; Canan, C.; Colla, E. Vinegar: Functional aspects. Científica 2015, 43, 302–308. [Google Scholar] [CrossRef]
- Samad, A.; Azlan, A.; Ismail, A. Therapeutic effects of vinegar: A review. Curr. Opin. Food Sci. 2016, 8, 56–61. [Google Scholar] [CrossRef]
- García-Parrilla, M.C.; Heredia, F.J.; Troncoso, A.M. Sherry wine vinegars: Phenolic composition changes during aging. Food Res. Int. 1999, 32, 433–440. [Google Scholar] [CrossRef]
- Palacios, V.; Valcárcel, M.; Caro, I.; Pérez, L. Chemical and biochemical transformations during the industrial process of sherry vinegar aging. J. Agric. Food. Chem. 2002, 50, 4221–4225. [Google Scholar] [CrossRef] [PubMed]
- Tagliazucchi, D.; Verzelloni, E.; Conte, A. Contribution of melanoidins to the antioxidant activity of traditional balsamic vinegar during aging. J. Food Biochem. 2010, 34, 1061–1078. [Google Scholar] [CrossRef]
- Lu, Z.M.; Xu, W.; Yu, N.H.; Zhou, T.; Li, G.Q.; Shi, J.S.; Xu, Z.H. Recovery of aroma compounds from Zhenjiang aromatic vinegar by supercritical fluid extraction. Int. J. Food Sci. Technol. 2011, 46, 1508–1514. [Google Scholar] [CrossRef]
- Yu, Y.J.; Lu, Z.M.; Yu, N.H.; Xu, W.; Li, G.Q.; Shi, J.S.; Xu, Z.H. HS-SPME/GC-MS and chemometrics for volatile composition of Chinese traditional aromatic vinegar in the Zhenjiang region. J. Inst. Brew. 2012, 118, 133–141. [Google Scholar] [CrossRef]
- Wang, Z.M.; Lu, Z.M.; Yu, Y.J.; Li, G.Q.; Shi, J.S.; Xu, Z.H. Batch-to-batch uniformity of bacterial community succession and flavor formation in the fermentation of Zhenjiang aromatic vinegar. Food Microbiol. 2015, 50, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Cocchi, M.; Lambertini, P.; Manzini, D.; Marchetti, A.; Ulrici, A. Determination of carboxylic acids in vinegars and in Aceto Balsamico Tradizionale di Modena by HPLC and GC methods. J. Agric. Food Chem. 2002, 50, 5255–5261. [Google Scholar] [CrossRef] [PubMed]
- Consonni, R.; Gatti, A. 1H NMR studies on Italian balsamic and traditional balsamic vinegars. J. Agric. Food Chem. 2004, 52, 3446–3450. [Google Scholar] [CrossRef] [PubMed]
- Consonni, R.; Cagliani, L.R.; Benevelli, F.; Spraul, M.; Humpfer, E.; Stocchero, M. NMR and chemometric methods: A powerful combination for characterization of balsamic and traditional balsamic vinegar of Modena. Anal. Chim. Acta 2008, 611, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Cirlini, M.; Caligiani, A.; Palla, L.; Palla, G. HS-SPME/GC-MS and chemometrics for the classification of balsamic vinegars of Modena of different maturation and ageing. Food Chem. 2011, 124, 1678–1683. [Google Scholar] [CrossRef]
- Bertelli, D.; Maietti, A.; Papotti, G.; Tedeschi, P.; Bonetti, G.; Graziosi, R.; Brandolini, V.; Plessi, M. Antioxidant activity, phenolic compounds, and NMR characterization of balsamic and traditional balsamic vinegar of Modena. Food Anal. Methods 2015, 8, 371–379. [Google Scholar] [CrossRef]
- Papotti, G.; Bertelli, D.; Graziosi, R.; Maietti, A.; Tedeschi, P.; Marchetti, A.; Plessi, M. Traditional balsamic vinegar and balsamic vinegar of Modena analyzed by nuclear magnetic resonance spectroscopy coupled with multivariate data analysis. LWT Food Sci. Technol. 2015, 60, 1017–1024. [Google Scholar] [CrossRef]
- Budak, H.N.; Guzel-Seydim, Z.B. Antioxidant activity and phenolic content of wine vinegars produced by two different techniques. J. Sci. Food Agric. 2010, 90, 2021–2026. [Google Scholar] [CrossRef] [PubMed]
- Budak, N.H.; Doguc, D.K.; Savas, C.M.; Seydim, A.C.; Tas, T.K.; Ciris, M.I.; Guzel-Seydim, Z.B. Effects of apple cider vinegars produced with different techniques on blood lipids in high-cholesterol-fed rats. J. Agric. Food Chem. 2011, 59, 6638–6644. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, I.; Caliskan, O.; Tornuk, F.; Ozcan, N.; Yalcin, H.; Baslar, M.; Sagdic, O. Antioxidant, antimicrobial, mineral, volatile, physicochemical and microbiological characteristics of traditional home-made Turkish vinegars. LWT Food Sci. Technol. 2015, 63, 144–151. [Google Scholar] [CrossRef]
- Lalou, S.; Hatzidimitriou, E.; Papadopoulou, M.; Kontogianni, V.G.; Tsiafoulis, C.G.; Gerothanassis, I.P.; Tsimidou, M.Z. Beyond traditional balsamic vinegar: Compositional and sensorial characteristics of industrial balsamic vinegars and regulatory requirements. J. Food Compos. Anal. 2015, 43, 175–184. [Google Scholar] [CrossRef]
- Liu, F.; He, Y.; Wang, L. Comparison of calibrations for the determination of soluble solids content and pH of rice vinegars using visible and short-wave near infrared spectroscopy. Anal. Chim. Acta 2008, 610, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Liu, F.; Kong, W.; Sun, D.W.; He, Y.; Qiu, Z. Measurement of soluble solid contents and pH of white vinegars using VIS/NIR spectroscopy and least squares support vector machine. Food Bioprocess Technol. 2014, 7, 54–61. [Google Scholar] [CrossRef]
- Willett, W.C.; Stampfer, M.J. Current evidence on healthy eating. Annu. Rev. Public Health 2013, 34, 77–95. [Google Scholar] [CrossRef] [PubMed]
- Chiu, Y.H.; Chavarro, J.F.; Souter, I. Diet and female fertility: Doctor, what should I eat? Fertil. Steril. 2018, 110, 560–569. [Google Scholar] [CrossRef] [PubMed]
- Falcone, P.M.; Chillo, S.; Giudici, P.; Nobile, M.A.D. Measuring rheological properties for applications in quality assessment of traditional balsamic vinegar: Description and preliminary evaluation of a model. J. Food Eng. 2007, 80, 234–240. [Google Scholar] [CrossRef]
- Wang, Z.; Li, T.; Liu, F.; Zhang, C.; Ma, H.; Wang, L.; Zhao, S. Effects of ultrasonic treatment on the maturation of Zhenjiang vinegar. Ultrason. Sonochem. 2017, 39, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Ding, J.; Cai, J.; Zhang, J. Rapid measurement of total acid content (TAC) in vinegar using near infrared spectroscopy based on efficient variables selection algorithm and nonlinear regression tools. Food Chem. 2012, 135, 590–595. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Zou, X.; Huang, X.; Zhao, J.; Li, Y.; Hao, L.; Zhang, J. Rapid detecting total acid content and classifying different types of vinegar based on near infrared spectroscopy and least-squares support vector machine. Food Chem. 2013, 138, 192–199. [Google Scholar] [CrossRef]
- Xu, W.; Huang, Z.; Zhang, X.; Li, Q.; Lu, Z.; Shi, J.; Xu, Z.; Ma, Y. Monitoring the microbial community during solid-state acetic acid fermentation of Zhenjiang aromatic vinegar. Food Microbiol. 2011, 28, 1175–1181. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; García, J.F.; Sun, D.W. Advances in wine aging technologies for enhancing wine quality and accelerating wine aging process. Crit. Rev. Food Sci. Nutr. 2014, 54, 817–835. [Google Scholar] [CrossRef]
- Maieves, H.A.; López-Froilán, R.; Morales, P.; Pérez-Rodríguez, M.L.; Ribani, R.H.; Cámara, M.; Sánchez-Mata, M.C. Antioxidant phytochemicals of Hovenia dulcis, Thunb. peduncles in different maturity stages. J. Funct. Foods 2015, 18, 1117–1124. [Google Scholar] [CrossRef]
- Chou, C.H.; Liu, C.W.; Yang, D.J.; Wu, Y.S.; Chen, Y. Amino acid, mineral, and polyphenolic profiles of black vinegar, and its lipid lowering and antioxidant effects in vivo. Food Chem. 2015, 168, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Cerezo, A.B.; Tesfaye, W.; Torija, M.J.; Mateo, E.; García-Parrilla, M.C.; Troncoso, A.M. The phenolic composition of red wine vinegar produced in barrels made from different woods. Food Chem. 2008, 109, 606–615. [Google Scholar] [CrossRef]
- Xie, X.; Zheng, Y.; Liu, X.; Cheng, C.; Zhang, X.; Xia, T.; Yu, S.; Wang, M. Antioxidant activity of Chinese Shanxi aged vinegar and its correlation with polyphenols and flavonoids during the brewing process. J. Food Sci. 2017, 82, 2479–2486. [Google Scholar] [CrossRef] [PubMed]
- Kawa-Rygielska, J.; Adamenko, K.; Kucharska, A.Z.; Piórecki, N. Bioactive compounds in cornelian cherry vinegars. Molecules 2018, 23, 379. [Google Scholar] [CrossRef] [PubMed]
- Verzelloni, E.; Tagliazucchi, D.; Conte, A. Relationship between the antioxidant properties and the phenolic and flavonoid content in traditional balsamic vinegar. Food Chem. 2007, 105, 564–571. [Google Scholar] [CrossRef]
- Shimoji, Y.; Tamura, Y.; Nakamura, Y.; Nanda, K.; Nishidai, S.; Nishikawa, Y.; Ishihara, N.; Uenakai, K.; Ohigashi, H. Isolation and identification of DPPH radical scavenging compounds in Kurosu (Japanese unpolished rice vinegar). J. Agric. Food. Chem. 2002, 50, 6501–6503. [Google Scholar] [CrossRef] [PubMed]
- Gülçin, İ. Antioxidant activity of food constituents: An overview. Arch. Toxicol. 2012, 86, 345–391. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.W.; Lazim, A.M.; Fazry, S.; Zaki, U.K.H.H.; Lim, S.J. Varieties, production, composition and health benefits of vinegars: A review. Food Chem. 2016, 221, 1621–1630. [Google Scholar] [CrossRef] [PubMed]
- Bastante, M.J.C.; Guerrero, E.D.; Mejías, R.C.; Marín, R.N.; Dodero, M.C.R.; Barroso, C.G. Study of the polyphenolic composition and antioxidant activity of new sherry vinegar-derived products by maceration with fruits. J. Agric. Food Chem. 2010, 58, 11814–11820. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Zhang, J.; Li, Z. Correlation of volatile and nonvolatile components with the total antioxidant capacity of tartary buckwheat vinegar: Influence of the thermal processing. Food Res. Int. 2012, 49, 65–71. [Google Scholar] [CrossRef]
- Liu, J.; Gan, J.; Nirasawa, S.; Zhou, Y.; Xu, J.; Zhu, S.; Cheng, Y. Cellular uptake and trans-enterocyte transport of phenolics bound to vinegar melanoidins. J. Funct. Foods 2017, 37, 632–640. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef] [PubMed]
- Candrawinata, V.I.; Golding, J.B.; Roach, P.D.; Stathopoulos, C.E. Optimisation of the phenolic content and antioxidant activity of apple pomace aqueous extracts. CyTA-J. Food 2015, 13, 293–299. [Google Scholar] [CrossRef]
- Rice-Evans, C.; Miller, N.; Paganga, G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997, 2, 152–159. [Google Scholar] [CrossRef]
- Le, B.C.; Renard, C.M. Interactions between polyphenols and macromolecules: Quantification methods and mechanisms. Crit. Rev. Food Sci. Nutr. 2012, 52, 213–248. [Google Scholar]
- Bindon, K.A.; Carew, A.L.; Mierczynska-Vasilev, A.; Kassara, S.; Kerslake, F.; Smith, P.A. Characterization of macromolecular complexes in red wine: Composition, molecular mass distribution and particle size. Food Chem. 2016, 199, 838–846. [Google Scholar] [CrossRef] [PubMed]
- Bakir, S.; Toydemir, G.; Boyacioglu, D.; Beekwilder, J.; Capanoglu, E. Fruit antioxidants during vinegar processing: Changes in content and in vitro bio-accessibility. Int. J. Mol. Sci. 2016, 17, 1658. [Google Scholar] [CrossRef] [PubMed]
- Hornedo-Ortega, R.; Álvarez-Fernández, M.A.; Cerezo, A.B.; Garcia-Garcia, I.; Troncoso, A.M.; Garcia-Parrilla, A.C. Influence of fermentation process on the anthocyanin composition of wine and vinegar elaborated from strawberry. J. Food Sci. 2017, 82, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Yang, M.; Dong, J.; Shen, R. Comparative analysis of the antioxidant capacities and phenolic compounds of oat and buckwheat vinegars during production processes. J. Food Sci. 2018, 83, 844–853. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zuo, Y. Ultrasound-assisted hydrolysis and gas chromatography-mass spectrometric determination of phenolic compounds in cranberry products. Food Chem. 2011, 128, 562–568. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Hu, Z.; Yu, Y.; Mou, R.; Zhu, Z.; Beta, T. Phenolic acids, anthocyanins, proanthocyanidins, antioxidant activity, minerals and their correlations in non-pigmented, red, and black rice. Food Chem. 2018, 239, 733–741. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Wu, W.; Sun, C.; Lei, Z.; Chen, H.; Liu, H.; Chen, W.; Ma, I.; Min, T.; Zhang, M.; et al. Comparison of releasing bound phenolic acids from wheat bran by fermentation of three aspergillus species. Int. J. Food Sci. Technol. 2017, 53, 1120–1130. [Google Scholar] [CrossRef]
- Alonso, A.M.; Guillen, D.A.; Barroso, C.G. Study of the antioxidant power of brandies and vinegars derived from Sherry wines and correlation with their content in polyphenols. Food Res. Int. 2004, 37, 715–721. [Google Scholar] [CrossRef]
- Kruger, N.J. The Bradford method for protein quantitation. Methods Mol. Biol. 1988, 32, 9–15. [Google Scholar]
Sample Availability: Samples of traditional and industrial Zhenjiang aromatic vinegars are available from the authors. |
| Samples | Aging Time (Year) | pH | Total Acidity (g/100 mL) | Soluble Solid (g/100 mL) | Protein (g/100 mL) | Crude Fat (g/100 mL) | Carbohydrate (g/100 mL) | Reducing Sugar (g/100 mL) | Amino Nitrogen (g/100 mL) |
|---|---|---|---|---|---|---|---|---|---|
| TZAV-1 | 0 | 3.59 ± 0.00 b | 5.80 ± 0.00 l | 17.81 ± 0.01 e | 0.86 ± 0.01 i | 0.31 ± 0.03 b | 5.36 ± 0.00 a | 2.70 ± 0.06 c | 0.26 ± 0.01 f |
| TZAV-2 | 2 | 3.61 ± 0.01 a | 6.82 ± 0.01 b | 17.23 ± 0.00 g | 0.90 ± 0.01 gh | 0.33 ± 0.02 b | 5.43 ± 0.07 a | 2.47 ± 0.01 e | 0.32 ± 0.00 c |
| TZAV-3 | 5 | 3.62 ± 0.00 a | 6.64 ± 0.00 d | 23.02 ± 0.00 a | 1.02 ± 0.02 b | 0.32 ± 0.00 b | 5.24 ± 0.08 b | 2.60 ± 0.03 d | 0.34 ± 0.00 b |
| TZAV-4 | 7 | 3.56 ± 0.00 c | 6.48 ± 0.01 f | 19.42 ± 0.00 b | 1.03 ± 0.02 b | 0.44 ± 0.02 a | 4.82 ± 0.04 c | 2.89 ± 0.06 b | 0.36 ± 0.00 a |
| IZAV-1 | 0 | 3.41 ± 0.01 g | 4.75 ± 0.01 m | 12.23 ± 0.00 l | 0.90 ± 0.01 gh | 0.22 ± 0.00 d | 4.35 ± 0.03 d | 1.35 ± 0.03 j | 0.21 ± 0.01 h |
| IZAV-2 | 1 | 3.47 ± 0.00 e | 6.13 ± 0.00 i | 12.56 ± 0.00 k | 0.95 ± 0.01 de | 0.15 ± 0.01 e | 4.35 ± 0.04 d | 2.13 ± 0.02 f | 0.26 ± 0.03 f |
| IZAV-3 | 2 | 3.49 ± 0.01 d | 6.09 ± 0.02 j | 15.79 ± 0.01 i | 0.88 ± 0.01 hi | 0.23 ± 0.01 d | 3.52 ± 0.12 f | 3.00 ± 0.03 a | 0.27 ± 0.00 ef |
| IZAV-4 | 3 | 3.41 ± 0.02 g | 5.95 ± 0.00 k | 14.18 ± 0.01 j | 0.89 ± 0.01 h | 0.43 ± 0.03 a | 5.16 ± 0.09 b | 2.40 ± 0.10 e | 0.24 ± 0.00 g |
| IZAV-5 | 4 | 3.45 ± 0.01 f | 6.41 ± 0.00 g | 18.42 ± 0.00 d | 0.97 ± 0.01 cd | 0.31 ± 0.02 b | 4.38 ± 0.00 d | 1.59 ± 0.08 h | 0.28 ± 0.00 def |
| IZAV-6 | 4.5 | 3.41 ± 0.01 g | 6.84 ± 0.00 a | 17.14 ± 0.01 h | 0.92 ± 0.01 fg | 0.21 ± 0.00 d | 3.64 ± 0.04 e | 1.78 ± 0.05 g | 0.29 ± 0.01 de |
| IZAV-7 | 5 | 3.49 ± 0.01 d | 6.32 ± 0.01 h | 17.37 ± 0.01 f | 0.98 ± 0.01 c | 0.27 ± 0.03 c | 3.62 ± 0.06 ef | 1.45 ± 0.06 i | 0.27 ± 0.01 ef |
| IZAV-8 | 6 | 3.44 ± 0.01 f | 6.69 ± 0.01 c | 17.81 ± 0.01 e | 1.06 ± 0.03 a | 0.22 ± 0.01 d | 4.74 ± 0.10 c | 2.67 ± 0.10 cd | 0.30 ± 0.02 d |
| IZAV-9 | 7 | 3.38 ± 0.01 h | 6.61 ± 0.00 e | 18.79 ± 0.00 c | 0.93 ± 0.01 ef | 0.43 ± 0.00 a | 5.37 ± 0.07 a | 2.72 ± 0.03 c | 0.29 ± 0.03 de |
| Mean values | |||||||||
| TZAV | 3.60 ± 0.26 A | 6.44 ± 0.40 A | 19.37 ± 2.36 A | 0.95 ± 0.08 A | 0.35 ± 0.06 A | 5.21 ± 0.25 A | 2.67 ± 0.16 A | 0.32 ± 0.04 A | |
| IZAV | 3.44 ± 0.39 B | 6.20 ± 0.59 A | 16.03 ± 2.39 B | 0.94 ± 0.06 A | 0.27 ± 0.10 B | 4.35 ± 0.65 B | 2.12 ± 0.58 B | 0.27 ± 0.03 B |
| Samples | Aging Time (Year) | Acetic Acid | Lactic Acid | Pyroglutamic Acid | Succinic Acid | Malic Acid | Oxalic Acid | Tartaric Acid |
|---|---|---|---|---|---|---|---|---|
| TZAV-1 | 0 | 4.22 ± 0.17 cd | 1.55 ± 0.03 j | 0.82 ± 0.03 g | 0.51 ± 0.02 de | 0.08 ± 0.01 g | 0.11 ± 0.00 e | 0.05 ± 0.00 d |
| TZAV-2 | 2 | 4.72 ± 0.15 ab | 2.44 ± 0.13 hi | 1.43 ± 0.05 d | 0.64 ± 0.01 c | 0.09 ± 0.01 g | 0.14 ± 0.01 cde | 0.06 ± 0.01 cd |
| TZAV-3 | 5 | 4.89 ± 0.22 a | 2.93 ± 0.06 d | 2.26 ± 0.17 a | 1.16 ± 0.05 b | 0.34 ± 0.01 a | 0.21 ± 0.00 a | 0.12 ± 0.01 a |
| TZAV-4 | 7 | 4.92 ± 0.17 a | 2.72 ± 0.03 ef | 2.05 ± 0.11 c | 1.37 ± 0.17 a | 0.30 ± 0.01 b | 0.20 ± 0.00 ab | 0.11 ± 0.01 ab |
| IZAV-1 | 0 | 1.58 ± 0.08 e | 1.16 ± 0.05 k | 0.22 ± 0.01 h | ND 4 | 0.01 ± 0.00 h | 0.01 ± 0.00 f | ND |
| IZAV-2 | 1 | 3.92 ± 0.10 cd | 2.77 ± 0.09 ef | 1.33 ± 0.05 de | 0.17 ± 0.00 g | 0.13 ± 0.01 f | 0.23 ± 0.01 a | 0.05 ± 0.01 d |
| IZAV-3 | 2 | 3.86 ± 0.15 d | 3.11 ± 0.08 c | 1.48 ± 0.08 d | 0.54 ± 0.04 d | 0.22 ± 0.03 d | 0.17 ± 0.01 bc | 0.07 ± 0.03 bcd |
| IZAV-4 | 3 | 4.45 ± 0.21 bc | 2.56 ± 0.10 gh | 1.45 ± 0.07 d | 0.09 ± 0.02 g | 0.27 ± 0.02 bc | 0.13 ± 0.01 de | 0.10 ± 0.03 abc |
| IZAV-5 | 4 | 4.03 ± 0.09 cd | 3.41 ± 0.06 a | 1.64 ± 0.05 c | 0.29 ± 0.01 f | 0.18 ± 0.01 e | 0.16 ± 0.01 cd | 0.06 ± 0.01 cd |
| IZAV-6 | 4.5 | 4.56 ± 0.16 b | 2.84 ± 0.05 de | 1.23 ± 0.04 ef | 0.30 ± 0.00 f | 0.23 ± 0.04 d | 0.12 ± 0.02 e | 0.08 ± 0.01 abcd |
| IZAV-7 | 5 | 4.09 ± 0.33 cd | 3.27 ± 0.09 b | 1.18 ± 0.04 f | 0.28 ± 0.00 f | 0.13 ± 0.03 f | 0.14 ± 0.05 cde | 0.10 ± 0.05 abc |
| IZAV-8 | 6 | 4.65 ± 0.11 ab | 2.37 ± 0.11 i | 1.75 ± 0.10 c | 0.33 ± 0.01 f | 0.23 ± 0.01 d | 0.17 ± 0.03 bc | 0.10 ± 0.02 abc |
| IZAV-9 | 7 | 4.12 ± 0.12 cd | 2.65 ± 0.06 fg | 2.13 ± 0.14 ab | 0.44 ± 0.00 e | 0.24 ± 0.01 cd | 0.13 ± 0.01 de | 0.09 ± 0.00 abcd |
| Mean values | ||||||||
| TZAV | 4.68 ± 0.33 A | 2.41 ± 0.55 A | 1.64 ± 0.60 A | 0.92 ± 0.38 A | 0.20 ± 0.12 A | 0.17 ± 0.04 A | 0.09 ± 0.03 A | |
| IZAV | 3.91 ± 0.90 B | 2.68 ± 0.64 A | 1.37 ± 0.51 A | 0.31 ± 0.14 B | 0.18 ± 0.08 A | 0.14 ± 0.06 A | 0.08 ± 0.03 A |
| Samples | Aging Time (Year) | TPC (mg GAE/mL) | TFC (mg RE/mL) | TAC (mmol TEAC/L) | ||
|---|---|---|---|---|---|---|
| DPPH | FRAP | ABTS | ||||
| TZAV-1 | 0 | 2.49 ± 0.03 g | 1.57 ± 0.03 f | 33.32 ± 1.94 cde | 11.31 ± 0.48 g | 14.29 ± 0.98 e |
| TZAV-2 | 2 | 2.82 ± 0.16 f | 1.64 ± 0.11 ef | 32.35 ± 1.35 cde | 9.83 ± 0.35 h | 11.96 ± 0.77 fg |
| TZAV-3 | 5 | 6.45 ± 0.19 a | 5.44 ± 0.09 a | 54.72 ± 0.79 a | 30.85 ± 0.14 a | 41.07 ± 1.06 a |
| TZAV-4 | 7 | 5.55 ± 0.19 b | 4.15 ± 0.09 b | 43.76 ± 1.90 abc | 24.03 ± 0.33 b | 37.49 ± 1.28 b |
| IZAV-1 | 0 | 2.07 ± 0.01 h | 1.21 ± 0.05 g | 17.82 ± 1.69 f | 9.25 ± 0.77 h | 10.47 ± 1.02 g |
| IZAV-2 | 1 | 2.58 ± 0.17 fg | 2.23 ± 0.03 d | 26.06 ± 0.79 ef | 13.44 ± 0.88 f | 10.74 ± 1.04 g |
| IZAV-3 | 2 | 2.65 ± 0.06 fg | 1.88 ± 0.05 e | 28.10 ± 2.08 def | 13.41 ± 1.25 f | 11.85 ± 2.28 fg |
| IZAV-4 | 3 | 3.34 ± 0.06 e | 2.26 ± 0.09 d | 37.08 ± 2.24 cde | 13.72 ± 0.35 f | 13.93 ± 0.67 ef |
| IZAV-5 | 4 | 3.34 ± 0.07 e | 2.40 ± 0.08 d | 38.99 ± 0.79 cd | 13.97 ± 0.47 f | 17.28 ± 0.18 d |
| IZAV-6 | 4.5 | 3.42 ± 0.19 e | 2.85 ± 0.09 c | 43.39 ± 1.91 abc | 19.80 ± 0.19 c | 20.74 ± 1.82 e |
| IZAV-7 | 5 | 3.35 ± 0.09 e | 2.38 ± 0.04 d | 44.51 ± 0.81 abc | 20.79 ± 0.56 c | 21.25 ± 1.49 c |
| IZAV-8 | 6 | 4.07 ± 0.11 c | 3.00 ± 0.07 c | 53.01 ± 1.81 ab | 15.54 ± 0.39 e | 21.52 ± 0.65 c |
| IZAV-9 | 7 | 3.76 ± 0.14 d | 2.77 ± 0.16 c | 40.65 ± 1.14 bcd | 16.65 ± 0.75 d | 19.35 ± 1.19 cd |
| Mean values | ||||||
| TZAV | 4.33 ± 1.79 A | 3.20 ± 1.73 A | 41.04 ± 12.45 A | 19.01 ± 9.18 A | 26.20 ± 13.78 A | |
| IZAV | 3.16 ± 0.61 B | 2.37 ± 0.60 A | 36.62 ± 11.18 A | 15.18 ± 3.45 A | 16.34± 4.60 B | |
| DPPH | FRAP | ABTS | ||||
|---|---|---|---|---|---|---|
| TZAV | IZAV | TZAV | IZAV | TZAV | IZAV | |
| TPC | 0.963 * | 0.960 ** | 0.988 * | 0.682 * | 0.987 * | 0.872 * |
| TFC | 0.985 * | 0.903 ** | 0.997 * | 0.724 * | 0.981 * | 0.824 * |
| Samples | Aging Time (Year) | P-hydroxybenzoic Acid | Chlorogenic Acid | Caffeic Acid | Vanillic Acid | Syringic Acid | Catechin | P-coumaric Acid | Ferulic Acid | Sinapic Acid | Rutin |
|---|---|---|---|---|---|---|---|---|---|---|---|
| TZAV-1 | 0 | 7.96 ± 1.05 b | 2.77 ± 0.38 fg | 4.46 ± 1.45 cd | 4.47 ± 0.97 d | 2.32 ± 0.18 ef | 44.66 ± 2.68 hi | 1.01 ± 0.09 a | 0.89 ± 0.06 h | 2.66 ± 0.30 def | 9.88 ± 0.98 c |
| TZAV-2 | 2 | 4.47 ± 0.27 ef | 3.75 ± 0.00 fg | 5.57 ± 0.96 abcd | 5.78 ± 0.82 cd | 2.08 ± 0.31 efg | 47.09 ±1.42 gh | 1.11 ± 0.01 a | 1.30 ± 0.08 h | 2.11 ±0.25 ef | 10.11 ±1.58 c |
| TZAV-3 | 5 | 10.90 ± 0.02 a | 15.09 ± 0.01 b | 7.37 ± 0.14 a | 5.82 ± 0.06 cd | 24.39 ± 0.01 a | 39.86 ± 0.09 i | 0.35 ± 0.00 b | 6.41 ± 0.00 b | 5.24 ± 0.03 ab | 18.50 ± 0.31 a |
| TZAV-4 | 7 | 6.52 ± 0.04 f bc | 6.23 ± 0.07 d | 3.66 ± 0.03 d | 6.97 ± 0.02 c | 22.46 ± 0.01 b | 33.81 ± 1.16 j | 0.19 ± 0.04 c | 17.98 ± 0.05 a | 4.43 ± 0.07 bc | 14.03 ± 0.19 b |
| IZAV-1 | 0 | 6.22 ± 2.15 cd | 4.32 ± 0.05 ef | 1.61 ± 0.21 e | 7.75 ± 0.09 bc | 2.83 ± 1.22 ef | 81.12 ± 1.26 a | ND 4 | 5.41 ± 0.23 c | 3.81 ± 1.36 bcd | ND |
| IZAV-2 | 1 | 6.20 ± 0.24 cd | 6.49 ± 1.75 d | 5.57 ± 0.36 abcd | 6.28 ± 0.25 cd | 2.69 ± 0.24 ef | 62.28 ± 1.43 de | ND | 2.91 ± 0.24 de | 6.54 ± 0.26 a | ND |
| IZAV-3 | 2 | 4.89 ± 0.13 def | 3.29 ± 0.07 fg | 5.08 ± 0.15 bcd | 4.40 ± 0.21 d | 3.00 ± 0.07 e | 67.49 ± 0.23 bc | ND | 4.90 ± 0.02 c | 2.83 ± 0.09 cdef | ND |
| IZAV-4 | 3 | 2.83 ± 0.09 g | 6.20 ± 0.15 d | 5.13 ± 1.79 bcd | 5.90 ± 2.43 cd | 2.76 ± 0.58 ef | 57.80 ± 0.84 ef | ND | 2.01 ± 0.15 g | 1.88 ± 0.10 ef | ND |
| IZAV-5 | 4 | 4.10 ± 0.25 efg | 8.23 ± 1.50 c | 6.04 ± 0.15 abc | 9.10 ± 0.26 ab | 5.38 ± 0.48 c | 63.37 ± 6.87 cd | ND | 2.69 ± 0.58 ef | 3.09 ± 1.74 cde | ND |
| IZAV-6 | 4.5 | 2.70 ± 0.10 g | 2.14 ± 0.25 g | 5.01 ± 0.59 bcd | 9.03 ± 0.90 ab | 4.00 ± 0.00 d | 57.06 ± 0.46 f | ND | 3.35 ± 0.08 d | 1.45 ± 0.04 ef | ND |
| IZAV-7 | 5 | 1.22 ± 0.05 h | 2.50 ± 0.03 g | 6.45 ± 0.58 abc | 6.24 ± 1.10 cd | 4.02 ± 0.05 d | 70.20 ± 0.09 b | ND | 1.90 ± 0.00 g | 1.59 ± 0.02 ef | ND |
| IZAV-8 | 6 | 3.65 ± 0.28 fg | 5.68 ± 0.26 de | 5.48 ± 1.72 abcd | 9.59 ± 0.49 ab | 1.89 ± 0.38 fg | 65.30 ± 0.86 bcd | ND | 2.26 ± 0.47 fg | 1.29 ± 0.06 f | ND |
| IZAV-9 | 7 | 5.54 ± 0.01 cde | 20.00 ± 0.75 a | 6.81 ± 0.12 ab | 10.31 ± 0.00 a | 1.17 ± 0.33 g | 51.87 ± 1.20 g | ND | 0.96 ± 0.10 h | 2.84± 0.99 cdef | ND |
| Mean values | |||||||||||
| TZAV | 7.46 ± 2.54 A | 6.96 ± 5.19 A | 5.27 ± 1.63 A | 5.76 ± 1.06 B | 12.81 ± 11.37 A | 41.35 ± 5.56 B | 0.66 ± 0.43 | 6.65 ± 7.37 A | 3.61 ± 1.37 A | 13.13 ± 3.82 | |
| IZAV | 4.15 ± 1.75 B | 6.54 ± 5.30 A | 5.24 ± 1.59 A | 7.62 ± 2.07 A | 3.08 ± 1.27 B | 64.17± 8.71 A | 2.83 ± 1.52 A | 2.81 ± 1.70 A |
| Phenolic Compounds | DPPH | FRAP | ABTS | |||
|---|---|---|---|---|---|---|
| TZAV | IZAV | TZAV | IZAV | TZAV | IZAV | |
| p-hydroxybenzoic acid | 0.784 | −0.693 * | 0.725 | −0.749 * | 0.626 | −0.674 * |
| Chlorogenic acid | 0.960 * | 0.111 | 0.903 | −0.039 | 0.814 | 0.135 |
| Caffeic acid | 0.554 | 0.664 | 0.414 | 0.673 * | 0.251 | 0.577 |
| Vanillic acid | 0.432 | 0.481 | 0.532 | 0.201 | 0.636 | 0.596 |
| Syringic acid | 0.929 * | 0.012 | 0.976* | 0.178 | 0.998 ** | 0.080 |
| Catechin | −0.631 | −0.492 | −0.749 | −0.454 | −0.855 | −0.386 |
| p-coumaric acid | −0.834 | - | −0.913 | - | −0.971 * | - |
| Ferulic acid | 0.475 | −0.711 * | 0.610 | −0.578 | 0.742 | −0.636 |
| Sinapic acid | 0.971 * | −0.717 * | 0.994 ** | −0.540 | 0.987 * | −0.725 * |
| Rutin | 0.998 ** | - | 0.979 * | - | 0.929 * | - |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, C.; Xia, T.; Du, P.; Duan, W.; Zhang, B.; Zhang, J.; Zhu, S.; Zheng, Y.; Wang, M.; Yu, Y. Chemical Composition and Antioxidant Characteristic of Traditional and Industrial Zhenjiang Aromatic Vinegars during the Aging Process. Molecules 2018, 23, 2949. https://doi.org/10.3390/molecules23112949
Zhao C, Xia T, Du P, Duan W, Zhang B, Zhang J, Zhu S, Zheng Y, Wang M, Yu Y. Chemical Composition and Antioxidant Characteristic of Traditional and Industrial Zhenjiang Aromatic Vinegars during the Aging Process. Molecules. 2018; 23(11):2949. https://doi.org/10.3390/molecules23112949
Chicago/Turabian StyleZhao, Chaoya, Ting Xia, Peng Du, Wenhui Duan, Bo Zhang, Jin Zhang, Shenghu Zhu, Yu Zheng, Min Wang, and Yongjian Yu. 2018. "Chemical Composition and Antioxidant Characteristic of Traditional and Industrial Zhenjiang Aromatic Vinegars during the Aging Process" Molecules 23, no. 11: 2949. https://doi.org/10.3390/molecules23112949
APA StyleZhao, C., Xia, T., Du, P., Duan, W., Zhang, B., Zhang, J., Zhu, S., Zheng, Y., Wang, M., & Yu, Y. (2018). Chemical Composition and Antioxidant Characteristic of Traditional and Industrial Zhenjiang Aromatic Vinegars during the Aging Process. Molecules, 23(11), 2949. https://doi.org/10.3390/molecules23112949





