The Inhibitory Effect of Flavonoid Aglycones on the Metabolic Activity of CYP3A4 Enzyme
Abstract
1. Introduction
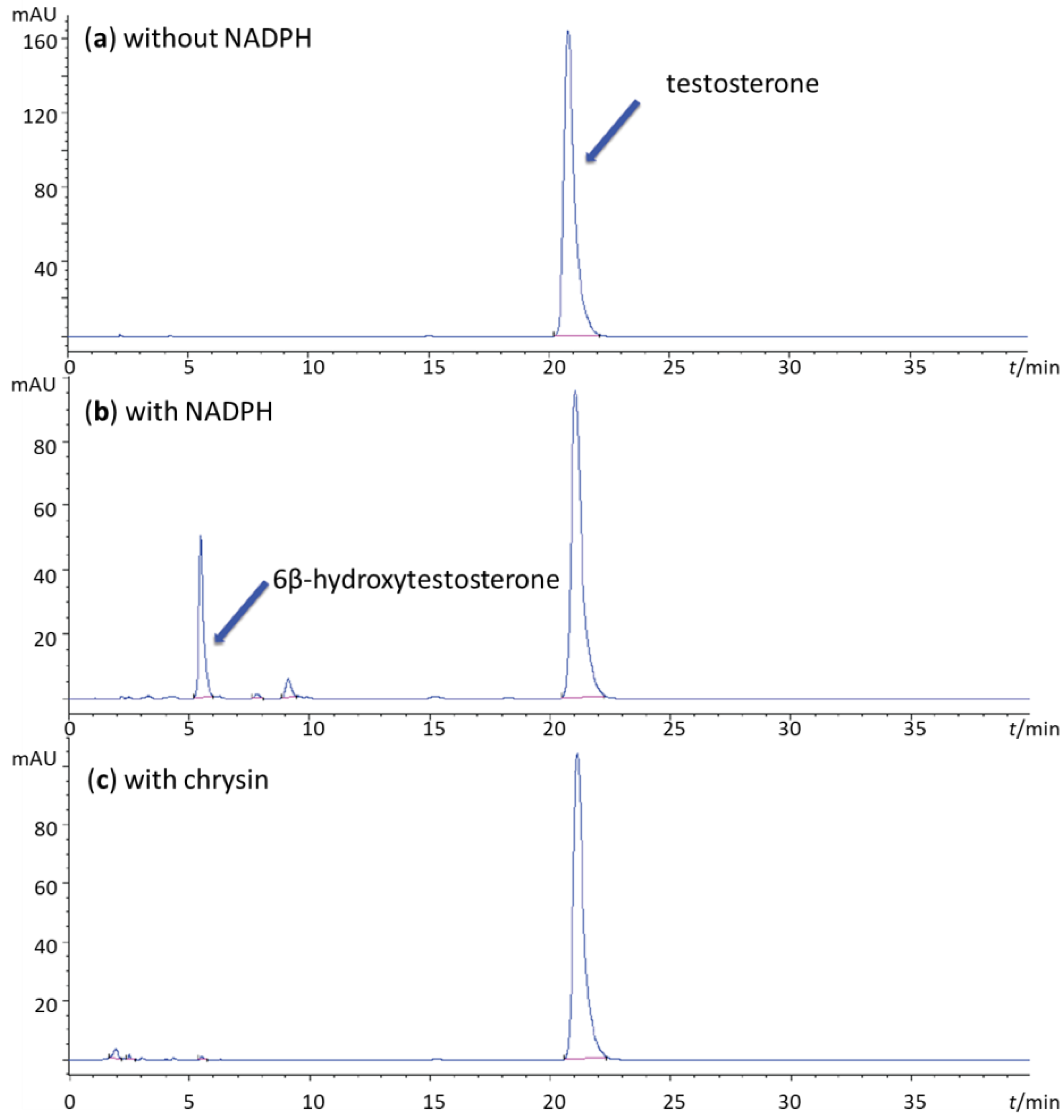
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. CYP3A4 Inhibition Assays
3.3. Determination of the Inhibition Type
3.4. HPLC-DAD Analysis
3.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Medić-Šarić, M.; Jasprica, I.; Smolčić-Bubalo, A.; Mornar, A. Optimization of chromatographic conditions in thin layer chromatography of flavonoids and phenolic acids. Croat. Chem. Acta 2004, 77, 361–366. [Google Scholar]
- Benavente-Garcia, O.; Castillo, J. Update on uses and properties of citrus flavonoids: New findings in anticancer, cardiovascular, and anti-inflammatory activity. J. Agric. Food Chem. 2008, 56, 6185–6205. [Google Scholar] [CrossRef] [PubMed]
- Bojić, M.; Debeljak, Ž.; Medić-Šarić, M.; Tomičić, M. Interference of selected flavonoid aglycons in platelet aggregation assays. Clin. Chem. Lab. Med. 2012, 50, 1403–1408. [Google Scholar] [CrossRef] [PubMed]
- Friščić, M.; Štibrić Baglama, M.; Milović, M.; Hazler Pilepić, K.; Maleš, Ž. Content of bioactive constituents and antioxidant potential of Galium L. species. Croat. Chem. Acta 2018, 91, 1–7. [Google Scholar] [CrossRef]
- Kale, A.; Gawande, S.; Kotwal, S. Cancer phytotherapeutics: Role for flavonoids at the cellular level. Phytother. Res. 2008, 22, 567–577. [Google Scholar] [CrossRef] [PubMed]
- Walle, T.; Ta, N.; Kawamori, T.; Wen, X.; Tsuji, P.A.; Walle, U.K. Cancer chemopreventive properties of orally bioavailable flavonoids--methylated versus unmethylated flavones. Biochem. Pharmacol. 2007, 73, 1288–1296. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Yang, X.; Coburn, R.A.; Morris, M.E. Structure activity relationships and quantitative structure activity relationships for the flavonoid-mediated inhibition of breast cancer resistance protein. Biochem. Pharmacol. 2005, 70, 627–639. [Google Scholar] [CrossRef] [PubMed]
- Martens, S.; Mithöfer, A. Flavones and flavone synthases. Phytochemistry 2005, 66, 2399–2407. [Google Scholar] [CrossRef] [PubMed]
- Csepanyi, E.; Szabados-Furjesi, P.; Kiss-Szikszai, A.; Frensemeier, L.M.; Karst, U.; Lekli, I.; Haines, D.D.; Tosaki, A.; Bak, I. Antioxidant Properties and oxidative transformation of different chromone derivatives. Molecules 2017, 22, 588. [Google Scholar] [CrossRef] [PubMed]
- Amić, D.; Davidović-Amić, D.; Beslo, D.; Rastija, V.; Lucić, B.; Trinajstić, N. SAR and QSAR of the antioxidant activity of flavonoids. Curr. Med. Chem. 2007, 14, 827–845. [Google Scholar] [CrossRef] [PubMed]
- Bubols, G.B.; Vianna Dda, R.; Medina-Remon, A.; von Poser, G.; Lamuela-Raventos, R.M.; Eifler-Lima, V.L.; Garcia, S.C. The antioxidant activity of coumarins and flavonoids. Mini Rev. Med. Chem. 2013, 13, 318–334. [Google Scholar] [PubMed]
- Chen, L.; Teng, H.; Xie, Z.; Cao, H.; Cheang, W.S.; Skalicka-Woniak, K.; Georgiev, M.I.; Xiao, J. Modifications of dietary flavonoids towards improved bioactivity: An update on structure-activity relationship. Crit. Rev. Food Sci. Nutr. 2018, 58, 513–527. [Google Scholar] [CrossRef] [PubMed]
- Machado, N.F.L.; Marques, M.P.M. Bioactive Chromone Derivatives – Structural Diversity. Bioact. Compd. 2010, 6, 76–89. [Google Scholar] [CrossRef]
- He, W.; Wu, J.J.; Ning, J.; Hou, J.; Xin, H.; He, Y.; Ge, G.B.; Xu, W. Inhibition of human cytochrome P450 enzymes by licochalcone A, a naturally occurring constituent of licorice. Toxicol. In Vitro 2015, 29, 1569–1576. [Google Scholar] [CrossRef] [PubMed]
- Henderson, M.; Miranda, C.; Stevens, J.; Deinzer, M.; Buhler, D. In vitro inhibition of human P450 enzymes by prenylated flavonoids from hops, Humulus lupulus. Xenobiotica 2000, 30, 235–251. [Google Scholar] [CrossRef] [PubMed]
- Obach, R.S. Inhibition of human cytochrome P450 enzymes by constituents of St. John’s Wort, an herbal preparation used in the treatment of depression. J. Pharmacol. Exp. Ther. 2000, 294, 88–95. [Google Scholar] [PubMed]
- Yuan, Y.; Qiu, X.; Nikolic, D.; Chen, S.N.; Huang, K.; Li, G.; Pauli, G.F.; van Breemen, R.B. Inhibition of human cytochrome P450 enzymes by hops (Humulus lupulus) and hop prenylphenols. Eur. J. Pharm. Sci. 2014, 53, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Zhai, S.; Dai, R.; Friedman, F.K.; Vestal, R.E. Comparative inhibition of human cytochromes P450 1A1 and 1A2 by flavonoids. Drug Metab. Dispos. 1998, 26, 989–992. [Google Scholar] [PubMed]
- Shimada, T. Inhibition of Carcinogen-Activating Cytochrome P450 Enzymes by Xenobiotic Chemicals in Relation to Antimutagenicity and Anticarcinogenicity. Toxicol. Res. 2017, 33, 79–96. [Google Scholar] [CrossRef] [PubMed]
- Hasler, J.A.; Estabrook, R.; Murray, M.; Pikuleva, I.; Waterman, M.; Capdevila, J.; Holla, V.; Helvig, C.; Falck, J.R.; Farrell, G. Human cytochromes P450. Mol. Aspects Med. 1999, 20, 1–137. [Google Scholar] [CrossRef]
- Spaggiari, D.; Geiser, L.; Daali, Y.; Rudaz, S. Phenotyping of CYP450 in human liver microsomes using the cocktail approach. Anal. Bioanal. Chem. 2014, 406, 4875–4887. [Google Scholar] [CrossRef] [PubMed]
- Guengerich, F.P.; Martin, M.V.; Beaune, P.H.; Kremers, P.; Wolff, T.; Waxman, D.J. Characterization of rat and human liver microsomal cytochrome P-450 forms involved in nifedipine oxidation, a prototype for genetic polymorphism in oxidative drug metabolism. J. Biol. Chem. 1986, 261, 5051–5060. [Google Scholar] [PubMed]
- Satoh, T.; Fujisawa, H.; Nakamura, A.; Takahashi, N.; Watanabe, K. Inhibitory Effects of Eight Green Tea Catechins on Cytochrome P450 1A2, 2C9, 2D6, and 3A4 Activities. J. Pharm. Pharm. Sci. 2016, 19, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Zanger, U.M.; Schwab, M. Cytochrome P450 enzymes in drug metabolism: Regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol. Ther. 2013, 138, 103–141. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, S.; Huang, H.; Foster, B.C.; Tam, T.W.; Xing, T.; Smith, M.L.; Arnason, J.T.; Akhtar, H. Antimicrobial and P450 inhibitory properties of common functional foods. J. Pharm. Pharm. Sci. 2014, 17, 254–265. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Tam, T.W.; Mao, J.; Saleem, A.; Krantis, A.; Arnason, J.T.; Foster, B.C. The effect of natural health products and traditional medicines on the activity of human hepatic microsomal-mediated metabolism of oseltamivir. J. Pharm. Pharm. Sci. 2010, 13, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Tam, T.W.; Akhtar, H.; Arnason, J.T.; Cvijovic, K.; Boon, H.; Cameron, D.W.; Drouin, C.E.; Jaeger, W.; Tsuyuki, R.T.; Vohra, S.; et al. Inhibition of human cytochrome p450 metabolism by blended herbal products and vitamins. J. Pharm. Pharm. Sci. 2011, 14, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Tam, T.W.; Liu, R.; Saleem, A.; Arnason, J.T.; Krantis, A.; Foster, B.C. Cytochrome P450 3A4 and 2D6-mediated metabolism of leisure and medicinal teas. J. Pharm. Pharm. Sci. 2014, 17, 294–301. [Google Scholar] [CrossRef] [PubMed]
- De Lima Toccafondo Vieira, M.; Huang, S.M. Botanical-drug interactions: A scientific perspective. Planta Med. 2012, 78, 1400–1415. [Google Scholar] [CrossRef] [PubMed]
- Hermann, R.; von Richter, O. Clinical evidence of herbal drugs as perpetrators of pharmacokinetic drug interactions. Planta Med. 2012, 78, 1458–1477. [Google Scholar] [CrossRef] [PubMed]
- Čović, D.; Bojić, M.; Medić-Šarić, M. Metabolism of flavonoids and phenolic acids. Farm. Glas. 2009, 65, 693–704. [Google Scholar]
- Wu, W.Y.; Li, Y.D.; Cui, Y.K.; Wu, C.; Hong, Y.X.; Li, G.; Wu, Y.; Jie, L.J.; Wang, Y.; Li, G.R. The natural flavone acacetin confers cardiomyocyte protection against hypoxia/reoxygenation injury via AMPK-mediated activation of Nrf2 signaling pathway. Front. Pharmacol. 2018, 15, 497. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.W.; Hu, J.J.; Fu, R.Q.; Liu, X.; Zhang, Y.H.; Li, J.; Liu, L.; Li, Y.N.; Deng, Q.; Luo, Q.S.; et al. Flavonoids inhibit cell proliferation and induce apoptosis and autophagy through downregulation of PI3Kγ mediated PI3K/AKT/mTOR/p70S6K/ULK signaling pathway in human breast cancer cells. Sci. Rep. 2018, 26, 11255. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Chang, E.; Uribe-Estanislao, G.V.; Martinez-Martinez, M.; Gálvez-Mariscal, A.; Romero, I. Anti-Helicobacter pylori Potential of Three Edible Plants Known as Quelites in Mexico. J. Med. Food 2018. [Google Scholar] [CrossRef] [PubMed]
- Doostdar, H.; Burke, M.D.; Mayer, R.T. Bioflavonoids: Selective substrates and inhibitors for cytochrome P450 CYP1A and CYP1B1. Toxicology 2000, 144, 31–38. [Google Scholar] [CrossRef]
- Tao, J.; Shen, C.; Sun, Y.; Chen, W.; Yan, G. Neuroprotective effects of pinocembrin on ischemia/reperfusion-induced brain injury by inhibiting autophagy. Biomed. Pharmacother. 2018, 106, 1003–1010. [Google Scholar] [CrossRef] [PubMed]
- Su, Q.; Sun, Y.; Ye, Z.; Yang, H.; Kong, B.; Li, L. Pinocembrin protects endothelial cells from oxidized LDL-induced injury. Cytokine 2018. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.; Ying, P.; Yan, B.; Xue, W.; Li, K.; Shi, A.; Sun, T.; Yan, J.; Hu, X. Pharmacokinetics, safety, and tolerability of single and multiple-doses of pinocembrin injection administered intravenously in healthy subjects. J. Ethnopharmacol. 2015, 168, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Makanjuola, S.B.L.; Ogundaini, A.O.; Ajonuma, L.C.; Dosunmu, A. Apigenin and apigeninidin isolates from the Sorghum bicolor leaf targets inflammation via cyclo-oxygenase-2 and prostaglandin-E2 blockade. Int. J. Rheum. Dis. 2018, 21, 1487–1495. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.A.; Ha, S.K.; Cho, E.; Choi, I. Resveratrol as a Bioenhancer to Improve Anti-Inflammatory Activities of Apigenin. Nutrients 2015, 7, 9650–9661. [Google Scholar] [CrossRef] [PubMed]
- Nabavi, S.M.; Habtemariam, S.; Daglia, M.; Nabavi, S.F. Apigenin and Breast Cancers: From Chemistry to Medicine. Anticancer Agents Med. Chem. 2015, 15, 728–735. [Google Scholar] [CrossRef] [PubMed]
- Mrazek, A.A.; Porro, L.J.; Bhatia, V.; Falzon, M.; Spratt, H.; Zhou, J.; Chao, C.; Hellmich, M.R. Apigenin inhibits pancreatic stellate cell activity in pancreatitis. J. Surg. Res. 2015, 196, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Thangaiyan, R.; Robert, B.M.; Arjunan, S.; Govindasamy, K.; Nagarajan, R.P. Preventive effect of apigenin against isoproterenol-induced apoptosis in cardiomyoblasts. J. Biochem. Mol. Toxicol. 2018, e22213. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ning, J.; Wang, Y.; Wang, C.; Sun, C.; Huo, X.; Yu, Z.; Feng, L.; Zhang, B.; Tian, X.; et al. Drug interaction study of flavonoids toward CYP3A4 and their quantitative structure activity relationship (QSAR) analysis for predicting potential effects. Toxicol. Lett. 2018, 294, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Kimura, Y.; Ito, H.; Ohnishi, R.; Hatano, T. Inhibitory effects of polyphenols on human cytochrome P450 3A4 and 2C9 activity. Food Chem. Toxicol. 2010, 48, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Tasdemir, D.; Kaiser, M.; Brun, R.; Yardley, V.; Schmidt, T.J.; Tosun, F.; Rüedi, P. Antitrypanosomal and antileishmanial activities of flavonoids and their analogues: In vitro, in vivo, structure-activity relationship, and quantitative structure-activity relationship studies. Antimicrob. Agents Chemother. 2006, 50, 1352–1364. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.K.; Park, S.H.; Choi, Y.J.; Shin, D.; Kang, Y.H. Chrysin inhibits diabetic renal tubulointerstitial fibrosis through blocking epithelial to mesenchymal transition. J. Mol. Med. (Berl.) 2015, 93, 759–772. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Zhang, G.; Liao, Y.; Pan, J. Inhibition of chrysin on xanthine oxidase activity and its inhibition mechanism. Int. J. Biol. Macromol. 2015, 81, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, X.; Liu, M.; Luan, H.; Ji, Y.; Guo, P.; Wu, C. Chrysin inhibits foam cell formation through promoting cholesterol efflux from RAW264.7 macrophages. Pharm. Biol. 2015, 53, 1481–1487. [Google Scholar] [CrossRef] [PubMed]
- Quintieri, L.; Palatini, P.; Nassi, A.; Ruzza, P.; Floreani, M. Flavonoids diosmetin and luteolin inhibit midazolam metabolism by human liver microsomes and recombinant CYP 3A4 and CYP3A5 enzymes. Biochem. Pharmacol. 2008, 75, 1426–1437. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.J.; Liu, Z.; Tang, W.; Wang, G.C.; Chung, H.Y.; Liu, Q.Y.; Zhuang, L.; Li, M.M.; Li, Y.L. Tangeretin from Citrus reticulate Inhibits Respiratory Syncytial Virus Replication and Associated Inflammation in Vivo. J. Agric. Food Chem. 2015, 63, 9520–9527. [Google Scholar] [CrossRef] [PubMed]
- Periyasamy, K.; Baskaran, K.; Ilakkia, A.; Vanitha, K.; Selvaraj, S.; Sakthisekaran, D. Antitumor efficacy of tangeretin by targeting the oxidative stress mediated on 7,12-dimethylbenz(a) anthracene-induced proliferative breast cancer in Sprague-Dawley rats. Cancer Chemother. Pharmacol. 2015, 75, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Lakshmi, A.; Subramanian, S.P. Tangeretin ameliorates oxidative stress in the renal tissues of rats with experimental breast cancer induced by 7,12-dimethylbenz[a]anthracene. Toxicol. Lett. 2014, 229, 333–348. [Google Scholar] [CrossRef] [PubMed]
- Obermeier, M.T.; White, R.E.; Yang, C.S. Effects of bioflavonoids on hepatic P450 activities. Xenobiotica 1995, 25, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Takanaga, H.; Ohnishi, A.; Yamada, S.; Matsuo, H.; Morimoto, S.; Shoyama, Y.; Ohtani, H.; Sawada, Y. Polymethoxylated flavones in orange juice are inhibitors of P-glycoprotein but not cytochrome P450 3A4. J. Pharmacol. Exp. Ther. 2000, 293, 230–236. [Google Scholar] [PubMed]
- Bojić, M. Preclinical cytochrome P450 inhibition and interaction studies of new drug candidates. Farm. Glas. 2015, 71, 229–242. [Google Scholar]
- Zhang, B.Y.; Wang, Y.M.; Gong, H.; Zhao, H.; Lv, X.Y.; Yuan, G.H.; Han, S.R. Isorhamnetin flavonoid synergistically enhances the anticancer activity and apoptosis induction by cis-platin and carboplatin in non-small cell lung carcinoma (NSCLC). Int. J. Clin. Exp. Pathol. 2015, 8, 25–37. [Google Scholar] [PubMed]
- AbdalDayem, A.; Choi, H.Y.; Kim, Y.B.; Cho, S.G. Antiviral effect of methylated flavonol isorhamnetin against influenza. PLoS ONE 2015, 10, e0121610. [Google Scholar]
- Wang, X.; Zhong, W. Isorhamnetin attenuates collagen-induced arthritis via modulating cytokines and oxidative stress in mice. Int. J. Clin. Exp. Med. 2015, 8, 16536–16542. [Google Scholar] [PubMed]
- Ding, L.L.; Zhang, J.J.; Dou, W. Effects of isorhamnetin on CYP3A4 and herb-drug interaction. Yao Xue Xue Bao 2012, 47, 1006–1010. [Google Scholar] [PubMed]
- Ekstrand, B.; Rasmussen, M.K.; Woll, F.; Zlabek, V.; Zamaratskaia, G. In vitro gender-dependent inhibition of porcine cytochrome p450 activity by selected flavonoids and phenolic acids. Biomed. Res. Int. 2015, 2015, 387918. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Lee, S.J.; Cho, J.; Gharbi, A.; Han, H.D.; Kang, T.H.; Kim, Y.; Lee, Y.; Park, W.S.; Jung, I.D.; et al. Tamarixetin exhibits anti-inflammatory activity and prevents bacterial sepsis by increasing IL-10 production. J. Nat. Prod. 2018, 81, 1435–1443. [Google Scholar] [CrossRef] [PubMed]
- Nicolini, F.; Burmistrova, O.; Marrero, M.T.; Torres, F.; Hernández, C.; Quintana, J.; Estévez, F. Induction of G2/M phase arrest and apoptosis by the flavonoid tamarixetin on human leukemia cells. Mol. Carcinog. 2014, 53, 939–950. [Google Scholar] [PubMed]
- Li, L.; Stanton, J.D.; Tolson, A.H.; Luo, Y.; Wang, H. Bioactive terpenoids and flavonoids from Ginkgo biloba extract induce the expression of hepatic drug-metabolizing enzymes through pregnane X receptor, constitutive androstane receptor, and aryl hydrocarbon receptor-mediated pathways. Pharm. Res. 2009, 26, 872–882. [Google Scholar] [CrossRef] [PubMed]
- Von Moltke, L.L.; Weemhoff, J.L.; Bedir, E.; Khan, I.A.; Harmatz, J.S.; Goldman, P.; Greenblatt, D.J. Inhibition of human cytochromes P450 by components of Ginkgo biloba. J. Pharm. Pharmacol. 2004, 56, 1039–1044. [Google Scholar] [CrossRef] [PubMed]
- Van Waterschoot, R.A.; Rooswinkel, R.W.; Sparidans, R.W.; van Herwaarden, A.E.; Beijnen, J.H.; Schinkel, A.H. Inhibition and stimulation of intestinal and hepatic CYP3A activity: Studies in humanized CYP3A4 transgenic mice using triazolam. Drug Metab. Dispos. 2009, 37, 2305–2313. [Google Scholar] [CrossRef] [PubMed]
- Bojić, M.; Barbero, L.; Dolgos, H.; Freisleben, A.; Gallemann, D.; Riva, S.; Guengerich, F.P. Time- and NADPH-dependent inhibition of cytochrome P450 3A4 by the cyclopentapeptide cilengitide: Significance of the guanidine group and accompanying spectral changes. Drug Metab. Dispos. 2014, 42, 1438–1446. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors and commercially. |


| Basic Skeleton of Flavonoids |  | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Flavonoid | R1 | R2 | R3 | R4 | R5 | R6 | R7 | R8 | Residual Activity (%) | p | |
| Flavanones | |||||||||||
| 1 | Acacetin | H | OH | H | OH | H | H | OCH3 | H | 5 ± 4 | 0.007 |
| 2 | Flavanone | H | H | H | H | H | H | H | H | 86 ± 46 | 0.361 |
| 3 | Hesperetin | H | OH | H | OH | H | H | OCH3 | OH | 49 ± 19 | 0.065 |
| 4 | Pinocembrin-7-methylether | H | OH | H | OCH3 | H | H | H | H | 83 ± 2 | 0.147 |
| 5 | Pinocembrin | H | OH | H | OH | H | H | H | H | 50 ± 15 | 0.003 |
| 6 | Sakuranetin | H | OH | H | OCH3 | H | H | OH | H | 94 ± 45 | 0.439 |
| Flavones | |||||||||||
| 7 | 6-hydroxyflavone | H | H | OH | H | H | H | H | H | 83 ± 14 | 0.220 |
| 8 | 7-hydroxyflavone | H | H | H | OH | H | H | H | H | 78 ± 14 | 0.172 |
| 9 | Apigenin | H | OH | H | OH | H | H | OH | H | 24 ± 3 | 0.013 |
| 10 | Chrysin | H | OH | H | OH | H | H | H | H | 17 ± 3 | 0.010 |
| 11 | Chrysin-dimethylether | H | OCH3 | H | OCH3 | H | H | H | H | 61 ± 21 | 0.049 |
| 12 | Diosmetin | H | OH | H | OH | H | OH | OCH3 | H | 172 ± 82 | 0.169 |
| 13 | Flavone | H | H | H | H | H | H | H | H | 81 ± 16 | 0.087 |
| 14 | Luteolin | H | OH | H | OH | H | OH | OH | H | 112 ± 31 | 0.356 |
| 15 | Naringenin | H | OH | H | OH | H | H | OH | H | 65 ± 24 | 0.155 |
| 16 | Tangeretin 1 | H | OCH3 | OCH3 | OCH3 | H | H | OCH3 | H | 42 ± 3 | 0.027 |
| 17 | Techtocrysin | H | OH | H | OCH3 | H | H | H | H | 102 ± 15 | 0.449 |
| Flavonoles | |||||||||||
| 18 | 3,6-dihydroxyflav. | OH | H | OH | H | H | H | H | H | 100 ± 14 | 0.220 |
| 19 | 3,7-dihydroxyflav. | OH | H | H | OH | H | H | H | H | 91 ± 27 | 0.375 |
| 20 | Galangin | OH | OH | H | OH | H | H | H | H | 48 ± 24 | 0.093 |
| 21 | Isohramnetin | OH | OH | H | OH | H | OCH3 | OH | H | 73 ± 6 | 0.048 |
| 22 | Kaempferol | OH | OH | H | OH | H | H | OH | H | 101 ± 14 | 0.449 |
| 23 | Morin | OH | OH | H | OH | OH | H | OH | H | 122 ± 8 | 0.061 |
| 24 | Myricetin | OH | OH | H | OH | H | OH | OH | OH | 133 ± 35 | 0.195 |
| 25 | Quercetin | OH | OH | H | OH | H | OH | OH | H | 126 ± 10 | 0.152 |
| 26 | Rhamnetin | OH | OH | H | OCH3 | H | OH | OH | H | 117 ± 84 | 0.386 |
| 27 | Tamarixetin | OH | OH | H | OH | H | OH | OCH3 | H | 195 ± 29 | 0.023 |
| Isoflavones | |||||||||||
| 28 | Genistein | H | OH | H | OH | H | H | OH | H | 72 ± 24 | 0.179 |
| 29 | Prunetin | H | OH | H | OCH3 | H | H | OH | H | 74 ± 14 | 0.149 |
| Catechins2 | |||||||||||
| 30 | Catechin | OH | OH | H | OH | H | H | OH | OH | 98 ± 10 | 0.441 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šarić Mustapić, D.; Debeljak, Ž.; Maleš, Ž.; Bojić, M. The Inhibitory Effect of Flavonoid Aglycones on the Metabolic Activity of CYP3A4 Enzyme. Molecules 2018, 23, 2553. https://doi.org/10.3390/molecules23102553
Šarić Mustapić D, Debeljak Ž, Maleš Ž, Bojić M. The Inhibitory Effect of Flavonoid Aglycones on the Metabolic Activity of CYP3A4 Enzyme. Molecules. 2018; 23(10):2553. https://doi.org/10.3390/molecules23102553
Chicago/Turabian StyleŠarić Mustapić, Darija, Željko Debeljak, Željan Maleš, and Mirza Bojić. 2018. "The Inhibitory Effect of Flavonoid Aglycones on the Metabolic Activity of CYP3A4 Enzyme" Molecules 23, no. 10: 2553. https://doi.org/10.3390/molecules23102553
APA StyleŠarić Mustapić, D., Debeljak, Ž., Maleš, Ž., & Bojić, M. (2018). The Inhibitory Effect of Flavonoid Aglycones on the Metabolic Activity of CYP3A4 Enzyme. Molecules, 23(10), 2553. https://doi.org/10.3390/molecules23102553






