Abstract
Two new phenolic glucosides, hostaflavanone A (1) and anti-1-phenylpropane-1,2-diol-2-O-β-d-glucopyranoside (2), together with six known compounds, anti-1-phenylpropane-1,2-diol (3), phenethyl-O-β-d-glucopyranoside (4), phenethanol-β-d-gentiobioside (5), phenethyl-O-rutinoside (6), (1S, 3S)-1-methyl-1,2,3,4-tetrahydro-β-carboline-3-carboxylic acid (7), and (1R, 3S)-1-methyl-1,2,3,4-tetrahydro-β-carboline-3-carboxylic acid (8), were isolated from the flower of Hosta plantaginea, and their structures were elucidated by nuclear magnetic resonance (NMR), high resolution electrospray ionization mass spectroscopy (HRESIMS), and circular dichroism (CD) analyses. The cyclooxygenases (COX-1 and COX-2) inhibition and antioxidant activities of compounds 1 and 4–6 were investigated, and they showed moderate cyclooxygenases inhibition activities. Moreover, only compound 1 exhibited moderate antioxidant activity, with an IC50 value of 83.2 μM, while 4–6 showed insignificant activity with IC50 values of 282, 257, and 275 μM, respectively. This is the first report of compounds 3 and 5–8 from the Liliaceae family. The chemotaxonomic significance of the isolated compounds was also summarized.
1. Introduction
The genus Hosta belongs to the family Liliaceae, with approximately 40 species distributed in the temperate and subtropical zones of Asia [1]. The ethnopharmacological and chemotaxonomic significance of the genus Hosta led us to investigate the chemical constituents of one of its species, namely Hosta plantaginea (Lam.) Aschers, which was a medicinal and ornamental plant in China. Its flowers are commonly used as a traditional Mongolian medicine in China for the treatment of sore throat, mute, lung heat, and toxic heat [2]. Previous phytochemical studies on H. plantaginea afforded structurally-diverse and biologically-active compounds, such as steroidals, alkaloids, flavonoids, and monoterpenes, some of them showed potent anti-inflammatory, cytotoxic, antibacterial, antiviral, and antioxidant activities [3,4,5,6,7,8]. These facts encouraged us to investigate new and bioactive secondary metabolites from H. plantaginea. In the present study, we had isolated and elucidated two new phenolic glucosides (1 and 2), and six known ones from the ethanol extract of the flowers of H. plantaginea. Herein, we report the isolation, structure elucidation, as well as the cyclooxygenases’ (COX-1 and COX-2) inhibition and antioxidant activities of compounds 1–8 (Figure 1). This is the first report of compounds 3 and 5–8 from the Liliaceae family. The chemotaxonomic significance of the isolated compounds was also summarized.
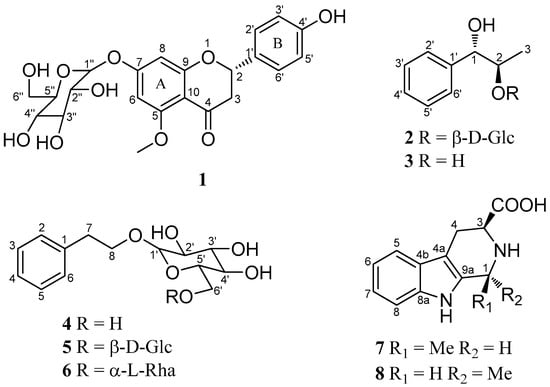
Figure 1.
Chemical structures of compounds 1–8.
2. Results and Discussion
2.1. Identification of Compounds 1–8
Compound 1 was isolated as a yellow oil, −48.2 (c 1.0, CH3OH), had a molecular formula of C22H24O10 on the basis of the HR-ESI-MS (m/z 449.14498, calcd. 449.14422 [M + H]+). The UV absorption bands at λmax 281 and 322 nm suggested the presence of a flavanone skeleton in 1 [9]. The 1H-NMR spectrum of 1 (Table 1, see the Supplementary Materials) exhibited a pair of meta-positioned aromatic protons at δH 6.28 and 6.24 (each, 1H, d, J = 2.5 Hz) in ring A, an AA’XX’ coupling system at δH 7.30 and 6.79 (each, 2H, d, J = 8.4 Hz) in ring B. The 13C-NMR spectrum of 1 (Table 1) combined with DEPT 135 spectrum displayed 22 resonances for a carbonyl carbon (δC 188.3), 12 aromatic carbons (δC 164.1, 163.3, 161.7, 157.7, 129.1, 128.3, 128.3, 115.2, 115.2, 106.1, 96.1, and 94.0), one oxymethine carbon (δC 78.3), one methoxyl group (δC 55.9), one methylene carbon (δC 44.7), and a d-glucosyl moiety (δC 99.7, 77.2, 76.5, 73.1, 69.7, and 60.7), which was supported by the result of the acid hydrolysis and HPLC analysis. Based on the above evidence, the aglycone of 1 was identified as flavanone. Additionally, the configuration of the anomeric carbon was deduced to be β based on the coupling constant of the anomeric proton (H-1′′ 7.8 Hz). The glucosidic linkage was established by the HMBC correlation (Figure 2) between H-1′′ (δH 4.99) and C-7 (δC 163.3), indicating that the glucosyl moiety was attached to C-7. Moreover, the methoxyl group was located at C-5 by the HMBC correlation from 5-OCH3 (δH 3.78) to C-5 (δC 161.7). With the aid of 1H-1H COSY, HSQC, and HMBC correlations allowed the established the planar structure and assigned all the 1H- and 13C-NMR signals of 1, which was isolated from Prunus cerasoides and named puddumin A [10]. However, only incomplete 1D-NMR data of 1 was given in the literature 10, which was also differ greatly from our data (Table 1). The electronic circular dichroism (CD) spectrum of 1 (Figure 3) showed a negative Cotton effect at 282 nm (π→π* electronic transition) and a positive Cotton effect at 338 nm (n→π* electronic transition), suggesting that the absolute configuration at C-2 was S [9,11]. Thus, the structure of 1 was fully elucidated, and it was named hostaflavanone A.

Table 1.
13C- and 1H-NMR data for compounds 1 and puddumin A in DMSO-d6 (δ in ppm).
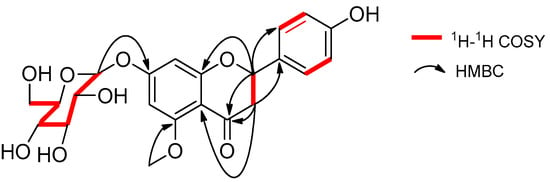
Figure 2.
Selected 1H-1H COSY and HMBC correlations of 1.
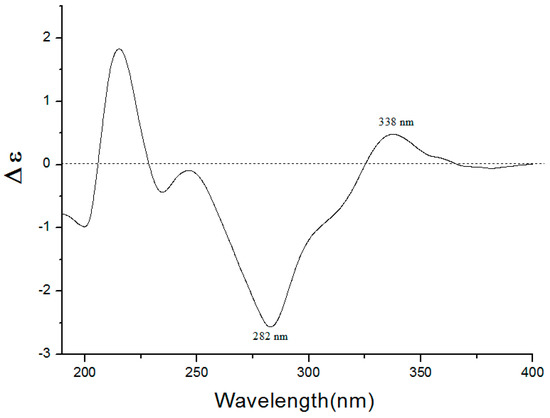
Figure 3.
CD spectrum of compound 1 in CH3OH.
Compound 2 was isolated as a yellow oil, had a molecular formula of C15H22O7 on the basis of the HR-ESI-MS (m/z 315.14283, calcd. 315.14383 [M + H]+). The UV absorption bands at λmax 224 and 274 nm. The 1H-NMR spectrum of 2 (Table 2) exhibited an AAʹXXʹ coupling system at δH 7.16–7.26 (5H, m), one methxyl at δH 1.16 (3H, d, J = 6.3 Hz). The 13C-NMR spectrum of 2 (Table 2) displayed 15 resonances for six aromatic carbons (δC 140.1, 129.3, 129.3, 127.9, 127.9, and 125.6), two oxymethine carbons (δC 78.6 and 73.7), one methxyl group (δC 16.5), and a glucosyl moiety (δC 102.9, 76.8, 76.4, 73.5, 70.1, and 61.0). The 1H and 13C-NMR spectra were similar to those of the known compound 3 [12], expect for the presence an additional glucosyl moiety signals. The major differences in the chemical shifts for C-1 (ΔC +2.5), C-2 (ΔC +4.3), and C-3 (ΔC -2.6) were ascribed to glycosylation, suggesting that the glucosyl moiety was located at C-2. Moreover, the configuration of the anomeric carbon was deduced to be β based on the coupling constant of the anomeric proton (H-1′′, 7.8 Hz). The small coupling constant 3J1,2 (5.1 Hz) of 2 and 3 (Table 2) indicated that 2 and 3 were in the anti-configuration. According to the literatures, the anti-phenylpropane-1,2-diol displayed smaller coupling constants of 3J1,2 (4.4–5.4 Hz) than syn-phenylpropane-1,2-diol (3J1,2 7–8 Hz) [12,13,14,15,16,17,18]. Due to the shortage of the sample, the two-dimensional (2D)-NMR experiments were not carried out. On the basis of the above evidence, the planar structure of 2 was deduced as anti-1-phenylpropane-1,2-diol-2-O-β-d-glucopyranoside.

Table 2.
13C- and 1H-NMR data for compounds 2 and 3 in DMSO-d6 (δ in ppm).
By comparison of the NMR and MS data with those reported, compounds 3–8 isolated from the flower of H. plantaginea were identified as anti-1-phenylpropane-1,2-diol (3) [12], phenethyl-O-β-d-glucopyranoside (4) [19], phenethanol-β-gentiobioside (5) [20], phenethyl-O-rutinoside (6) [21], (1S,3S)-1-methyl-1,2,3,4-tetrahydro-β-carboline-3-carboxylic acid (7) [22], and (1R,3S)-1-methyl-1,2,3,4-tetrahydro-β-carboline-3-carboxylic acid (8) [22], respectively.
2.2. Biological Activities
Compounds 1 and 4–6 exhibited moderate activity to that of the standard reference drug, and were tested for their inhibitory activity against ovine COX-1 and COX-2 (Table 3), with IC50 values of 15.5–41.2 and 31.7–45.4 μM, while the IC50 values of the positive control celecoxib were 9.0 and 1.0 μM, respectively. While compounds 1 and 4 were more active against COX 1 (SI values <<1), compounds 5 and 6 were about equally potent against both COX enzymes with SI values of about 1.

Table 3.
In vitro COX-1/COX-2 inhibition and antioxidant activities of isolated compounds.
The antioxidant activity of compounds 1, and 4–6 was measured by the 1,1-diphenyl-2-picrylhydrazyl (DPPH) method and the results are summarized in Table 3. Only compound 1 exhibited moderate antioxidant activity, with an IC50 value of 83.2 μM, while 4–6 showed insignificant activity with IC50 values of 282, 257, and 275 μM, respectively. The IC50 value of the positive control l-ascorbic acid was 33.9 μM. These compounds may thus, possibly together with further constituents, contribute to the biological activity of H. plantaginea.
2.3. Chemotaxonomic Significance
In our present study, eight compounds including one flavanone (1), two phenylpropanoids (2 and 3), three phenethanols (4–6), and two β-carboline alkaloids (7 and 8) were isolated from the flowers of H. plantaginea. Hostaflavanone A (1) and anti-1-phenylpropane-1,2-diol-2-O-β-d-glucopyranoside (2) were identified as two new ones, and this is the first report of compounds 3, and 5–8 from the Liliaceae family. Additionally, the structure types of flavanone and β-carboline alkaloid from Liliaceae family for the first time.
The phenylpropanoids have been previously isolated from the Hosta species, including trans-p-hydroxy-cinnamic acid from H. ventricosa [23], p-coumaramide, trans-N-p-coumaroyltyramine, and cis-N-coumaroyltyramine from H. longipes [24], feruloyltyramine, and lyciumide A from H. ensata [25]. In addition, the phenethanols 4 and α-hydroxyacetovanillone were isolated from H. plantaginea and H. ventricosa [23], respectively. Thus, compounds 2–6 from H. plantaginea, suggesting that their occurrence could be used to verify the chemotaxonomic relationship of H. plantaginea and other species of Hosta, and also might sever as valuable chemotaxonomic makers for the identification of H. plantaginea. Further comprehensive phytochemical investigations involving an expand series of compounds could help define the chemotaxonomic significance of species belonging to genus Hosta.
3. Experimental Section
3.1. General Procedures
Optical rotations were measured using a JASCO P-1020 polarimeter (JASCO Corporation, Tokyo, Japan). The UV spectra were recorded in CH3OH using a JASCO V-550 UV-VIS spectrophotometer (JASCO Corporation, Tokyo, Japan). 1H (600 MHz), 13C (150 MHz), DEPT 135 (150 MHz), and 2D (1H-1H COSY, HSQC, and HMBC) NMR spectra were recorded on a Bruker AV 600 spectrometer (Bruker Corporation, Fallanden, Switzerland). HR-ESI-MS was measured on a Waters Synapt G2 TOF mass spectrometer (Waters Corporation, Manchester, UK). Column chromatographies (CCs) were carried out on silica gel (200–300 mesh, Marine Chemical Group Corporation, Qingdao, China) and ODS (60–80 µm, YMC, Tokyo, Japan). Silica gel GF254 (Marine Chemical Group Corporation, Qingdao, China) was used for analytical TLC. 2,2-Di-phenyl-1-picrylhydrazyl (DPPH) (Sigma Corporation, Ronkonkoma, New York, NY, USA), l-cysteine methyl ester and o-tolyl isothiocyanate (Meilun Biotech. Co. Ltd., Dalian, China), d-glucose, and l-glucose (Energy Chemical, Shanghai, China) were used. COX inhibitor screening assay kit was purchased from Cayman Chemical Company (Ann Arbor, MI, USA). The analytical HPLC was performed on a Shimadzu HPLC system equipped with an LC-20AB pump, and a SPD-20A diode array detector (Shimadzu, Kyoto, Japan), using a Phenomenex Gemini C18 column (5 μm, 4.6 mm × 250 mm, Phenomenex Inc., Los Angeles, CA, USA). The preparative HPLC was performed on a Shimadzu LC-6AD system equipped with an LC-6AD pump and an SPD-M20A detector (Shimadzu, Kyoto, Japan), using an RP-18 column (5 μm, 21.2 × 250 mm, Gemini, Phenomenex Inc., Los Angeles, CA, USA; detector set at 220 and 254 nm).
3.2. Plant Materials
The flowers of Hosta plantaginea (Lam.) Aschers were collected in Shanquan town, Nanchuan district, Chongqing, People’s Republic of China, in September 2014, and were identified by one of authors (Guo-yue Zhong). A voucher specimen (no. YZH201409) was deposited at the Research Center of Natural Resources of Chinese Medicinal Materials and Ethnic Medicine, Jiangxi University of Traditional Chinese Medicine, Nanchang, China.
3.3. Extraction and Isolation
The air-dried and powdered flowers of H. plantaginea (16.5 kg) were extracted three times with 80% EtOH (40 L) by maceration at room temperature for three days. After filtration, combination, and solvent evaporation the residue (6.60 kg) was dissolved in water and successively partitioned with petroleum ether, ethyl acetate, and n-BuOH to afford petroleum ether (A, 363 g), ethyl acetate (B, 127 g), n-BuOH (C, 804 g), and water (5.27 kg) extracts, respectively. The n-BuOH extract (760 g) was subjected to HP20 macroporous adsorption resin column chromatography (CC) eluting with H2O, 20%, 50%, and 95% aqueous EtOH to give four fractions, c1, c2, c3, and c4, respectively. Fr. c2 (32.8 g) was subjected to MCI CC using a EtOH/H2O gradient elution (10%, 20%, 30%, and 95%) to give four fractions (c21 to c2d). The subfraction c2c (6.02 g) was applied to silica gel CC eluting with dichloromethane–CH3OH (5:1, 3:1, 1;1, 0:100, v/v) to afford four subfractions (c2c1–c2c4). c2c2 (1.04 g) was purified by pre-HPLC eluting with CH3OH/H2O (v/v, 35:65, flow rate: 10 mL/min) to afford compound 1 (53.7 mg, tR 22.7 min). The subfraction c2b (15.1 g) was applied to polyamide CC using a EtOH/H2O gradient elution (10%, 20%, and 95%) to afford five subfractions (c2b1–c2b5). The subfraction c2b1 (7.12 g) was applied to silica gel CC eluting with dichloromethane–CH3OH (10:1, 5:1, 1:5, v/v) to afford four subfractions (c2b11–c2b14). c2b11 (1.81 g) was purified by pre-HPLC eluting with CH3CN/H2O (v/v, 20:80, flow rate: 2 mL/min) to afford compounds 2 (1.0 mg, tR 15.0 min), 3 (0.7 mg, tR 25.3 min), and 4 (1.45 g, tR 17.2 min). c2b12 (1.19 g) was purified by pre-HPLC eluting with CH3OH/H2O (v/v, 30:70, flow rate: 10 mL/min) to afford compound6 6 (1.10 g, tR 31.0 min). The subfraction c2a (8.05 g) was applied to silica gel CC eluting with dichloromethane/CH3OH (1:1, 1:3, 1:10, v/v) to afford three subfractions (c2a1–c2a3). c2a1 (3.89 g) was subjected to Sephadex LH-20 CC eluting with CH3OH to afford three subfractions (c2a11–c2a13). c2a12 (2.59 g) was purified by pre-HPLC eluting with CH3OH/H2O (v/v, 25:75, flow rate: 10 mL/min) to afford compounds 5 (329 mg, tR 32.8 min), 7 (1.9 mg, tR 23.2 min), and 8 (1.4 mg, tR 27.3 min).
3.4. Acid Hydrolysis and HPLC Analysis
The absolute configurations of the sugar moieties in the structures were determined by the previously described method with minor modifications [9]. Compound 1 (3 mg) was hydrolyzed with 2 mL of 2 M HCl for 3 h at 90 °C. The mixture was evaporated to dryness in vacuo, and the residue was dissolved in H2O and extracted with CHCl3. After the aqueous layer was dried in vacuo, the residue was dissolved in pyridine (1 mL) containing l-cysteine methyl ester (1 mg) and heated at 60 °C for 1 h. o-Tolyl isothiocyanate (5 μL) was added, and the mixture was heated at 60 °C for 1 h and directly analyzed by HPLC. Analytical HPLC was performed on a reversed-phase C18 column (5 μm, 4.60 × 250 mm; Intertsutain, Shimadzu) at 30 °C with isocratic elution using 25% CH3CN containing 0.1% formic acid for 40 min at a flow rate 0.8 mL/min. The peaks were detected with a UV detector at 250 nm. The standard monosaccharides, d-glucose, and l-glucose, were subjected to the same process.
3.5. In Vitro COX-1 and COX-2 Inhibitory Assay
Inhibitory activities of the compounds towards COX-1 and COX-2 activity was determined using colorimetric COX (ovine) inhibitor screening assay kit (Cayman, no. 760111) following the manufacturer’s instructions, using celecoxib as a positive control [26]. The 50% inhibitory concentration (IC50) values were calculated from the concentration-inhibition response curve.
3.6. Antioxidant Assay
DPPH radical-scavenging activity of the sample was measured as previously described with minor modifications [27]. In a 96-well microplate, 150 μL of DPPH solution (200 μM) was added to 50 μL of the test sample in methanol at different concentrations. The OD values of the reaction mixtures was recorded at 517 nm using a Multiskan Go (Thermo Fisher Scientific, Inc., Waltham, MA, USA) for 40 min at 30 °C. The DPPH radical scavenging activity was calculated by the following equation: DPPH scavenging activity % = (Asample – Ablank)/Acontrol × 100, where Asample represents the absorbance of sample and DPPH, Ablank represents the absorbance of sample and CH3OH, Acontrol represents the absorbance of DPPH and CH3OH. IC50 value was calculated as the concentration required to scavenge 50% DPPH free radicals and was obtained by plotting the DPPH-scavenging percentage of each sample against the sample concentration. l-ascorbic acid was used as the positive control in this experiment. All tests were run in triplicate, and values obtained from experiments were averaged.
4. Conclusions
In summary, one new flavanone (1) and one new phenylpropanoid (2), together with six known ones, one phenylpropanoid (3), three phenethanols (4–6), and two β-carboline alkaloids (7 and 8), were isolated from the flowers of H. plantaginea. This is the first report of compounds 3 and 5–8 from the Liliaceae family. Additionally, the structure types of flavanone and β-carboline alkaloid from the Liliaceae family for the first time. Moreover, compounds 2–6 from H. plantaginea suggest that their occurrence could be used to verify the chemotaxonomic relationship of H. plantaginea and other species of Hosta, and also might serve as valuable chemotaxonomic makers for the identification of H. plantaginea. The cyclooxygenases’ (COX-1 and COX-2) inhibition and antioxidant activities of compounds 1 and 4–6 were investigated, and they showed moderate cyclooxygenase inhibition activities. Moreover, only compound 1 exhibited moderate antioxidant activity, with an IC50 value of 83.2 μM, while 4–6 showed insignificant activity with IC50 values of 282, 257, and 275 μM, respectively. These compounds may, possibly together with further constituents, contribute to the biological activity of H. plantaginea.
Supplementary Materials
The 1H-NMR, 13C-NMR, DEPT-135, 1H-1H COSY, HSQC, and HMBC spectra of 1 and 2 are available as supplementary materials available online.
Acknowledgments
This work was financially supported by grants from the National Natural Science Foundation of China (No. 81503357), the Natural Science Foundation of Jiangxi Province (No. 20161BAB215209 and 20171BBH80026), the China Postdoctoral Science Foundation (No. 2016M600514), and the Research Project of Jiangxi Health Department (No. 2016A058).
Author Contributions
The list authors contributed to this work as follows: L.Y. and J.-W.H. conceived and designed the experiments; S.-T.J., Q.-G.Z., L.Y., and J.-W.H. performed the experiments and analyzed the data; L.Y. and J.-W.H. wrote the paper; and G.-Y. Z. and J.-W.H. acquired funding for the research. All authors read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Liu, J.X.; Zhao, C.H.; Liu, X.R.; Xi, Y.Z.; Zhang, Y.L. Pollen morphology of Hosta Tratt. in China and its taxonomic significance. Plant Syst. Evol. 2011, 294, 99–107. [Google Scholar] [CrossRef]
- State Administration of Traditional Chinese Medicine. Chinese Materia Medica; Shanghai Science and Technology Press: Shanghai, China, 1999; Volume VIII, pp. 107–108. [Google Scholar]
- He, J.W.; Yang, L.; Zhong, G.Y. Research progress in chemical constituents, pharmacological activities, clinical practices and quality control of folk medicine Hosta plantaginea. Chin. Tradit. Herb. Drugs 2016, 47, 4295–4300. [Google Scholar]
- Wang, Q.H.; Han, J.J.; Bao, B.Y. Antibacterial effects of two monoterpene glycosides from Hosta plantaginea (lam.) Aschers. J. Food Biochem. 2017, 41, e12320. [Google Scholar] [CrossRef]
- He, J.W.; Yang, L.; Zhu, J.X.; Wang, X.M.; Zhou, Z.R.; He, W.W.; Zhong, G.Y. Comparison of anti-inflammatory effects and HPLC detection on different extracts from the flower of Hosta plantaginea in mice. J. Jiangxi Norm. Univ. (Nat. Sci.) 2016, 40, 183–185. [Google Scholar]
- Wang, Y.H.; Zhang, Z.K.; Yang, F.M.; Sun, Q.Y.; He, H.P.; Di, Y.T.; Mu, S.Z.; Lu, Y.; Chang, Y.; Zheng, Q.T.; et al. Benzylphenethylamine Alkaloids from Hosta plantaginea with inhibitory activity against tobacco mosaic virus and acetylcholinesterase. J. Nat. Prod. 2007, 70, 1458–1461. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.X.; Zhang, J.H.; Zhang, H.G.; Xue, P.F. Studies on chemical constituents from Hosta plantaginea (Lam.) Asehers, a Mongolia medieine. Chin. Pharm. J. 2009, 44, 733–735. [Google Scholar]
- Mimaki, Y.; Kameyama, A.; Kuroda, M.; Sashida, Y.; Hirano, T.; Oka, K.; Koike, K.; Nikadio, T. Steroidal glycosides from the underground parts of Hosta plantainea var. Japonica and their cytostatic activity on leukamia HL-60 cells. Phytochemistry 1997, 44, 305–310. [Google Scholar] [CrossRef]
- Li, B.; Ni, Y.; Zhu, L.J.; Wu, F.B.; Yan, F.; Zhang, X.; Yao, X.S. Flavonoids from matteuccia struthiopteris and their anti-influenza virus (H1N1) activity. J. Nat. Prod. 2015, 78, 987–995. [Google Scholar] [CrossRef] [PubMed]
- Bahuguna, R.P.; Jangwan, J.S.; Kaiya, T.; Sakakibara, J. Puddumin A, a new flavanone glucoside from Prunus cerasoides. J. Nat. Prod. 1987, 50, 232–234. [Google Scholar] [CrossRef]
- Hanáková, Z.; Hošek, J.; Kutil, Z.; Temml, V.; Landa, P.; Vanĕk, T.; Schuster, D.; Dall’Acqua, S.; Cvačka, J.; Polansky, O.; Šmejkal, K. Anti-inflammatory activity of natural geranylated flavonoids: Cyclooxygenase and lipoxygenase inhibitory properties and proteomic analysis. J. Nat. Prod. 2017, 80, 999–1006. [Google Scholar] [CrossRef] [PubMed]
- Andreu, C.; Olmo, M. Potential of some yeast strains in the stereoselective synthesis of (R)-(−)-phenylacetylcarbinol and (S)-(+)-phenylacetylcarbinol and their reduced 1,2-dialcohol derivatives. Appl. Microbiol. Biotechnol. 2014, 98, 5901–5913. [Google Scholar] [CrossRef] [PubMed]
- Hao, B.; Gunaratna, M.J.; Zhang, M.; Weerasekara, S.; Seiwald, S.N.; Nguyen, V.T.; Meier, A.; Hua, D.H. Chiral-substituted poly-N-vinylpyrrolidinones and bimetallic nanoclusters in catalytic asymmetric oxidation reactions. J. Am. Chem. Soc. 2016, 138, 16839–16848. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.K.; Chen, Y.Z.; Xu, Y.; Li, A.T.; Xu, Q.S.; Glieder, A.; Li, Z. Enantioselective trans-dihydroxylation of aryl olefins by cascade biocatalysis with recombinant Escherichia coli coexpressing monooxygenase and epoxide hydrolase. ACS Catal. 2014, 4, 409–420. [Google Scholar] [CrossRef]
- Kingsbury, C.A.; Cowles, C.R. Conformations of vicinal diesters. J. Org. Chem. 1975, 40, 1302–1308. [Google Scholar] [CrossRef]
- Kihumbu, D.; Stillger, T.; Hummel, W.; Liese, A. Enzymatic synthesis of all stereoisomers of 1-phenylpropane-1,2-diol. Tetrahedron Asymmetry 2002, 13, 1069–1072. [Google Scholar] [CrossRef]
- Brambilia, U.; Nasini, G.; Pava, O.V. Secondary mold metabolites, part 49. isolation, structural elucidation, and biomimetic synthesis of trametol, a new 1-arylpropane-1,2-diol produced by the fungus Trametes sp. J. Nat. Prod. 1995, 58, 1251–1253. [Google Scholar] [CrossRef]
- Mayorga, H.; Knapp, H.; Winterhalter, P.; Duque, C. Glycosidically bound flavor compounds of cape gooseberry (Physalis Peruviana L.). J. Agric. Food Chem. 2001, 49, 1904–1908. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, C.J.; Yang, J.Z.; Ma, J.; Zhang, D.M. Chemical constituents from stems of Clausena lansium. Chin. Tradit. Herb. Drugs 2016, 47, 32–37. [Google Scholar]
- Ma, S.J.; Mizutani, M.; Hiratake, J.; Hayashi, K.; Yagi, K.; Watanabe, N.; Sakata, K. Substrate specificity of β-primeverosidase, a key enzyme in aroma formation during oolong tea and black tea manufacturing. Biosci. Biotechnol. Biochem. 2001, 65, 2719–2729. [Google Scholar] [CrossRef] [PubMed]
- Hamerski, L.; Bomm, M.D.; Silva, D.H.; Young, M.C.M.; Furlan, M.; EBerlin, M.N.; Castro-Gamboa, I.; Cavalherio, A.J.; Bolzani, V.S. Phenylpropanoid glucosides from leaves of Coussarea hydrangeifolia (Rubiaceae). Phytochemistry 2005, 66, 1927–1932. [Google Scholar] [CrossRef] [PubMed]
- Li, G.Q.; Deng, Z.W.; Li, J.; Fu, H.Z.; Lin, W.H. Chemical constituents from starfish Asterias rollestoni. J. Chin. Pharm. Sci. 2004, 13, 81–86. [Google Scholar]
- Yang, S.X.; Zhao, F.W.; Wang, H.; Huang, Q.Q.; Xu, J.J.; Wang, Y.H.; Long, C.L. Chemical constituents of Hosta ventricosa, an ornamental medicinal plant. J. Yunnan Agric. Univ. 2011, 26, 662–667. [Google Scholar]
- Kim, C.S.; Kim, K.Y.; Lee, K.R. Phytochemical constituents of the leaves of Hosta longipes. Nat. Prod. Sci. 2014, 20, 86–90. [Google Scholar]
- Liu, H.X.; Sun, Q.Y.; Yang, F.M.; Zhao, F.W.; Wang, Y.H.; Long, C.L. A new sesquiterpene lactone from H. ensata. Chem. Nat. Compd. 2012, 48, 580–582. [Google Scholar] [CrossRef]
- Ochieng, G.O.; Opiyo, S.A.; Mureka, E.W.; Ishola, I.O. Cyclooxygenase inhibitory compounds from Gymnosporia heterophylla aerial parts. Fitoterapia 2017, 119, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.T.; Li, C.Y.; Wang, C.H.; Wang, Y.F.; Wang, X.D.; Wang, H.T.; Zhu, Y.; Jiang, M.M.; Gao, X.M. Phenolic compounds from the roots of Rhodiola crenulata and their antioxidant and inducing IFN-γ production activities. Molecules 2015, 20, 13725–13739. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).