Selective Extraction of Flavonoids from Sophora flavescens Ait. by Mechanochemistry
Abstract
:1. Introduction
2. Results and Discussion
2.1. Preliminary Experiments
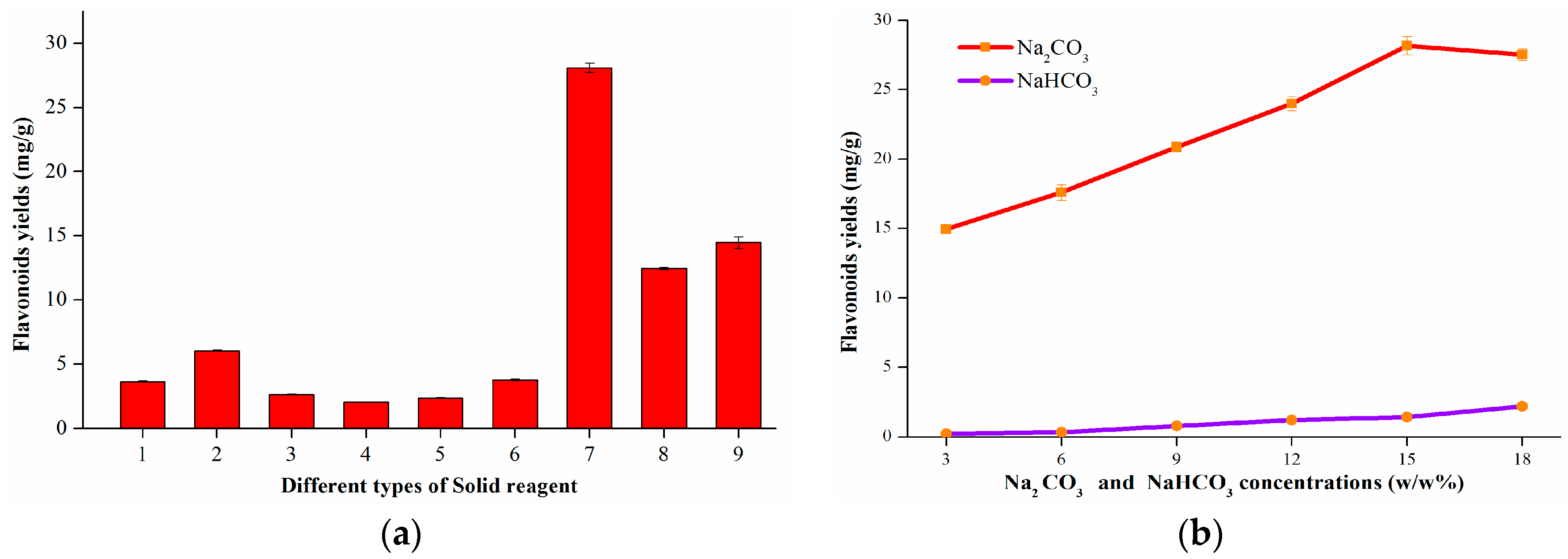
2.1.1. Screening of Solid Reagents
2.1.2. Acidification pH
2.2. Optimization of the Operating Parameters
2.2.1. Model Fitting
2.2.2. Analysis of Variance
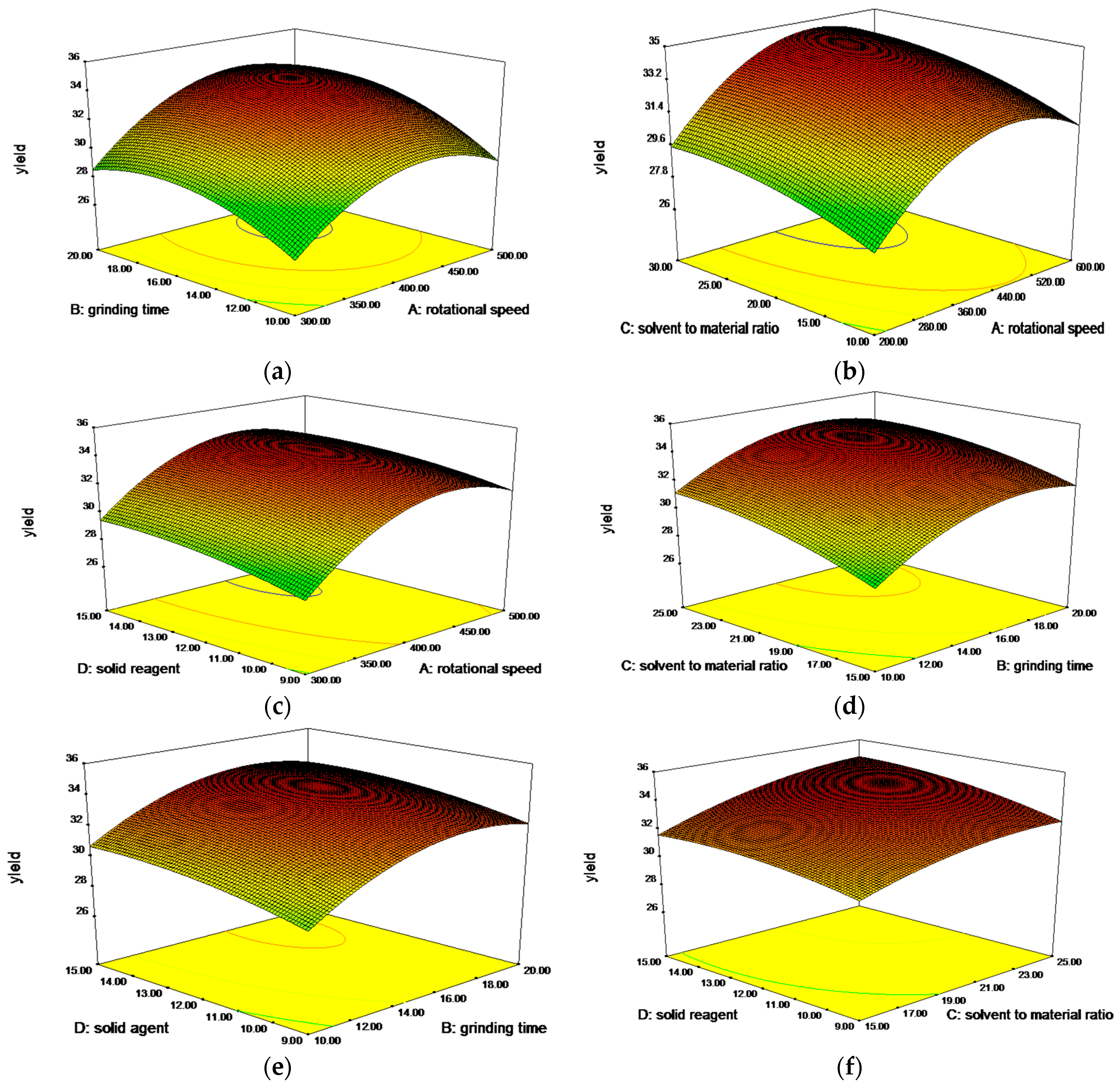
2.2.3. Analysis of Response Surfaces and Optimal Processing Conditions
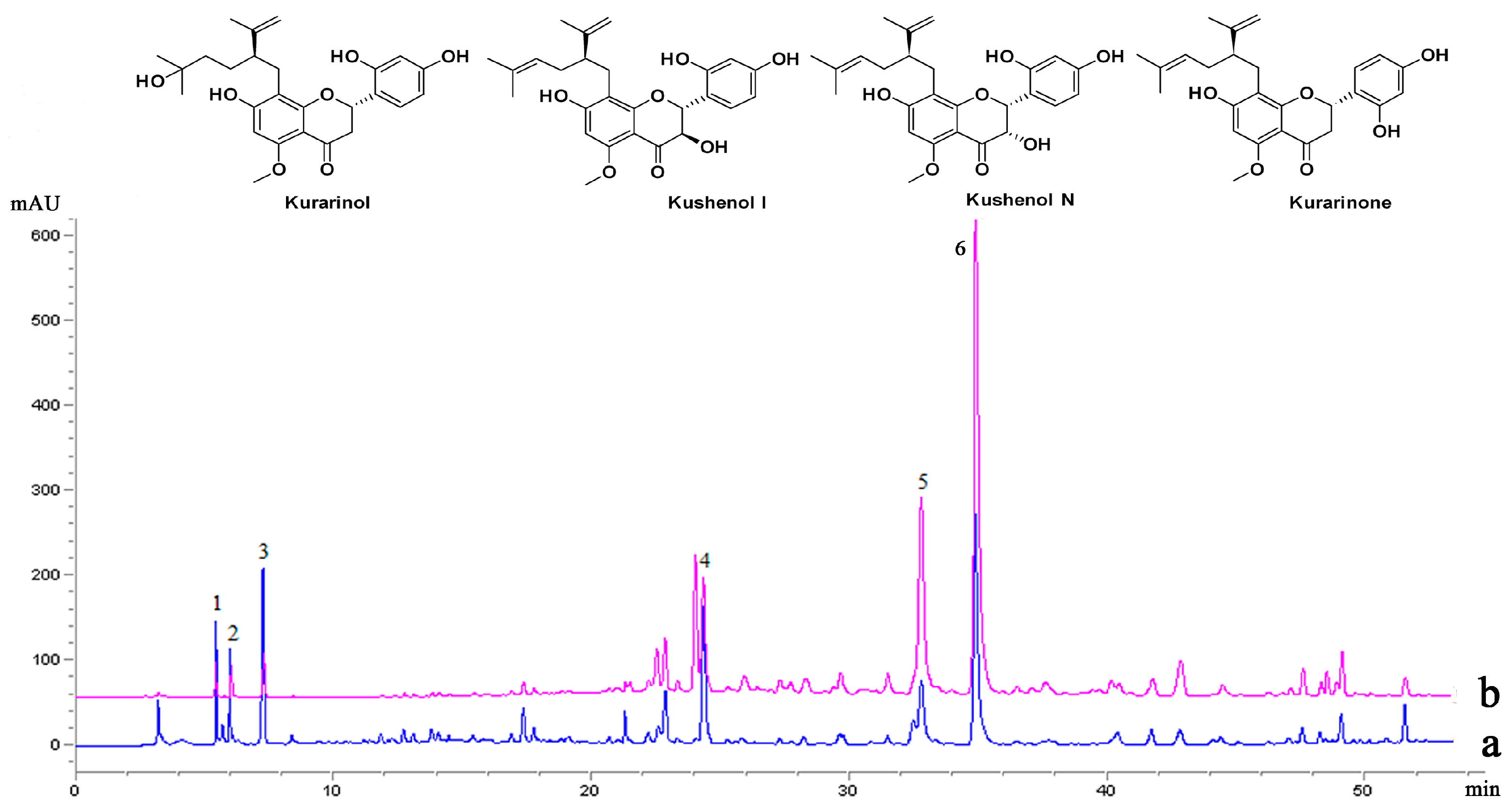
2.3. The Selectivity Analysis by HPLC-MS/MS
2.4. Antioxidant Activities in Vitro
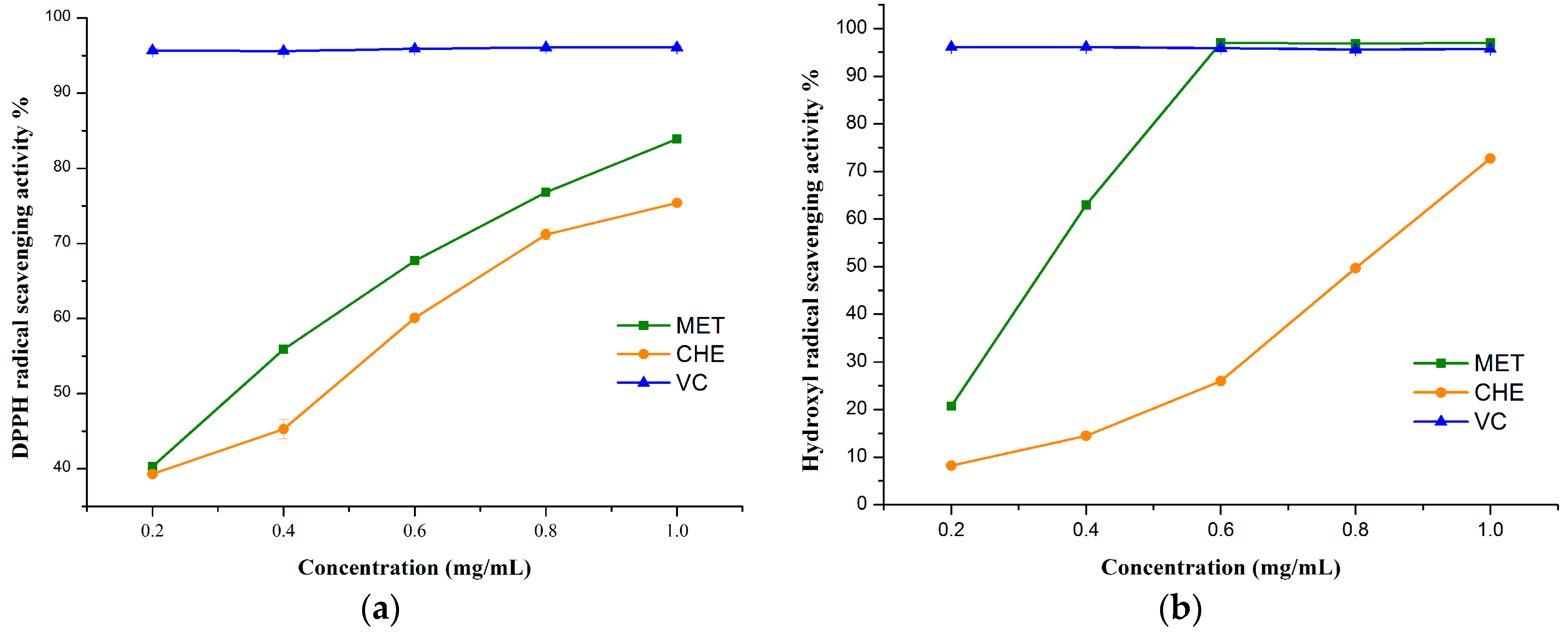
2.4.1. The DPPH Radical Scavenging Activity
2.4.2. Hydroxyl Radical Scavenging Activity
2.5. Physicochemical Property Changes during Mechanochemical Milling
2.5.1. Morphology Analysis
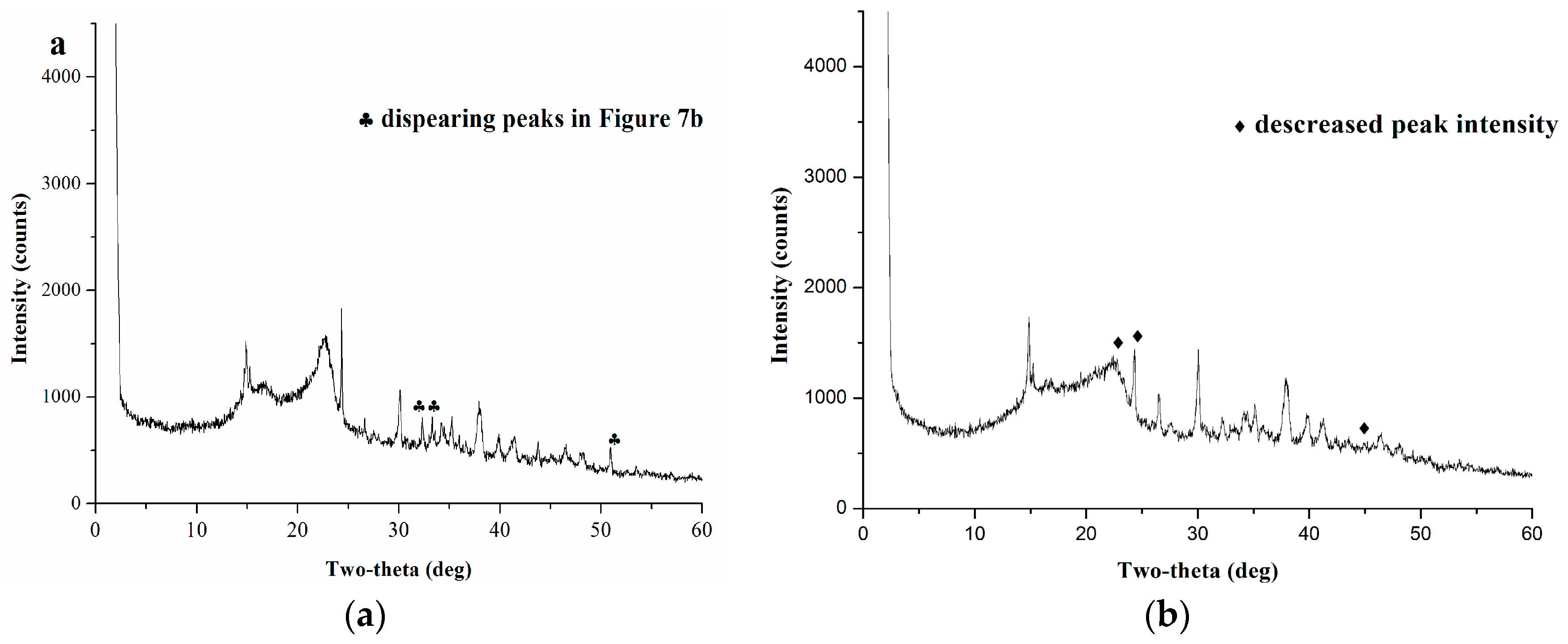
2.5.2. XRD Analysis
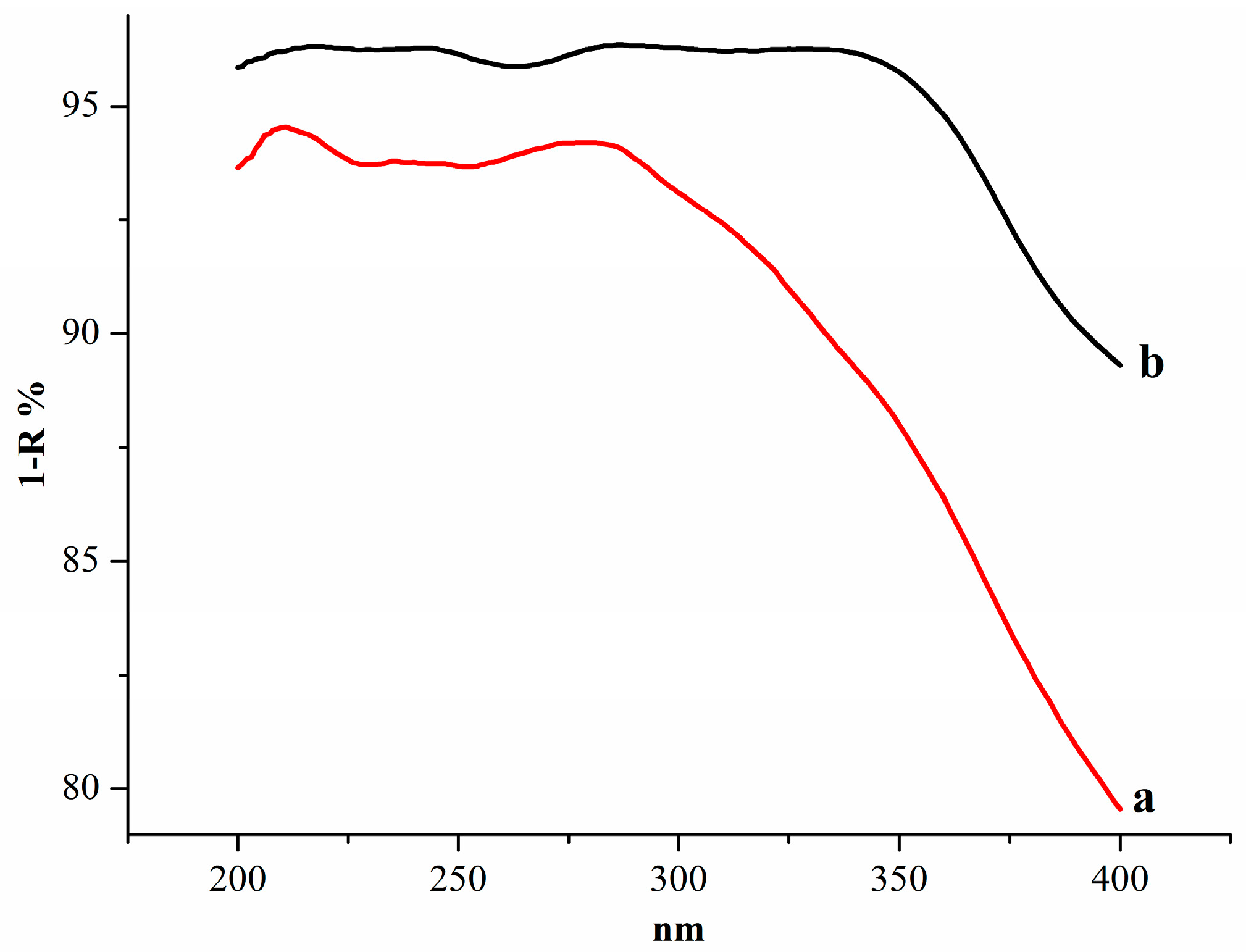
2.5.3. UV-Vis Diffuse-Reflectance Analysis
2.6. Comparison with Different Methods in Flavonoids Extraction
3. Materials and Methods
3.1. Materials and Reagents
3.2. Mechanochemical-Promoted Extraction Technology (MPET)
3.3. Conventional Heating Extraction (CHE)
3.4. Experimental Design
3.5. Total Flavonoids Content
3.6. HPLC-MS Analysis
3.7. Antioxidant Activities
3.7.1. Assay of DPPH Radical Scavenging Activity
3.7.2. Assay of Hydroxyl Radical Scavenging Activity
3.8. Physicochemical Property Analysis
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| Sophora flavescens | S. flavescens |
| MPET | Mechanochemical-promoted extraction technology |
| CHE | Conventional heating extraction |
| RSM | Response surface methodology |
References
- Matsuo, K.; Ito, M.; Honda, G.; Qui, T.K.; Kiuchi, F. Trypanocidal Flavonoids from Sophora flavescens. Nat. Med. 2003, 57, 253–255. [Google Scholar]
- Kuroyanagi, M.; Arakawa, T.; Hirayama, Y.; Hayashi, T. Antibacterial and antiandrogen flavonoids from Sophora flavescens. J. Nat. Prod. 1999, 62, 1595–1599. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.J.; Cao, M.A.; Li, W.H.; Shen, C.S.; Yan, S.Q.; Yuan, C.S. Alkaloids from Sophora flavescens Aition. Fitoterapia 2010, 81, 524–527. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Huang, Q. Studies on effective components of treating sugar diabetes by Chinese crude drug Sophora. Strait. Pharm. J. 1999, 10, 9–11. [Google Scholar]
- Son, J.K.; Park, J.S.; Kim, J.A.; Kim, Y.; Chung, S.R.; Lee, S.H. Prenylated flavonoids from the roots of Sophora flavescens with tyrosinase inhibitory activity. Planta Med. 2003, 69, 559–561. [Google Scholar] [PubMed]
- Jin, J.H.; Kim, J.S.; Kang, S.S.; Son, K.H.; Chang, H.W.; Kim, H.P. Anti-inflammatory and anti-arthritic activity of total flavonoids of the roots of Sophora flavescens. J. Ethnopharmacol. 2010, 127, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.C.; Lin, T.W.; Huang, Y.L. Phenolic constituents of the roots of Sophora flavescens. J. Nat. Prod. 2006, 69, 1237–1240. [Google Scholar] [CrossRef] [PubMed]
- Wan, Z.M.; Shen, L.L. Extraction and Antioxidant Activity of Flavoniods from Sophora flavescens. Food Sci. 2009, 30, 137–140. [Google Scholar]
- Zhang, R.J.; Zhang, G.Y.; Sun, H.B.; Ling, J.Y.; Wang, G. Extraction of Total Flavonoids in Sophora flavescens by Supercritical CO2 Fluid. Food Drug 2009, 11, 19–21. [Google Scholar]
- Zhang, R.J.; Zhang, G.Y.; Sun, H.B.; Ling, J.Y.; Wang, G. Research on microwave extraction for flavones in Sophora flavescens. Food Res. Dev. 2007, 28, 43–46. [Google Scholar]
- Li, H.P.; Li, Y.F.; Liu, Y.Z.; Wang, Y.J. Optimization of Ultrasonic-Assisted Extraction Technology of Total Phenol from the Sophora flavescens by Response Surface Methodology. Food Res. Dev. 2015, 5, 24–28. [Google Scholar]
- Enayati, M.H.; Mohamed, F.A. Application of mechanical alloying/milling for synthesis of nanocrystalline and amorphous materials. Int. Mater. Rev. 2014, 59, 394–416. [Google Scholar] [CrossRef]
- Stolle, A.; Ranu, B. Ball Milling Towards Green Synthesis: Applications, Projects, Challenges, 3rd ed.; RSC: Cambridge, UK, 2015; pp. 55–57. [Google Scholar]
- Dushkin, A.V.; Meteleva, E.S.; Tolstikova, T.G.; Tolstikov, G.A.; Polyakov, N.E.; Neverova, N.A.; Medvedeva, E.N.; Babkin, V.A. Mechanochemical preparation and pharmacological activities of water-soluble intermolecular complexes of arabinogalactan with medicinal agents. Russ. Chem. Bull. 2008, 57, 1299–1307. [Google Scholar] [CrossRef]
- Golovko, A.K.; Davaatseren, B.; Dneprovsky, K.S.; Surkov, V.G.; Lomovsky, O.I. Mechanochemical Treatment of Petroleum Residues and their Mixtures with Brown Coal. Oil Gas Sci. Technol. Rev. de l'IFP 2007, 63, 49–55. [Google Scholar] [CrossRef]
- Lomovsky, O.; Korolyov, K.; Kwon, Y.S. Mechanochemical solubilization and mechanochemically assisted extraction of plant bioactive substances. In Proceedings of the 7th Korea-Russia International Symposium on Science and Technology, Ulsan, Korea, 28 June–6 July 2003; Volume 1, pp. 7–20.
- Goncharov, A.E.; Politov, A.A.; Pankrushina, N.A.; Lomovskii, O.I. Isolation of lappaconitine from Aconitum septentrionale roots by adsorption. Chem. Nat. Compd. 2006, 42, 336–339. [Google Scholar] [CrossRef]
- Liu, Y.; Jin, L.J.; Li, X.Y.; Xu, Y.P. Application of Mechanochemical Pretreatment to Aqueous Extraction of Isofraxidin from Eleutherococcus senticosus. Ind. Eng. Chem. Res. 2007, 46, 6584–6589. [Google Scholar] [CrossRef]
- Wu, L.J. Natural Medicinal Chemistry, 3rd ed.; People’s Medical Publishing House: Beijing, China, 2007; pp. 187–192. [Google Scholar]
- Wang, Y.Q.; Wu, Z.F.; Ke, G.; Yang, M. An Effective Vacuum Assisted Extraction Method for the Optimization of Labdane Diterpenoids from Andrographis paniculata by Response Surface Methodology. Molecules 2015, 20, 430–445. [Google Scholar] [CrossRef] [PubMed]
- Lei, Z.; Liang, X.; Xiao, S.S. Characterization of flavonoids in the extract of Sophora flavescens Ait. by high-performance liquid chromatography coupled with diode-array detector and electrospray ionization mass spectrometry. J. Pharm. Biomed. Anal. 2007, 44, 1019–1028. [Google Scholar]
- Zhang, Y.; Zhang, P.; Cheng, Y. Structural characterization of isoprenylated flavonoids from Kushen by electrospray ionization multistage tandem mass spectrometry. J. Mass Spectrom. 2008, 43, 1421–1431. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Ma, Y.; Gao, H.M.; Zhang, Q.M.; Wang, Z.M. Advance on Chemical Constituents in Sophora flavescens. Chin. J. Exp. Tradit. Med. Formulae 2014, 20, 205–214. [Google Scholar]
- Dong, L.L.; Fu, Y.J.; Zu, Y.G.; Li, J. Negative pressure cavitation accelerated processing for extraction of main bioactive flavonoids from Radix scutellariae. Chem. Eng. Process. 2011, 50, 780–789. [Google Scholar] [CrossRef]
- Jing, C.L.; Dong, X.F.; Tong, J.M. Optimization of Ultrasonic-Assisted Extraction of Flavonoid Compounds and Antioxidants from Alfalfa Using Response Surface Method. Molecules 2015, 20, 15550–15571. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Bi, H.; Gao, T.; Li, Z.; Li, J.; Wei, Y.; Iteku, B.J.; Liu, E.; Zhou, Y. Structural elucidation and antioxidant activity of a water-soluble polysaccharide from the fruit bodies of Bulgaria inquinans (Fries). Food Chem. 2013, 138, 1470–1475. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds are available from the authors.


| Experiment Number | Coded Variables | Actual Variables | Yield (mg/g) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | −1 | −1 | −1 | −1 | 300 | 10 | 15 | 10 | 23.62 |
| 2 | 1 | −1 | −1 | −1 | 500 | 10 | 15 | 10 | 25.65 |
| 3 | −1 | 1 | −1 | −1 | 300 | 20 | 15 | 10 | 25.95 |
| 4 | 1 | 1 | −1 | −1 | 500 | 20 | 15 | 10 | 30.44 |
| 5 | −1 | −1 | 1 | −1 | 300 | 10 | 25 | 10 | 24.99 |
| 6 | 1 | −1 | 1 | −1 | 500 | 10 | 25 | 10 | 28.76 |
| 7 | −1 | 1 | 1 | −1 | 300 | 20 | 25 | 10 | 27.13 |
| 8 | 1 | 1 | 1 | −1 | 500 | 20 | 25 | 10 | 32.08 |
| 9 | −1 | −1 | −1 | 1 | 300 | 10 | 15 | 20 | 24.31 |
| 10 | 1 | −1 | −1 | 1 | 500 | 10 | 15 | 20 | 27.17 |
| 11 | −1 | 1 | −1 | 1 | 300 | 20 | 15 | 20 | 26.97 |
| 12 | 1 | 1 | −1 | 1 | 500 | 20 | 15 | 20 | 30.95 |
| 13 | −1 | −1 | 1 | 1 | 300 | 10 | 25 | 20 | 27.82 |
| 14 | 1 | −1 | 1 | 1 | 500 | 10 | 25 | 20 | 30.15 |
| 15 | −1 | 1 | 1 | 1 | 300 | 20 | 25 | 20 | 29.42 |
| 16 | 1 | 1 | 1 | 1 | 500 | 20 | 25 | 20 | 33.78 |
| 17 | −2 | 0 | 0 | 0 | 200 | 15 | 20 | 15 | 19.41 |
| 18 | 2 | 0 | 0 | 0 | 600 | 15 | 20 | 15 | 27.20 |
| 19 | 0 | −2 | 0 | 0 | 400 | 5 | 20 | 15 | 24.08 |
| 20 | 0 | 2 | 0 | 0 | 400 | 25 | 20 | 15 | 30.24 |
| 21 | 0 | 0 | −2 | 0 | 400 | 15 | 10 | 15 | 28.37 |
| 22 | 0 | 0 | 2 | 0 | 400 | 15 | 30 | 15 | 33.94 |
| 23 | 0 | 0 | 0 | −2 | 400 | 15 | 20 | 5 | 30.03 |
| 24 | 0 | 0 | 0 | 2 | 400 | 15 | 20 | 25 | 33.38 |
| 25 | 0 | 0 | 0 | 0 | 400 | 15 | 20 | 15 | 33.15 |
| 26 | 0 | 0 | 0 | 0 | 400 | 15 | 20 | 15 | 33.63 |
| 27 | 0 | 0 | 0 | 0 | 400 | 15 | 20 | 15 | 33.45 |
| 28 | 0 | 0 | 0 | 0 | 400 | 15 | 20 | 15 | 33.56 |
| 29 | 0 | 0 | 0 | 0 | 400 | 15 | 20 | 15 | 33.74 |
| 30 | 0 | 0 | 0 | 0 | 400 | 15 | 20 | 15 | 33.65 |
| Source | df | Sum of Squares | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 14 | 425.74 | 30.41 | 279.18 | <0.0001 ** |
| 1 | 81.96 | 81.96 | 752.39 | <0.000 1** | |
| 1 | 55.72 | 55.72 | 511.57 | <0.0001 ** | |
| 1 | 38.03 | 38.03 | 349.11 | <0.0001 ** | |
| 1 | 14.49 | 14.49 | 133.05 | <0.0001 ** | |
| 1 | 2.88 | 2.88 | 26.45 | 0.0001 ** | |
| 1 | 0.26 | 0.26 | 2.41 | 0.1413 | |
| 1 | 0.18 | 0.18 | 1.68 | 0.2148 | |
| 1 | 0.51 | 0.51 | 4.73 | 0.0461 * | |
| 1 | 0.052 | 0.052 | 0.48 | 0.5012 | |
| 1 | 1.25 | 1.25 | 11.46 | 0.0041 ** | |
| 1 | 185.28 | 185.28 | 1701.00 | <0.0001 ** | |
| 1 | 73.35 | 73.35 | 673.40 | <0.0001 ** | |
| 1 | 11.11 | 11.11 | 102.04 | <0.0001 ** | |
| 1 | 6.83 | 6.83 | 62.72 | <0.0001 ** | |
| Residual | 15 | 1.63 | 0.11 | ||
| Lack od fit | 10 | 1.41 | 0.14 | 3.21 | 0.1050 |
| Pure error | 5 | 0.22 | 0.044 | ||
| Cor total | 29 | 427.37 | |||
| R2 = 0.9962 | |||||
| Adj-R2 = 0.9926 |
| Method | Extraction Solvent | Extraction Temperature (°C) | Extraction Time (min) | Content (%) | Extraction Yield (mg/g) |
|---|---|---|---|---|---|
| MPET | water | 25 | 20 | 4.76 ± 0.14 | 35.17 ± 0.06 |
| Ultrasound | 44.20% ethanol | 57 | 29.5 | 1.98 ± 0.09 | 33.56 ± 0.11 |
| Microwave | 60% ethanol | - a | 25 | 1.73 ± 0.08 | 35.35 ± 0.09 |
| Conventional heating | 80% ethanol | 85 | 60 + 60 | 2.01 ± 0.13 | 33.87 ± 0.10 |
| Independent Variables | Symbol | Coded Factor Level | ||||
|---|---|---|---|---|---|---|
| −2 | −1 | 0 | 1 | 2 | ||
| Rotational speed (rpm) | 1 | 200 | 300 | 400 | 500 | 600 |
| Grinding time (min) | 2 | 5 | 10 | 15 | 20 | 25 |
| Solvent to material ratio | 3 | 10:1 | 15:1 | 20:1 | 25:1 | 30:1 |
| Solid reagents (%) | 4 | 6 | 9 | 12 | 15 | 18 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Q.; Yu, J.; Wang, Y.; Su, W. Selective Extraction of Flavonoids from Sophora flavescens Ait. by Mechanochemistry. Molecules 2016, 21, 989. https://doi.org/10.3390/molecules21080989
Zhang Q, Yu J, Wang Y, Su W. Selective Extraction of Flavonoids from Sophora flavescens Ait. by Mechanochemistry. Molecules. 2016; 21(8):989. https://doi.org/10.3390/molecules21080989
Chicago/Turabian StyleZhang, Qihong, Jingbo Yu, Yingyao Wang, and Weike Su. 2016. "Selective Extraction of Flavonoids from Sophora flavescens Ait. by Mechanochemistry" Molecules 21, no. 8: 989. https://doi.org/10.3390/molecules21080989
APA StyleZhang, Q., Yu, J., Wang, Y., & Su, W. (2016). Selective Extraction of Flavonoids from Sophora flavescens Ait. by Mechanochemistry. Molecules, 21(8), 989. https://doi.org/10.3390/molecules21080989






