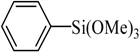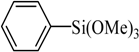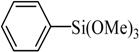Abstract
A palladium (II) complex {[(PhCH2O)2P(CH3)2CHNCH(CH3)2]2PdCl2} catalyzed Hiyama cross-coupling reaction between aryl bromides and arylsilanes has been developed. The substituted biaryls were produced in moderate to high yields, regardless of electron-withdrawing or electron-donating.
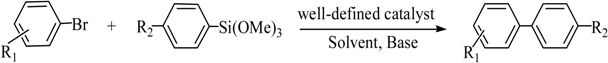
1. Introduction
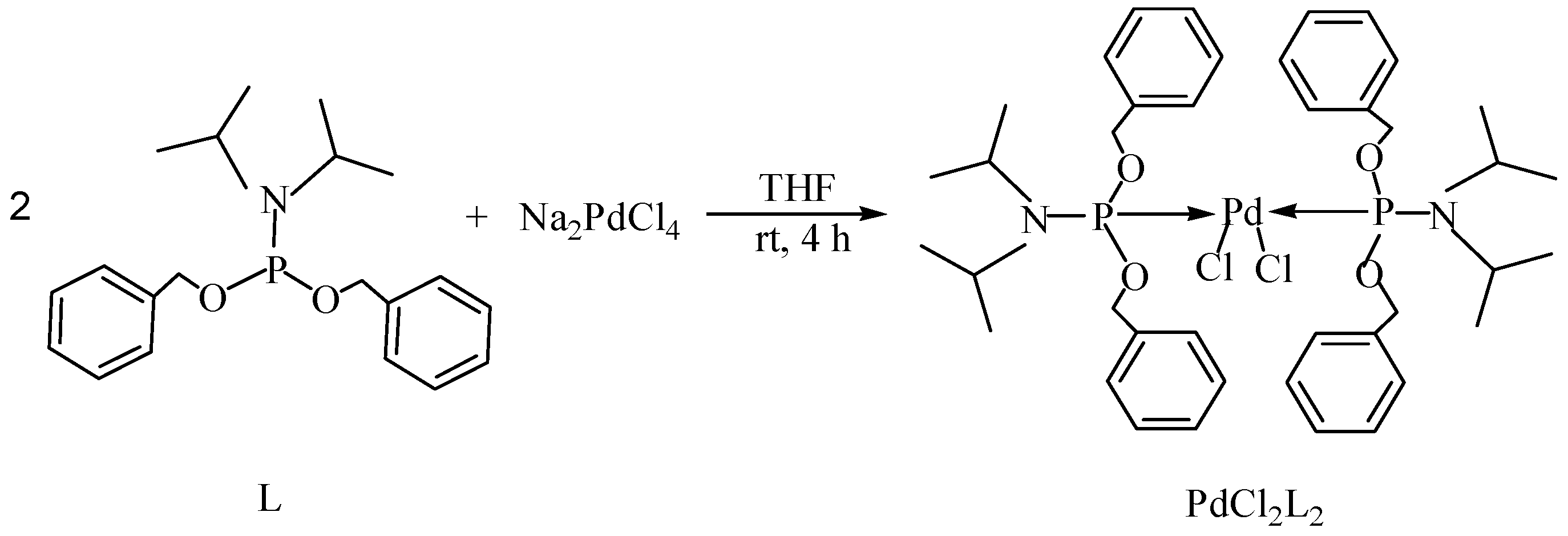
Transition metal-mediated cross-coupling reactions is one of the most powerful and versatile methods for the generation of unsymmetrical biaryls which are widely found as important structural units in pharmaceuticals [1], natural products [2], bioactive products [3], herbicides [4], conducting polymers [5], liquid crystal materials [6] and microelectrode arrays [7]. These cross-coupling transformations include organozinc (Negishi) [8] reaction, organotin (Stille) [9] reaction, organoboron (Suzuki-Miyaura) [10] reaction, and organomagnesium (Kumada) reaction [11]. Aryl halides have been widely used for a variety of cross-coupling reactions to form C-C bond. Recently, “comparatively unreactive” organosilane (Hiyama) reagents have been proposed as potential coupling partners due to their low cost and toxicity [12,13] ease of handling and stability in various chemical media [14]. Generally, great successes have been obtained using in situ catalytic systems as catalysts for Hiyama cross-coupling reactions [15,16,17,18]. However, the use of well-defined catalysts is still rare [14,19,20,21,22]. Herein, we have synthesized an air- and moisture-stable Pd (II) complex [(PhCH2O)2P(CH3)2CHNCH(CH3)2]2PdCl2 (PdCl2L2) containing electron-rich, sterically bulky phosphane (L), and it has been proved to be a highly efficient catalyst for Suzuki reaction with low Pd-catalyst loading (0.01%) [23]. Herein, this catalyst (Figure 1) is used for palladium-catalyzed Hiyama coupling reactions of arylsilanes with aryl bromides under ambient atmosphere.
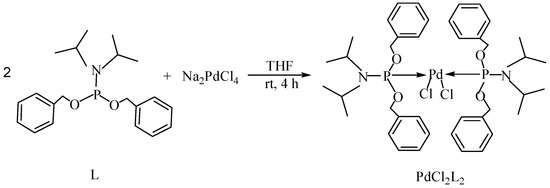
Figure 1.
Synthesis of catalyst.
2. Results and Discussion
2.1. Optimization of the Hiyama Reaction Condition
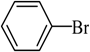
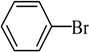
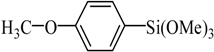
The catalyst PdCl2L2, which was inert to air and moisture, was composed of Na2PdCl4 with 2 equiv of the ligand L in THF at room temperature (Figure 1). The X-ray crystal structure of PdCl2L2 was shown as reported in [23]. The model reaction of bromobenzene with phenyltrimethoxysilane was initiated to optimize the cross-coupling conditions. Solvents used in the reaction were environmentally friendly and cheap and it avoid the troublesome solvents (NMP, DMF, etc.) that were conventionally applied in similar Hiyama reactions [14]. Results showed that no cross-coupled product was obtained when only H2O was used as solvent (Table 1, entry 1). However, it was exciting that a low yield (36%) was obtained in PEG solvent (Table 1, entry 2). Inspired by this, we explored different proportions of PEG and H2O (Table 1, entries 3–5), and it was discovered that PEG:H2O = 1:1 (volume ratio) was the efficient reaction system in this reaction (Table 1, entry 4) while the mixed solvent CH3CH2OH and H2O was not suitable for this reaction (Table 1, entry 6). Consequently, PEG:H2O = 1:1 (volume ratio) was chosen as the best solvent. To the best our knowledge, the base TBAF•3H2O played a key role in this reaction because TBAF•3H2O may show the function of the activation of arylsilane [24]. In order to avoid the use of TBAF•3H2O, we started to investigate a variety of inorganic salt base which should show the capability to favor the removal of silicon groups. Intriguingly, it gave a high yield of biphenyls (85%) when NaOH was applied as the base (Table 1, entry 7). Furthermore, the reaction did not proceed well when carried out with other base such as KOH, Et3N, Na2CO3, K3PO4 and NaOAc•3H2O (Table 1, entries 8–12). According to the above results, PEG:H2O = 1:1 (volume ratio), as the solvent, and NaOH as the base gave the best results. Since this catalytic system was not sensitive to oxygen, the reactions were carried out under air atmosphere without the protection of nitrogen.

Table 1.
Optimization of the Hiyama reaction condition a. 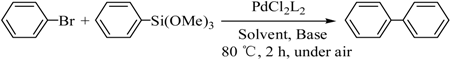
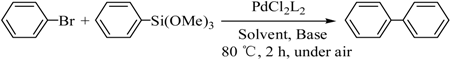
2.2. Scope of the Substrates
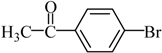
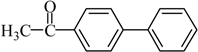
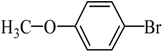
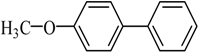
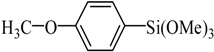
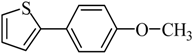
The optimized reaction conditions were used in the Hiyama coupling reaction of various aryl bromides and arylsilanes with PdCl2L2 as a catalyst. The results were shown in Table 2. As expected, activated aryl bromide was smoothly converted into the corresponding products in 92% yields and 85% yields (Table 2, entries 2, 5). However, the electron donating group of aryl bromide would slightly decrease the reaction efficiency (Table 2, entries 3, 6). We also examined the electron donating group of arylsilane base on the resulting yields of the reactions. Electron donating group of arylsilane could be afforded biphenyl compounds at a higher temperature for 100 °C (Table 2, entries 4–8) but with lower yields. If aryl bromide and arylsilane both contained an electron donating group, the biphenyl yield clearly declined (Table 2, entry 6). Importantly, 1-bromonaphthalene was also applicable to these reaction conditions in moderate to good yield (Table 2, entry 7). The reaction system was also sufficiently stable for halogenated heterocyclic, so that 2-bromothiophene could be coupled with good efficiency (Table 2, entry 8).

Table 2.
Scope and Limitations of the Substrates a. 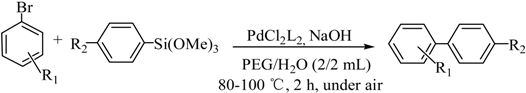

3. Experimental Section
3.1. Reagents and Equipment
NMR spectra were recorded using 400 MHz in DMSO-d6 solutions at room temperature (tetramethylsilane (TMS) was used as an internal standard) on a Bruker Avance III spectrometer (Billerica, MA, USA, see Supplementary Materials). All chemicals employed in the reaction were analytical grade, obtained commercially from Aldrich or Alfa Aesar and were used as received without any prior purification.
3.2. Synthesis of the Catalyst
The palladium complex PdCl2L2 was prepared using a method previously reported elsewhere [21]. A solution of 1 mmol (0.345 g) was added dropwise to a suspension of 0.5 mmol Na2PdCl4 (0.147 g) in THF (20.0 mL) and the reaction mixture was stirred at ambient temperature for 4 h (Figure 1). The volume was reduced to 5.0 mL and diethyl ether was added to precipitate a yellow powder which was then filtered off and washed with diethyl ether. The complex PdCl2L2 was obtained in 92% yield.
3.3. General Procedure for the Synthesis
A mixture of aryl bromide (1.0 mmol), arylsilane (1.2 mmol), NaOH (3.0 mmol), 4.0 mL solvent, PEG:H2O = 1:1 (volume ratio) and catalyst (0.02 mmol) was stirred at 80–100 °C for 2 h under air. The reaction was quenched with brine (15 mL) and extracted three times with ethyl acetate (3 × 10 mL). The organic phase was dried with MgSO4 for 4 h, filtered and concentrated under reduced pressure using a rotary evaporator. The crude products were re-crystallized by dichloromethane (2 mL) at −10 °C for 24 h. Filtered and dried, the purified products were identified by 1H-NMR and 13C-NMR spectroscopy.
3.4. Analytical Data of Representative Products
Biphenyl Yield 88%: mp: 70–71 °C; 1H-NMR (DMSO-d6): δ 7.66 (d, J = 7.2 Hz, 4H), 7.47 (d, J = 7.6 Hz, 4H), 7.37 (t, J = 7.2 Hz, 2H). 13C-NMR (DMSO-d6): δ 127.1, 127.8, 129.3, 140.7.
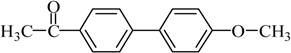
4-Acetyl-4′-methoxybiphenyl Yield 85%: mp: 153–154 °C; 1H-NMR (DMSO-d6): δ = 7.99 (d, J = 8.0 Hz, 2H), 7.79(d, J = 7.5 Hz, 2H), 7.77 (d, J = 8.0 Hz, 2H), 7.05 (d, J = 7.5 Hz, 2H), 3.81 (s, 3H), 2.59 (s, 3H). 13C-NMR (DMSO-d6): δ 27.1, 55.7, 115.0, 126.6, 128.6, 129.3, 131.5, 135.4, 144.6, 160.1, 197.8.
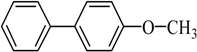
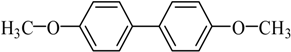
4-Methoxybiphenyl Yield 74% and Yield 72%: mp: 90 °C; 1H-NMR (DMSO-d6): δ = 7.61 (m, 4H), 7.43 (m, 2H), 7.32 (d, J = 7.2 Hz, 1H), 7.01 (d, J = 8.2 Hz, 2H), 3.79 (s, 3H, CH3). 13C-NMR (DMSO-d6): δ 55.6, 114.8, 126.6, 127.1, 128.2, 129.3, 133.0, 140.3, 159.3.
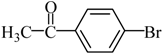
4-Acetylbiphenyl Yield 92%: mp: 121 °C; 1H-NMR (DMSO-d6): δ = 8.04 (d, J = 8.0 Hz, 2H), 7.82 (d, J = 8.0 Hz, 2H), 7.75 (d, J = 8.0 Hz, 2H), 7.51 (m, 1H), 7.44 (d, J = 8.0 Hz, 2H), 2.61 (s, 3H). 13C-NMR (DMSO-d6): δ 27.2, 127.3, 127.4, 128.8, 129.3, 129.5, 136.1, 139.3, 145.0, 197.9.
4. Conclusions
In conclusion, complex PdCl2L2 was demonstrated to be a highly active catalyst for the Hiyama coupling reaction of a range of aryl bromides with arylsilanes, affording the coupling products with moderate to high yields. This method is consistent with the concept of green chemistry, and further studies on the applicability of this catalyst system in other coupling reactions such as Sonogashira and amination are currently under investigation in our laboratory.
Supplementary Materials
Supplementary materials can be accessed at: http://www.mdpi.com/1420-3049/21/8/987/s1.
Acknowledgments
Financial support from the National Natural Science Foundation of China (No. 21063015, No. 21363026) and the Jiangxi Provincial Natural Science Foundation of China (No. 20114BAB203012), the Key Science and Technology plan of Yichun City (No. (2010) 24) is gratefully acknowledged.
Author Contributions
M.G. and L.F. conceived and designed research. J.L., L.Z. and Y.K. performed the experiments. L.F. wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bringmann, G.; Rudenauer, S.; Bruhn, L.; Benson, L.; Brun, R. Total synthesis of the antimalarial naphthylisoquinoline alkaloid 5-epi-4′-O-demethylancistrobertsonine C by asymmetric Suzuki cross-coupling. Tetrahedron 2008, 64, 5563–5568. [Google Scholar] [CrossRef]
- Pouilhes, A.; Amado, A.F.; Vidals, A.; Langlois, Y.; Kouklovsky, C. Enantioselective total synthesis of pyrinodemin A. Org. Biomol. Chem. 2008, 6, 1502–1510. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Karisch, R.; Lautens, M. Efficient syntheses of KDR kinase inhibitors using a Pd-catalyzed tandem CN/Suzuki coupling as the key step. J. Org. Chem. 2007, 72, 1341–1346. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, P.; Hamprecht, G.; Puhl, M.; Westphalen, K.-O.; Zagar, C. Synthesis and Herbicidal Activity of Phenylpyridines—A New Lead. CHIMIA Int. J. Chem. 2003, 57, 715–719. [Google Scholar] [CrossRef]
- Iraqi, A.; Barker, G.W. Synthesis and characterisation of telechelic regioregular head-to-tail poly(3-alkylthiophenes). J. Mater. Chem. 1998, 8, 25–29. [Google Scholar] [CrossRef]
- Hassan, J.; Sevignon, M.; Gozzi, C.; Schulz, E.; Lemaire, M. Aryl-aryl bond formation one century after the discovery of the Ullmann reaction. Chem. Rev. 2002, 102, 1359–1470. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Maurer, K.; Moeller, K.D. Building addressable libraries: Site-selective Suzuki reactions on microelectrode arrays. Org. Lett. 2009, 11, 1273–1276. [Google Scholar] [CrossRef] [PubMed]
- Dubbaka, S.R.; Vogel, P. Palladium-catalyzed desulfinylative Negishi C–C bond forming cross-couplings of sulfonyl and organozinc chlorides. Tetrahedron Lett. 2006, 47, 3345–3348. [Google Scholar] [CrossRef]
- Dubbaka, S.R.; Vogel, P. Palladium-catalyzed stille cross-couplings of sulfonyl chlorides and organostannanes. J. Am. Chem. Soc. 2003, 125, 15292–15293. [Google Scholar] [CrossRef] [PubMed]
- Dubbaka, S.R.; Vogel, P. Palladium-catalyzed Suzuki-Miyaura cross-couplings of sulfonyl chlorides and boronic acids. Org. Lett. 2004, 6, 95–98. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, W.A.; Bohm, V.P.W.; Reisinger, C.P. Application of palladacycles in Heck type reactions. J. Organomet. Chem. 1999, 576, 23–41. [Google Scholar] [CrossRef]
- Franzen, R.G. Utilization of Grignard reagents in solid-phase synthesis: A review of the literature. Tetrahedron 2000, 56, 685–691. [Google Scholar] [CrossRef]
- Ashby, E.C.; Laemmle, J.; Neumann, H.M. Mechanisms of Grignard reagent addition to ketones. Acc. Chem. Res. 1974, 7, 272–280. [Google Scholar] [CrossRef]
- Abdol, R.H.; Fatemeh, R.; Narges, N. Hiyama cross-coupling reaction catalyzed by a palladium salt of 1-benzyl-4-aza-1-azoniabicyclo[2.2.2]octane chloride under microwave irradiation. Appl. Organomet. Chem. 2014, 28, 217–220. [Google Scholar]
- Peñafiel, I.; Pastor, I.M.; Yus, M. NHC-Ligand Effectiveness in the Fluorine-Free Hiyama Reaction of Aryl Halides. Eur. J. Org. Chem. 2013, 1479–1484. [Google Scholar] [CrossRef]
- Lee, J.Y.; Fu, G.C. Room-temperature Hiyama cross-couplings of arylsilanes with alkyl bromides and iodides. J. Am. Chem. Soc. 2003, 125, 5616–5617. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Yang, M.; Li, H.; Qi, Y.-X. Application of Cyclic Thiourea as an Efficient Ligand in Palladium-Catalyzed Hiyama Cross-Coupling Reactions. Synthesis 2008, 2008, 1415–1419. [Google Scholar]
- Raders, S.M.; Kingston, J.V.; Verkade, J.G. Advantageous Use of tBu2P-N=P(iBuNCH2CH2)3N in the Hiyama Coupling of Aryl Bromides and Chlorides. J. Org. Chem. 2010, 75, 1744–1747. [Google Scholar] [CrossRef] [PubMed]
- Hajipour, A.R.; Rafiee, F. Microwave-assisted Stille and Hiyama cross-coupling reactions catalyzed by ortho-palladated complexes of homoveratrylamine. Tetrahedron Lett. 2012, 53, 4661–4664. [Google Scholar] [CrossRef]
- Yang, J.; Li, P.H.; Zhang, Y.C.; Wang, L. Dinuclear NHC-palladium complexes containing phosphine spacers: Synthesis, X-ray structures and their catalytic activities towards the Hiyama coupling reaction. Dalton Trans. 2014, 43, 7166–7175. [Google Scholar] [CrossRef] [PubMed]
- Gao, T.T.; Jin, A.P.; Shao, L.X. N-Heterocyclic carbine-palladium(II)-1-methylimidazole complex catalyzed Mizoroki-Heck reaction of aryl chlorides with styrenes. Beilstein J. Organ. Chem. 2012, 8, 1916–1919. [Google Scholar] [CrossRef] [PubMed]
- Hajipour, A.R.; Rafiee, F. Synthesis of substituted biaryls via Suzuki, Stille and Hiyama cross-coupling and homo-coupling reactions by CN-dimeric and monomeric ortho-palladated catalysts. Appl. Organomet. Chem. 2013, 27, 412–418. [Google Scholar] [CrossRef]
- Guo, M.P.; Zhang, Q.C. An inexpensive and highly stable palladium(II) complex for room temperature Suzuki coupling reactions under ambient atmosphere. Tetrahedron Lett. 2009, 50, 1965–1968. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, F.; Li, K.; Zhao, B.L. Pd-catalyzed desulfitative Hiyama coupling with sulfonyl chlorides. Appl. Organomet. Chem. 2014, 28, 379–381. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds {[(PhCH2O)2P(CH3)2CHNCH(CH3)2]2PdCl2} are available from the authors.
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).