Abstract
Traditional Chinese Medicine (TCM) has been used to treat diseases in China for thousands of years. TCM compositions are complex, using as their various sources plants, animals, fungi, and minerals. Polysaccharides are one of the active and important ingredients of TCMs. Polysaccharides from TCMs exhibit a wide range of biological activities in terms of immunity- modifying, antiviral, anti-inflammatory, anti-oxidative, and anti-tumor properties. With their widespread biological activities, polysaccharides consistently attract scientist's interests, and the studies often concentrate on the extraction, purification, and biological activity of TCM polysaccharides. Currently, numerous studies have shown that the modification of polysaccharides can heighten or change the biological activities, which is a new angle of polysaccharide research. This review highlights the current knowledge of TCM polysaccharides, including their extraction, purification, modification, and biological activity, which will hopefully provide profound insights facilitating further research and development.
1. Introduction
For thousands of years, Traditional Chinese Medicine (TCM) has been the most important therapeutic method in China and even the whole of East Asia. As we know, the compositions of traditional Chinese medicines are complex, and their active ingredients are often polysaccharides, saponins, flavonoids, polyphenols, and polypeptides [1]. Many extracts from TCM, such as artemisinin, arsenic trioxide, and curcumin display important roles in medicine and have been used in the clinic [2]. Polysaccharides from TCM have been a research hotspot recently. They are a kind of biomacromolecule composed of ten or more monosaccharides (Figure 1) whose structure and sugar composition vary. It is reported that at least thirty polysaccharides have been studied in standard clinical trials [3].
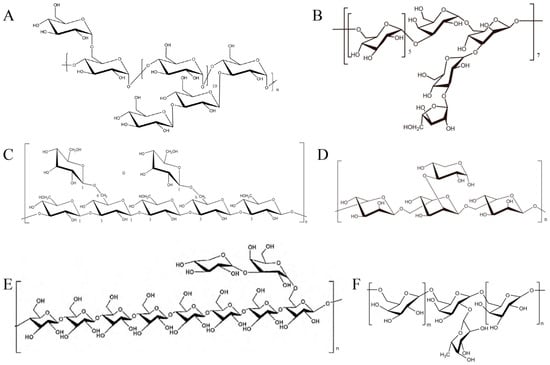
Figure 1.
Examples of structures of some TCM polysaccharides. (A) Bush sophora root polysaccharide, whose average molecular weight is 2.24 × 104 Da [4]; (B) Euphorbia fischeriana polysaccharide, whose average molecular weight is 1.12 × 104 Da [5]; (C) Lentinula edodes polysaccharide [3]; (D) Lactarius deliciosus Gray polysaccharide, whose average molecular weight is 1.1 × 104 Da [6]; (E) Tricholoma matsutake polysaccharide, whose average molecular weight is 8.89 × 104 Da [7]; and (F) Hericium erinaceus polysaccharide, m + n = 3, whose average molecular weight is 1.5 × 104 Da [8].
Studies on TCM polysaccharides generally focus on their extraction, purification, structure, modification, and biological activity. Extraction methods of TCM polysaccharides are various and always influence the variety and characteristics of the final extracted products [3]. The most widely used method is hot water extraction [3,9], although some polysaccharides are not soluble in hot water and therefore not extracted by this technique. Consequently, many new methods and techniques have been applied to develop efficient extractions (Figure 2). No matter the extraction method, the purity of the polysaccharide is generally low. Consequently, purification and structural determination studies are important (Figure 2). The biological activities of polysaccharides from TCMs are varied and, in general, one polysaccharide always expresses several biological activities. For example, Astragalus polysaccharide exhibits immunoregulatory [10], anti-oxidative [11], antiviral [12], and anti-tumor [13] activities. Currently, many studies have focused on the immunity-modifying, antiviral, anti-inflammatory, anti-oxidative, and anti-tumor activities of TCM polysaccharides [14,15,16]. Moreover, chemical modifications can heighten or change the biological activities of TCM polysaccharides [17,18,19] and have attracted more and more attention. As we know, the extraction and purification of polysaccharides are so complicated that it is quite difficult to obtain pure active polysaccharides. From another perspective, this suggests that the extraction and purification also have significant influence on the modification and biological activity of polysaccharides. Different extraction methods and purified fractions may lead to different biological activities of polysaccharides, thereby influencing the modification. Thus, extraction and purification are the necessary prerequisites for the analysis of biological activity and modification. Here, we summarize the current knowledge on the extraction, purification, modification, and biological activities of polysaccharides from TCMs.
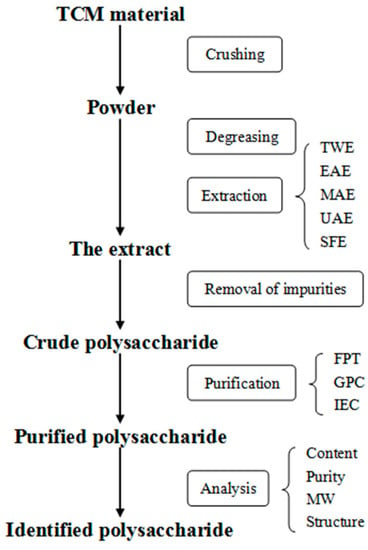
Figure 2.
The flowchart of the extraction, purification, and analysis of polysaccharides. TWE: traditional water extraction, EAE: enzyme-assisted extraction, MAE: microwave-assisted extraction, UAE: ultrasonic-assisted extraction, SFE: supercritical fluid extraction, FPT: fractionated precipitation technique, GPC: gel permeation chromatography, IEC: ion exchange chromatography, MW: molecular weight.
2. Extraction of TCM Polysaccharides
2.1. Extraction Pre-Processing
TCM polysaccharides are classified into two types according to their locations in the plant: intracellular polysaccharides (IPSs) and extracellular polysaccharides (exopolysaccharides, EPSs) [20]. To facilitate the release of IPSs, the first step of extraction is mechanical crushing of the TCM material. Next is degreasing, as many plant seeds and animal products are rich in lipids, which largely surround the cell wall. Usually this is accomplished by Soxhlet extraction using an organic solvent because of the hydrophilicity of the polysaccharides. Subsequently, the polysaccharides are extracted by means of various methods.
2.2. Extraction Method
The basic extraction principle is to destroy and degrade the cell wall under mild conditions so that the intrinsic properties of polysaccharides remain unchanged [21,22]. Traditional water extraction (TWE) is a common and popular approach for polysaccharide extraction, especially hot water extraction (HWE), which is the most traditional and classic one. Recently, various novel techniques have been developed, including enzyme-assisted extraction (EAE) [23], microwave-assisted extraction (MAE) [24], ultrasonic-assisted extraction (UAE) [25], and supercritical fluid extraction (SFE) [26]. An appropriate extraction method is not only a means to increase the extraction yield, but also contribute to the high biological activity of the resulting polysaccharide extract [27]. Every extraction method has its strengths and weaknesses, so a comprehensive understanding of these effects on the physical and chemical properties of the polysaccharide is necessary.
2.2.1. Traditional Water Extraction
In general, TCM polysaccharides are polar and hydrophilic macromolecules. Polysaccharides can be extracted with a strong polar solvent, and water is an ideal solvent which is popularly used in practice. High temperature can accelerate the dissolution of polysaccharide from the cell wall and make it easier to dissolve in water [28]. Thus, hot water extraction (HWE) has long been the traditional and classic method for polysaccharide extraction [22]. Many studies have demonstrated that the extraction yield of HWE is largely affected by extraction time, temperature, ratio of water to raw material, and the number of extraction steps [29,30,31,32]. Especially, HWE of long duration and at high temperature may result in the degradation of the polysaccharides and decrease the biological activity [22,27]. For example, the yield of polysaccharides from the roots of Codonopsis pilosula increases up to its maximum amount at 94°C, but beyond this level, the yield decreases with increasing extraction temperature [33]. Usually, the common temperature range for HWE is between 70 °C and 90 °C, and the extraction time is between 2 h and 6 h, which are suitable for most polysaccharides [3]. In a word, HWE is the most common and convenient method to extract polysaccharides, and some of its major drawbacks are the high temperatures, long times, and low efficiency.
To improve the efficiency and purity, dilute alkaline and acidic solution methods based on HWE have come into use. Some acidic polysaccharides are not easily dissolved in hot water, and therefore an alkaline solution is applied to increase their solubility [3]. An acidic polysaccharide could be isolated from Morinda officinalis by alkaline solvent extraction [34]. However, the glycosidic bonds of polysaccharides easily break under acidic conditions, so acidic solutions are seldom used. The extraction pH, time, and temperature should be limited to a certain range, or the structure of the polysaccharide could be destroyed.
2.2.2. Enzyme-Assisted Extraction
Owing to the relatively mild reaction conditions, EAE possesses the advantages of environmental friendliness, high efficiency, ease of operation, low investment cost and energy requirements [23,35]. EAE involves the use of enzymes, such as proteases, cellulases, amylases, glucanases, or endoproteases [35], which effectively and mildly catalyze the degradation of the cell wall and facilitate the release of IPSs [36,37]. However, the enzyme is characterized by specificity and selectivity while, at the same time, the biological activity is influenced by several factors, such as enzyme concentration, temperature, time, and pH, which cooperatively affect the extraction [38,39]. Thus, only one kind of enzyme usually cannot satisfy the extraction objectives. Composite enzymes or a cocktail of enzymes which has a broad spectrum of activity is one of the best methods to disrupt the cell wall [40]. Moreover, it can lead to a higher level of hydrolysis because of synergistic effects. It has been reported that an efficient complex enzyme-assisted extraction technology has been developed and optimized to extract polysaccharides from alfalfa [41]. As is known to all, every enzyme has optimum reaction conditions where the activity is the highest, while the optimal condition of composite enzymes could be different from that of any single enzyme [42]. Thus, the synergistic effects, types of substrate and the presence of any enzyme inhibitors also should be considered in EAE. EAE is seldom used alone, and is usually combined with other extraction methods to increase the yield of polysaccharide.
2.2.3. Physical Technique-Assisted Extraction
Physical techniques have demonstrated their extraction ability. In comparison to traditional extraction methods, the yield of polysaccharides increases with lower cost and shorter time [43]. There are three important methods commonly used in research and practice.
MAE has drawn significant attention in the analysis and extraction of active compounds from TCM materials [44]. Some studies have suggested a mechanism whereby microwave power rapidly ruptures the cell structure and releases the intracellular products into the solvent [45,46,47]. On the other hand, microwaves can also penetrate into products and interact with polar components to generate heat [44]. The resulting kinetic energy and high temperature will accelerate the mass transfer in a short time [44,48]. Due to its special mechanism, MAE has noticeable advantages, such as shorter extraction times, higher extraction yields, lower cost, and less solvent consumption compared to TWE [49,50,51,52,53], and factors including microwave power, extraction time, ratio of solid to solvent, and microwave application duration may influence the efficiency of MAE [54,55]. Previous studies have reported the possibility of polysaccharide decomposition during MAE [56,57]. Furthermore, there are some drawbacks associated with MAE, such as the requirement of additional clean up steps and the restriction to polar solvent application [58]. However, it is apparent that the technique of MAE has been continuously improved to enhance its performance.
The use of UAE as an alternative and promising method has been on the rise. As a tool for extraction intensification, the mechanism of UAE is not yet well understood [59,60]. Many studies have reported that the possible benefits of ultrasound may be due to an enhancement of the mass transfer between the plant and solvent [61,62,63], intensification of shear forces arising from acoustic cavitation [20,64], and improved penetration and capillary effects [65]. All of these lead to an increase of polysaccharides’ extractability by destruction of the cell wall [54,62]. On the other hand, ultrasonic treatment could affect the structure and molecular weight (MW) of polysaccharides to some extent, which would inevitably cause a change in the biological activity [66,67,68,69]. Several parameters are involved in UAE, such as the type of ultrasonic device, the frequency and power of the ultrasound, and the sonication time [43].
SFE is a new method developed in recent years to extract polysaccharides. Supercritical fluids are a special kind of solvent, and has the characteristics of both liquid and gas which can effuse through solids like a gas, and dissolve materials like a liquid. SFE increases the solubility of polysaccharides and enables their better extraction [70]. CO2 is often used as the solvent for SFE because its critical conditions is easy to achieve. Pachyman in Poriacocos (Schw.) wolf has been extracted by SFE under optimal conditions involving an extraction temperature of 35 °C and CO2 pressure 20 MPa [71]. The main advantages of SFE over TWE are the improvement of extraction efficiency and shorter production cycle, as well as the elimination of polluting solvents and expensive post-processing [26]. However, the problems of SFE are complex equipment requirements, high operational cost, and limited applicability.
3. Purification of Polysaccharides
3.1. Removal of Impurities
Generally the extract is a mixture which contains proteins, pigments, small molecules, and other impurities. Therefore, a subsequent processing step should be performed to remove these impurities. The common deproteinization methods are the Sevag method [72], the trichlorotrifluoroethane method [3], and the trichloroacetic acid method [73]. In these methods, the reagents used for denaturation and precipitation of protein hardly work for polysaccharides, so the protein should be removed. For example, after addition of the Sevag reagent (chloroform: n-butanol = 4:1) to polysaccharide aqueous solution, the mixture is violently shaken and centrifuged. Usually this process is repeated five times or more to get rid of the protein. In order to obtain a better removal rate, several methods can be combined together to reduce time and solvent use at the same time. Pigments always affect the purification and property analysis of polysaccharides. They can be absorbed with activated carbon, resins, or destroyed by hydrogen peroxide treatment [73,74]. However, activated carbon can absorb the polysaccharide causing a yield loss, and hydrogen peroxide treatment can also cause partial hydrolysis of the polysaccharide [75,76]. In general, these two methods are seldom used for decolorization. A polysaccharide is macromolecule, so small molecules, like oligosaccharides and inorganic salt, can be easily removed using dialysis against water. After these treatments, the resulting extract still needs to be purified further to obtain pure polysaccharide in composition and properties.
3.2. Purification Method
Most purification methods are based on solubility differences, and the fractionated precipitation technique is considered as a unique and convenient approach to selectively yield polysaccharides with large differences in solubility in lower alcohols or ketones [77]. In this method, the lower alcohol or ketone is gradually added to the extract. Then the precipitated polysaccharides at each concentration of the lower alcohol or ketone can be collected by centrifugation. With the increasing concentration, the MW of obtained polysaccharides gradually reduces. It has been reported that three main polysaccharide fractions from Asparagus officinalis, AOP-4, AOP-6, and AOP-8 are obtained by fractional precipitation using gradient concentrations of ethanol (40%, 60%, and 80%) [78]. The salting-out method is also able to separate different polysaccharides out in the form of a precipitate [3]. Furthermore, long-chain quaternary ammonium salts and various metal ions can form coordination compounds with polysaccharides to achieve separation and purification.
On the other hand, the chromatography technique is one of the most effective separation and analysis methods. This technique can separate out individual polysaccharides from the extract with high sensitivity, selectivity, and separation resolution. The most used methods to purify polysaccharides are gel permeation chromatography and ion exchange chromatography. Gel permeation chromatography (size exclusion chromatography) is based on the simple principle of the separation of molecules due to size [79]. Briefly, the large molecules simply pass by the pores of particular gels because those molecules are too large to enter the pores, while the small molecules are trapped in the pores. Therefore, high MW polysaccharide is eluted out first, and then low MW polysaccharide is eluted. Various gels have been used, like Sephacryl S-400 HR, Sephadex G-200, and Sepharose 6B [80]. Most TCM polysaccharides are weak acids, so they are charged by ionic complex formation in aqueous media [81]. Ion exchange chromatography is able to separate acidic and neutral polysaccharides based on their net charge differences. The ionizable molecules can bind to oppositely charged moieties by forming covalent bonds to the insoluble stationary phase, and then these molecules can be gradually eluted under different elution conditions. In this way, the polysaccharides can be separated and quantified. For example, the polysaccharides extracted from the dried fruiting bodies of Pithecellobium dulce have been isolated by ion exchange chromatography and afford three water-soluble polysaccharides PDP-1, PDP-2, and PDP-3 [82].
Furthermore, other techniques like ultracentrifugation, ultrafiltration [83], and preparative zone electrophoresis can also be applied to the purification of polysaccharides. To achieve high purity of polysaccharides, many researchers advise using two or more methods simultaneously.
4. Analysis of Polysaccharides
It is generally accepted that the biological activity of polysaccharides often depends on the molecular structure and other physicochemical properties, including water solubility, MW, monosaccharide composition, glycosidic bonds of the main chain, etc. [84], so the composition of polysaccharides is critical to their biological activity. Since many kinds of proteins, lipids, biological bases, and other ingredients usually exist together with polysaccharides in TCM materials, the analysis is very difficult. Therefore, the extraction and purification can greatly influence the analysis. At present, there are various methods for the analysis. The total content of polysaccharide is often determined by colorimeter with different chromogenic systems, such as phenol-sulfuric acid [85,86], anthracenone-sulfuric acid [87], and carbazole-sulfuric acid [88]. As the strongest separation technique, chromatographic methods have been extensively applied to the compositional and structural analysis of polysaccharides when combined with other structural analytical techniques, including IR, NMR, MS, and so on [81]. According to the previous studies [80,81,89,90], the different chromatography techniques are shown in Table 1. TCM polysaccharides are complicated and the pretreatments must be done before analysis. In general, there are a number of ways to pretreat polysaccharides, including Smith degradation, periodate oxidation, methylation analysis, etc., and then the various analysis techniques mentioned above can be applied to the compositional and structural determination. Moreover, the electromigration method has very high separation efficiency and is also extensively used for analysis of polysaccharides. With the emergence and development of modern analysis techniques, more and more methods will be created and introduced to the analysis of polysaccharides.

Table 1.
The comparison of different chromatography techniques.
5. Modification of Polysaccharides
Chemical modification is an important approach to tailor polysaccharide structure and properties [91]. In the last decades, much attention has been focused on the modification of polysaccharides which can heighten or change the biological activities. Among the various modification methods, sulfation, phosphorylation, and carboxymethylation are the most common and important.
5.1. Sulfation
It is well known that sulfation of polysaccharides is an effective method to modify their biological activity and function. In general, the hydroxyl groups of TCM polysaccharides are substituted by sulfate groups [92], and it has been reported that the hydroxyl groups of the monosaccharide residues at C-6 are more active owing to the steric hindrance effect and the absence of a hydrogen bond, therefore substitution at C-6 easily occurs [93,94]. There are several methods for polysaccharide sulfation, using different reagents such as sulfuric acid, sulfur trioxide-pyridine [95], chlorosulfonic acid-pyridine (CSA-Pyr), and so on [96]. The first two are less used because of low degree of esterification and recovery. CSA-Pyr, by contrast, is the most common and widely used one since it is characterized by high yield and convenient isolation of the product [97], and it has been applied in the sulfation of polysaccharides from Cyclocarya paliurus [85], Cyclina sinensis [98], Borojoa sorbiliscuter [99], etc. The sulfation can affect the MW, charge, solubility, and conformation of the polysaccharide [100]. As a result, the biological activity will be heightened or changed. There are many reports demonstrating the influence of sulfation on the biological activities, including antioxidant, immunostimulant, antitumor, antiviral, anticoagulant, hypoglycemic, and cytotoxic properties [101,102,103,104,105,106,107,108]. For example, sulfated Angelica polysaccharides-1 could not only inhibit virus replication, but also improve the immune function. Additionally, the activity of sulfated polysaccharide also depends on structural modification, such as the degree of substitution (DS), the sulfation position, the type of polysaccharide, and the structure of the main chains and branches [99,109,110,111]. In particular, the DS which represents the average number of sulfate groups on each monosaccharide residue [92,112] is significantly important to the activity. Previous studies have shown that there is an optimum DS of sulfation to achieve a maximal biological response [101]. The antiviral activity of sulfated polysaccharides reaches the best value when the DS was within the range of 1.5–2.0 [113].
After sulfation, it is also needed to confirm whether the modification was successful. For sulfate estimation, the common method is the turbidometric barium chloride method [114,115], and the sulfur content is estimated using the benzidine method [116] and elemental analysis [92]. The DS of sulfation can subsequently be calculated using the formula: DS= 1.62 × S/(32 −1.02 × S), where S is the sulfur content of sulfated polysaccharide [117]. Infrared spectroscopy (IR) and nuclear magnetic resonance (NMR) are often used to analyze the structure changes caused by modification. Generally, the signals of the polysaccharide backbone chain still exist after modification, which indicates that the main structure of the derivative is preserved, and some new or changed signals appear, suggesting the modification changes. Compared with Artemisia sphaerocephala polysaccharide, two characteristic absorption bands appear in the IR-spectrum of sulfated Artemisia sphaerocephala polysaccharide near 1250 and 810 cm−1 indicating the sulfation reaction has occurred. As for the NMR results, the new signal at 67.0 ppm of sulfated Artemisia sphaerocephala polysaccharide could be assigned to the O-6 substituted carbons, suggesting sulfation at O-6 [118].
5.2. Phosphorylation
The phosphorylation of polysaccharides is essentially an esterification process [112], and it is also a kind of covalent modification, during which the hydroxyl groups of the branched chain re substituted by a phosphate group [119]. Until now, many methods have been reported to synthetize phosphorylated derivatives, such as the polyphosphoric acid/tributylamine/dimethyl formamide method [120], H3PO4/fatty acid esters method [121], and the H3PO4/palmitic acid/tertiary amines method [122]. As for TCM polysaccharides, the common reagents used are phosphate, phosphorus oxychloride, and phosphoric anhydride, and the phosphorylation of different TCM polysaccharides is listed in Table 2. After modification, the physicochemical and biological properties of polysaccharides are changed [123]. For example, phosphorylation may contribute to the improvement of water solubility. A water-soluble polysaccharide is generated through phosphorylation of Dictyophora indusiata polysaccharide which is, conversely, water-insoluble [123]. It is noted that phosphorylation can enhance the anti-oxidative [124], anti-tumor [93], and antiviral [125] activities. The studies on other activities of phosphorylated TCM polysaccharides, like anti-inflammatory, antibacterial, and anticoagulant abilities [126], are relatively scarce. However, the phosphorylation does not always improve the activity of the polysaccharide. Phosphorylated porphyran shows weaker inhibitory effect on the superoxide radical than porphyran [127].

Table 2.
The phosphorylation of TCM polysaccharides.
Like sulfation, the extent of phosphorylation also needs to be tested. Firstly, the total phosphate content in the derivative can be determined by the ascorbic acid method [128], the phosphate group content can be determined by the molybdenum blue method [129,130], and the phosphorus contents can also be determined by ICP-AES [131]. Moreover, the structures of phosphorylated derivatives can also be analyzed by IR and NMR spectroscopy. The bands in the IR-spectrum polysaccharide from Porphyra haitanensis at 1268 cm−1 and 988 cm−1 indicate P=O stretching vibrations and P–O vibrations, respectively [127]. To confirm the introduction of the phosphate group, 31P-NMR and 13C-NMR are widely applied to the study of polysaccharide structure [123].
5.3. Carboxymethylation
Carboxymethylation is another versatile modification method. Due to the substitution of one hydrogen atom from the OH group by a carboxymethyl group [132,133,134], it is able to change the biological activity of TCM polysaccharides. The common method for carboxymethylation is to dissolve the polysaccharide in an alkaline solution, and add a certain amount of chloroacetic acid to react under proper conditions, then adjust to neutral pH using hydrochloric acid or acetic acid. After purification, the carboxymethylated derivative is obtained. The introduction of the carboxymethylated group brings new activity or enhances the intrinsic activity of the polysaccharide [135,136,137]. For example, Poria sclerotium polysaccharide without anti-gastric adenocarcinoma activity shows strong anti-gastric adenocarcinoma activity after carboxymethylation [138], and the carboxymethylated derivative exhibits stronger antioxidant activity compared to the polysaccharide from Auricularia auricular [139]. Additionally, there are other studies on the improvement of antitumor, immune enhancement, etc. [140].
To confirm the success of carboxymethylation, DS can be determined using the colorimetric method [139,141], conductometric titration [142], the acid-washed method, and neutralization titration. In addition, DS is able to be affected by chloroacetic acid concentration, reaction time, and temperature [143]. As for the structure, IR and NMR spectroscopy can also be applied. Two new intense absorption bands of carboxymethylated Ganoderma applanatum polysaccharide in the IR-spectrum at 1623 cm−1 and 1333 cm−1 correspond to the asymmetrical and symmetrical stretching vibrations of the COO− group, and a new resonance signal appearing at 176.90 ppm in the NMR spectrum is assigned to the carboxylate group, which provides strong evidence of carboxymethylation [144].
5.4. Other Modifications
In addition to the above three modifications, other methods, including acetylation, selenylation, alkylation, and benzoylation [145] are also used to modify TCM polysaccharides. These methods not only improve the water solubility but also heighten or change the biological activities. For example, selenylation can improve the immune-enhancing activity of natural polysaccharides [146]. However, the reagents used, like Na2SeO3 and SeOCl2, are toxic and are threats to human health. Since these modifications have certain limitations or are little studied, the details will not be covered in this review.
6. Biological Activities of TCM Polysaccharides
6.1. Immunity
In TCM theory, disease prevention treatment is the most important objective and it strengthens our bodies’ disease resistance by kinesiotherapy and TCM, which relate to immunity. Almost all of TCM polysaccharides possess immunoregulatory effects [3,147,148,149,150], and various biological activities of TCM polysaccharides are seemingly related to their immunoregulation effects. This may be because polysaccharides are one of the main identification objects of the immune system.
6.1.1. Immune Cell
T-lymphocytes are an important type of immune cell which play a pivotal role in the immune system through cellular immunity. A lot of TCM polysaccharides are able to increase the number and activity of T-lymphocytes. Grifola frondosa polysaccharide D [151] is able to increase the activity and the amount of CD8+ T cells. In the meantime, it induces the TH1 immune pathway and increases IL-2 secretion. Bush sophora root polysaccharide accelerates the proliferation of T lymphocytes and its sulfate enhances such ability [4].
TCM polysaccharides are also able to improve the amount and activity of another important immune cell type, B lymphocytes. Astragalus polysaccharide [152], epimedium polysaccharide [153], and Bush sophora root polysaccharide [4] all accelerate the proliferation of B-lymphocytes. The binding of Acanthopanax senticosus polysaccharide and toll-like receptor stimulates mitogen-activated protein kinases and then promotes the growth and differentiation of B-lymphocytes [154].
Natural killer cells and macrophagocytes are also vital immune system cells. Similarly, TCM polysaccharides, such as Lentinus edodes polysaccharide [155] and Tinospora cordifolia polysaccharide [156], are able to stimulate the activities of natural killer cells and macrophagocytes.
6.1.2. Cytokines
Cytokines are secreted by immune and non-immune cells and play significant roles in immunoregulation. TCM polysaccharides regulate the immunity by their influence on the secretion of cytokines. Ganoderma lucidum polysaccharide is able to promote the secretion of IL-1, IL-6, IFN-γ, TNF-α, GM-CSF, GVCSF, M-CSF, and IL-12 [157]. Astragalus polysaccharide modulates the expression of TNF-α, IL-1β, and NFATc4 in a rat model of experimental colitis [10]. Angelica sinensis polysaccharide stimulates the production of IFN-γ, IL-2, IL-6, and TNF-α [158]. Cordyceps militaris is a rare kind of Chinese traditional medicine; its polysaccharide can up-regulate the expression of TNF-α in mouse peritoneal macrophages and RAW264.7 macrophages [159].
6.1.3. Antibody
Antibodies, including IgM, IgG, IgA, IgE, and IgD, are important kinds of immunoglobulins. Often, the antiviral activity of a TCM polysaccharide is due to its promoting effect on antibodies. Bush sophora root polysaccharide reduces the amount of duck hepatitis A virus by stimulating the antibodies [4]. Both Lycium barbarum polysaccharide [160] and Rehmannia glutinosa polysaccharide [161] exhibit anti-porcine circovirus type 2 effects by promoting the specific IgG.
6.1.4. Complement System
Activation of the complement system enhances immunity. Some TCM polysaccharides exhibit complement activation. Polysaccharides from Vernonia kotschyana Sch. Bip. ex Walp exhibit strong complement activation abilities [162]. Though the complement system is one of the important immune defense systems, excessive activation can result in too strong an inflammatory response and diseases, such as rheumatoid arthritis. Polysaccharides, like those from the root of Bupleurum chinense [163] and the stem of Ephedra sinica Stapf [164], exhibit anti-complement effects.
6.2. Antiviral Activity
Antiviral activities of polysaccharides are well known and such activities of TCM polysaccharides are affirmed by many studies (Table 3). As far back as 1958, Gerber et al. [165] reported the anti-influenza B and mumps virus activities of polysaccharides extracted from Gelidium cartilagenium, which is called “qilincai” in TCM. Astragalus polysaccharide shows antiviral activities against infectious bursal disease virus [166], porcine circovirus virus type 2 [12], duck hepatitis A virus [167], human hepatitis B virus, porcine reproductive and respiratory syndrome virus, and classical swine fever virus [22].

Table 3.
Antiviral activities of TCM polysaccharides.
It is generally recognized that the antiviral activities of TCM polysaccharides are related to the activation of the immune system, such as activating macrophagocytes to promote their phagocytic ability and inducing the secretion of IL-2, IFN-γ, and antibodies [4,155,157,160,167]. Antiviral activities of TCM polysaccharides are also related to sulfate radicals. It is reported that sulfate modification of Astragalus polysaccharide [167] and Bush sophora root polysaccharide [17] increases the inhibitory rate of duck hepatitis A virus, and Chuanminshen violaceum polysaccharide exhibits no anti-duck enteritis virus activity. After sulfate modification, sulfated Chuanminshen violaceum polysaccharide possesses significant antiviral activity against duck enteritis virus, ranging from 77.12 μg/mL to 104.81 μg/mL [169]. Additionally, TCM polysaccharides are able to block different stages of viral life. Sulfated Chuanminshen violaceum polysaccharide inhibits the adsorption of duck enteritis virus on duck embryo fibroblasts [169]. Bush sophora root polysaccharide inhibits the replication of duck hepatitis A virus, and its sulfate inhibits replication and release [17].
6.3. Anti-Inflammatory Activity
Inflammation is a common and important pathological process. It is body’s defense response to stimulus. The anti-inflammatory activity of TCM polysaccharides is mainly due to the inhibition of the expression of the chemotactic factor and adherence factor, as well as the activities of key enzymes in the inflammation process. CCl4 causes an inflammatory response by increasing TNF-α and IL-1β. Treatment with Ganoderma lucidum polysaccharides in pre-treatment and post-treatment modes attenuates the increases of TNF-α and IL-1β [175]. Lycium barbarum polysaccharide alleviates the inflammatory reaction in the kidneys of diabetic rabbits through reducing the expression of MCP-1mRNA and ICAM-1mRNA by restraining the expression of NF-κB and AngII [176]. Astragalus polysaccharide reduces cell accumulation and swelling, the arthritic index of joints, and serum concentrations of TNF-α and IL-1βin adjuvant-induced arthritic rats [22].
6.4. Anti-Oxidative Activity
Under normal circumstances, free radicals regulate cell growth as well as inhibit viruses and bacteria. However, many free radicals, including superoxide anion, hydrogen peroxide, and NO, also cause various types of diseases, such as cancer, arteriosclerosis, and aging [177]. Biological antioxidants can prevent and cure these diseases by breaking peroxide chain reactions. A lot of studies show that TCM polysaccharides possess anti-oxidative abilities [12,178,179,180,181,182,183].
TCM polysaccharides are able to scavenge free ROS [12,182,183]. Each monosaccharide unit possesses active hydroxyl groups which can combine with hydroxyl radicals or superoxide anion free radicals to form water. In addition to this, it is also related to some other mechanisms. Astragalus polysaccharide [12] and Rhizoma Dioscoreae Nipponicae polysaccharide [184] inhibit ROS generation by blocking the NF-κB pathway. Inhibition of ROS induced by angiotensin II of Bletilla striata polysaccharide is involved in the NOX4 pathway [182]. Cordyceps sinensis polysaccharide inhibits PDGF-BB-induced inflammation and ROS production via the ERK/Akt pathways [183].
The anti-oxidative activity of TCM polysaccharides is also due to their promoting effect on antioxidant enzyme activity. The main antioxidant enzymes in bodies are SOD, CAT, and GSH-Px. Bush sophora root polysaccharide is able to increase the activities of SOD, CAT, and GSH-Px [178]. Lycium barbarum polysaccharides increase the activities of GSH-Px and SOD in chickens [181]. The anti-oxidative activity of Astragalus polysaccharide has been widely studied [12,167], and the results show that the anti-oxidative activity of Astragalus polysaccharide is mainly attributable to the enhancement of antioxidant enzyme activity [12,167,170].
6.5. Anti-TumorActivity
Cancer is a severe human disease. Chemotherapy and radiotherapy, as is well-known, are the primary treatment methods, however, normal cells are injured when tumor cells are killed by chemotherapy or radiotherapy. Therefore, research on anti-tumor drugs with low toxicity and high efficiency is vitally important. A lot of studies in recent years show that many TCM polysaccharides possess anti-tumor activities with low toxicity (Table 4).

Table 4.
Anti-tumor activities of TCM polysaccharides.
TCM polysaccharides play anti-tumor roles through two aspects. One is by inducing cell apoptosis or inhibiting the expression of cellular oncogenes to kill tumors directly. Lycium barbarum polysaccharide [185] and Angelica sinensis polysaccharide [186] kill tumor cells by inducing apoptosis. Astragalus polysaccharide [187,188] directly reduces the mass of tumors in vivo. Radix Hedysari polysaccharide and Achyranthes bidentata polysaccharide are able to arrest the cell cycle and thus play anti-tumor roles. Another anti-tumor mechanism is by enhancing the immunity to kill tumor cells indirectly. In this mechanism, TCM polysaccharides do not act on tumor cells, but rather they play the role by activating the immune system. For example, Sophora flavescens polysaccharide [189], Sanguisorba officinalis polysaccharide [190], Curcuma kwangsiensis polysaccharide [191], Schisandra chinensis polysaccharide [192], and Ganoderma lucidum polysaccharide [193] all enhance the immunity system so that the growth of tumors is inhibited. As TCM polysaccharides possess characteristics of low toxicity and side effects, as well as high anti-tumor efficiency, they are expected to be a new kind of anti-tumor drug.
6.6. OtherActivities
TCM polysaccharides also possess hypoglycemic, neuroprotective, hair growth promoting, anti-vomiting, anti-glaucoma, hepatoprotective, hematopoietic, growth-promoting, antiatherosclerotic activities, and so on [3,170].
7. Summary
Conspicuously, TCM polysaccharides possess various abilities to enhance resistance or treat diseases, and the potential applications of TCM polysaccharides in the clinic are excellent. However, the extraction, purification, and modification often affect the activities of homogeneous TCM polysaccharides. Thus, studies must choose appropriate methods of extraction, purification, and modification, and the particular action mechanisms of specific TCM polysaccharides still need to be studied further.
Acknowledgments
The project was supported by the Special Fund for Agro-scientific Research in the Public Interest (201303040, 201403051), National Natural Science Foundation of China (Grant Nos. 31172355, 31572557), and the Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD). We are grateful to all other staff in the Institute of Traditional Chinese Veterinary Medicine of Nanjing Agricultural University for their assistances to this review.
Author Contributions
Y.C., F.Y. and J.L. conceived and designed the review; Y.C. and F.Y. wrote the paper; K.M., D.W. and Y.H. contributed to the modification of the paper. J.L. supervised the work and critically revised the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Shi, M.M.; Piao, J.H.; Xu, X.L.; Zhu, L.; Yang, L.; Lin, F.L.; Chen, J.; Jiang, J.G. Chinese medicines with sedative–hypnotic effects and their active components. Sleep Med. Rev. 2016, 29, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Feng, N.P. Nanocarriers for the delivery of active ingredients and fractions extracted from natural products used in traditional Chinese medicine (TCM). Adv. Colloid Interface 2015, 221, 60–76. [Google Scholar] [CrossRef] [PubMed]
- Shi, L. Bioactivities, isolation and purification methods of polysaccharides from natural products: A review. Int. J. Biol. Macromol. 2016, 92, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wu, Y.; Xian, L.T.; Song, M.Y.; Zeng, L.; Xiong, W.; Liu, J.G.; Sun, W.D.; Wang, D.Y.; Hu, Y.L. Effects of Bush Sophora Root polysaccharide and its sulfate on immuno-enhancing of the therapeutic DVH. Int. J. Biol. Macromol. 2015, 80, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Sun, Y.; Yu, C.; Liu, L. Chemical structure of one low molecular weight and water-soluble polysaccharide (EFP-W1) from the roots of Euphorbia fischeriana. Carbohydr. Polym. 2012, 87, 1236–1240. [Google Scholar] [CrossRef]
- Ding, X.; Hou, Y.; Hou, W. Structure feature and antitumor activity of a novel polysaccharide isolated from Lactarius deliciosus, Gray. Carbohydr. Polym. 2012, 89, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Tang, J.; Cao, M.; Guo, C.X.; Zhang, X.; Zhong, J.; Zhang, J.; Sun, Q.; Feng, S.; Yang, Z.R.; Zhao, J. Structure elucidation and antioxidant activity of a novel polysaccharide isolated from Tricholoma matsutake. Int. J. Biol. Macromol. 2010, 47, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.Z.; Wu, D.; Zhou, S.; Liu, Y.F.; Li, Z.P.; Feng, J.; Yang, Y. Structure elucidation of a bioactive polysaccharide from fruiting bodies of Hericium erinaceus in different maturation stages. Carbohydr. Polym. 2016, 144, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.Z.; Jiang, N.; Liu, L.; Sheng, X.J.; Shi, A.M.; Hu, H.; Yang, Y.; Wang, Q. Extraction, purification and primary characterization of polysaccharides from Defatted Peanut (Arachis hypogaea) Cakes. Molecules 2016, 21, 716. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Lin, H.B.; Gong, S.; Chen, P.Y.; Geng, L.L.; Zeng, Y.M.; Li, D.Y. Effect of Astragalus polysaccharides on expression of TNF-α, IL-1β and NFATc4 in a rat model of experimental colitis. Cytokine 2014, 70, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Pu, X.Y.; Ma, X.L.; Liu, L.; Ren, J.; Li, H.B.; Li, X.Y.; Yu, S.; Zhang, W.J.; Fan, W.B. Structural characterization and antioxidant activity in vitro of polysaccharides from Angelica and Astragalus. Carbohydr. Polym. 2016, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Xue, H.X.; Gan, F.; Zhang, Z.Q.; Hu, J.F.; Chen, X.X.; Huang, K.H. Astragalus polysaccharides inhibits PCV2 replication by inhibiting oxidative stress and blocking NF-κB pathway. Carbohydr. Polym. 2015, 81, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Xiao, B.; Sun, T.Y. Antitumor and immunomodulatory activity of Astragalus membranaceus polysaccharides in H22 tumor-bearing mice. Int. J. Biol. Macromol. 2013, 62, 287–290. [Google Scholar] [CrossRef] [PubMed]
- Kamarudin, F.; Gan, C.Y. Molecular structure, chemical properties and biological activities of Pinto bean pod polysaccharide. Int. J. Biol. Macromol. 2016, 88, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.H.; Tang, W.; Jin, M.L.; Li, J.E.; Xie, M.Y. Recent advances in bioactive polysaccharides from Lycium barbarum L., Zizyphus jujuba Mill, Plantago spp., and Morus spp.: Structures and functionalities. Food Hydrocolloids 2016, 60, 148–160. [Google Scholar] [CrossRef]
- Zhang, F.; Lin, L.H.; Xie, J.H. A mini-review of chemical and biological properties of polysaccharides from Momordica charantia. Int. J. Biol. Macromol. 2016, 92, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xiong, W.; Zeng, L.; Wang, D.Y.; Liu, J.G.; Wu, Y.; Hu, Y.L. Comparison of Bush Sophora Root polysaccharide and its sulfate's anti-duck hepatitis A virus activity and mechanism. Carbohydr. Polym. 2014, 102, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Qin, T.; Chen, J.; Wang, D.Y.; Hu, Y.L.; Zhang, J.; Wang, M.; Qiu, S.L.; Gao, Z.Z.; Liu, R.R.; Yu, Y.; Huang, Y.E.; Wang, Q.C.; Wang, Q.X. Selenylation modification can enhance immune-enhancing activity of Chinese angelica polysaccharide. Carbohydr. Polym. 2013, 95, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Ni, Y.Y.; Hu, X.S.; Li, Q.H. Effect of phosphorylation on antioxidant activities of pumpkin (Cucurbita pepo, Lady godiva) polysaccharide. Int. J. Biol. Macromol. 2015, 81, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.K.; Wang, W.Q.; Wu, J.Y. Recent advances in Cordyceps sinensis polysaccharides: Mycelial fermentation, isolation, structure, and bioactivities: A review. J. Funct. Foods 2014, 6, 33–47. [Google Scholar] [CrossRef]
- Zhang, M.; Cui, S.W.; Cheung, P.C.K.; Wang, Q. Antitumor polysaccharides from mushrooms: A review on their isolation process, structural characteristics and antitumor activity. Trends Food. Sci. Technol. 2007, 18, 4–19. [Google Scholar] [CrossRef]
- Jin, M.L.; Zhao, K.; Huang, Q.S.; Xu, C.L.; Shang, P. Isolation, structure and bioactivities of the polysaccharides from Angelica sinensis (Oliv.) Diels: A review. Carbohydr. Polym. 2012, 89, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.L.; You, Q.H.; Jiang, Z.H. Optimization of enzyme assisted extraction of polysaccharides from Tricholoma matsutake by response surface methodology. Carbohydr. Polym. 2011, 86, 1358–1364. [Google Scholar] [CrossRef]
- Wang, J.L.; Zhang, J.; Zhao, B.T.; Wang, X.F.; Wu, Y.Q.; Yao, J. A comparison study on microwave-assisted extraction of Potentilla anserina L. polysaccharides with conventional method: Molecule weight and antioxidant activities evaluation. Carbohydr. Polym. 2010, 8, 84–93. [Google Scholar] [CrossRef]
- Hromadkova, Z.; Ebringerova, A.; Valachovic, P. Comparison of classical and ultrasound-assisted extraction of polysaccharides from Salvia officinalis L. Ultrason. Sonochem. 1999, 5, 163–168. [Google Scholar] [CrossRef]
- Reverchon, E.; Marco, I. Supercritical fluid extraction and fractionation of natural matter. J. Supercrit. Fluid. 2006, 38, 146–166. [Google Scholar] [CrossRef]
- Ma, T.T.; Sun, X.Y.; Tian, C.R.; Luo, J.Y.; Zheng, C.P.; Zhan, J.C. Polysaccharide extraction from Sphallerocarpus gracilis roots by response surface methodology. Int. J. Biol. Macromol. 2016, 88, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Nie, S.P.; Zhang, H.; Li, W.J.; Xie, M.Y. Current development of polysaccharides from Ganoderma: Isolation, structure and bioactivities. Bioact. Carbohydr. Dietary Fibre 2013, 1, 10–20. [Google Scholar] [CrossRef]
- Wang, N.; Zhang, Y.; Wang, X.; Huang, X.; Fei, Y.; Yu, Y.; Shou, D. Antioxidant property of water-soluble polysaccharides from Poriacocos Wolf using different extraction methods. Int. J. Biol. Macromol. 2016, 83, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Govender, S.; Pillay, V.; Chetty, D.J.; Essack, S.Y.; Dangor, C.M.; Govender, T. Optimisation and characterisation of bioadhesive controlled release tetracycline microspheres. Int. Pharm. J. 2005, 306, 24–40. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.A.; Mafra, I.; Soares, M.R.; Evtuguin, D.V.; Coimbra, M.A. Dimeric calcium complexes of arabinan-rich pectic polysaccharides from Olea europaea L. cell walls. Carbohydr. Polym. 2006, 65, 535–543. [Google Scholar]
- Yin, G.H.; Dang, Y.L. Optimization of extraction technology of the Lycium barbarum polysaccharides by Box–Behnken statistical design. Carbohydr. Polym. 2008, 74, 603–610. [Google Scholar]
- Sun, Y.X.; Liu, J.C.; Kennedy, J.F. Application of response surface methodology for optimization of polysaccharides production parameters from the roots of Codonopsis pilosula by a central composite design. Carbohydr. Polym. 2010, 80, 949–953. [Google Scholar] [CrossRef]
- Zhang, H.L.; Li, J.; Xia, J.M.; Lin, S.Q. Antioxidant activity and physicochemical properties of an acidic polysaccharide from Morinda officinalis. Int. J. Biol. Macromol. 2013, 58, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Li, Q.; Mao, G.H.; Zou, Y.; Feng, W.W.; Zheng, D.H.; Wang, W.; Zhou, L.L.; Zhang, T.X.; Yang, J.; Yang, L.Q.; Wu, X.Y. Optimization of enzyme-assisted extraction and characterization of polysaccharides from Hericium erinaceus. Carbohydr. Polym. 2014, 101, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Li, B.B.; Smith, B.; Hossain, M.M. Extraction of phenolics from citrus peels: II. Enzyme-assisted extraction method. Sep. Purif. Technol. 2006, 48, 189–196. [Google Scholar] [CrossRef]
- Pan, L.H.; Wang, J.; Ye, X.Q.; Zha, X.Q.; Luo, J.P. Enzyme-assisted extraction of polysaccharides from Dendrobium chrysotoxum and its functional properties and immunomodulatory activity. LWT-Food Sci. Technol. 2015, 60, 1149–1154. [Google Scholar] [CrossRef]
- Li, F.; Yang, L.Q.; Zhao, T.; Zhao, J.L.; Zou, Y.M.; Zou, Y.; Wu, X.Y. Optimization of enzymatic pretreatment for n-hexane extraction of oil from Silybum marianum seeds using response surface methodology. Food Bioprod. Process. 2012, 90, 87–94. [Google Scholar] [CrossRef]
- Zhang, J.; Jia, S.Y.; Liu, Y.; Wu, S.H.; Ran, J.Y. Optimization of enzyme-assisted extraction of the Lycium barbarum polysaccharides using response surface methodology. Carbohydr. Polym. 2011, 86, 1089–1092. [Google Scholar] [CrossRef]
- Thomas, L.; Parameswaran, B.; Pandey, A. Hydrolysis of pretreated rice straw by an enzyme cocktail comprising acidic xylanase from Aspergillus sp. for bioethanol production. Renew. Energ. 2016, 98, 9–15. [Google Scholar] [CrossRef]
- Wang, S.P.; Dong, X.F.; Tong, J.M. Optimization of enzyme-assisted extraction of polysaccharides from alfalfa and its antioxidant activity. Int. J. Biol. Macromol. 2013, 62, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Passos, C.P.; Yilmaz, S.; Silva, C.M.; Coimbra, M.A. Enhancement of grape seed oil extraction using a cell wall degrading enzyme cocktail. Food Chem. 2009, 115, 48–53. [Google Scholar] [CrossRef]
- Guillard, C.L.; Bergé, J.P.; Donnay-Moreno, C.; Bruzac, S.; Ragon, J.Y.; Baron, R.; Fleurence, J.; Dumay, J. Soft liquefaction of the red seaweed Grateloupia turuturu Yamada by ultrasound-assisted enzymatic hydrolysis process. J. Appl. Phycol. 2016, 28, 2575–2585. [Google Scholar] [CrossRef]
- Chan, C.H.; Yusoff, R.; Ngoh, G.C.; Kung, F.W.L. Microwave-assisted extractions of active ingredients from plants. J. Chromatogr. A 2011, 1218, 6213–6225. [Google Scholar] [CrossRef] [PubMed]
- Ferhat, M.A.; Tigrine-Kordjani, N.; Chemat, S.; Meklati, B.Y.; Chemat, F. Rapid extraction of volatile compounds using a new simultaneous microwave distillation: solvent extraction device. Chromatographia 2007, 65, 217–222. [Google Scholar] [CrossRef]
- Liu, T.T.; Sui, X.Y.; Zhang, R.R.; Yang, L.; Zu, Y.G.; Zhang, L.; Zhang, Y.; Zhang, Z.H. Application of ionic liquids based microwave-assisted simultaneous extraction of carnosic acid, rosmarinic acid and essential oil from Rosmarinus officinalis. J. Chromatogr. A 2011, 1218, 8480–8489. [Google Scholar] [CrossRef] [PubMed]
- Ng, L.K.; Hupe, M. Effects of moisture content in cigar tobacco on nicotine extraction: Similarity between Soxhlet and focused open-vessel microwave-assisted techniques. J. Chromatogr. A 2003, 1011, 213–219. [Google Scholar] [CrossRef]
- Chen, F.L.; Du, X.Q.; Zu, Y.G.; Yang, L.; Wang, F. Microwave-assisted method for distillation and dual extraction in obtaining essential oil, proanthocyanidins and polysaccharides by one-pot process from Cinnamomi Cortex. Sep. Purif. Technol. 2016, 164, 1–11. [Google Scholar] [CrossRef]
- Perez-Serradilla, J.A.; Luque de Castro, M.D. Microwave-assisted extraction of phenolic compounds from wine lees and spray-drying of the extract. Food Chem. 2011, 124, 1652–1659. [Google Scholar] [CrossRef]
- Gallo, M.; Ferracane, R.; Graziani, G.; Ritieni, A.; Fogliano, V. Microwave assisted extraction of phenolic compounds from four different spices. Molecules 2010, 15, 6365–6374. [Google Scholar] [CrossRef] [PubMed]
- Dahmoune, F.; Boulekbache, L.; Moussi, K.; Aoun, O.; Spigno, G.; Madani, K. Valorization of Citrus limon residues for the recovery of antioxidants: Evaluation and optimization of microwave and ultrasound application to solvent extraction. Ind. Crops Prod. 2013, 50, 77–87. [Google Scholar] [CrossRef]
- Pan, Y.M.; Wang, K.; Huang, S.Q.; Wang, H.S.; Mu, X.M.; He, C.H.; Ji, X.W.; Zhang, J.; Huang, F.J. Antioxidant activity of microwave-assisted extract of longan (Dimocarpus Longan Lour.) peel. Food. Chem. 2008, 106, 1264–1270. [Google Scholar] [CrossRef]
- Pan, Y.M.; He, C.H.; Wang, H.S.; Ji, X.W.; Wang, K.; Liu, P.Z. Antioxidant activity of microwave-assisted extract of Buddleia officinalis and its major active component. Food Chem. 2010, 121, 497–502. [Google Scholar] [CrossRef]
- Jia, X.J.; Ma, L.S.; Li, P.; Chen, M.W.; He, C.W. Prospects of Poriacocos polysaccharides: Isolation process, structural features and bioactivities. Trends Food. Sci. Technol. 2016, 54, 52–62. [Google Scholar] [CrossRef]
- Dahmoune, F.; Nayak, B.; Moussi, K.; Remini, H.; Madani, K. Optimization of microwave-assisted extraction of polyphenols from Myrtus communis L. leaves. Food. Chem. 2015, 166, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.Z.; Jin, C.G.; Tong, Z.G.; Lu, J.; Tan, L.; Tian, L.; Chang, Q.Q. Optimization extraction, characterization and antioxidant activities of pectic polysaccharide from tangerine peels. Carbohydr. Polym. 2016, 136, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Fishman, M.L.; Chau, H.K.; Cooke, P.H.; Yadav, M.P.; Hotchkiss, A.T. Physico-chemical characterization of alkaline soluble polysaccharides from sugar beet pulp. Food Hydrocolloids 2009, 23, 1554–1562. [Google Scholar] [CrossRef]
- Dean, J.R.; Xiong, G.H. Extraction of organic pollutants from environmental matrices: selection of extraction technique. TrAC-Trends Anal. Chem. 2000, 19, 553–564. [Google Scholar] [CrossRef]
- Delgado-Povedano, M.M.; Luque de Castro, M.D. A review on enzyme and ultrasound: A controversial but fruitful relationship. Ana. Chim. Acta 2015, 889, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.J.; Li, X.; Wan, M.J.; Chen, J.P.; Li, S.J.; Cao, M.; Zhang, D.Y. Effect of extraction methods on property and bioactivity of water-soluble polysaccharides from Amomum villosum. Carbohydr. Polym. 2015, 117, 632–635. [Google Scholar] [CrossRef] [PubMed]
- Vinatoru, M.; Toma, M.; Mason, T.J. Ultrasonically assisted extraction of bioactive principles from plants and their constituents. Adv. Sonochem. 1999, 5, 216. [Google Scholar]
- Fu, L.; Chen, H.; Dong, P.; Zhang, X.; Zhang, M. Effects of Ultrasonic Treatment on the Physicochemical Properties and DPPH Radical Scavenging Activity of Polysaccharides from Mushroom Inonotus obliquus. J. Food Sci. 2010, 75, C322–C327. [Google Scholar] [CrossRef] [PubMed]
- Glisic, S.B.; Ristic, M.; Skala, D.U. The combined extraction of sage (Salvia officinalis L.): Ultrasound followed by supercritical CO2 extraction. Ultrason. Sonochem. 2011, 18, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Velickovic, D.T.; Milenovic, D.M.; Ristic, M.S.; Veljkovic, V.B. Kinetics of ultrasonic extraction of extractive substances from garden (Salvia officinalis L.) and glutinous (Salvia glutinosa L.) sage. Ultrason. Sonochem. 2006, 13, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Toma, M.; Vinatoru, M.; Paniwnyk, L.; Mason, T.J. Investigation of the effects of ultrasound on vegetal tissues during solvent extraction. Ultrason. Sonochem. 2001, 8, 137–142. [Google Scholar] [CrossRef]
- Mislovicova, D.; MasArovA, J.; Bendzalova, K.; Soltes, L.; Machova, E. Sonication of chitin–glucan, preparation of water-soluble fractions and characterization by HPLC. Ultrason. Sonochem. 2000, 7, 63–68. [Google Scholar] [CrossRef]
- Zhou, C.S.; Ma, H.L. Ultrasonic Degradation of Polysaccharide from a Red Algae (Porphyrayezoensis). J. Agric. Food Chem. 2006, 54, 2223–2228. [Google Scholar] [CrossRef] [PubMed]
- Ebringerova, A.; Z Hromadkova, Z. The effect of ultrasound on the structure and properties of the water-soluble corn hull heteroxylan. Ultrason. Sonochem. 1997, 4, 305–309. [Google Scholar] [CrossRef]
- Liu, H.; Bao, J.G.; Du, Y.M.; Zhou, X.; Kennedy, J.F. Effect of ultrasonic treatment on the biochemphysical properties of chitosan. Carbohydr. Polym. 2006, 64, 553–559. [Google Scholar] [CrossRef]
- Glisic, S.; Ivanovic, J.; Ristic, M.; Skala, D. Extraction of sage (Salvia officinalis L.) by supercritical CO2: Kinetic data, chemical composition and selectivity of diterpenes. J. Supercrit. Fluid. 2010, 52, 62–70. [Google Scholar] [CrossRef]
- Zhao, Z.J.; Lian, Y.; Wang, G.Q.; Li, W.W. Orthogonal test for optimization of CO2-SFE process of pachyman in Poria cocos(Schw.) wolf. Lishizhen Med. Mater. Med. Res. 2008, 19, 1628–1629. [Google Scholar]
- Staub, A.M. Removal of protein-Sevag method. Methods Carbohydr. Chem. 1965, 5, 5–6. [Google Scholar]
- Qu, C.L.; Yu, S.C.; Jin, H.L.; Wang, J.S.; Luo, L. The pretreatment effects on the antioxidant activity of jujube polysaccharides. Spectrochim. Acta A. 2013, 114, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.R.; Xu, H.L.; Xie, L.L.; Sun, J.; Sun, T.T.; Wu, X.Y.; Fu, Q.B. Purification, characterization and in vitro anticoagulant activity of polysaccharides from Gentiana scabra Bunge roots. Carbohydr. Polym. 2016, 140, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Luo, J.G.; Sun, Y.; Ye, H.; Lu, Z.X.; Zeng, X.X. A simple method for the simultaneous decoloration and deproteinization of crude levan extract from Paenibacillus polymyxa EJS-3 by macroporous resin. Bioresour. Technol. 2010, 101, 6077–6083. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Meng, D.M.; Song, Y.; Li, J.; Zhang, Y.Y.; Hu, X.S.; Ni, Y.Y.; Li, Q.H. Simultaneous Decoloration and Deproteinization of Crude Polysaccharide from Pumpkin Residues by Cross-Linked Polystyrene Macroporous Resin. J. Agric. Food Chem. 2012, 60, 8450–8456. [Google Scholar] [CrossRef] [PubMed]
- Li, H.L.; Dai, Q.Q.; Ren, J.L.; Jian, L.F.; Peng, F.; Sun, R.C.; Liu, G.L. Effect of structural characteristics of corncob hemicelluloses fractionated by graded ethanol precipitation on furfural production. Carbohydr. Polym. 2016, 136, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.S.; Xie, B.X.; Yan, J.; Zhao, F.C.; Xiao, J.; Yao, L.Y.; Zhao, B.; Huang, Y.X. In vitro antioxidant and antitumor activities of polysaccharides extracted from Asparagus officinalis. Carbohydr. Polym. 2012, 87, 392–396. [Google Scholar] [CrossRef]
- Morris, G.A.; Adams, G.G.; Harding, S.E. On hydrodynamic methods for the analysis of the sizes and shapes of polysaccharides in dilute solution: A short review. Food Hydrocolloid. 2014, 42, 318–334. [Google Scholar] [CrossRef]
- Paulsen, B.S.; Olafsdottir, E.S.; Ingolfsdottir, K. Chromatography and electrophoresis in separation and characterization of polysaccharides from lichens. J. Chromatogr. A 2002, 967, 163–171. [Google Scholar] [CrossRef]
- Wang, Q.J.; Fang, Y.Z. Analysis of sugars in traditional Chinese drugs. J. Chromatogr. B. 2004, 812, 309–324. [Google Scholar] [CrossRef]
- Preethi, S.; Saral, M. Screening of natural polysaccharides extracted from the fruits of Pithecellobium dulce as a pharmaceutical adjuvant. Int. J. Biol. Macromol. 2016, 92, 347–356. [Google Scholar]
- Xu, Z.; Li, X.; Feng, S.L.; Liu, J.; Zhou, L.J.; Yuan, M.; Ding, C.B. Characteristics and bioactivities of different molecular weight polysaccharides from camellia seed cake. Int. J. Biol. Macromol. 2016, 91, 1025–1032. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.S.; Chen, H.X.; Zhang, Y.; Zhang, N.; Fu, L.L. Chemical modification and antioxidant activities of polysaccharide from mushroom Inonotus obliquus. Carbohydr. Polym. 2012, 89, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.M.; Yin, Y.; Yang, W.; Ma, M.L.; Yang, L.; Chen, X.J.; Zhang, Z.; Ye, B.; Song, L.Y. Structural elucidation and biological activity of a novel polysaccharide by alkaline extraction from cultured Cordyceps militaris. Carbohydr. Polym. 2009, 75, 166–171. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Xu, X.B.; Gu, Z.X.; Liu, S.; Gao, N.; He, X.Z.; Xin, X. Purification and characterization of a glucan from Bacillus calmette guerin and the antitumor activity of its sulfated derivative. Carbohydr. Polym. 2015, 128, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Li, X.Z.; Wu, Z.P.; Kuang, C.T. Research progress on extraction, purification and content determination of plant polysaccharides. Chem. Ind. Forest Prod. 2009, 29, 238–242. [Google Scholar]
- Yang, L.Q.; Zhang, L.M. Chemical structural and chain conformational characterization of some bioactive polysaccharides isolated from natural sources. Carbohydr. Polym. 2009, 76, 349–361. [Google Scholar] [CrossRef]
- Wei, W.L.; Zeng, R.; Gu, C.M.; Qu, Y.; Huang, L.F. Angelica sinensis in China-A review of botanical profile, ethnopharmacology, phytochemistry and chemical analysis. J. Ethnopharmacol. 2016, 190, 116–141. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.T.; Edgar, K.J. “Click” reactions in polysaccharide modification. Prog. Polym. Sci. 2016, 53, 52–85. [Google Scholar] [CrossRef]
- Xie, J.H.; Wang, Z.J.; Shen, M.Y.; Nie, S.P.; Gong, B.; Li, H.S.; Zhao, Q.; Li, W.J.; Xie, M.Y. Sulfated modification, characterization and antioxidant activities of polysaccharide from Cyclocarya paliurus. Food Hydrocolloids 2016, 53, 7–15. [Google Scholar] [CrossRef]
- He, Y.L.; Ye, M.; Jing, L.Y.; Du, Z.Z.; Surahio, M.; Xu, H.M.; Li, J. Preparation, characterization and bioactivities of derivatives of an exopolysaccharide from Lachnum. Carbohydr. Polym. 2015, 117, 788–796. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.H.; Jia, M.; Zhou, S.Y.; Pan, F.; Mei, Q.B. Antivirus and immune enhancement activities of sulfated polysaccharide from Angelica sinensis. Int. J. Biol. Macromol. 2012, 50, 768–772. [Google Scholar] [CrossRef] [PubMed]
- Alban, S.; Kraus, J.; Franz, G. Synthesis of laminarin sulfates with anticoagulant activity. Arzneimittelforschung 1992, 42, 1005–1008. [Google Scholar] [PubMed]
- Lu, Y.; Wang, D.Y.; Hu, Y.L.; Huang, X.Y.; Wang, J.M. Sulfated modification of epimedium polysaccharide and effects of the modifiers on cellular infectivity of IBDV. Carbohydr. Polym. 2008, 71, 180–186. [Google Scholar] [CrossRef]
- Baba, M.; Snoeck, R.; Pauwels, R.; Clercq, E. Sulfated polysaccharides are potent and selective inhibitors of various enveloped viruses, including herpes simplex virus, cytomegalovirus, vesicular stomatitis virus, and human immunodeficiency virus. Antimicrob Agents Chemother. 1988, 32, 1742–1745. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.X.; Xiong, Q.Q.; Li, S.L.; Zhao, X.R.; Zeng, X.X. Structural characterization, sulfation and antitumor activity of a polysaccharide fraction from Cyclina sinensis. Carbohydr. Polym. 2015, 15, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.F.; Liao, K.S.; Wu, Y.S.; Pan, Q.; Wu, L.L.; Jiao, H.; Guo, D.A.; Li, B.; Liu, B. Optimization, characterization, sulfation and antitumor activity of neutral polysaccharides from the fruit of Borojoa sorbilis cuter. Carbohydr. Polym. 2016, 151, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, S.S.; Passos, C.P.; Madureira, P.; Vilanova, M.; Coimbra, M.A. Structure-function relationships of immunostimulatory polysaccharides: A review. Carbohydr. Polym. 2015, 132, 378–396. [Google Scholar] [CrossRef] [PubMed]
- Cardozo, F.T.G.S.; Camelini, C.M.; Cordeiro, M.N.S.; Mascarello, A.; Malagoli, B.G.; Larsen, I.; Rossi, M.J.; Nunes, R.J.; Braga, F.C.; Brandt, C.R.; Simoes, C.M.O. Characterization and cytotoxic activity of sulfated derivatives of polysaccharides from Agaricus brasiliensis. Int. J. Biol. Macromol. 2013, 57, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Costa, L.S.; Fidelis, G.P.; Cordeiro, S.L.; Oliveira, R.M.; Sabry, D.A.; Câmara, R.B.G. Biological activities of sulfated polysaccharides from tropical seaweeds. Biomed. Pharmacother. 2010, 64, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Telles, C.B.S.; Sabry, D.A.; Almeida-Lima, J.; Costa, M.S.S.P.; Melo-Silveira, R.F.; Trindade, E.S. Sulfation of the extracellular polysaccharide produced by the edible mushroom Pleurotus sajor-caju alters its antioxidant, anticoagulant and antiproliferative properties in vitro. Carbohydr. Polym. 2011, 85, 514–521. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Y.J.; Park, H.S.; Xia, Y.M.; Kim, G.S. Antitumor activity of sulfated extracellular polysaccharides of Ganoderma lucidum from the submerged fermentation broth. Carbohydr. Polym. 2012, 87, 1539–1544. [Google Scholar] [CrossRef]
- Wijesekara, I.; Pangestuti, R.; Kim, S.K. Biological activities and potential health benefits of sulfated polysaccharides derived from marine algae. Carbohyd. Polym. 2011, 84, 14–21. [Google Scholar] [CrossRef]
- Wang, J.M.; Hu, Y.L.; Wang, D.Y.; Zhang, F.; Zhao, X.N.; Abula, S.F.D.; Fan, Y.P.; Guo, L.W. Lycium barbarum polysaccharide inhibits the infectivity of Newcastle disease virus to chicken embryo fibroblast. Int. J. Biol. Macromol. 2010, 46, 212–216. [Google Scholar] [CrossRef] [PubMed]
- Dace, R.; Bride, E.M.; Brooks, K.; Gander, J.; Buszko, M.; Doctor, V.M. Comparison of the Anticoagulant Action of Sulfated and phosphorylated polysaccharides. Thromb. Res. 1997, 87, 113–121. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, H.; Wang, Y.X.; Nie, S.P.; Li, C.; Xie, M.Y. Sulfated modification of the polysaccharides from Ganoderma atrum and their antioxidant and immunomodulating activities. Food. Chem. 2015, 186, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Cong, Q.F.; Xiao, F.; Liao, W.F.; Dong, Q.; Ding, K. Structure and biological activities of an alginate from Sargassum fusiforme: and its sulfated derivative. Int. J. Biol. Macromol. 2014, 69, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.L.; Niu, S.F.; Zhao, B.T.; Wang, X.F.; Yao, J.; Zhang, J.; Zhao, W.W.; Zhao, Y.T. Regioselective synthesis of sulfated guar gum: Comparative studies of structure and antioxidant activities. Int. J. Biol. Macromol. 2013, 62, 734–740. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.L.; Yang, W.; Yang, T.; Zhang, X.N.; Zuo, Y.; Tian, J.; Yao, J.; Zhang, J.; Lei, Z.Q. Catalytic synthesis of sulfated polysaccharides I: Characterization of chemical structure. Int. J. Biol. Macromol. 2015, 74, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Zhu, L.; Liu, X.Y.; Li, Y.Y.; Zhao, C.D.; Xu, Z.G.; Yan, W.F.; Zhang, H.X. Synthesis and antioxidant activity of phosphorylated polysaccharide from Portulaca oleracea L. with H 3PW 12O 40 immobilized on polyamine functionalized polystyrene bead as catalyst. J. Mol. Catal. A Chem. 2011, 342–343, 74–82. [Google Scholar] [CrossRef]
- Wang, C.Y.; Guan, H.S. Advances of researches on antiviral activities of polysaccharides-II. Antiviral activities of sulfated polysaccharides. Prog. Biotechnol. 2000, 20, 3–8. [Google Scholar]
- Chattopadhyay, K.; Mateu, C.G.; Mandal, P.; Pujol, C.A.; Damonte, E.B.; Ray, B. Galactan sulfate of Grateloupia indica: Isolation, structural features and antiviral activity. Phytochemistry 2007, 68, 1428–1435. [Google Scholar] [CrossRef] [PubMed]
- Dodgson, K.S.; Price, R.G. A note on the determination of the ester sulphate content of sulphated polysaccharides. Biochem. J. 1962, 84, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Antonopoulos, C.A.; Daniel, A.F.; Havanka, R.; Haslewood, G.A.D. A modification for the determination of sulfate in mucopolysaccharides by the benzidine method. Acta Chem. Scand. 1962, 16, 1521–1522. [Google Scholar] [CrossRef]
- Wang, J.L.; Yang, W.; Wang, J.C.; Wang, X.; Wu, F.; Yao, J.; Zhang, J.; Lei, Z.Q. Regioselective sulfation of Artemisia sphaerocephala polysaccharide: Characterization of chemical structure. Carbohydr. Polym. 2015, 133, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.L.; Guo, H.Y.; Zhang, J.; Wang, X.F.; Zhao, B.T.; Yao, J.; Wang, Y.P. Sulfated modification, characterization and structure–antioxidant relationships of Artemisia sphaerocephala polysaccharides. Carbohydr. Polym. 2010, 81, 897–905. [Google Scholar] [CrossRef]
- Blennow, A.; Nielsen, T.H.; Baunsgaard, L.; Mikkelsen, R.; Engelsen, S.B. Starch phosphorylation: A new front line in starch research. Trends Plant. Sci. 2002, 7, 445–450. [Google Scholar] [CrossRef]
- Heinze, T.; Liebert, T.; Heublein, B.; Hornig, S. Functional polymers base on dextran. Adv. Polym. Sci. 2006, 205, 199–291. [Google Scholar]
- Ye, M.; Yuan, R.Y.; He, Y.L.; Du, Z.Z.; Ma, X.J. Phosphorylation and anti-tumor activity of exopolysaccharide from Lachnum YM120. Carbohydr. Polym. 2013, 97, 690–694. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.M.; Zhang, Z.S.; Yao, Q.; Zhao, M.X.; Qi, H.M. Phosphorylation of low-molecular-weight polysaccharide from Enteromorpha linza with antioxidant activity. Carbohydr. Polym. 2013, 96, 371–375. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.; Fu, H.T.; Xu, J.J.; Shang, J.Y.; Cheng, Y.M. Physiochemical and biological properties of phosphorylated polysaccharides from Dictyophora indusiata. Int. J. Biol. Macromol. 2015, 72, 894–899. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.M.; Zhang, W.W.; Li, X.G.; Lü, X.X.; Li, N.; Gao, X.L.; Song, J.M. Preparation and in vitro antioxidant activity of κ-carrageenan oligosaccharides and their oversulfated, acetylated, and phosphorylated derivatives. Carbohydr. Res. 2005, 340, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.X.; Wan, Z.J.; Shi, L.; Lu, X.X. Preparation and antiherpetic activities of chemically modified polysaccharides from Polygonatum cyrtonema Hua. Carbohydr. Polym. 2011, 83, 737–742. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Q.B.; Zhang, Z.S.; Zhang, J.J.; Li, P.C. Synthesized phosphorylated and aminated derivatives of fucoidan and their potential antioxidant activity in vitro. Int. J. Biol. Macromol. 2009, 4, 170–174. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.S.; Zhang, Q.B.; Wang, J.; Zhang, H.; Niu, X.Z.; Li, P.C. Preparation of the different derivatives of the low-molecular-weight porphyran from Porphyra haitanensis and their antioxidant activities in vitro. Int. J. Biol. Macromol. 2009, 45, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Lowry, O.H.; Roberts, N.R.; Leiner, K.Y.; Wu, M.L.; Farr, A.L. The quantitative histochemistry of brain Chemical Methods. J. Biol. Chem. 1954, 207, 1–17. [Google Scholar] [PubMed]
- Sun, X.; Pan, D.D.; Zeng, X.Q.; Cao, J.X. Phosphorylation modification of Polysaccharides from Enteromorpha. J. Food Sci. 2011, 24, 17. [Google Scholar]
- Holman, W.I.M. A new technique for the determination of phosphorus by the molybdenum blue method. Biochem. J. 1943, 37, 256–259. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.Y.; Xu, X.J.; Zhang, L.N.; Zeng, F.B. Chain conformation and anti-tumor activities of phosphorylated (1→3)-β-d-glucan from Poriacocos. Carbohydr. Polym. 2009, 78, 581–587. [Google Scholar] [CrossRef]
- Pal, S.; Sen, G.; Mishra, S.; Dey, R.K.; Jha, U. Carboxymethyl tamarind: Synthesis, characterization and its application as novel drug-delivery agent. J. Appl. Polym. Sci. 2008, 110, 392–400. [Google Scholar] [CrossRef]
- Pal, S.; Pal, A. Carboxymethyl guar: Its synthesis and macromolecular characterization. J. Appl. Polym. Sci. 2009, 111, 2630–2636. [Google Scholar] [CrossRef]
- Chakravorty, A.; Barman, G.; Mukherjee, S.; Sa, B. Effect of carboxymethylation on rheological and drug release characteristics of locust bean gum matrix tablets. Carbohydr. Polym. 2016, 144, 50–58. [Google Scholar] [CrossRef] [PubMed]
- El-Sherbiny, I.M. Synthesis, characterization and metal uptake capacity of a new carboxymethyl chitosan derivative. Eur. Polym. J. 2009, 45, 199–210. [Google Scholar] [CrossRef]
- Magnani, M.; Calliari, C.M. Optimized methodology for extraction of (1→3)(1→6)-β-d-glucan from Saccharomyces cerevisiae and in vitro evaluation of the cytotoxicity and genotoxicity of the corresponding carboxymethyl derivative. Carbohydr. Polym. 2009, 78, 658–665. [Google Scholar] [CrossRef]
- Wu, Y.N.; Ye, M.; Du, Z.Z.; Jing, L.Y.; Surahio, M.; Yang, L. Carboxymethylation of an exopolysaccharide from Lachnum and effect of its derivatives on experimental chronic renal failure. Carbohydr. Polym. 2014, 114, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Q.; Hou, X.H.; Wang, Y.F.; Zhang, L.N. Correlation between structure and anti-gastric adenocarcinoma activity of β-d-glucan isolated from Poria cocos sclerotium. World Chin. J. Digestol. 2012, 20, 1277–1283. [Google Scholar]
- Yang, L.Q.; Zhao, T.; Wei, H.; Zhang, M.; Zou, Y.; Mao, G.H.; Wu, X.Y. Carboxymethylation of polysaccharides from Auricularia auricula and their antioxidant activities in vitro. Int. J. Biol. Macromol. 2011, 49, 1124–1130. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.B.; Zhao, C.; Pan, W.; Wang, J.P.; Wang, W.J. Carboxylate groups play a major role in antitumor activity of Ganoderma applanatum polysaccharide. Carbohydr. Polym. 2015, 123, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.M.; Zhang, Z.S.; Zhao, M.X. Carboxymethylation of polysaccharides from Tremella fuciformis for antioxidant and moisture-preserving activities. Int. J. Biol. Macromol. 2015, 72, 526–530. [Google Scholar] [CrossRef] [PubMed]
- Capitani, D.; Porro, F.; Segre, A.L. High field NMR analysis of the degree of substitution in carboxymethyl cellulose sodium salt. Carbohydr. Polym. 2000, 42, 283–286. [Google Scholar] [CrossRef]
- Shi, M.J.; Wei, X.Y.; Xu, J.; Chen, B.J.; Zhao, D.Y.; Cui, S.; Zhou, T. Carboxymethylated degraded polysaccharides from Enteromorpha prolifera: Preparation and in vitro antioxidant activity. Food. Chem. 2017, 215, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.B.; Jin, X.Z.; Pan, W.; Wang, J.J. Syntheses of new rare earth complexes with carboxymethylated polysaccharides and evaluation of their in vitro antifungal activities. Carbohydr. Polym. 2014, 113, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.S.; Zhang, Q.B.; Wang, J.; Shi, X.L.; Song, H.F.; Zhang, J.J. In vitro antioxidant activities of acetylated, phosphorylated and benzoylated derivatives of porphyran extracted from Porphyra haitanensis. Carbohydr. Polym. 2009, 78, 449–453. [Google Scholar] [CrossRef]
- Feng, H.B.; Fan, J.; Bo, H.Q.; Tian, X.; Bao, H.; Wang, X.H. Selenylation modification can enhance immune-enhancing activity of Chuanminshen violaceum polysaccharide. Carbohydr. Polym. 2016, 153, 302–311. [Google Scholar]
- Cui, G.T.; Zhang, W.X.; Wang, Q.J.; Zhang, A.; Mu, H.B.; Bai, H.J.; Duan, J.Y. Extraction optimization, characterization and immunity activity of polysaccharides from Fructus Jujubae. Carbohydr. Polym. 2014, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.; Sakwiwatkul, K.; Zhang, C.R.; Wang, Y.M.; Zhai, L.J.; Hu, S.H. Atractylodis macrocephalae Koidz. polysaccharides enhance both serum IgG response and gut mucosal immunity. Carbohydr. Polym. 2013, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Pan, K.; Jiang, Q.G.; Liu, G.Q.; Miao, X.Y.; Zhong, D.W. Optimization extraction of Ganoderma lucidum polysaccharides and its immunity and antioxidant activities. Int. J. Biol. Macromol. 2013, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.Y.; Wang, C.L.; Wang, Y.R.; Li, Z.J.; Chen, M.H.; Li, F.J.; Sun, Y.P. Pleurotus nebrodensis polysaccharide (PN-S) enhances the immunity of immunosuppressed mice. Chin. J. Nat. Med. 2015, 760–766. [Google Scholar] [CrossRef]
- Kodama, N.; Murata, Y.; Asakawa, A.; Inui, A.; Hayashi, M.; Sakai, N.; Nanba, H. Maitake D-fraction enhances antitumor effects and reduces immunosuppression by mitomycin-C in tumor-bearing mice. Nutrition 2005, 21, 624–629. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.P.; Ma, L.; Zhang, W.M.; Cui, X.Q.; Zhen, Y.; Suolangzhaxi; Song, X.P. Liposome can improve the adjuvanticity of astragalus polysaccharide on the immune response against ovalbumin. Int. J. Biol. Macromol. 2013, 60, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.Y.; Chen, X.L.; Qiu, S.L.; Hu, Y.L.; Wang, D.Y.; Liu, X.; Zhao, X.J.; Liu, C.; Chen, X.H. Adjuvanticity of compound astragalus polysaccharide and sulfated epimedium polysaccharide per os. Int. J. Biol. Macromol. 2013, 62, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Han, S.B.; Yoona, Y.D.; Ahna, H.J.; Lee, H.S.; Lee, C.W.; Yoon, W.K.; Park, S.K.; Kim, H.M. Toll-like receptor-mediated activation of B Cells and bacrophages by polysaccharide isolated from cell culture of Acanthopanax senticosus. Int. J. Immunopharmacol. 2003, 3, 1301–1312. [Google Scholar] [CrossRef]
- Ruan, Z.; Su, J.; Dai, H.C.; Wu, M.C. Characterization and immunomodulating activities of polysaccharide from Lentinus edodes. Int. J. Immunopharmacol. 2005, 5, 811–820. [Google Scholar]
- Raveendran, P.K.; Rodrigueza, S.; Ramachandran, R.; Alamo, A.; Melnick, S.J.; Escalon, E.; Garcia, P.I., Jr.; Wnuk, S.F.; Ramachandran, C. Immune stimulating properties of a novel polysaccharide from the medicinal plant Tinospora cordifolia. Int. J. Immunopharmacol. 2004, 4, 1645–1659. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.S.; Tsai, Y.F.; Lin, S.; Lin, C.C.; Hhoo, K.H.; Lin, C.H.; Wong, C.H. Studies on the immuno-modulating and anti-tumor activities of Ganoderma lucidum (Reishi) polysaccharides. Bioorg. Med. Chem. 2004, 12, 5595–5601. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.M.; Ge, B.L.; Li, Z.H.; Guan, F.X.; Li, F.F. Structural analysis and immunoregulation activity comparison of five polysaccharides from Angelica sinensis. Carbohydr. Polym. 2016, 140, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Hong, E.K. Immunostimulating activity of the polysaccharides isolated from Cordyceps militaris. Int. Immunopharmacol. 2011, 11, 1226–1233. [Google Scholar] [CrossRef] [PubMed]
- Bo, R.N.; Zheng, S.S.; Xing, J.; Luo, L.; Niu, Y.L.; Huang, Y.E.; Liu, Z.G.; Hu, Y.L.; Liu, J.G.; Wu, Y.; Wang, D.Y. The immunological activity of Lycium barbarum polysaccharides liposome in vitro and adjuvanticity against PCV2 in vivo. Int. J. Biol. Macromol. 2016, 85, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.E.; Liu, Z.G.; Bo, R.N.; Xing, J.; Luo, L.; Zhen, S.S.; Niu, Y.L.; Hu, Y.L.; Liu, J.G.; Wu, Y.; Wang, D.Y. The enhanced immune response of PCV-2 vaccine using Rehmannia glutinosa polysaccharide liposome as an adjuvant. Int. J. Biol. Macromol. 2016, 86, 929–936. [Google Scholar] [CrossRef] [PubMed]
- Cecilie, S.N.; Drissa, D.; Terje, E.M.; Malterud, K.E.; Kiyohara, H.; Matsumoto, T.; Yamada, H.; Paulsen, B.S. Isolation, partial characterisation and immunomodulating activities of polysaccharides from Vernonia kotschyana Sch. Bip. ex Walp. J. Ethnopharmacol. 2004, 91, 141–152. [Google Scholar]
- Di, H.Y.; Zhang, Y.Y.; Chen, D.F. Isolation of an anti-complementary polysaccharide from the root of Bupleurum chinense and identification of its targets in complement activation cascade. Chin. J. Nat. Med. 2013, 11, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.G.; Kuang, H.X.; Yang, B.Y.; Wang, Q.H.; Liang, J.; Sun, Y.P.; Wang, Y.H. Optimum extraction of acidic polysaccharides from the stems of Ephedra sinica Stapf by Box-Behnken statistical design and its anti-complement activity. Carbohydr. Polym. 2011, 84, 282–291. [Google Scholar] [CrossRef]
- Gerber, P.; Dugene, J.D.; Adams, E.V.; Sherman, J.H. Protective effect of seaweed extracts for chicken embryos infected with influenza B or Mumps virus. Proc. Soc. Exp. Biol. Med. 1958, 99, 590–593. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.Y.; Wang, D.Y.; Hu, Y.L.; Lu, Y.; Guo, Z.H.; Kong, X.F.; Sun, J.L. Effect of sulfated astragalus polysaccharide on cellular infectivity of infectious bursal disease virus. Int. J. Biol. Macromol. 2008, 42, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Song, M.Y.; Wang, Y.X.; Xiong, W.; Zeng, L.; Zhang, S.B.; Xu, M.Y.; Du, H.X.; Liu, J.G.; Wang, D.Y.; Wu, Y.; Hu, Y.L. The anti-DHAV activities of astragalus polysaccharide and its sulfate compared with those of BSRPS and its sulfate. Carbohydr. Polym. 2015, 117, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.J.; Wei, Y.Y.; Yin, D.; Shuai, X.H.; Zeng, Y.; Hu, T.J. Effect of Sophora subprosrate polysaccharide on oxidative stress induced by PCV2 infection in RAW264.7 cells. Int. J. Biol. Macromol. 2013, 62, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Yin, Z.Q.; Li, L.; Cheng, A.C.; Jia, R.Y.; Xu, J.; Wang, Y.; Yao, X.P.; Lv, C.; Zhao, X.H. Antiviral activity of sulfated Chuanminshen violaceum polysaccharide against duck enteritis virus in vitro. Antivir. Res. 2013, 98, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.L.; Zhao, K.; Huang, Q.S.; Shang, P. Structural features and biological activities of the polysaccharides from Astragalus membranaceus. Int. J. Biol. Macromol. 2014, 64, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.H.; Song, J.; Shi, Y.Q.; Wang, C.M.; Chen, B.; Xie, D.H.; Jia, X.B. Anti-hepatitis B virus activity of chickweed [Stellaria media (L.) Vill.] extracts in HepG2.2.15 cells. Molecules 2012, 17, 8633–8646. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.M.; Chen, H.J.; Chen, K.; Gao, Y.F.; Gao, S.; Liu, X.F.; Li, J.G. Sulfated modification can enhance antiviral activities of Achyranthes bidentata polysaccharide against porcine reproductive and respiratory syndrome virus (PRRSV) in vitro. Int. J. Biol. Macromol. 2013, 52, 21–24. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Zhang, Y.T.; Yin, Z.Q.; Zhao, X.H.; Liang, X.X.; He, C.L.; Yin, L.Z.; Lv, C.; Zhao, L.; Gang, Y.; Shi, F.; Shu, G.; Jia, R.Y. Antiviral effect of sulfated Chuanminshen violaceum polysaccharide in chickens infected with virulent Newcastle disease virus. Virology 2015, 476, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.P.; Wang, D.Y.; Liu, J.G.; Hu, Y.L.; Zhao, X.J.; Han, G.C.; Nguyen, T.L.; Chang, S.S. Adjuvantcity of epimedium polysaccharide-propolis flavone on inactivated vaccines against AI and ND virus. Int. J. Biol. Macromol. 2012, 51, 1028–1032. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.J.; Du, J.L.; Cao, L.P.; Jia, R.; Shen, Y.J.; Zhao, C.Y.; Xu, P.; Yin, G.J. Anti-inflammatory and hepatoprotective effects of Ganoderma lucidum polysaccharides on carbon tetrachloride-induced hepatocyte damage in common carp (Cyprinus carpio L.). Int. Immunopharmacol. 2015, 25, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.H.; Li, J.J.; Yan, J.; Liu, S.; Guo, Y.L.; Chen, D.J.; Luo, Q. Lycium barbarum polysaccharides ameliorates renal injury and inflammatory reaction in alloxan-induced diabetic nephropathy rabbits. Life Sci. 2016, 157, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, A.P.; Melissa, D.H. Effect of in vitro generation of oxygen free radicals on T cell function in young and old rats. Free Radic. Bio. Med. 1998, 25, 903–913. [Google Scholar]
- Chen, Y.; Xiong, W.; Zeng, L.; Wang, Y.; Zhang, S.B.; Xu, M.Y.; Song, M.Y.; Wang, Y.X.; Du, H.X.; Liu, J.G.; Wang, D.Y.; Wu, Y.; Hu, Y.L. Bush Sophora Root polysaccharide and its sulfate can scavenge free radicals resulted from duck virus hepatitis. Int. J. Biol. Macromol. 2014, 66, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Olatunji, O.J.; Feng, Y.; Olatunji, O.O.; Tang, J.; Wei, Y.; Ouyang, Z.; Su, Z.L. Polysaccharides purified from Cordyceps cicadae protects PC12 cells against glutamate-induced oxidative damage. Carbohydr. Polym. 2016, 153, 187–195. [Google Scholar] [CrossRef] [PubMed]
- 171 Liu, Y.; Liu, F.; Yang, Y.; Li, D.; Lv, J.; Ou, Y.J.; Sun, F.J.; Chen, J.H.; Shi, Y.; Xia, P.Y. Astragalus polysaccharide ameliorates ionizing radiation-induced oxidative stress in mice. Int. J. Biol. Macromol. 2014, 68, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.L.; Chen, J.; Chen, X.; Fan, Q.; Zhang, C.S.; Wang, D.Y.; Li, X.P.; Chen, X.Y.; Chen, X.L.; Liu, C.; Gao, Z.Z.; Li, H.Q.; Hu, Y.L. Optimization of selenylation conditions for lyceum barbarum polysaccharide based on antioxidant activity. Carbohydr. Polym. 2014, 103, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Yue, L.; Wang, W.W.; Wang, Y.; Du, T.; Shen, W.P.; Tang, H.L.; Wang, Y.; Yin, H.P. Bletilla striata polysaccharide inhibits angiotensin II-induced ROS and inflammation via NOX4 and TLR2 pathways. Int. J. Biol. Macromol. 2016, 89, 376–388. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Y.; Liu, D.; Wang, W.; Zhao, H.; Wang, M.; Yin, H.P. Cordyceps sinensis polysaccharide inhibits PDGF-BB-induced inflammation and ROS production in human mesangial cells. Carbohydr. Polym. 2015, 125, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Liu, K.X.; Peng, J.Y.; Wang, C.Y.; Kang, L.; Chang, N.; Sun, H.J. Rhizoma Dioscoreae Nipponicae polysaccharides protect HUVECs from H2O2-induced injury by regulating PPARγ factor and the NADPH oxidase/ROS–NF-κB signal pathway. Toxicol. Lett. 2015, 232, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Du, G. Lycium barbarum polysaccharide stimulates of MCF-7 cells by the ERK pathway. Life Sci. 2012, 91, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Li, X.Q.; Wang, X.; Fan, H.T.; Zhang, X.N.; Hou, Y.; Liu, S.B.; Mei, K.B. A novel polysaccharide, isolated from Angelica sinensis (Oliv.) Diels induces the apoptosis of cervical cancer HeLa cells through an intrinsic apoptotic pathway. Phytomedicine 2010, 17, 598–605. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.Y.; Liu, R.Q.; Si, C.L.; Zhou, F.; Wang, Y.X.; Ding, L.N.; Jing, C.; Liu, A.J.; Zhang, Y.M. Structural analysis and anti-tumor activity comparison of polysaccharides from Astragalus. Carbohydr. Polym. 2011, 85, 895–902. [Google Scholar] [CrossRef]
- Li, S.G.; Wang, D.G.; Tian, W.; Wang, X.X.; Zhao, J.X.; Liu, Z.; Chen, R. Characterization and anti-tumor activity of a polysaccharide from Hedysarum polybotrys Hand.-Mazz. Carbohydr. Polym. 2008, 73, 344–350. [Google Scholar] [CrossRef]
- Bai, L.; Zhu, L.Y.; Yang, B.S.; Shi, L.J.; Liu, Y.; Jiang, A.M.; Zhao, L.L.; Song, G.; Liu, T.F. Antitumor and immunomodulating activity of a polysaccharide from Sophora flavescens Ait. Int. J. Biol. Macromol. 2012, 51, 705–709. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.B.; Li, W.; Wang, H.T.; Yan, W.; Zhou, Y.; Wang, G.; Cui, J.; Wang, F. Anti-tumor and immunomodulating activities of a polysaccharide from the root of Sanguisorba officinalis L. Int. J. Biol. Macromol. 2012, 51, 484–488. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.H.; Dai, P.; Ren, L.Y.; Song, B.; Chen, X.; Wang, X.; Wang, J.; Zhang, T.; Zhu, W. Apoptosis-induced anti-tumor effect Curcuma kwangsiensis polysaccharides against human nasopharyngeal carcinoma cells. Carbohyd. Polym. 2012, 89, 1067–1072. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.L.; Li, Y.H.; Dong, M.; Wu, X.; Wang, X.C.; Xiao, Y.S. Inhibitory effect of Schisandra chinensis leaf polysaccharide against L5178Y lymphoma. Carbohydr. Polym. 2012, 88, 21–25. [Google Scholar] [CrossRef]
- Zhao, L.Y.; Dong, Y.H.; Chen, G.T.; Hu, Q.H. Extraction, purification, characterization and antitumor activity of polysaccharides from Ganoderma lucidum. Carbohydr. Polym. 2010, 80, 783–789. [Google Scholar] [CrossRef]
- Wei, D.; Wei, Y.; Cheng, W.; Zhang, L. Sulfated modification, characterization and antitumor activities of Radix hedysari polysaccharide. Int. J. Biol. Macromol. 2012, 51, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Li, Y.; Wang, Y.; Zhang, J.; Ouyang, X.; Peng, R.; Yang, J. Antitumor and immunostimulatory activity of a polysaccharide-protein complex from Scolopendra subspinipes mutilans L. Koch in tumor-bearing mice. Food Chem. Toxicol. 2012, 50, 2648–2655. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.Q.; Zheng, Z.J.; Peng, Y.; Li, W.X.; Chen, X.M.; Lu, J.X. Opposite effects on tumor growth depending on dose of Achyranthes bidentata polysaccharides in C57BL/6 mice. Int. Immunopharmacol. 2007, 7, 568–577. [Google Scholar] [CrossRef] [PubMed]
- Ji, N.F.; Yao, L.S.; Li, Y.; He, W.; Yi, K.S.; Huang, M. Polysaccharide of Cordyceps sinensis enhances cisplatin cytotoxicity in non-small cell lung cancer H157 cell line. Integr. Cancer Ther. 2011, 10, 359–367. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).