Abstract
Lawsonia inermis Linn (Lythraceae), also known as henna, is a small shrub or tree distributed throughout Taiwan’s Lanyu Island, in North Africa, and in Australia. Its leaves are used as a folk medicine for the treatment of external hemorrhage and fingernail abscesses. Investigation of the ethyl acetate (EtOAc)-soluble fractions from methanol extract of the aerial part of Lawsonia inermis has led to the isolation of a new diphenol, (Z)-4,4′-(prop-1-ene-1,3-diyl)diphenol (1), two new isocoumarin carbonates, inermiscarbonates A (2) and B (3), and six known compounds, 4′-hydroxyflavanone (4), apigenine (5), kampferol (6), luteolin (7), quercetin (8), and (-)-catechin (9). Their structures were determined by detailed analysis of spectroscopic data and comparison with the data of known analogues. Compounds 1 and 4–9 were evaluated for the inhibition of nitric oxide production in lipopolysaccharide (LPS)-stimulated product of nitrite in RAW 264.7 cells with IC50 values of 5.63, 15.72, 8.67, 6.67, 6.17, 7.61, and 14.52 μg/mL, respectively.
1. Introduction
Lawsonia inermis Linn (Lythraceae) is a tall shrub or small tree native to northern Africa, western and southern Asia, and northern Australasia [1]. Lawsonia inermis is a folk herbal medicine used in Taiwan for skin diseases and wounds [2]. Flavonoids, quinoids, naphthalene derivatives, triterpenoids, coumarins [3], and their derivatives are widely distributed in plants of the genus Lythraceae. Many of these compound derivatives exhibit anti-inflammatory [4], antibacterial, antifungal, antimycotic, and antiparasitic activities [5]. A continuing chemical investigation of the secondary metabolites of this plant resulted in the isolation and identification of a new diphenol, (Z)-4,4′-(prop-1-ene-1,3-diyl)diphenol (1), two new isocoumarin carbonates, inermiscarbonates A (2) and B (3) (Figure 1), as well as six flavonoids (4–9) from the Lawsonia inermis, and their structures are depicted in Figure 1. The isolation and detailed structural elucidation of the (Z)-4,4′-(prop-1-ene-1,3-diyl)diphenol (1), inermiscarbonates A (2), B (3), and the anti-inflammatory activities of all isolates are described herein.

Figure 1.
The chemical structures of compounds 1–9.
2. Results and Discussion
2.1. Isolation and Structural Elucidation
The MeOH extract of the aerial part of Lawsonia inermis was concentrated to give a brown-green residue, which was suspended in water and partitioned with EtOAc and H2O, successively. The combined ethyl acetate (EtOAc)-soluble fraction was purified by repeated silica gel column chromatography and normal phase semipreparative high-performance liquid chromatography (HPLC) to obtain a new diphenol, (Z)-4,4′-(prop-1-ene-1,3-diyl)diphenol (1), two new isocoumarin carbonates, inermiscarbonates A (2) and B (3), and six known flavonoids, 4–9. The identification of the known compounds was established through direct comparison with the published physical and spectral data.
Compound 1 was obtained as a pale yellow powder. Its molecular formula was calculated as C15H14O2 from the analysis of its high resolution electron spray mass spectrometry (HR-ESI-MS) data, corresponding to nine degrees of unsaturation. The UV spectrum exhibited conjugated absorption at λ (log ε) 285 (4.32) and 296 (4.24) nm. Its IR spectrum showed absorption bands for hydroxyl (3554 cm−1), olefinic (1652 cm−1), and aromatic (1602 and 1500 cm−1) functionalities. Two 1,4-disubstituted benzene rings were suggested from the 1H-NMR signals at δH 7.25 (2H, d, J = 8.5 Hz, H-2′, H-6′) and δH 6.73 (2H, d, J = 8.5 Hz, H-3′, H-5′), along with δH 7.04 (2H, d, J = 8.3 Hz, H-2″, H-6″) and δH 6.68 (2H, d, J = 8.3 Hz, H-3″, H-5″). The proton resonances of two olefinic methine (δH 6.00 (1H, dt, J = 10.5, 7.5 Hz, H-2: δC 142.6) and δH 5.70 (1H, d, J = 10.5 Hz, H-1: δC 110.5)) and one methylene (δH 3.57 (2H, d, J = 7.5 Hz, H-3)) indicated a partial structure of cis-double bond with an adjacent methylene group. The UV absorption at λmax 285 and 296 nm with larger log ε value suggested the conjugated olefinic and phenyl groups. The presence of a 1-propenyl moiety linked between two phenyl moieties in 1 was predicted from the COSY correlations of H-2 (δH 6.00)/H-1 (δH 5.70) and H2-3 (δH 3.57)/H-2 (δH 6.00), as well as the HMBC correlations (Figure 2), as follows: H-1 (δH 5.70)/C-2′,6′ (δC 134.1), H-1 (δH 5.70)/C-3 (δC 36.8), H2-3 (δH 3.57)/C-1 (δC 110.5), and H2-3 (δH 3.57)/C-2 (δC 142.6) (Table 1). The connection of the two phenol groups (1,4-disubstituted benzene rings) was determined by the HMBC correlations of H-2″, H-6″ (δH 7.04)/C-3 (δC 36.8), H2-3 (δH 3.57)/C-2″, C-6″ (δC 130.6), and H-2′, H-6′ (δH 7.25)/C-1 (δC 110.5). Thus, compound 1 was assigned as (Z)-4,4′-(prop-1- ene-1,3-diyl)diphenol.
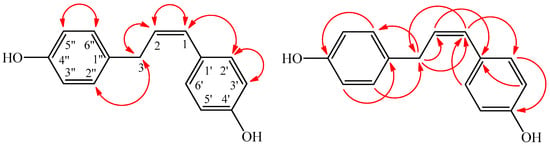
Figure 2.
Significant NOESY (  ) and HMBC (
) and HMBC (  ) correlations of 1.
) correlations of 1.
 ) and HMBC (
) and HMBC (  ) correlations of 1.
) correlations of 1.

Table 1.
NMR data (CD3OD) of 1. δ in ppm, J in Hz.
Inermiscarbonate A (2) was isolated as an amorphous powder with molecular formula C11H8O5 as determined by positive-ion HR-ESI-MS, showing an [M + H]+ ion at m/z 221.1864 (calcd. for C11H9O5, 221.1861) and eight degrees of unsaturation. The IR spectrum demonstrated the presence of carbonate carbonyl (1793 cm−1) and isocoumarin carbonyl groups (1716 cm−1) [6]. The UV absorptions at λmax (log ε) 244 (4.42), 255 (4.35), 272 (4.46), 281 (4.23), and 350 (3.11) nm indicated that compound 2 possessed isocoumarin with a 3-oxygenated skeleton as 3-methoxy-1H-isochromen-1-one (2a) [6]. The 1H- and 13C-NMR data of 2 were similar to those of 3-methoxy-1H-isochromen-1-one (2a) [7], except that the methyl carbonate group (δH 3.85 (3H, s, OCOCH3); δC 52.0 (OCOCH3), 164.1(OCOCH3)) at C-3 of 2 replaced the 3-methoxy group of 2a. By the aid of HSQC, HMBC (Figure 3), 1H–1H COSY, and NOESY (Figure 3) techniques, and the full 1H- and 13C-NMR signals of 2 were unambiguously assigned (Table 2). Therefore, the structure of 2 was determined as methyl (4-oxo-1H-isochromen-3-yl)carbonate.
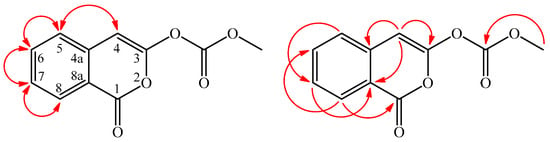
Figure 3.
Significant NOESY (  ) and HMBC (
) and HMBC (  ) correlations of 2.
) correlations of 2.
 ) and HMBC (
) and HMBC (  ) correlations of 2.
) correlations of 2.

Table 2.
NMR data (CDCl3) of 2. δ in ppm, J in Hz.
Inermiscarbonate (3) was isolated as an amorphous powder. Compound 3 shows the molecular formula to be C12H11O5 due to the HR-ESI-MS molecular [M + H]+ ion at m/z 235.2129 (calcd. 235.2127), implying eight degrees of unsaturation. The IR spectrum demonstrated the presence of carbonate carbonyl (1772 cm−1) and isocoumarin carbonyl groups (1720 cm−1) [6]. The UV absorptions at λmax (log ε) 244, 255, 272, 280, and 350 nm were similar to those of 2 and 3-methoxy-1H-isochromen-1-one (2a), and were characteristic of the 3-oxygenated isocoumarin skeleton [6]. The 13C-NMR spectrum of 3 showed signals for 12 carbons which were one more carbon signal than compound 2. Comparison of the 1H-, 13C-NMR, and MS data of 3 with those of 2 suggested that their structures were closely related, except that the ethoxy group (δH 1.36 (t, J = 7.3 Hz, OCOCH2CH3), 4.30 (q, J = 7.3 Hz, OCOCH2CH3); δC 14.3 (OCOCH2CH3), 61.0 (OCOCH2CH3)) of 3 replaced the methoxy group of 2. This was supported by HMBC correlation (Figure 4) between OCOCH2CH3 (δH 4.30) and OCOCH2CH3 (δC 163.6). The full assignment of 1H- and 13C-NMR resonances was supported by 1H–1H COSY, DEPT, HSQC, NOESY, and HMBC spectral analyses (Table 3). According to the above data, the structure of 3 was elucidated as ethyl (1-oxo-1H-isochromen-3-yl)carbonate. This is the first isolation of inermiscarbonate B from a natural source, although inermiscarbonate B was synthesized by Schnekenburger [8].
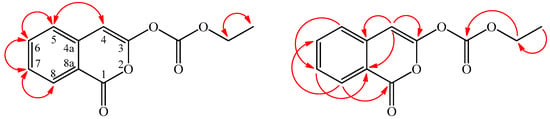
Figure 4.
Significant NOESY (  ) and HMBC (
) and HMBC (  ) correlations of 3.
) correlations of 3.
 ) and HMBC (
) and HMBC (  ) correlations of 3.
) correlations of 3.

Table 3.
NMR data (CDCl3) of 3. δ in ppm, J in Hz.
2.2. Structure Identification of the Known Isolates
The known isolates were readily identified by comparison of their physical and spectroscopic data (UV, IR, 1H-NMR, , and MS) with those of the corresponding authentic samples or literature values. They include six flavonoids: 4′-hydroxyflavanone (4) [9], apigenine (5) [10], kampferol (6) [11], luteolin (7) [10], quercetin (8) [12], and, (−)-catechin (9) [12].
2.3. Inhibitory Activity against Nitric Oxide Production
Nitric oxide (NO) is derived from the oxidation of l-arginine by NO synthase (NOS), and is a mediator in the inflammatory response involved in host defense [13]. In inflammation and carcinogenesis conditions, there is an increased production of NO by inducible NO synthase (iNOS) [14]. The anti-inflammatory effects of the compounds isolated from the Lawsonia inermis were also evaluated for the suppression of lipopolysaccharide (LPS)-induced NO generation in murine macrophage. In this study, the inhibitory activity of three new compounds (1–3) and six flavonoids (4–9) toward NO production was evaluated by the measurement of nitrite/nitrate in LPS-stimulated RAW 264.7 cells. To search for the appropriate concentrations for the above assay, these nine compounds were first tested for their cytotoxic activity against the RAW 264.7 cells, and no significant cytotoxic activities were observed under all tested concentrations. From the results of our anti-inflammatory tests, the following conclusions could be drawn: (a) The high cell viability (>92%) indicated that the inhibitory activities of compounds 1 and 4–9 on LPS-induced NO production did not result from their cytotoxicities; (b) Compounds 1, 6, and 7 exhibited inhibitory effects on lipopolysaccharide (LPS)-induced nitric oxide production in RAW 264.7 cells with IC50 values of 5.63, 6.67, and 6.17 μg/mL, respectively (Table 4). (c) (Z)-4,4′-(Prop-1-ene-1,3-diyl)diphenol (1) is the most effective among the isolated compounds against LPS-induced NO generation, with IC50 = 5.63 ± 3.64 μg/mL.

Table 4.
Inhibitory effect of compounds 1–9 on overproduction of nitric oxide in lipopolysaccharide (LPS)-stimulated RAW 264.7 cells.
3. Experimental Section
3.1. General
UV spectra were obtained with a Shimadzu Pharmaspec-1700 UV-Visible spectrophotometer (Shimadzu, Kyoto, Japan). Infrared spectra were obtained with a Shimadzu IR prestige-21 Fourier transform (FT) infrared spectrophotometer (Shimadzu). 1D- and 2D-NMR spectra were recorded with a Bruker DRX-500 FT-NMR spectrometer (Bruker, Bremen, Germany). Mass spectrometric (HR-EI-MS) data were generated at the Mass Spectrometry Laboratory of the Chung Hsing University (Taichung, Taiwan). Column chromatography was performed using LiChroCART Si gel (5 μM; Merck, Darmstadt, Germany), and TLC analysis was carried out using aluminum pre-coated Si plates (Merck & Co., Inc.), and the spots were visualized using a UV lamp at λ = 254 nm.
3.2. Chemicals
The solvents used to open column isolation (Sephadex LH 20 and silica gel column) in the study, such as n-hexane, chloroform, ethyl acetate, acetone, and methanol were ACS grade. The HPLC grade n-hexane, ethyl acetate, and acetone for HPLC isolation, and the deuterated solvent for NMR measurement (CDCl3, CD3OD) were purchased from the branch of Merck in Taipei, Taiwan. LPS (endotoxin from Escherichia coli, serotype 0127:B8), Carr (type IV), indomethacin, MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide), and other chemicals were purchased from Sigma Chemical Co. (St. Louis, MO, USA).
3.3. Plant Material
Lawsonia inermis was collected from Neipu Township, Pingtung, Taiwan, in February 2009 and identified by I.-S. Chen (Emeritus Professor, School of Pharmacy, College of Pharmacy, Kaohsiung Medical University, Kaohsiung, Taiwan). A voucher specimen (CMU-LIY-090711) was deposited at the School of Chinese Pharmaceutical Sciences and Chinese Medicine Resources.
3.4. Extraction and Isolation
The dried aerial part (5.0 kg) of Lawsonia inermis was extracted three times with MeOH (50 L each) for 7 days. The extract was concentrated under reduced pressure at 35 °C, and the residue (440 g) was partitioned between EtOAc and H2O (1:1) to provide the EtOAc-soluble fraction (fraction A; 132.5 g). Fraction A (132.5 g) was purified by column chromatography (CC) (6.0 kg of SiO2, 70–230 mesh; n-hexane/EtOAc/methanol gradient) to afford 14 fractions: A1–A14.
Fraction A2 (32.8 g) was re-separated by silica gel column chromatography (n-hexane:ethyl acetate = 10:1) and semi-preparative normal phase HPLC (n-hexane:acetone = 8:1 to afford pure compounds 1 (37.5 mg), 2 (16.8 mg), and 3 (24.1 mg). Fraction 9 (16.45 g) was re-separated by Sephadex LH 20 column chromatography (chloroform:methanol = 3:7), silica gel column chromatography (n-hexane:ethyl acetate = 5:2), and then semi-preparative HPLC (n-hexane:acetone = 3:1) to afford pure compounds 4 (12.1 mg), 5 (8.5 mg), 6 (16.0 mg), 7 (14.6 mg), 8 (22.6 mg), and 9 (16.3 mg).
(Z)-4,4′-(Prop-1-ene-1,3-diyl)diphenol (1). Pale yellow powder; HR-ESI-MS: C15H14O2, found: 227.2787 [M + H]+, calcd.: 227.2784; UV (MeOH) λmax (log ε): 285 (4.32), 296 (4.24) nm. IR (KBr) νmax: 3554 (OH), 1652, 1602, 1500, 1439 cm−1. 1H-NMR and 13C-NMR (500/125 MHZ, in CDOD) were shown on Table 1.
Methyl (1-oxo-1H-isochromen-3-yl)carbonate (Inermiscarbonate A) (2). Amorphous powder; HR-ESI-MS: C11H9O5, found: 221.1864 [M + H]+, calcd.: 221.1861; UV (Dioxane) λmax (log ε): 244 (4.42), 255 (4.35), 272 (4.46), 281 (4.23), 350 (3.11) nm. IR (KBr) νmax: 1793 (C=O), 1716 (C=O), 1599 cm−1. 1H-NMR and 13C-NMR (500/125 MHZ, in CDCl3) were shown on Table 2.
Ethyl (1-oxo-1H-isochromen-3-yl)carbonate (Inermiscarbonate B) (3). Amorphous powder; HR-ESI-MS: C12H11O5, found: 235.2129 [M + H]+, calcd.: 235.2127; UV (Dioxane) λmax (log ε): 244 (4.48), 255 (4.43), 272 (4.55), 280 (4.49), 350 (3.42) nm. IR (KBr) νmax: 1772 (C=O), 1720 (C=O), 1598 cm−1. 1H-NMR and 13C-NMR (500/125 MHZ, in CDCl3) were shown on Table 3.
3.5. Cell Culture
A murine macrophage cell line RAW 264.7 (BCRC No. 60001) was purchased from the Bioresources Collection and Research Center (BCRC, Hsinchu, Taiwan) of the Food Industry Research and Development Institute (Hsinchu, Taiwan). Cells were cultured in plastic dishes containing Dulbecco’s Modified Eagle Medium (DMEM, Sigma) supplemented with 10% fetal bovine serum (FBS, Sigma) in a CO2 incubator (5% CO2 in air) at 37 °C and subcultured every 3 days at a dilution of 1:5 using 0.05% trypsin-0.02% EDTA in Ca2+-, Mg2+-free phosphate-buffered saline (DPBS).
3.6. Cell Viability
Cells (2 × 105) were cultured in 96-well plates containing DMEM supplemented with 10% FBS for 1 day to become nearly confluent. Then cells were cultured with compounds 1–9 in the presence of 100 ng/mL LPS (lipopolysaccharide) for 24 h. After that, the cells were washed twice with DPBS and incubated with 100 μL of 0.5 mg/mL MTT for 2 h at 37 °C, testing for cell viability. The medium was then discarded, and 100 μL dimethyl sulfoxide (DMSO) was added. After 30-min incubation, absorbance at 570 nm was read using a microplate reader (Molecular Devices, Sunnyvale, CA, USA).
3.7. Measurement of Nitric Oxide/Nitrite
NO production was indirectly assessed by measuring the nitrite levels in the cultured media and serum determined by a colorimetric method based on the Griess reaction. The cells were incubated with different concentrations of samples in the presence of LPS (100 ng/mL) at 37 °C for 24 h. Then, cells were dispensed into 96-well plates, and 100 μL of each supernatant was mixed with the same volume of Griess reagent (1% sulfanilamide, 0.1% naphthylethylenediamine dihydrochloride and 5% phosphoric acid) and incubated at room temperature for 10 min, and the absorbance was measured at 540 nm with a Micro-Reader (Molecular Devices, SpectraMax® M2e, Sunnyvale, CA, USA). By using sodium nitrite to generate a standard curve, the concentration of nitrite was measured from absorbance at 540 nm.
3.8. Statistical Analysis
The data is expressed as means ± standard errors (SE). The IC50 values were calculated from the dose curves using a non-linear regression algorithm (SigmaPlot 8.0; SPSS Inc., Chicago, IL, USA, 2002). Statistical evaluation was carried out by one-way analysis of variance (ANOVA followed by Scheffe’s multiple range tests).
4. Conclusions
Nine compounds, including three new compounds (1–3), were isolated from the aerial part of Lawsonia inermis. The structures of these compounds were established on the basis of spectroscopic data. New compounds 2 and 3 are carbonate derivatives, with very unique structures from natural sources. Compounds 1 and 4–9 showed effective anti-inflammatory activities by decreasing nitrate of LPS-stimulated production in RAW 264.7 cell with IC50 values of 5.63 ± 3.64 μg/mL 15.72 ± 2.52 μg/mL, 8.67 ± 3.84 μg/mL, 6.67 ± 3.48 μg/mL, 6.17 ± 2.86 μg/mL, 7.61 ± 3.34 μg/mL, and 14.52 ± 3.31 μg/mL, respectively, wherein compounds 2 and 3 exhibited no inhibition. (Z)-4,4′-(Prop-1-ene-1,3-diyl)diphenol (1) is a new compound and the most effective among the isolated compounds, with IC50 values of 5.63 ± 3.64 μg/mL against LPS-stimulated nitrite generation. Our study suggests Lawsonia inermis and its isolates (especially 1, 6, and 7) could be further developed as potential candidates for the treatment or prevention of various inflammatory diseases.
Supplementary Materials
Supplementary materials can be accessed at: http://www.mdpi.com/1420-3049/21/10/1299/s1.
Acknowledgments
Financial was supported from CMU under the Aim for Top University Plan of the Ministry of Education, Taiwan, and Taiwan Ministry of Health and Welfare Clinical Trial and Research Center of Excellence (MOHW105-TDU-B-212-133019).
Author Contributions
Chang-Syun Yang and Jin-Jung Chen performed the isolation and structure elucidation of the constituents, and manuscript writing. Ping-Jyun Sung and Hui-Chi Huang contributed to the structure elucidation and also part of the preparation of the manuscript. Sheng-Yang Wang and Guan-Jhong Huang conducted the bioassay and analyzed the data. Yueh-Hsiung Kuo planned, designed, and organized the whole research of this study and the preparation of the manuscript. All authors read and approved the final version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chen, H.Y.; Qian, C. Flora of China; Editorial Committee of the Flora of China: Beijing, China, 2007; pp. 274–288. [Google Scholar]
- Lin, Y.X.; Chang, Y.S.; Chen, I.S.; Ou, J.C. The Catalogue of Medicinal Plant Resources in Taiwan; Committee on Chinese Medicine and Pharmacy: Taipei, Taiwan, 2003.
- Ahmed, S.; Rahman, A.; Alam, A.; Saleem, M.; Athar, M.; Sultana, S. Evaluation of the efficacy of Lawsonia alba in the alleviation of carbon tetrachloride-induced oxidative stress. J. Ethnopharmacol. 2000, 69, 157–164. [Google Scholar] [CrossRef]
- Liou, J.R.; Mohamed, E.S.; Du, Y.C.; Tseng, C.N.; Hwang, T.L.; Chuang, Y.L.; Hsu, Y.M.; Hsieh, P.W.; Wu, C.C.; Chen, S.L.; et al. 1,5-Diphenylpent-3-en-1-ynes and methyl naphthalene carboxylates from Lawsonia inermis and their anti-inflammatory activity. Phytochemistry 2013, 88, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Babu, P.D.; Subhasree, R.S. Antimicrobial activities of Lawsonia inermis—A review. Acad. J. Plant Sci. 2009, 2, 231–232. [Google Scholar]
- Neuman, J.R.C.; Behar, J.V. 2-Carbomethoxybenzocyclobutenone. Synthesis of a photochemically sensitive small-ring system by a pyrolytic Wolff rearrangement. J. Am. Chem. Soc. 1967, 89, 4550–4551. [Google Scholar]
- Sevil, Ö.; Metin, B. The chemistry of homophthalic acid: A new synthetic strategy for construction of substituted isocoumarin and indole skeletons. Tetrahedron 2008, 64, 5531–5540. [Google Scholar]
- Schnekenburger, J. Acylierung von Homophthalsäureanhydrid mit kohlensäureäthylesterchlorid. J. Arch. Pharm. 1965, 298, 405–410. [Google Scholar] [CrossRef]
- Kagawa, H.; Shigematsu, A.; Ohta, S.; Harigaya, Y. Preparative monohydroxyflavanone syntheses and a protocol for gas chromatography-mass spectrometry analysis of monohydroxyflavanones. Chem. Pharm. Bull. 2005, 53, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Parveen, S.; Riaz, N.; Saleem, M.; Khan, J.; Ahmad, S.; Ashraf, M.; Ejaz, S.A.; Tareen, R.B.; Jabbar, A. Bioactive phenolics from Launaea intybacea. J. Chem. Soc. Pak. 2012, 34, 1–7. [Google Scholar]
- Liu, M.; Yang, S.; Jin, L.; Hu, D.; Wu, Z.; Yang, S. Chemical constituents of the ethyl acetate extract of Belamcanda chinensis (L.) DC roots and their antitumor activities. Molecules 2012, 17, 6156–6169. [Google Scholar] [CrossRef] [PubMed]
- Qi, S.H.; Wu, D.G.; Ma, Y.B.; Luo, X.D. A novel flavane form Carapa guianensis. Acta Bot. Sin. 2003, 45, 1129–1133. [Google Scholar]
- Geller, D.A.; Billiar, T.R. Molecular biology of nitric oxide synthases. Cancer Metastasis Rev. 1998, 17, 7–23. [Google Scholar] [CrossRef] [PubMed]
- Moncada, S.; Palmer, R.M.; Higgs, E.A. Nitric oxide: Physiology, pathophysiology, and pharmacology. Pharmcol. Rev. 1991, 43, 109–142. [Google Scholar]
- Sample Availability: Samples of the compounds are available from the authors.
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).