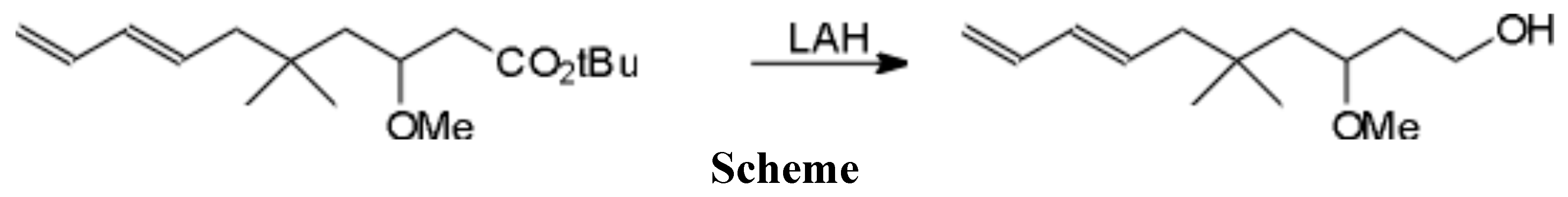
To a slurry of LAH (65 mg, 1.7 mmol) in ether (4 ml) at 0 ƒC under Ar, the diene ester [1] (487 mg, 1.7 mmol) in ether (5 ml) was added dropwise. After addition was complete, the reaction was quenched with sat aq Na2SO4. The solution was filtered and the solids were washed with ether (5 x 10 ml). The combined organic layers were dried over Na2SO4 and concentrated to give, after flash chromatography (1 : 1 pet ether : ether) the title alcohol as a colorless oil, 354 mg, in 97 percent yield.
1H NMR (CDCl3): d: 6.29 (dt, J = 16.9, 10.3Hz, 1H), 6.01 (dd, J = 15.3, 10.7Hz, 1H), 5.71 (dt, J = 15.3, 7.5Hz, 1H), 5.12 (d, J = 16.9Hz, 1H), 4.93 (d, J = 10.3Hz, 1H), 3.81 (m, 1H), 3.69 (m, 1H), 3.48 (m, 1H), 3.30 (s, 3H), 2.37 (bm, 2H), 1.99 (d, J = 7.6Hz, 2H), 1.88 (m, 1H), 1.60 (m, 2H), 1.28 (dd, J - 14.7, 4.0Hz, 1H), 0.90 (s, 3H), 0.88 (s, 3H).
IR (CH2Cl2): 3610, 3490, 2930, 1645, 1600, 1460, 1420, 1385, 1075, 1005, 895.
MS (m/e): 212 (M+, 8), 197, 180, 167, 143, 136, 124, 108, 89 (100).
HRMS: calc. for C13H24O2: 212.1776; found: 212.1774.
Supplementary materials
Supplementary File 1Supplementary File 2References and Notes
- Smith, D. tert-Butyl 3-methoxy-5,5-dimethyl-7(E),9-decadienoate. Molecules 1997, 2, M34. [Google Scholar] [CrossRef]
- Sample Availability: No sample available.
© 1997 MDPI. All rights reserved