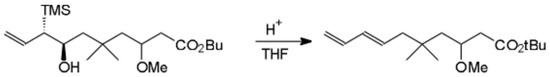
Scheme.
The diastereomeric alcohols [1], either separately or as a mixture, were converted under acidic Peterson olefination conditions to the title E-diene.
Typically, a mixture of alcohols in dry THF was stirred with a catalytic amount of H2SO4 at room temperature for 19 hours, then washed with sat aq NaHCO3. The aq washings were combined and back extracted with ether. The combined organic layers were washed with sat aq NaCl, dried over Na2SO4 and concentrated to give the title E-diene ester as a colorless oil in 73 percent yield.
1H NMR (CDCl3): δ: 6.29 (dt, J = 16.9, 10.3Hz, 1H), 6.01 (dd, J = 15.3, 10.7Hz 1H), 5.71 (dt, J = 15.3, 7.5Hz, 1H), 5.12 (d, J = 16.9 Hz, 1H), 4.93 (d, J = 10.3Hz, 1H), 3.63 (m, 1H), 3.28 (s, 3H), 2.51 (dd, J = 14.6, 5.4Hz, 1H), 2.21 (dd, J = 14.6, 7.3Hz, 1H), 1.99 (bt, J = 6.2Hz, 2H), 1.6 - 1.3 (m, 1H), 1.43 (s, 9H), 1.27 (dd, J = 14.7, 2.7Hz, 1H), 0.90 (s, 3H), 0.88 (s, 3H).
IR (CDCl3): 2965, 2930, 2830, 1715, 1640, 1600, 1450, 1365, 1295, 1260, 1150.
MS (m/e): 226, 195, 157, 127 (100), 117, 108, 103, 73, 57.
HRMS: calc. for C13H22O3 (M - C4H8): 226.1569; found: 226.1569.
Supplementary materials
Supplementary File 1Supplementary File 2References and Notes
- Smith, D. tert-Butyl-3-methoxy-5,5-dimethyl-7-hydroxy-8-trimethylsilyl-9-decenoate. Molecules 1997, 2, M33. [Google Scholar] [CrossRef]
- Sample Availability: No sample available.
© 1997 MDPI. All rights reserved