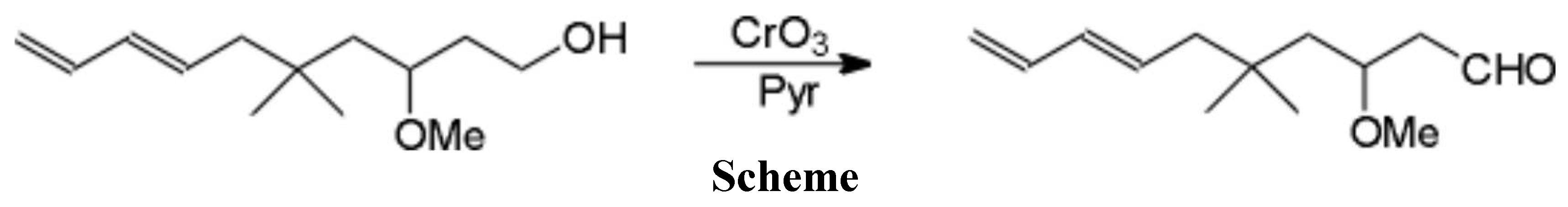
Using the Collins oxidation procedure previously described [1], the title aldehyde was prepared from the diene alcohol [2] as a colorless oil in 76% yield.
1H NMR (CDCl3): d: 9.78 (t, J = 2.2Hz, 1H), 6.29 (dt, J = 16.9, 10.3Hz, 1H), 6.01 (dd, J = 15.3, 10.7Hz, 1H), 5.71 (dt, J = 15.3, 7.5Hz, 1H), 5.12 (d, J = 16.9Hz, 1H), 4.93 (d, J = 10.3Hz, 1H), 3.76 (m, 1H), 3.29 (s, 3H), 2.59 (bs, 1H), 2.56 (bs, 1H), 2.0 (dd, J = 7.3, 2.9Hz, 1H), 1.57 (dd, J = 14.6, 7.9Hz, 1H), 1.26 (dd, J = 14.6, 3.3Hz, 1H), 0.92 (s, 3H), 0.89 (s, 3H).
IR (CDCl3): 2955, 2930, 2825, 1720, 1600, 1460, 1360, 1105, 1070, 1005, 880.
MS (m/e): 210 (M+, 3), 192, 141, 134, 111, 108, 99, 93, 87 (100), 59.
HRMS: calc. for C13H22O2: 210.1620; found: 210.1619.
Supplementary materials
Supplementary File 1Supplementary File 2References and Notes
- Smith, D. tert-Butyl-3-methoxy-5,5-dimethyl-7-oxo-heptanoate. Molecules 1997, 2, M32. [Google Scholar] [CrossRef]
- Smith, D. 3-Methoxy-5,5-dimethyl-7(E),9-decadien-1-o1. Molecules 1997, 2, M35. [Google Scholar] [CrossRef]
- Sample Availability: No sample available.
© 1997 MDPI. All rights reserved