Abstract
Aminoanthraquinones were successfully synthesized via two reaction steps. 1,4-Dihydroxyanthraquinone (1) was first subjected to methylation, reduction and acylation to give an excellent yield of anthracene-1,4-dione (3), 1,4-dimethoxyanthracene-9,10-dione (5) and 9,10-dioxo-9,10-dihydroanthracene-1,4-diyl diacetate (7). Treatment of 1, 3, 5 and 7 with BuNH2 in the presence of PhI(OAc)2 as catalyst produced seven aminoanthraquinone derivatives 1a, b, 3a, and 5a–d. Amination of 3 and 5 afforded three new aminoanthraquinones, namely 2-(butylamino)anthracene-1,4-dione (3a), 2-(butylamino)anthracene-9,10-dione (5a) and 2,3-(dibutylamino)anthracene-9,10-dione (5b). All newly synthesised aminoanthraquinones were examined for their cytotoxic activity against MCF-7 (estrogen receptor positive human breast) and Hep-G2 (human hepatocellular liver carcinoma) cancer cells using MTT assay. Aminoanthraquinones 3a, 5a and 5b exhibited strong cytotoxicity towards both cancer cell lines (IC50 1.1–13.0 µg/mL).
Keywords:
methylation; reduction; acylation; amination; substitution; aminoanthraquinone; mechanism; cytotoxic; MCF-7; Hep-G2 1. Introduction
Natural and synthetic anthraquinones have attracted the interest of researchers due to their significant biological activities such as antitumour [1,2,3,4], anti-inflammatory [5], antimalarial [5,6,7], antimicrobial [5,8], antifungal [9], antileukemic [10,11], antiviral and anti-HIV properties [12,13,14,15]. Anthraquinone and its derivatives are also used as antioxidants [16], dyes [17,18,19,20] or in photoimaging [20].
It has also been reported that amino-substituted anthraquinones show significantly increased antiproliferative activities against human/mammalian cancer cell lines [21,22] and are known to have potential antitumor activity, but are less toxic to normal cells and display low cardiotoxicity [23,24,25,26]. A study on 4-(N-cyclohexylamino)-emodin implied that it can discriminate well between hepatoma cells and primary hepatocytes and it retained the capacity to reverse the multi-drug-resistance phenotype [27]. Other aminoanthraquinone derivatives such as Reactive Blue 2 (RB-2), Acid Blue 25 (AB-25) and Acid Blue 129 (AB-129) also known as a good nucleotide-binding proteins [28] where RB-2 is one of the most widely used P2-receptor antagonists [29]. The potential of RB-2 was driven by its hydrophobic interactions of aromatic π-electron systems, and the hydrogen bonds with nitrogen as donor and acceptor atoms as these properties could block the P2-recptor.
Based on these promising bioactivities, the aim of this research was therefore to synthesise some new aminoantharaquinone derivatives with high potential as anticancer and antimicrobial agents. The effects of substrate amount, catalyst amount, reaction time and reaction temperature were studied. According to a study, shorter amines would result in lower cytotoxic effects [24] whereas the use of diamines or longer amine chains allow the possibility of forming side products due to their reactive properties [30]. Therefore, a simple straight chain amine containing four carbons (BuNH2) was chosen for this study, while diacetoiodobenzene [PhI(OAc)2] was used as catalyst because of it selectivity towards the substitution reaction [24]. Synthesised aminoanthraquinones were then further tested for cytotoxicity and antimicrobial activity.
2. Results and Discussion
The synthesis of aminoanthraquinone derivatives was achieved through two simple reaction steps. 1,4-Dihydroxyanthraquinone (1) was first subjected to methylation, reduction, and acylation to produce anthracene-1,4-dione (3) 1,4-dimethoxyanthracene-9,10-dione (5) and 9,10-dioxo-9,10-dihydro-anthracene-1,4-diyl diacetate (7). Compounds 1, 3, 5 and 7 were then treated with butylamine (BuNH2) using iodobenzenediacetate [PhI(OAc)2] as catalyst to produce the desired aminoanthraquinones. All the compounds were elucidated using mp, IR, MS, 1D NMR, 2D NMR and comparison with data in the literature. The proposed mechanisms for the amination are also presented.
2.1. Reduction, Methylation and Acylation
The reduction of compound 1 using NaBH4 (1:1 equiv.) was performed successfully in 30 min to give a mixture of 4-hydroxyanthracene-1,10-dione (2) and anthracene-1,4-dione (3) in 69% and 21% yields, respectively. Increasing the amount of NaBH4 to 3 equiv. led to the formation of only compound 3 in excellent yield (Scheme 1 and Table 1). Further NaBH4 increases resulted in a lower yield of 3 as the single product obtained (Table 1, Entry I, II and III).
The methylation of compound 1 with (CH3)2SO4 in the presence of K2CO3 was achieved in acetone under reflux at 60 °C [31,32]. The used of (CH3)2SO4 as methylating agent is proven to give higher yields [33]. A mixture of 1-hydroxy-4-methoxyanthracene-9,10-dione (4) and 1,4-dimethoxy-anthracene-9,10-dione (5) were obtained as yellow and orange solids, respectively (Table 1, Entry IV and V). The 1H-NMR spectra of compounds 4 and 5 showed singlets at δ 3.76 and δ 3.94 ppm, respectively, which were attributed to the methoxy protons.
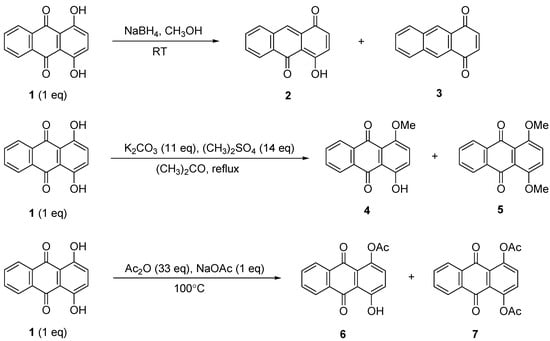
Scheme 1.
Reduction, methylation and acylation of 1.

Table 1.
Some effects on the reduction, methylation and acylation of 1.
| Entry | NaBH4 (equiv.) | Reaction Time | Product (% Yield) |
|---|---|---|---|
| I | 1 | 30 min | 2 (69), 3 (21) |
| II | 3 | 30 min | 3 (90) |
| III | 15 | 30 min | 3 (60) |
| IV | - | 3 h | 4 (5), 5 (85) |
| V | - | 4 h | 5 (96) |
| VI | - | 2 h | 6 (26), 7 (60) |
| VII | - | 3 h | 6 (15), 7 (75) |
| VII | - | 9 h | 7 (90) |
The acylation of compound 1 by using excess acetic acid anhydride in the presence of sodium acetate was completed in 2 h to obtain a mixture of 4-hydroxy-9,10-dioxo-9,10-dihydroanthracen-1-yl acetate (6) and 9,10-dioxo-9,10-dihydroanthracene-1,4-diyl diacetate (7) (Scheme 1). Increasing the reaction time for both methylation and acylation seemed to increase the dimethylated and diacetylated products, 5 and 7 (Table 1, Entry V, VI, VII and VIII). It was believed that the product of 3, 5 and 7 were produced through the intermediate compounds 2, 4 and 6, respectively.
2.2. Amination
Amination reactions were attempted using procedure of Teich et al. [24]. It was stated that the formation of aminoanthraquinones can be achieved in higher yield using 1.1 equiv. of the catalyst PhI(OAc)2. Compounds 1, 3, 5, and 7 (1 equiv.) were then used in a substitution reaction to produce aminoanthraquinones derivatives. The reaction was performed with excess BuNH2 in the presence of PhI(OAc)2 for 15 min to 6 d of reaction, depending on the hindrance of the starting material. BuNH2 also acted as a solvent for the reaction (Scheme 2). The catalytic effect was studied by performing the reaction either with or without the catalyst to observe what is the possible reaction or product that might occur or form. Therefore, direct amination of 1 with higher equivalents of butylamine in the presence of 1.1 equiv. of catalyst at RT gave a mixture of anthraquinone butylamines 1a and 1b in 1:1 ratio (78% yield). Reducing the equivalents of amine to half either with or without the catalyst produced a single product 1a in higher yield (70%–90%, Table 2, Entry I, II and III). However, increasing the reaction temperature gave a mixture of products again, where 1a was isolated as the major one (70%). The butylamino group was observed to substitute at the ortho position or replace the OH group. The results proved that the amination occurred via a nucleophilic substitution reaction and in agreement with reported works [21,25]. The 1H- and 13C-NMR spectra of 1a and 1b are also consistent with their structures and the reported data.
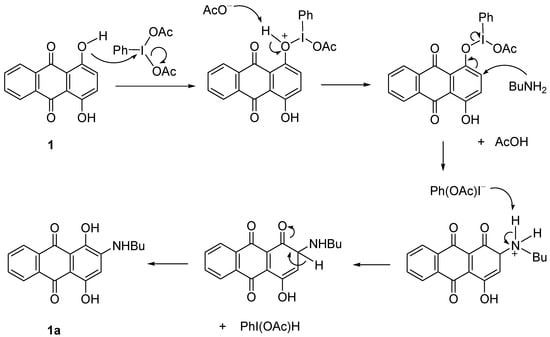
Scheme 2.
Proposed mechanism of formation of 1a in the presence of catalyst.

Table 2.
Effect of amine equivalence, catalyst and temperature on the amination of 1, 3, 5 and 7.
| Entry | BuNH2 (equiv.) | PhI(OAc)2 (equiv.) | Temperature | Product (% Yield) |
|---|---|---|---|---|
| I | 450 | 1.1 | RT | 1a (40), 1b (38) |
| II | 225 | 1.1 | RT | 1a (90) |
| III | 225 | - | RT | 1a (70) |
| IV | 225 | - | 80 °C | 1a (70), 1b (15) |
| V | 225 | 1.1 | RT | 3a (60) |
| VI | 225 | - | RT | 3a (51) |
| VII | 112 | 1.1 | RT | 3a (46) |
| VIII | 225 | 1.1 | RT | 5a (7), 5b (10), 5 (78) |
| IX | 225 | 1.1 | 80 °C | 5c (10), 5d (73) |
| X | 225 | 1.1 | RT | 1a (83) |
| XI | 225 | - | RT | 1a (55) |
| XII | 225 | - | 80 °C | 1a (46) |
In the presence of PhI(OAc)2, the reaction proceeded with the interaction of the OH group of compound 1 with the catalyst to give the intermediate, O-IPh(OAc). Further attack by BuNH2 at the next carbon was produced C=O and removed the PhIOAc. Proton elimination then restored the double bond and OH of 1a (Scheme 2). In the absence of catalyst, the electron-donating nature of the OH group together with the electron delocalization made compound 1 easily attacked by the BuNH2 at the ortho-position to form 1a (Scheme 3).
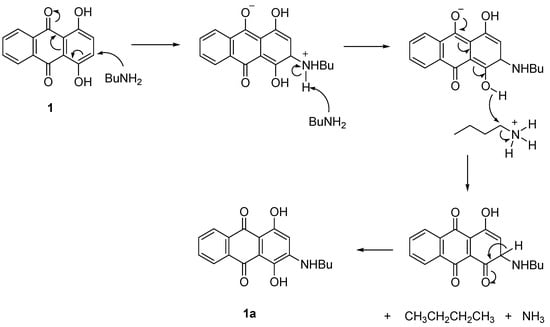
Scheme 3.
Proposed mechanism of formation of 1a without using a catalyst.
The compounds 1a, 1b and the optimised amount of BuNH2 (225 equiv.) and PhI(OAc)2 (1.1 equiv.) were used as a comparison of nucleophilic substitution for amination of compounds 3, 5 and 7. Therefore, the effect of different 1,4-disubstituted of anthraquinones and the mechanisms were further investigated.
2.2.1. Amination of 3
Amination of compound 3 produced a higher yield of 2-(butylamino)anthracene-1,4-dione (3a) when treated with 255 equiv. of BuNH2 (Scheme 4). It was observed that the yield decreased when the reaction was performed without a catalyst or if equivalents of amine were reduced (Table 2, Entry V, VI and VII).
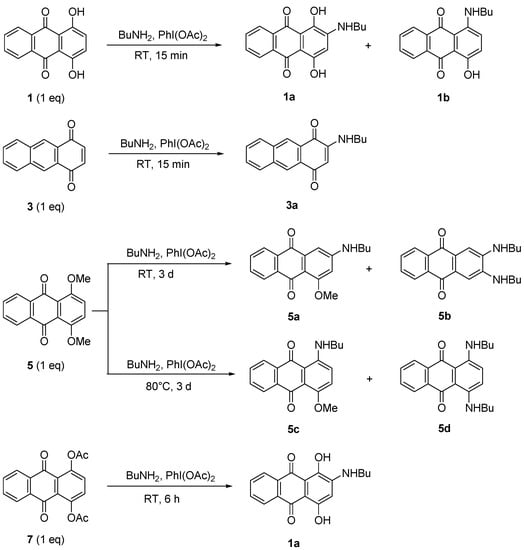
Scheme 4.
Amination of 1, 3, 5, and 7.
The 1H-NMR spectrum of 3a showed aromatic proton singlets at δ 8.50 and δ 8.48 ppm that are attributed to the aromatic carbons at positions C9 and C10, respectively. A proton singlet observed at δ 5.80 ppm indicated the presence of an unsaturated proton, whereas a broad singlet observed at δ 5.99 ppm referred to the chelated proton of the amine. The 13C-NMR spectrum showed the presence of 18 signals correspond to the 18C in the structure. Higher chemical shifts were observed at δ 182.7 and δ 181.4 ppm that represent the two C=O groups, whereas lower signals for C2 and C3 were observed at δ 130.2 and δ 134.0 ppm. In comparison to the starting material, only one signal was observed for both C=O (δ184.7), and for C2 and C3 (δ140.1) due to the symmetrical structure of 3. The position of the butylamino group of 3a was confirmed by an HMBC experiment which showed a 3J correlation as indicated in Figure 1.
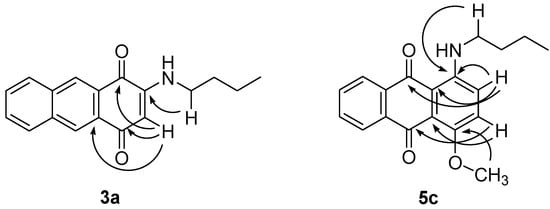
Figure 1.
Selected HMBC correlation of 3a and 5c.
The proposed mechanism showed that C3 is positively charged and readily attacked by BuNH2 due to the resonance that drives by the interaction between the oxygen at C1 with the iodine of the catalyst. Further elimination of a proton at position C3, PhI and AcOH then restored the double bonds between C-C and C-O to give the product 3a (Scheme 5). A similar resonance effect is assumed to drive the BuNH2 to attack the C3 to produce a negatively charged oxygen for the reaction without catalyst. The reaction proceeded through a keto-enol tautomerism followed by elimination of H to give 3a (Scheme 6).
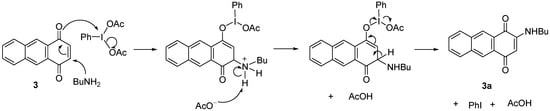
Scheme 5.
Proposed mechanism of formation of 3a by using a catalyst.
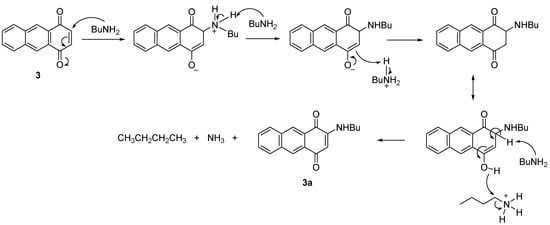
Scheme 6.
Proposed mechanism for the formation of 3a (without catalyst).
2.2.2. Amination of 5
Treatment of dimethoxyanthraquinone 5 with BuNH2 in the presence of PhI(OAc)2 produced four aminoathraquinones. At RT, a mixture of 2-(butylamino)-4-methoxyanthracene-9,10-dione (5a) and 2,3-(dibutylamino)anthracene-9,10-dione (5b) was obtained in low yields (17%) together with the unreacted starting material (78%). A mixture of 1-(butylamino)-4-methoxyanthacene-9,10-dione (5c) and 1,4-(dibutylamino)anthracene-9,10-dione (5d) was produced at 80 °C, in higher combined yield (83%, Scheme 2). The diaminosubstituted products 5b and 5d were isolated as a major product (Table 2, Entry VIII and IX). It appears that the higher the temperature, the more the nucleophilic substitution selectivity. All four amino derivatives were easily separated by column chromatography (dichloromethane-petroleum ether/3:1). It seems that compound 5a and 5c which had same Rf values and appearance (Rf 0.33, dichloromethane-petroleum ether, 4:1; dark pink solid) were produced first as intermediates and then reacted further to form diaminoanthraquinones 5b and 5d. Surprisingly, compounds 5b and 5d also shared same Rf values 0.67 (dichloromethane-petroleum ether, 4:1) and both were obtained as dark blue solids.
The 1H-NMR spectra of 5a and 5c displayed signals attributed to methoxy protons at δ 3.98 and δ 3.98 ppm, respectively. A broad singlet signal at δ 7.27 ppm in the spectrum of 5a was assigned to the non-chelated amine proton, whereas for 5c, the spectrum show a higher chemical shift at δ 9.84 ppm which is due to the hydrogen bond between the amine hydrogen and carbonyl oxygen. Both compounds shared the same molecular formulae, C19H19NO3, as the mass spectra exhibited a molecular ion peak at m/z 309. Selected HMBC correlations were shown in Figure 1.
The 1H-NMR of 5d showed the disappearance of the signal for the methoxy protons, which clearly indicated that nucleophilic aromatic substitution had occurred. The broad singlet at δ 10.73 ppm was assigned to the chelated amine proton. The 13C-NMR showed the presence of 11 signals attributed to 22 carbons which are symmetrical. Four quaternary carbons were observed at δ 182.0 (C=O), 146.2 (C-C=O), 134.6 (O=C-C) and 109.5 ppm (C-NH). Three peaks at δ 123.5, 126.0 and 131.8 ppm were assigned to methine aromatic carbons.
The reaction occurred as the methoxy group interacts with the catalyst. The positive charge on oxygen is then stabilized by the C1-O bond cleavage driven by the formation of PhI(OAc)(OCH3) and leaves the aromatic carbocation. With increasing heat, the carbocation is readily attacked by BuNH2 to produce 5c and 5d. In contrast a slow reaction at RT favored the carbocation rearrangement to produce 5a and 5b (Scheme 7). This aromatic substitution of methoxy groups by amines is also supported by literature precedents [21,25,28].
2.2.3. Amination of 7
2-(Butylamino)-1,4-dihydroxyanthracene-9,10-dione (1a) was successfully obtained in 83% yield under a similar amination approach applied to the acylated anthraquinone 7 (Scheme 4). The mechanism suggested that the acetate groups were reduced by the conditions and reagent used to give 1,4-dihydroxyanthraquinone (1) as an intermediate before it further reacted with BuNH2/PhI(OAc)2 to give 1a (Scheme 8). It was observed that 1a can still be obtained when the reaction was performed without catalyst or at 80 °C but gave lower yield. Heat applied to the reaction induced the reaction to be faster as the reaction time reduced from 6 h to 4 h (Table 2, Entry X, XI and XII).
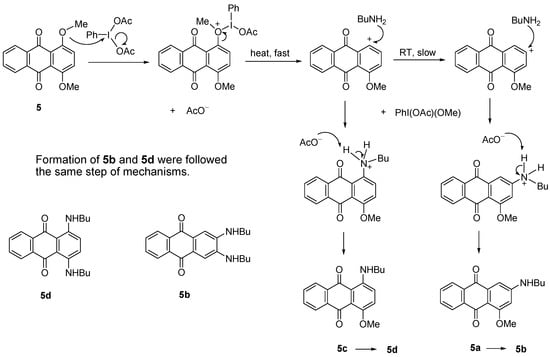
Scheme 7.
Proposed mechanism of formation of 5a, 5b, 5c and 5d.
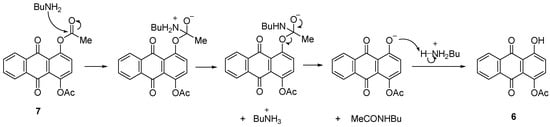
Scheme 8.
Reduction of acyl group of 7 by butylamine.
The 1H-NMR for 1a showed the disappearance of the COOCH3 signal which proved that the acyl groups were reduced. This is also proven by the existence of strong chemical shifts at δ 14.23 and δ 13.90 ppm which represented the two chelated OH. A broad singlet proton at δ 5.60 ppm was attributed to the amine proton.
All three new aminoanthraquinones 3a, 5a and 5b were subjected to cytotoxic activity assays against MCF-7 (estrogen receptor positive human breast) and Hep-G2 (human hepatocellular liver carcinoma) cancer cell lines with compounds 3a and 5a being the most cytotoxic (IC50 1.1–3.0 g/mL). Increasing the number of amino substituents (compound 5b) on the structure of anthraquinone basically reduced the activity against both MCF-7 and Hep-G2 cancer cell lines with IC50 of 3.0 and 13.0 g/mL, respectively (Table 3). Saha et al. [34] reported that the starting material, quinizarin 1 (free hydroxy groups) was not active towards MCF-7 but the methylated hydroxyl derivative of quinizarin showed some potential with IC50 values 70–80 M. The results of this study also supported their finding where the presence of methoxy or amino groups enhanced the cytotoxic activity of the compounds.

Table 3.
Cytotoxic activity of aminoanthraquinones.
| Sample | Cytotoxic Activity IC50 (µg/mL) | |
|---|---|---|
| MCF-7 | Hep-G2 | |
| 3a | 1.1 | 1.2 |
| 5a | 1.1 | 3.0 |
| 5b | 3.0 | 13.0 |
Compounds 3a, 5c and 5d have been screened for antimicrobial activity using four different types of microbe, methicillin-resistant Staphylococcus aureus (MRSA), Pseudomonas aeruginosa, Candida albicans and Escherichia coli. The other two new compounds, 5a and 5b were replaced by compounds 5c and 5d due to insufficient materials and based on the similarity of their structures. All four compounds have the same methoxy and amino substituents and only differ at their amino positions. Therefore, it was assumed that all four compounds might give similar activity results. However, none of compounds had any effect on the growth of all four bacteria or the fungus.
3. Experimental
3.1. General
All chemicals are commercially available and were of analytical grade and used without purification unless otherwise stated. All reactions were monitored by TLC, using Silica gel 60 F245 (Merck KGaA) precoated aluminium backed plates and visualised by UV and H2SO4 solution. All organic extracts were dried over Na2SO4 and evaporated using rotary evaporator. Column chromatography was performed on silica gel 60. Melting points were recorded by digital melting point equipment (Electrothermal IA9000 Series). The IR spectra were obtained by using Perkin-Elmer FT-IR Model Spectrum 100 series spectrophotometer using UATR techniques and the adsorption bands were measured in the range of 280–4000 cm−1. MS spectra were recorded using equipped Shimadzu model QP5050A series. The 1D NMR and 2D NMR spectra were run on a JEOL machine at 400 MHz or 500 MHz. All chemical shifts, δ were recorded in ppm relative to TMS signal. The coupling constants J are given in Hz.
3.2. Reduction
1,4-Dihydroxyanthraquinone (1, 1 equiv.) was dissolved in methanol (3 mL) and stirred homogenously under a nitrogen atmosphere. Sodium borohydride (1, 3 or 15 equiv.) was added slowly to the reaction mixture and stirred for 30 min. Water (0.5 mL) was added to the reaction mixture and the pH was adjusted to 5–6 with 1 M HCl. Methanol was evaporated and the residue of aqueous layer was extracted with CH2Cl2 (3 × 10 mL). The combined organic layer were dried, filtered and evaporated under vacuum. The crude product was purified by column chromatography (CH2Cl2-petroleum ether, 3:2) to obtain compounds 2 (NaBH4: 1 equiv.) and 3 (NaBH4: 1, 3 and 15 equiv.).
4-Hydroxyanthracene-1,10-dione (2). Dark orange powder (65.0 mg, 69%); mp 205.8–206.1 °C; vmax (UATR) 3317, 3056, 1662, 1568, 1271 cm−1; δH (500 MHz, CDCl3) 13.74 (1H, s, OH), 8.44 (1H, d, J = 8.0, CH=C), 8.03 (1H, s, CH=C), 7.91 (1H, d, J = 8.0, CH=C), 7.64–7.69 (2H, m, H-aromatic), 7.00 (1H, d, J = 10.3, H-aromatic), 6.96 (1H, d, J 10.3, H-aromatic); δC (125 MHz, CDCl3) 189.2, 184.1, 162.6, 140.8, 140.0, 136.0, 131.4, 130.6, 129.3, 127.7, 127.4, 124.9, 121.9, 108.9; m/z (EI) 224 (M+, C14H8O3 requires 224).
Anthracene-1,4-dione (3). Orange powder (78.4 mg, 90%); mp 216.4–217.2 °C; vmax (UATR) 3319, 3053, 1662, 1596 cm−1; δH (400 MHz, CDCl3) 8.55 (2H, s, CH=C), 8.01 (2H, dd, J = 2.8, 6.4, H-aromatic), 7.66 (2H, dd, J = 2.8, 6.4, H-aromatic), 7.03 (2H, s, H-aromatic); δC (100 MHz, CDCl3) 184.7, 140.1, 134.9, 130.3, 129.7, 128.9, 128.4; m/z (EI) 208 (M+, C14H8O3 requires 208).
3.3. Methylation
A mixture of 1 (1 equiv.), K2CO3 (11 equiv.) and (CH3)2SO4 (14 equiv.) in acetone (5 mL) was refluxed for 3 h. The reaction mixture was added to 25 mL of 2 M HCl and extracted with EtOAc (3 × 25 mL). The combined organic layers were dried, filtered and evaporated under vacuum. The crude product was purified by column chromatography (CH2Cl2-petroleum ether, 4:1) to produce compounds 4 and 5.
1-Hydroxy-4-methoxyanthracene-9,10-dione (4). Orange powder (5.3 mg, 5%); mp 162–163 °C; vmax (UATR) 3073, 2924, 1667, 1590, 1440, 1234, 1178 cm−1; δH (400 MHz, CDCl3) 12.71 (1H, s, OH), 8.01 (2H, t, J = 6.4, H-aromatic) 7.52 (2H, dt, J = 19.3, 6.4, H-aromatic), 7.15 (1H, d, J = 9.2, H-aromatic), 7.06 (1H, d, J = 10.1, H-aromatic), 3.76 (3H, s, OCH3); δC 188.8, 181.5, 157.2, 154.1, 134.9, 134.6, 133.1, 132.1, 127.2, 126.2, 126.2, 123.5, 119.0, 115.8, 56.9 (100 MHz, CDCl3); m/z (EI) 254 (M+, C15H10O4 requires 254).
1,4-Dimethoxyanthracene-9,10-dione (5). Yellow powder (108.1 mg, 96%); mp 198–200 °C; vmax (UATR) 3089, 2998, 1668, 1566, 1404, 1243 cm−1; δH (400 MHz, CDCl3) 8.10 (2H, dd, J = 5.5, 3.6 H-aromatic), 7.65 (2H, dd, J = 5.5, 3.6 H-aromatic), 7.28 (2H, s, H-aromatic), 3.94 (6H, s, OCH3); δC (100 MHz, CDCl3) 183.5, 154.2, 134.3, 133.4, 126.5, 123.1, 120.3, 57.1; m/z (EI) 268 (M+, C16H12O4 requires 268).
3.4. Acylation
A mixture of compound 1 (1 equiv.), Ac2O (33 equiv.), and NaOAc (1 equiv) was stirred at 100 °C for 2 h. The reaction mixture were added to ice cold water and extracted with CH2Cl2 (3 × 25 mL). The combined organic layers was dried and evaporated under vacuum. The crude product was purified by column chromatography (CH2Cl2-petroleum ether, 3:1) to produce compounds 6 and 7.
4-Hydroxy-9,10-dioxo-9,10-dihydroanthracen-1-yl acetate (6). Light orange powder (31.8 mg, 26%); mp 189.1–189.5 °C; vmax (UATR) 3535, 2935, 1772, 1667, 1587, 1360, 1168, 1011 cm−1; δH (400 MHz, CDCl3) 13.04 (1H, s, OH), 8.28 (1H, t, J = 4.6, H-aromatic), 8.21 (1H, t, J = 4.6, H-aromatic), 7.79 (2H, t, J = 3.7, H-aromatic), 7.33 (2H, s, H-aromatic), 2.46 (3H, s, OCH3 ); δC (100 MHz, CDCl3) 188.5, 181.2, 169.9, 161.3, 143.2, 135.0, 134.1, 133.5, 132.5, 131.22, 127.4, 126.8, 126.0, 123.5, 115.9, 21.2; m/z (EI) 282 (M+, C16H10O5 requires 282).
9,10-Dioxo-9,10-dihydroanthracene-1,4-diyl diacetate (7). Light yellow powder (122.4 mg, 90%); mp 236.2–237.0 °C; vmax (UATR) 3081, 1762, 1667, 1582, 1437, 1177 cm−1; δH (400 MHz, CDCl3) 8.14 (2H, dd, J = 5.5, 3.7 H-aromatic), 7.73 (2H, dd, J = 5.5, 3.7, H-aromatic), 7.41 (1H, s, H-aromatic), 2.47 (6H, s, OCCH3 ); δC (100 MHz, CDCl3) 181.6, 169.6, 148.3, 134.2, 133.4, 131.1, 127.0, 126.2, 21.3; m/z (EI) 324 (M+, C16H10O5 requires 324).
3.5. Amination—General Procedure
Butylamine (112, 225 or 450 equiv.) was added dropwise to a mixture of 1, 3, 5, or 7 (1 equiv.) and PhI(OAc)2 (0.41 mmol, or without catalyst) at RT (or heated at 80 °C) and stirred for 15 min to 3 d. The reaction mixture, 25 mL 10 M HCl and NaHCO3 (84 mL) were added to ice cold water (84 mL) successively. The resulting solution was extracted with EtOAc (3 × 25 mL). The organic layer was washed with H2O (3 × 25 mL), dried and evaporated. The crude product was then chromatographed on silica gel (DCM-petroleum ether, 4:1) to give a mixture of amine derivatives of anthraquinone 1a, 1b, 3a, 5a–d.
2-(Butylamino)-1,4-dihydroxyanthracene-9,10-dione (1a). Dark pink powder (176.0 mg, 90%); mp 158.0–158.4 °C; vmax (UATR) 3378, 3308, 2949, 1635, 1565, 1516, 1459, 1417, 1260, 1156 cm−1; δH (500 MHz, CDCl3) 14.23 (1H, s, OH) 13.90 (1H, s, OH), 8.28 (1H, d, J = 6.9, H-aromatic), 8.25 (1H, d, J = 6.9, H-aromatic), 7.75 (1H, t, J = 5.8, H-aromatic), 7.68 (1H, t, J = 5.8, H-aromatic), 6.05 (1H, s, H-aromatic), 5.57 (1H, br. s, NH), 3.21 (2H, q, J = 6.9, NHCH2CH2), 1.65–1.72 (2H, m, CH2CH2CH2), 1.42–1.50 (2H, m, CH2CH2CH3), 0.98 (3H, t, J 6.9, CH2CH3); δC (125 MHz, CDCl3) 183.5, 178.0, 166.5, 153.6, 147.7, 134.7, 134.0, 132.4, 132.3, 126.5, 126.3, 110.4, 102.9, 100.3, 42.8, 30.7, 20.3, 13.7; m/z (EI) 311 (M+, C18H17NO4 requires 311).
1-(Butylamino)-4-hydroxyanthracene-9,10-dione (1b). Dark purple powder (31.5 mg, 38%); mp 127.2–127.9 °C; vmax (UATR) 3170, 2954, 2926, 2853, 1616, 1580, 1463, 1233, 1162 cm−1; δH (500 MHz, CDCl3) 13.71 (1H, s, OH) 10.29 (1H, br. s, NH), 8.30 (2H, dd, J = 8.0, 16.0, H-aromatic), 7.77 (1H, t, J = 5.7, H-aromatic), 7.70 (1H, t, J = 5.8, H-aromatic), 7.21 (1H, s, H-aromatic), 7.20 (1H, s, H-aromatic), 3.36 (2H, t, J = 6.9, NHCH2CH2), 1.70–1.77 (2H, m, CH2CH2CH2), 1.47–1.55 (2H, m, CH2CH2CH3), 0.99 (3H, t, J = 6.9, CH2CH3); δC (125 MHz, CDCl3) 187.5, 182.0, 156.8, 147.7, 135.5, 134.2, 132.7, 132.5, 129.0, 126.7, 126.4, 124.1, 113.8, 108.4, 42.7, 31.6, 20.4, 13.9; m/z (EI) 311 (M+, C18H17NO3 requires 295).
2-(Butylamino)anthracene-1,4-dione (3a). Yellow powder, (70.1 mg, 60%); mp 173.4–173.9 °C; vmax (UATR) 3325, 2926, 1677, 1573, 1504, 1456, 1406, 1319, 1250 cm−1; δH (500 MHz, CDCl3) 8.50 (1H, s, H-aromatic), 8.48 (1H, s, H-aromatic), 7.93 (2H, t, J = 8.0, H-aromatic), 7.59 (1H, t, J = 5.8, H-aromatic), 7.55 (1H, d, J = 8.1, H-aromatic), 5.99 (1H, br. s, NH), 5.80 (1H, s, CH=C), 3.16 (2H, q, J = 6.9, NHCH2CH2), 1.62–1.69 (2H, m, CH2CH2CH2), 1.37–1.46 (2H, m, CH2CH2CH3), 0.94 (3H, t, J = 6.9, CH2CH3); δC (125 MHz, CDCl3) 182.7, 181.4, 149.1, 135.7, 134.0, 130.2, 130.0, 129.8, 129.6, 129.0, 128.7, 127.7, 127.6, 102.6, 42.4, 30.4, 20.3, 13.8; m/z (EI) 279 (M+, C18H17NO2 requires 279).
2-(Butylamino)-4-methoxyanthracene-9,10-dione (5a). Dark purple powder (12.5 mg, 10%); mp 87.8–88.2 °C; vmax (UATR) 3535, 2936, 1630, 1590, 1506, 1461, 1354, 1250, 1180 cm−1; δH (400 MHz, CDCl3) ) 8.23 (1H, t, J = 2.8, H-aromatic), 8.21 (1H, t, J = 2.8, H-aromatic), 7.70 (2H, t, J = 3.6, H-aromatic), 7.37 (1H, d, J = 9.2, H-aromatic), 7.27 (1H, d, J = 10.1, H-aromatic), 5.29 (1H, br. s, NH), 3.98 (3H, s, OCH3), 3.33 (2H, t, J = 7.3, NHCH2CH2), 1.73–1.82 (2H, m, CH2CH2CH2), 1.46–1.57 (2H, m, CH2CH2CH3), 0.99 (3H, t, J = 7.3, CH2CH3); δC (100 MHz, CDCl3) 185.3, 183.3, 152.7, 145.6, 134.5, 134.0, 133.3, 133.2, 126.8, 126.2, 124.1, 121.7, 121.4, 114.0, 57.6, 44.1, 31.1, 20.4, 13.9; m/z (EI) 309 (M+, C19H19NO3 requires 309).
2,3-(Dibutylamino)anthracene-9,10-dione (5b). Dark blue powder (10.6 mg, 7%); mp 113.0–113.6 °C; vmax (UATR) 3539, 2929, 1669, 1583, 1522, 1364, 1249, 1172 cm−1; δH (500 MHz, CDCl3) 8.29 (2H, dd, J = 5.7, 3.4, H-aromatic), 7.76 (2H, d, J = 5.7, 3.4, H-aromatic), 7.50 (2H, s, H-aromatic), 5.29 (2H, s, NH), 3.36 (4H, t, J = 6.9, NHCH2CH2), 1.79–1.85 (4H, m, CH2CH2CH2), 1.47–1.54 (4H, m, CH2CH2CH3), 0.99 (6H, t, J = 6.9, CH2CH3); δC (500 MHz, CDCl3) 184.5, 141.6, 133.8, 133.7, 126.8, 125.4, 114.7 , 45.9, 30.6, 20.3, 13.8; m/z (EI) 350 (M+, C22H26N2O2 requires 350).
1-(Butylamino)-4-methoxyanthacene-9,10-dione (5c). Dark purple powder (11.4 mg, 10%); mp 96.3–96.9 °C; vmax (UATR) 3536, 2929, 1647, 1591, 1506, 1461, 1356, 1248, 1180 cm−1; δH (500 MHz, CDCl3) 9.84 (1H, s, NH), 8.17 (2H, dd, J = 16.9, 2.3, H-aromatic), 7.60–7.67 (2H, m, H-aromatic), 7.27 (1H, d, J = 9.2, H-aromatic), 7.02 (1H, d, J = 9.2, H-aromatic), 3.92. (3H, s, OCH3), 3.23 (2H, q, J = 6.9, NHCH2CH2), 1.66–1.71 (2H, m, CH2CH2CH2), 1.43–1.51 (2H, m, CH2CH2CH3), 0.96 (3H, t, J = 6.9, CH2CH3); δC (125 MHz, CDCl3) 184.8, 183.4, 151.6, 147.5, 134.4, 134.2, 133.0, 132.8, 126.6, 126.1, 124.3, 121.1, 120.2, 112.3, 57.5, 42.8, 31.4, 20.5, 13.9; m/z (EI) 309 (M+, C19H19NO3 requires 309).
1,4-(Dibutylamino)anthracene-9,10-dione (5d). Dark blue powder (94.5 mg, 73%); mp 113.5–114.2 °C; vmax (UATR) 3747, 2927, 1641, 1570, 1518, 1461, 1364, 1258 cm−1; δH (500 MHz, CDCl3) 10.73 (2H, br. s, NH), 8.28 (2H, dd, J 5.7, 3.4, H-aromatic), 7.62 (2H, dd, J = 5.7, 3.4, H-aromatic), 7.04 (2H, s, H-aromatic), 3.25–3.29 (4H, m, NHCH2CH2), 1.65–1.71 (4H, m, CH2CH2CH2), 1.43–1.51 (4H, m, CH2CH2CH3), 0.96 (6H, t, J = 6.9, CH2CH3); δC (125 MHz, CDCl3) 182.0, 146.2, 134.6, 131.8, 126.0, 123.5, 109.5 , 42.6, 31.8, 20.5, 14.0; m/z (EI) 350 (M+, C22H26N2O2 requires 350).
3.6. Cytotoxic Assays
The cytotoxic assay was carried out according to the method described by Sukari et al. [35]. The MCF-7 (estrogen receptor positive human breast) and Hep-G2 (human hepatocellular liver carcinoma) cancer cells were purchased from ATCC. The cells were grown and maintained in RPMI 1640 media, supplemented with 10% fetal calf serum (FCS) and 1% antibiotic penicillin-streptomycin in an atmosphere of 5% CO2 at 37 °C. The medium was used to dilute the cells to a concentration of 5 × 105 cells/mL. From this cells suspension, 100 μL of various concentrations of the synthesised compounds were pipetted into a 96-well micro titer plate and incubated in 37 °C, 5% CO2 incubator for 72 h. The various concentration used were 100, 50, 25, 12.5, 6.25, 3.125, 1.56 μg/mL. The assay of each concentration of synthesised compounds was performed in triplicate and the control wells of untreated population were also included. After three d, the fraction of surviving cells was determined relative to the untreated cells population by the colorimetric MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) method where the viability of cells was measured by 20 μL of blue formazan crystals of MTT solution (5 mg/mL in phosphate-buffered saline, PBS) added to each well followed by incubation in 37 °C, 5% CO2 incubator for 3–4 h. 100 μL of cells suspension or cells monolayer in each micro titer was removed from each well. The plate was left at room temperature for 30 min before reading the absorbance. The absorbance was read with the multiwell scanning spectrophotometer (ELISA reader) test wavelength of 570 nm and reference wavelength of 630 nm. The cytotoxic index used was IC50 which is the concentration that yield 50% inhibition of the cells compared with untreated control.
3.7. Antimicrobial Assay
The modified disc diffusion method was followed a procedure by Garba and Okeniyi [36]. Nutrient agar (20 g) was suspended in 1 L of distilled water and stirred. It was boiled to dissolve homogenously and autoclaved at 121 °C for 20 min. The agar was allowed to cool to 50 °C and poured into sterile disposable petri dishes. Four microbes, methicillin-resistant Staphylococcus aureaus (MRSA), Pseudomonas aeruginosa, Candida albicans and Escherichia coli were inoculated into prepared nutrient broth and incubated at 37 °C for overnight. The suspension of the microbes in the broth was inoculated on the nutrient agar using sterile cotton bud. The sterile 6 mm paper discs were impregnated with the synthesised compounds in concentrations of 20, 10, 5, 2, 1, 0.5 and 0.1 mg/mL and allowed to soak for 1 min. The paper discs were removed, dried and placed on the surface of agar plates inoculated with the microbial cultures. Each synthesised compound was tested in triplicate. Paper discs impregnated with acetone were used as a negative control. The petri dishes were incubated in inverted position at 37 °C for 24 h. The zones of inhibitions (clear area without bacterial growth) were measured in cm.
4. Conclusions
Aminoantraquinone derivatives were synthesised through methylation, reduction or acylation then followed by amination. In total, seven aminoanthraquinones (compounds 1a, 1b, 3a, and 5–d) were produced, including three new compounds: 2-(butylamino)anthracene-1,4-dione (3a), 2-(butyl- amino)anthracene-9,10-dione (5a) and 2,3-(dibutylamino)anthracene-9,10-dione (5b). All amino-anthraquinones were produced via nucleophilic substitution mechanisms. Aminoanthraquinones 3a, 5a and 5b were found to demonstrate strong cytotoxic activity against both MCF-7 (IC50 1.1, 1.1 and 3.0 µg/mL respectively) and Hep-G2 cancer cell lines (IC50 1.2, 3.0 and 13.0 µg/mL, respectively).
Acknowledgments
We are grateful to FRGS (Vot. No. 5523918) and RUGS (Vot. No. 9342700) for financial support, Department of Chemistry, Faculty of Science, UPM for the research facilities, Zainal for MS spectra and Rusnani for the IR spectra.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ashnagar, A.; Bruce, M.; Dutton, L.; Prince, R.C. One- and two-electron reduction of hydroxy-1,4-naphthoquinones and hydroxy-9,10-anthraquinones: The role of internal hydrogen bonding and its bearing on the redox chemistry of the anthracycline antitumour quinones. BBA-Gen Subjects 1984, 801, 351–359. [Google Scholar] [CrossRef]
- Ge, P.; Russel, R.A. The synthesis of anthraquinone derivatives as potential anticancer agent. Tetrahedron 1997, 53, 17469–17476. [Google Scholar] [CrossRef]
- Hua, D.H.; Lou, K.; Havens, J.; Perchellet, E.M.; Wang, Y.; Perchellet, J.P.; Iwamotoc, T. Synthesis and in vitro antitumor activity of substituted anthracene-1,4-diones. Tetrahedron 2004, 60, 10155–10163. [Google Scholar] [CrossRef]
- Chang, P.; Chen, C. Isolation and characterisation of antitumour anthraquinones from Morinda umbellata. Chin. Pharm. J. (Taipei) 1995, 47, 347–353. [Google Scholar]
- Yadav, J.P.; Arya, V.; Yadav, S.; Panghal, M.; Kumar, S.; Dhankhar, S. Cassia occidentalis L.: A review on its ethnobotany, phytochemical and pharmacological profile. Fitoterapia 2010, 81, 223–230. [Google Scholar] [CrossRef]
- Osman, C.P.; Ismail, N.H.; Ahmad, R.; Ahmat, N.; Awang, K.; Jaafar, F.M. Anthraquinones with antiplasmodial activity from the roots of Rennellia elliptica Korth. (Rubiaceae). Molecules 2010, 15, 7218–7226. [Google Scholar] [CrossRef]
- Sittie, A.A.; Lemmich, E.; Olsen, C.E.; Hviid, L.; Kharazmi, A.; Nkrumah, F.K.; Christensen, S.B. Structure-Activity studies: In vitro antileishmanial and antimalarial activities of anthranones from Morinda lucida. Planta Med. 1999, 65, 259–261. [Google Scholar] [CrossRef]
- Xiang, W.; Song, Q.S.; Zhang, H.J.; Guo, S.P. Antimicrobial anthraquinones from Morinda angustifolia. Fitoterapia 2008, 79, 501–504. [Google Scholar] [CrossRef]
- Rath, G.; Ndonzao, M.; Hostettmann, K. Antifungal anthraquinones from Morinda lucida. Int. J. Pharmacogn. 1995, 33, 107–114. [Google Scholar] [CrossRef]
- Chang, P.; Lee, K.H. Cytotoxic antileukemic anthraquinones from Morinda parvifolia. Phytochemistry 1984, 23, 1733–1736. [Google Scholar] [CrossRef]
- Ismail, N.H.; Ali, A.M.; Aimi, N.; Kitajima, M.; Takayama, H.; Lajis, N.H. Anthraquinone from Morinda ellintica. Phytochem. 1997, 45, 1723–1725. [Google Scholar] [CrossRef]
- Schinazi, R.F.; Chu, C.K.; Babu, J.R.; Oswald, B.J.; Saalmann, V.; Cannon, D.L.; Eriksson, B.F. H.; Nasr, M. Anthraquinones as a new class of antiviral agents against human immunodeficiency virus. Antiviral Res. 1990, 13, 265–272. [Google Scholar] [CrossRef]
- Alves, D.S.; Pérez-Fons, L.; Estepa, A.; Micol, V. Membrane-related effects underlying the biological activity of the anthraquinones emodin and barbaloin. Biochem. Pharmacol. 2004, 68, 549–561. [Google Scholar] [CrossRef]
- Andersen, D.O.; weber, N.D.; Wood, S.G.; Hughes, B.G.; Murray, B.K.; North, J.A. In vitro virucidal activity of selected anthraquinones and anthraquinones derivatives. Antiviral Res. 1991, 16, 185–196. [Google Scholar] [CrossRef]
- Barnard, D.L.; Huffman, J.H.; Morris, J.L.; Wood, S.G.; Hughes, B.G.; Sidwell, R.W. Evaluation of the antiviral activity of anthraquinones, anthrones and anthraquinones derivatives against human cytomegalovirus. Antiviral Res. 1992, 17, 63–77. [Google Scholar]
- Yen, G.C.; Duh, P.D.; Chuang, D.Y. Antioxidant activity of anthraquinones and anthrones. Food Chem. 2000, 70, 437–441. [Google Scholar] [CrossRef]
- Sokolyuk, N.T.; V.V. Romanov, L.P.; Pisulina, L.P. Naphthacenequinones: Synthesis and properties. Russ. Chem. Rev. 1993, 62, 1005–1024. [Google Scholar] [CrossRef]
- Bechtold, T.; Burtscher, E.; Turcanu, A. Anthraquinones as mediators for the indirect cathodic reduction of dispersed organic dyestuffs. J. Electroanal Chem. 1999, 465, 80–87. [Google Scholar] [CrossRef]
- Guo, J.; Zhou, J.; Wang, D.; Tian, C.; Wang, P.; Salah Uddin, M.; Yu, H. Biocatalyst effects of immobilised anthraquinone on the anaerobic reduction of azo dyes by the salt-tolerant bacteria. Water Res. 2007, 41, 426–432. [Google Scholar] [CrossRef]
- Ahn, K.D.; Yoo, K.W.; Soh, J.H.; Kang, J.H. Fluorescent photoimaging with polymers having protected quinizarin dye precursors by a dry process based on chemical amplification. React. Funct. Polym. 2009, 69, 111–116. [Google Scholar] [CrossRef]
- Shchekotikhin, A.E.; Glazunova, V.A.; Dezhenkova, L.G.; Shevtsova, E.K.; Traven, V.F.; Balzarini, J.; Huang, H.S.; Shtil, A.A.; Preobrazhenskaya, M.N. The first series of 4,11-bis[(2-aminoethyl)amino]anthra[2,3-b]furan-5,10-diones: Synthesis and anti-proliferative characteristics. Eur. J. Med. Chem. 2011, 46, 423–428. [Google Scholar] [CrossRef]
- Lee, C.C.; Huang, K.F.; Lin, P.Y.; Huang, F.C.; Chen, C.L.; Chen, T.C.; Guh, J.H.; Lin, J.J.; Huang, H.S. Synthesis, antiproliferative activities and telomerase inhibition evaluation of novel asymmetrical 1,2-disubstituted amidoanthraquinone derivatives. Eur. J. Med. Chem. 2012, 47, 323–336. [Google Scholar] [CrossRef]
- Jin, G.Z.; Song, G.Y.; Zheng, X.G.; Kim, Y.; Sok, D.E.; Ahn, B.Z. 2-(1 -Oxyalkyl)-1,4-dioxy-9,10-anthraquinones: Synthesis and evaluation of antitumor activity. Arch. Pharm. Res. 1998, 21, 198–206. [Google Scholar] [CrossRef]
- Teich, L.; Daub, K.S.; Krugel, V.; Nissler, L.; Gebhardt, R.; and Eger, K. Synthesis and biological evaluation of new derivatives of emodin. Bioorg. Med. Chem. 2004, 12, 5961–5971. [Google Scholar] [CrossRef]
- Shchekotikhin, A.E.; Glazunova, V.A.; Luzikov, Y.N.; Buyanov, V.N.; Susova, O.Y.; Shtil, A.A.; Preobrazhenskaya, M.N. Synthesis and structure-activity relationship studies of 4,11-diaminophtho[2,3-f]indole-5,10-diones. Bioorg. Med. Chem. 2006, 14, 5241–5251. [Google Scholar] [CrossRef]
- Shchekotikhin, A.E.; Shtil, A.A.; Luzikov, Y.N.; Bobrysheva, T.V.; Buyanov, V.N.; Preobrazhenskaya, M.N. 3-Aminomethyl derivatives of 4,11-dihydroxynaphtho[2,3-f]indole-5,10-dione for circumvention of anticancer drug resistance. Bioorg. Med. Chem. 2005, 13, 2285–2291. [Google Scholar] [CrossRef]
- Krapcho, A.P.; Shaw, K.J.; Landi, J.J., Jr.; Phinney, D.J. Synthesis of unsymmetrical 1,4-Bis[(aminoalkyl)amino]anthracene-9,10-diones for Antineoplastic Evaluation. J. Org. Chem. 1984, 49, 5253–5255. [Google Scholar]
- Glänzel, M.; Bültmann, R.; Starke, K.; Frahm, A.W. Structure-activity relationships of novel P2-receptor antagonists structurally related to reactive blue 2. Eur. J. Med. Chem. 2005, 40, 1262–1276. [Google Scholar] [CrossRef]
- Baqi, Y.; Müller, C.E. Efficient and mild deamination procedure for 1-Aminoanthraquinones yielding a diverse library of novel derivatives with potential biological activity. Tetrahedron Lett. 2012, 53, 6739–6742. [Google Scholar] [CrossRef]
- Jin, G.Z.; Jin, H.S.; Jin, L.L. Synthesis and antiproliferative activity of 1,4-Bis(dimethylamino)-9,10-anthraquinone derivatives against P388 mouse leukemic tumor cells. Arch Pharm. Res. 2011, 34, 1071–1076. [Google Scholar] [CrossRef]
- Sugimoto, N.; Kawasaki, Y.; Sato, K.; Aoki, H.; Ichi, T.; Koda, T.; Yamazaki, T.; Maitani, T. Structure of Acid-Stable Carmine. J. Food Hyg. Soc.Jpn. 2002, 43, 18–23. [Google Scholar] [CrossRef]
- Zielske, A.G. (Tosyloxy)anthraquinones: Versatile synthones for the preparation of various aminoanthraquinones. J. Org. Chem. 1987, 52, 1305–1309. [Google Scholar]
- Camara, C.A.; Pinto, A.C.; Rosa, M.A.; Vargas, M.D. Secondary amine and unexpected 1-Aza-anthraquinone from 2-Methoxylapachol. Tetrahedron 2001, 57, 9569–9574. [Google Scholar]
- Saha, K.; Lam, K.W.; Abas, F.; Hamzah, A.S.; Stanslas, J.; Hui, L.S.; Lajis, N.H. Synthesis of damnacanthal, a naturally occurring 9,10-Anthraquinone and its analogues, and its biological evaluation against five cancer cell lines. Med. Chem. Res. 2013, 22, 2093–2104. [Google Scholar] [CrossRef]
- Sukari, M.A.; Tang, S.W.; Neoh, B.K.; Ee, G.C.L.; Rahmani, M. Antileukemic activity and chemical constituents of some Zingiberaceae species. Asian J. Chem. 2010, 22, 7891–7896. [Google Scholar]
- Garba, S.; Okenyi, S.O. Antimicrobial activities of total alkaloids extracted from some nigerian medicinal plants. J. Microbiol. Antimicrob. 2012, 4, 60–63. [Google Scholar]
- Sample Availability: Samples of the compounds 3a and 5d are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).