Abstract
In this work, we report the synthesis and characterization of a novel series of first and second generation Fréchet type dendrons bearing amino-nitro substituted azobenzene units and tetra(ethylene glycol) spacers. These compounds were fully characterized by FTIR, 1H and 13C-NMR spectroscopies, and their molecular weights were determined by MALDI-TOF-MS. The thermal properties of the obtained dendrons were studied by TGA and DSC and their optical properties by absorption spectroscopy in solution and cast film. Molecular calculations were performed in order to determine the optimized geometries of these molecules in different environments. Besides, Langmuir and Langmuir Blodgett films were prepared with the first generation dendrons that were shown to be amphiphilic. Finally, some of the dendrons showed a liquid crystalline behaviour, which was studied by light polarized microscopy as a function of the temperature in order to determine the transition temperatures and the structure of the mesophase.
1. Introduction
Nowadays, dendrimers and dendrons are considered one of the most attractive research fields in polymer chemistry, due to their sophisticated structures and potential applications [1,2,3,4]. These molecules can be modified by introducing functional groups and specific units at different levels of their structure: core, branches or surface [5], giving rise to well-defined and highly functionalized molecules. Depending on the type of functional groups present in dendrimers, various properties have been already studied such as response to light, which has a wide variety of potential applications. Many reviews include the first examples of photo-responsive dendrimers [6,7,8,9] taking into account various examples of azo-dendrimers. The most recent review covering the most important aspects of azobenzene-containing dendrons and dendrimers reported until 2009 has been published by Caminade and Deloncle [10].
In the beginning azobenzenes had been exclusively incorporated as terminal groups of dendrimers and dendrons; the first examples were described by Vögtle and co-workers [11]. The first reported structures were obtained from poly(propyleneimine) (PPI) dendrimers built from either ethylenediamine [12] or 1,4-diaminobutane [1,2,3,4,5,6,7,8,9,10,11,12,13,14] cores. Except for the first example, all the terminal groups were generally azobenzenes [15,16,17,18].
The most popular types of dendrimers: poly(amidoamine) (PAMAM) dendrimers [19] and poly(arylether) dendrimers [20] have been rarely used as supports for azobenzene moieties. The first example of Fréchet-type azo-dendrimers was prepared by grafting through their core poly(arylether) dendrons bearing a single azobenzene group on the surface, leading to original dendrimers [21,22], having azobenzene units as terminal groups. Other types of poly(ether) dendrimers having long alkyl chains and azobenzene groups from generation 0 to generation 3 have been reported in the literature [23].
Unlike dendrimers, dendrons have been infrequently functionalized with azobenzene units on their surface. The first example was synthesized to be used as building block for dendrimers [21,22]. Also, more sophisticated systems such as polyether dendrons linked to a fullerene as core have been prepared [24].
Rau classified azobenzenes into three main groups, based on their photochemical behaviour [25]. Unsubstituted photochromic azobenzenes makes up the first group, known as “azobenzenes”. The thermally stable trans isomer exhibits a strong π-π* transition at 350 nm and a weak n-π* transition at 440 nm, whereas the cis isomer undergoes similar transitions but with a more intense n-π* band. In addition, “azobenzenes” have a relatively poor π-π* and n-π* overlap. The second group, known as “aminoazobenzenes” typically includes azobenzenes that are substituted by an electron-donor group and are characterized by the overlapping of the π-π* and n-π* bands. Finally, azobenzenes bearing both electron-donor and electron-acceptor groups belong to the third category, “pseudostilbenes”, where the π-π* and n-π* bands are practically superimposed and inverted on the energy scale with respect to the “azobenzenes” bands [25].
When donor-acceptor substituted azobenzenes are incorporated into a polymer backbone or side-chain, they provide very versatile materials from the applications point of view. In particular, “pseudostilbene” azobenzenes undergo rapid trans-cis-trans photoisomerization when they are irradiated with linear polarized light. The use of polarized light allows the selective activation of “pseudostilbenes” with polarization axis parallel to the absorbing radiation [26,27,28,29,30,31,32]. Azobenzene molecules are also known to undergo chromic changes through aggregation in various media including solution, spin-cast films and Langmuir-Blodgett layers. Both H-type and J-type aggregates have been observed [33]. On the other hand, azobenzene and poly(ethylene glycol) have been employed in the synthesis of amphiphilic azo-dyes, copolymers [34,35], nanomaterials [36,37], cellulose derivatives [38,39] and cyclodextrin polymers [40,41], sometimes forming supramolecular complexes with interesting properties [42]. Poly(ethylene glycol) segments provide flexibility and water solubility to the systems to which they are incorporated [43,44].
Previously, we have published the synthesis and characterization of four novel azo-dyes bearing terminal hydroxyl groups (RED-PEG series), the preparation of grafted azo-polymer films containing oligo(ethylene glycol) segments (AC-g-PE-RED-PEG series) [45], and the synthesis and characterization of a new series of azo-polymers bearing RED-PEG units in their structure (pnPEGMAN series) [46]. Very recently, we reported the synthesis and characterization of a series of liquid crystalline dyes bearing two amino-nitro-substituted azobenzene units linked by well defined oligo(ethylene glycol) spacers (DIRED-PEG series) [47].
In the last years, our research group has worked on the synthesis and characterization of amphiphilic azo-dyes and azo-polymers bearing oligo(ethylene glycol) segments with different architectures. Herein, we report the incorporation of the RED-PEG dyes into Fréchet type dendrons in order to obtain new liquid crystalline materials bearing azobenzene units. The thermal and optical properties of these dendrons were studied in detail. Some of them exhibited a liquid crystalline behaviour that was studied by DSC and Light Polarized Microscopy as a function of the temperature. Finally, the viability of these compounds to form Langmuir and Langmuir-Blodgett films was also investigated.
2. Results and Discussion
2.1. Synthesis of the Dendrons
Six novel Fréchet type dendrons bearing azobenzene groups have been synthesized using 3,5 dihydroxybenzylic alcohol as building unit, using the classical methodology described in the literature [45,48]. First and second generation dendritic molecules were prepared according to the synthetic sequences illustrated in Scheme 1, Scheme 2, respectively.
First RED-PEG-4 (1) was treated in the presence of iodine and imidazole to give the corresponding alkyl iodide 2. Then 3,5-dihydroxybenzylic alcohol (3) was reacted with 2 using K2CO3 as base and DMF as solvent, in the presence of 18-crown-6 to give the first generation dendron 4G1OH. On the other hand, when 3 was treated with 1-dodecyl bromide (1 eq) under the same reaction conditions it gave the monoalkylated compound 5. This intermediate was reacted with 2 (1 equiv.) in the presence of K2CO3, DMF and 18-crown-6 to give 6G1OH. Similarly, 3 was reacted with 2 equiv. of 1-dodecyl bromide under the same reaction conditions to yield the symmetric compound 7G1OH.
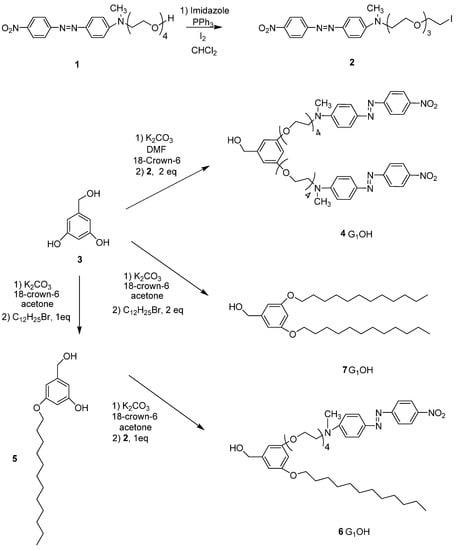
Scheme 1.
Synthesis of the first generation dendritic molecules.
First generation dendron 4G1OH and 6G1OH were treated with iodine and imidazole to give the corresponding halogenated compounds 4G1I and 6G1I. In the case of 7G1OH, this compound was brominated in the presence of CBr4 and triphenylphosphine to give 7G1Br. Once the activated first generation dendrons were obtained, 3 was reacted with 2 eq of 4G1I or 6G1I in the presence of K2CO3 and 18-crown-6 using DMF as solvent, to give the corresponding symmetric second generation dendritic molecules 8G2OH and 9G2OH, respectively. Similarly, 3 was reacted first with 7G1Br and then with 4G1I under the same reaction conditions to give the asymmetric dendron 10G2OH.
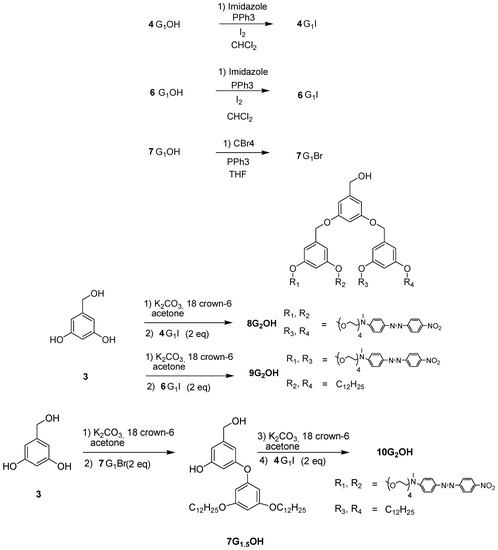
Scheme 2.
Synthesis of the second generation dendritic molecules.
2.2. Characterization of the Dendrons
All dendritic compounds were fully characterized by FTIR, 1H- and 13C-NMR spectroscopies and their molecular weights and purity were confirmed by MALDI-TOF-MS. The spectroscopic characterization of these compounds and that of the intermediates involved in the synthesis is included in the Experimental part, and in this section we explain in detail the assignment of the signals only for 4G1OH and 8G2OH.
The FTIR spectra of the azo-dendrons (not shown) were quite similar and exhibited the signals corresponding to the functional groups present in the molecules. For instance 4G1OH and 8G2OH exhibited a series of signals at 3442 (O-H), 2920, 2852 (C-H), 1594 (C=C), 1514, 1445 (N=N), 1375 (C-H), 1336 (NO2), 1292, 1102, 1067 (C-O of aryl and alkyl ethers), 857 and 826 (=C-H out of plane) cm−1.
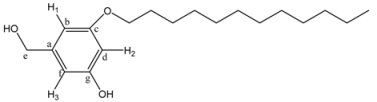
The 1H-NMR spectrum of 4G1OH is shown in Figure 1a (the assignment of the signals is indicated in the experimental part). As we can see, in the aromatic region there are five signals corresponding to the protons present in the azobenzene unit and the phenyl group, which appear at 8.31 ppm (H4), 7.93 ppm (H3), 7.88 ppm (H2), 6.77 ppm (H1), 6.51 ppm (H5) and 6.37 ppm (H6). In the aliphatic region, we can observe five signals: a singlet at 4.59 ppm (CH2-OH), two triplets at 4.06 ppm and 3.82 ppm corresponding to protons OCH2 and NCH2), followed by a multiplet at 3.68 ppm due to the protons of all other OCH2 present in the tetra(ethylene glycol) segments. Finally, a singlet related to the methyl groups NCH3 was observed at 3.06 ppm.
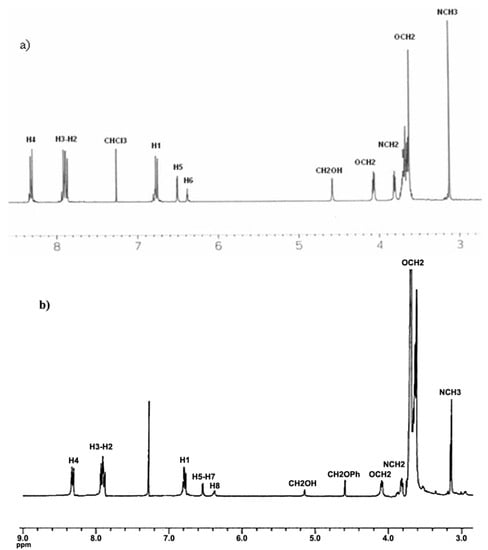
Figure 1.
1H-NMR of: (a) First generation dendron 4G1OH and (b) Second generation dendron 8G2OH.
In the 13C-NMR spectrum of this dendron (not shown) (the assignment of the signals is indicated in the experimental part), we can observe 12 signals in the aromatic region at 160.20, 157.50, 152.7, 147.50, 143.87, 143.54, 126.05, 124.56, 122.52, 111.61, 106.74, 101.10 ppm due to the 12 types of aromatic carbons present in the structure. In the aliphatic zone, we can see a series of signals at 71.07, 71.04, 70.64, 69.75, 68.57 ppm corresponding to all OCH2 carbons present in the tetra(ethylene glycol) segment. Finally, we can observe three more signals at 67.56 (CH2-OH), 52.16 (CH2N) and 39.10 ppm (CH3N). The molecular weight of 4G1OH was confirmed by MALDI-TOF mass spectrometry and the base peak was observed at m/z = 969.5 as expected.
The 1H-NMR spectrum of 8G2OH is shown in Figure 1b (the assignment of the signals is indicated in the experimental part). In the aromatic region, one can see six signals at 8.34 ppm (H4), 7.95 ppm (H3), 7.89 ppm (H2), 6.87 ppm (H1), 6.54 ppm (H5-H7), 6.41 ppm (H8), corresponding to the protons present in the azobenzene units and the phenyl groups of the dendron. In the aliphatic zone, we can observe two singlets at 5.14 ppm (CH2OH) and 4.59 ppm (CH2OPh), followed by two triplets at 4.08 and 3.81 ppm due to protons OCH2 and NCH2, respectively. Finally, a multiplet at 3.66 related to protons OCH2 present in the tetra(ethylene glycol) segment as well as a singlet at 3.14 ppm due to the NCH3 groups, were also seen.
The 13C-NMR spectrum of this dendron (not shown) (see experimental part) exhibits 16 signals in the aromatic zone at 160.15, 160.12, 156.89, 152.63, 147.48, 143.95, 143.46, 143.43, 126.05, 124.89, 122.79, 111.98, 105.98, 105.90, 101.11, 100.02 ppm, due to the 16 types of aromatic carbons present in the structure of the dendron. Moreover, in the aliphatic zone, we can observe different signals at 71.01, 70.99, 69.98, 68.82 and 67.87 ppm, due to carbons (CH2O) present in the tetra(ethylene glycol) segments. Finally, 3 more signals were perceived at 65.98 ppm (CH2OH), 52.18 ppm (CH2N) and 39.98 ppm (CH3N). The molecular weight of 8G2OH was confirmed by MALDI-TOF mass spectrometry and the base peak was clearly observed at m/z = 2042.2, as expected.
2.3. Thermal Properties of the Dendritic Molecules
The thermal properties of the obtained dendritic compounds were studied by Thermogravimetric Analysis (TGA) and Differential Scanning Calorimetry (DSC), and the results are summarized in Table 1. TGA curves of the dendrons are shown in Figure 2. All dendritic compounds exhibited good thermal stability, with T10 values ranging from 123 and 302 °C, and showed drastic degradation between 270 and 470 °C. As we can observe, dendrons 4G1OH and 8G2OH containing exclusively peripheral azobenzene groups are more susceptible towards degradation than those containing also side alkyl chains. We believe that this can be due to the presence of intramolecular H-aggregates resulting from the proximity of the azobenzene units, which make these compounds more succeptible towards degradation. After thermal fragmentation an azobenzene aggregate is more stabilized than a non-paired azobenzene unit. In contrast, the other dendritic compounds showed a higher thermal stability, which slightly decreases as the number of azobenzene units present in their structure augments. That is why first generation dendrons were shownto be thermally more stable than those of second generation. If we make a comparison between second generation dendrons 9G2OH and 10G2OH, it is worth pointing out that the latter, where the azobenzene groups are located in the same branch, showed a lower thermal stability. This fact revealed the formation of H-aggregates, which are more susceptible towards degradation than azobenzene units themselves. The presence of such kind of aggregates was further confirmed by absorption spectroscopy in solid state (vide infra).

Table 1.
Thermal and optical properties of the dendrons.
| Dendrons | T10 (°C) a | Tm (°C) a | λmax (nm) b | Cut off (nm) b | Dipole Moment μ (D) [M062x] | Dipole Moment μ (D) [BPW91] |
|---|---|---|---|---|---|---|
| 6G1OH | 302 | 36 | 478 | 628 | 14.5253 | 13.9576 |
| 4G1OH | 172 | 71 | 482 | 610 | 9.4828 | 9.1611 |
| 9G2OH | 295 | 29 | 480 | 615 | 29.9523 | 29.4126 |
| 8G2OH | 124 | 68 | 480 | 602 | 17.2420 | 15.5955 |
| 10G2OH | 283 | 51 | 476 | 624 | 17.0627 | 15.7889 |
(a) Under nitrogen atmosphere using a heating rate of 10 °C/min; (b) In chloroform solution at room temperature.
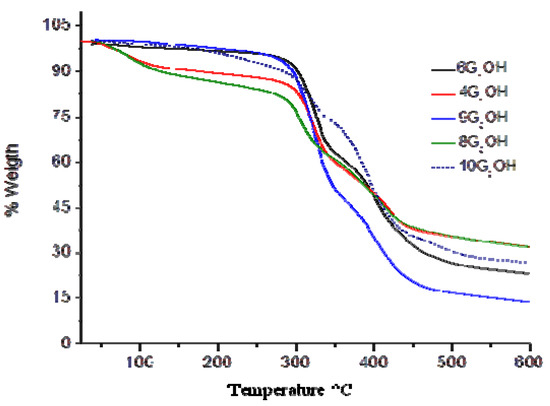
Figure 2.
TGA of the obtained dendritic compounds.
Melting points (Tm) of the dendritic compounds were determined by DSC and the values are included in Table 1. All compounds exhibited Tm values between 29 and 71 °C. In particular 4G1OH (Figure 3), exhibited a well defined mesophase, which clearly indicates the presence of liquid-crystalline domains.
DSC of 6G1OH, 9G2OH, 8G2OH and 10G2OH (not shown) exhibited broad asymmetric endotherms at 36, 30, 68 and 51 °C, respectively. The broadness of the curves indicates the presence of a discrete mesophase, which appears prior to the melting point. This behaviour was confirmed by repeating the DSC experiments after cooling down at a heating rate of 10 °C/min. A similar broadness in the DSC curves was reported in the literature for some dendrons containing azobenzene units, triazole, alkyl chains and poly(ethylene glycol) segments in their structure [63]. In the particular case of 4G1OH (Figure 3), a well defined endotherm can be observed at 71 °C, revealing the presence of mesophase, followed by a second endotherm at 80 °C, due to the melting point of the dendron. In contrast, 9G2OH only showed a symmetric endotherm at 30 °C, which is related to the melting point of this compound. In this particular case, no mesophase was detected because despite the presence of an azobenzene unit, the long oligo(ethylene glycol) spacers jointly with the alkyl chains make the structure of this dendritic compound too flexible to favour the formation of liquid crystalline domains.
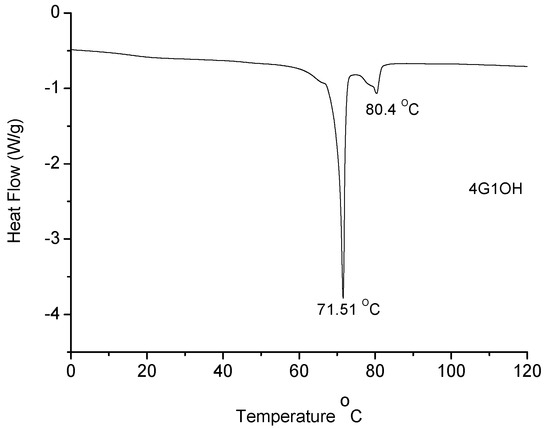
Figure 3.
DSC of dendron 4G1OH.
2.4. Liquid Crystalline Behaviour of the Dendrons
In order to confirm the liquid-crystalline (LC) behaviour of 4G1OH, light polarized optical microscopy studies in function of the temperature were carried out [64,65] with a heating rate of 1 °C/min. Thus, the samples were gradually heated, which allowed us to observe LC behaviour in the range of temperatures of the mesophase. The images along the heating process are illustrated in Figure 4.
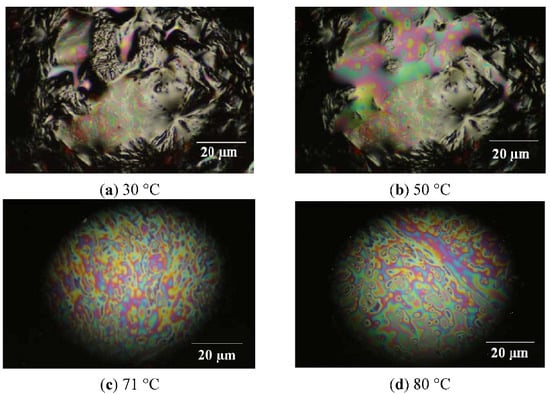
Figure 4.
Liquid crystalline behaviour monitored by Light Polarized Microscopy Images for dendron 4G1OH.
Along gradual heating, the formation of structural arrangements was seen in the range of temperatures of the mesophase. For 4G1OH (Figure 4 picture a, T = 30 °C), we can notice the presence of a crystalline structure. After heating, the dendron starts to soften and exhibits the presence of coloured domains (picture b, T = 50 °C). Furthermore, in the range of temperatures of the mesophase, we can notice the appearance of a LC phase with a well defined structure (picture c, T= 71°C), which persist until melting point was reached at 80 °C (picture d). Beyond this temperature, 4G1OH passes from the LC to the isotropic phase, so that only darkness can be observed (not shown). During the cooling process this dendron adopts again a LC structure and recrystallises again at 40 °C. Experiments performed with light polarized microscopy allowed us to detect the C-LC and CL-I transitions for this compound at 71 °C and 82 °C, respectively.
2.5. Optical Properties of the Dendrons
Absorption spectra of the obtained compounds in chloroform solution are shown in Figure 5 and their optical properties are summarized in Table 1. All these compounds except 7G1OH showed maxima absorption wavelength in the range between λ = 476–482 nm in CHCl3 solution [32,43,44,45,46,47]. Since these compounds contain high dipole moment azobenzene units in their structure, they exhibit the typical photochemical behaviour of azobenzenes belonging to the “pseudostilbenes” category. According to Rau, these compounds exhibit a total overlap of the π-π* and n-π* bands, which are inverted in the energy scale, so that only one band can be observed in their absorption spectra [25,32]. In fact, the higher the generation of the dendron is the more red-shifted the absorption band appears, which is an indication of the high conjugation degree in these molecules. On the other hand, in solid state these dyes behave differently. The absorption spectra of all these compounds in cast film are very similar. The UV-vis spectrum of 4G1OH in cast film is shown in Figure 5b.
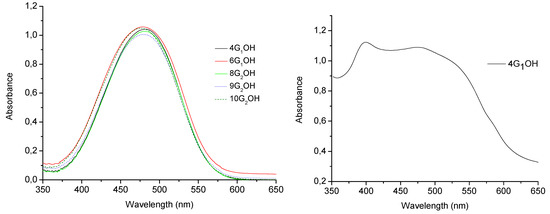
Figure 5.
Absorption spectra of the obtained compounds: in solution (left) and in cast film (right).
As we can see, the absorption spectra of the dendritic molecules in cast film showed a bathochromic shift of the maxima absorption bands. Besides, we can observe the presence of additional blue-shifted bands at ca. λ = 400 nm for both 4G1OH and 6G1OH, which reveals the presence of H-aggregates (Figure 5b, parallel interactions) [33]. A similar behaviour was observed with the second generation dendrons 9G2OH and 10G2OH. In this case, an additional blue shifted band appears at higher wavelength values due to formation of H-aggregates between the azobenzene chromophores [33,64]. Moreover, in the absorption spectra of these compounds in cast film, we can notice the presence of red-shifted absorption tails due to the presence of traces of J-aggregates (Figure 5b, head to tail interactions) [33].
2.6. Theoretical Results
Optimized structures of the dendrons in chloroform solution are shown in Figure 6. As we can see in the case of dendrons bearing more than one azobenzene units, these chromophores stay away from each other, which avoid the formation of aggregates in solution. This results match well with those observed in the absorption spectra of the dendrons in chloroform solution. The dihedral angles (C-C-N-C) are equal to 90 degrees in 4G1OH, 8G1OH, 9G2OH and 10G2OH whereas 6G1OH presents dihedral angles close to 180 degrees. For 7G1OH all the carbon atoms of the aliphatic chains are quasi-linear due to the sp3 hybridization.
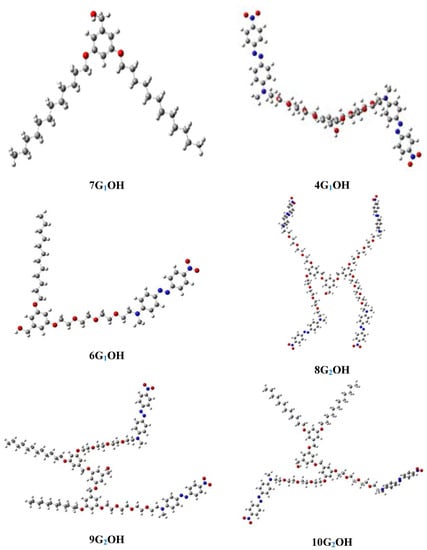
Figure 6.
Optimized geometry of the first and second generation dendritic molecules in solution.
Theoretical UV-Vis spectra of the dendrons were calculated and the predicted λmax values are listed in Table 2. Moreover, available experimental results are included for comparison. As it can be seen, there is a very good agreement between theoretical and experimental results. With these data it is possible to say that the methodology is appropriate and the structures correspond with the experimental ones. Analyzing the values, the most important difference is the change in the λmax of the compounds with and without nitrogen functional groups. The molecule without azobenzene (7G1OH) is colorless and the λmax appears in the UV region. In contrast, azobenzene compounds present a λmax located in the blue region of the visible spectrum. It is important to note that there is no effect of the azobenzene content of the molecule since all the azobenzene units exhibit almost the same λmax value.

Table 2.
UV-vis λmax in nm obtained at M062x/LANL2DZ level in chloroform are reported. Oscillator strengths (f) in parenthesis are included.
| Dendrons | λ (f) | λ (exp) | Error (%) |
|---|---|---|---|
| 7G1OH | 189 (0.83) | - | - |
| 4G1OH | 469 (1.86) | 482 | 3 |
| 6G1OH | 470 (1.40) | 478 | 3 |
| 8G2OH | 469 (3.07) | 482 | 3 |
| 9G2OH | 469 (2.14) | 480 | 2 |
| 10G2OH | 471 (1.48) | 476 | 1 |
Azobenzene dendrons have a small shoulder at lower λ close to the UV region with an absorption lambda similar to the value of the compound without nitrogen functional groups. Compound 6G1OH present the most intense shoulder, while 10G2OH present a weak intensity shoulder. Apparently, this second signal is correlated with the number of azobenzene units, i.e. and the intensity of the shoulder decreases as the number of azobenzene groups increases.
Table 3 reports the results of vertical ionization energy (I), vertical electron affinity (A) and the HOMO-LUMO gap (the energy difference between the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO)). The presence of the azobenzene groups in the dendrons generate a π-conjugated system that decreases slightly the ionization energy and increases dramatically the electron affinity. As a consequence, all azo-dendrons are better electron acceptors than compound 7G1OH. The HOMO-LUMO gap is in agreement with the absorption spectra. Small values of the HOMO-LUMO gap indicate lower excitation energy and correspond to a greater λ values.

Table 3.
Ionization energy (I), electron affinity (A) and the HOMO-LUMO gap in eV.
| Dendrons | 7G1OH | 4G1OH | 6G1OH | 8G2OH | 9G2OH | 10G2OH |
|---|---|---|---|---|---|---|
| I | 6.8 | 6.2 | 6.1 | 6.1 | 6.1 | 6.1 |
| A | 0.2 | 3.4 | 3.4 | 3.4 | 3.4 | 3.4 |
| HOMO-LUMO | 7.9 | 4.1 | 4.1 | 4.1 | 4.1 | 4.1 |
The atomic charge of the azobenzene groups is analyzed and reported in Table 4. There is no difference in the atomic charge between the complexes. In all compounds, the N atoms have practically the same charge. The N atom of the tertiary amine is negative (N1), while one atom of the azo-group is negative (N3) and the other is positive (N2). Finally de N atom of the nitro group is positive (N4).

Table 4.
Atomic charges.
| Atom | Atomic Charge | |
|---|---|---|
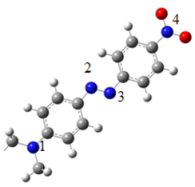 | N1 | −1.2 |
| N2 | 0.7 | |
| N3 | −0.7 | |
| N4 | 1.6 |
2.7. Preparation of Langmuir and Langmuir-Blodgett Films
It has been possible to prepare Langmuir films with some of these compounds and to plot the corresponding isotherms. Langmuir films of compounds 6G1OH and 4G1OH have been characterized by their surface pressure versus molecular area (π/A) isotherms and Brewster angle microscopy (BAM). The π/A isotherms obtained with 4G1OH and 6G1OH are shown in Figure 7a. The two main parameters of these isotherms, final molecular area A0 extrapolated at zero pressure and collapse pressure πc, have been calculated from this graph. The good quality of the obtained films was confirmed by BAM images. In the film obtained with 4G1OH (Figure 7b), when the molecular area reaches A ≈ 188 Å2 (πc ≈ 15 mN/m), a change of slope appears in the surface pressure curve, thereby indicating a greater compressibility of the film. At the same time, defects can be seen in the BAM picture showing that the film is collapsing at this point. In the case of 6G1OH (not shown), the film is not continuous at large molecular areas and shows holes through which water can be seen. These domains smoothly weld together when the molecular area goes below A ≈ 100 Å2, and as long as the film does not enter the collapse regime only defectless surfaces are observed. This clearly indicates the formation of a homogeneous monomolecular layer.
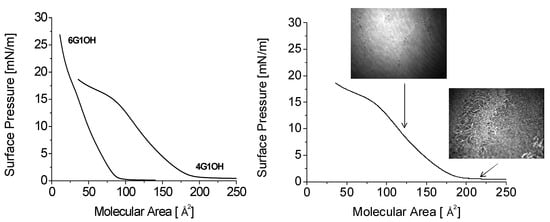
Figure 7.
Isotherms of the Langmuir monolayers of 4G1OH and 6G1OH with BAM images.
Finally Langmuir-Blodgett films were prepared with 4G1OH and 6G1OH by transferring the monolayer on quartz slides. Film transfers were performed at surface pressures close to the collapse point, corresponding to the most condensed phase in the monolayer. The vertical dipping method with a dipping speed of 5 mm/min allowed us to get 2, 10, 14 and 24 multilayer films (Figure 8). The obtained multilayers films exhibited good quality and were analyzed by absorption spectroscopy. Figure 8 shows the UV-vis spectra of 4G1OH in solution and LB film (24 layers). As we can observe the LB film of this dendron shows a maximum absorption wavelength at 468 nm. Moreover, we can perceive a blue shifted shoulder at λ = 425 nm, which reveals the formation of H-aggregates with the azobenzene units after the compression process. The amphiphilicity of 4G1OH and 6G1OH allows the preparation of LB films which can be useful for the future elaboration of NLO devices.
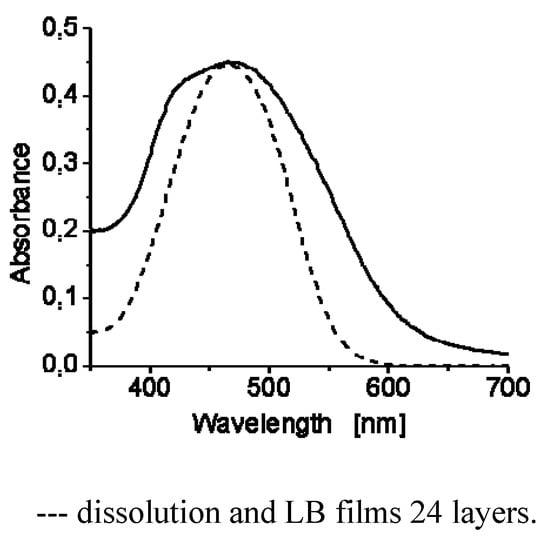
Figure 8.
UV-Visible spectra of 4G1OH.
3. Experimental
3.1. General Conditions
All reagents used in the synthesis of the dendrons were purchased from Aldrich and used as received without further purification. Acetone and dichloromethane were dried by distillation over calcium hydride. Precursor dye RED-PEG-4 was synthesized according to the method previously reported by us [45] and the poly(aryl ether) dendrons were prepared as described in the literature [48].
FTIR spectra of the compounds were carried out on a Spectrum 100 (Perkin Elmer PRECISELY) spectrometer in solid state. 1H and 13C-NMR spectra of these compounds were recorded in CDCl3 solution at room temperature on a Bruker Avance 400 MHz spectrometer, operating at 400 MHz and 100 MHz for 1H and 13C, respectively.
Thermal properties of the obtained azo-dendrons were studied by determining T10 (10% weight loss temperature) and Tm (melting point). Thermogravimetric Analysis (TGA) was conducted on a Hi-Res TGA 2950 Instrument (from 20 to 800 °C) under inert atmosphere and Differential Scanning Calorimetry (DSC) was carried out in a DSC 2910 Instrument (from 20 to 250 °C), in both cases with a heating rate of 10 °C/min.
All dendritic compounds were dissolved in spectral quality solvents purchased from Aldrich, and their absorption spectra were recorded on a Varian Cary 1 Bio UV-vis (model 8452A) spectrophotometer at room temperature, using 1 cm quartz cuvettes. Absorption spectra of these compounds were also recorded in cast films, using the same instrument. Cast films were prepared from a saturated solution of the compounds in CHCl3, which was deposited over a glass substrate with further evaporation of the solvent.
3.2. Synthesis of the Dendrons
Precursor azo-dye RED-PEG-4 (1) was prepared according to the method previously reported by us [45]. The synthesis of first and second generation dendritic molecules is shown in Scheme 1, Scheme 2, respectively.
3-Dodecyloxy-5-hydroxybenzyl alcohol (5). A mixture of 1-bromododecane (1.12 g, 8.02 mmol), 3,5-dihydroxybenzyl alcohol (3, 1 g, 4.01 mmol), K2CO3 (4.43 g, 32 mmol) and 18-crown-6 in 500 mL of acetone was heated to reflux with vigorous stirring for 48 h. The reaction mixture was filtrated and concentrated at reduced pressure. The resulting product was purified by column chromatography using ethyl acetate/hexane 4:6, 5:5, and 6:4 as eluent to yield (5). Yield: 68%. 1H-NMR (CDCl3): δ (ppm) = 6.35 (s, 2H, H1-H3), 6.28 (s, 1H, H2), 4.46 (s, 2H, CH2OH), 3.80 (t, 2H, CH2OPh), 1.69 (m, 2H, CH2CH2OPh), 1.26 (m, 18H, other CH2 of the aliphatic chain), 0.88 (t, 3H, CH3) ppm. 13C-NMR (CDCl3): δ = 160.41 (1C, Cg), 157.13 (1C, Cc), 142.67 (1C, Ca), 106.32 (1C, Cf), 105.49 (1C, Cb), 101.28 (1C, Cd), 68.13 (1C, Ce), 64.91 (1C, CH2OPh), 31.88 (1C, CH2CH2OPh), 29.61, 29.41, 29.32, 29.18, 25.98, 22.64 (9C, other CH2 of the aliphatic chain), 14.05 (1C, CH3) ppm.
(2-{2-[2-(2-Iodo-ethoxy)-ethoxy]-ethoxy}-ethyl)-methyl-[4-(4-nitro-phenylazo)-phenyl]-amine (2). The intermediate 1 was treated with imidazole (0.89 g, 13.1 mmol), triphenylphosphine (3.44 g, 13.1 mmol) and iodine (3.34 g, 13.1 mmol) in anhydrous dichloromethane (50 mL) at room temperature. The resulting solution was stirred for 6 h, filtered and concentrated at reduced pressure. The crude product was purified by column chromatography over silica gel using mixtures of ethyl acetate/hexane 4:6, 5:5 and 6:4 as eluent. Since this intermediate is very unstable it has to be immediately employed in the next reaction. Relative yield: 80% [the formation of this compound was confirmed by FTIR spectroscopy, due to the absence of the band at 3400 cm−1 (OH) and the appearance of a new band at 600 cm−1(C-I)].
[3-Dodecyloxy-5-(2-{2-[2-(2-{methyl-[4-(4-nitro-phenylazo)-phenyl]-amino}-ethoxy)-ethoxy]-ethoxy}-ethoxy)-phenyl]-methanol (6G1OH). A mixture of 5 (0.706 g, 229 mmol), 2 (1.14 g, 210 mmol), K2CO3 (1.58 g, 149 mmol) and 18-crown-6 dissolved in anhydrous DMF (50 mL) was heated at 80 °C with vigorous stirring for 48 h. The reaction mixture was filtered and evaporated at reduced pressure. The crude product was purified by column chromatography in silica gel using mixtures ethyl acetate/hexane 5:5 and 6:4 as eluent, to yield 6G1OH. Yield: 62%. 1H-NMR (CDCl3): δ (ppm) = 8.24 (d, J = 9.02 Hz, 2H, H4), 7.84 (d, J = 9.05 Hz, 2H, H3), 7.81 (d, J = 9.21 Hz, 2H, H2), 6.70 (d, J = 9.23 Hz, 2H, H1), 6.44 (s, 2H, H5), 6.31 (s, 1H, H6), 4.53 (s, 2H, CH2OH), 4.03 (t, 2H, CH2-OPh), 3.84 (t, 2H, CH2N), 3.75 (t, 2H, OCH2R), 3.64-3.57 (m, 12H, all other OCH2), 3.06 (s, 3H, CH3N), 1.68 (m, 2H, OCH2CH2), 1.35 (m, 2H, OCH2CH2CH2), 1.30–1.19 (m, 16H, other CH2 groups of the dodecyl chain), 0.81 (t, 3H, CH3 of the aliphatic chain) ppm. 13C-NMR (CDCl3): δ = 160.63 (1C, Ck), 160.22 (1C, Cm), 156.89 (1C, Ce), 152.67 (1C, Ca), 147.53 (1C, Ch), 143.92 (1C, Cd), 143.37 (1C, Ci), 126.08 (2C, Cc), 124.62 (2C, Cg), 122.59 (2C, Cf), 111.60 (2C, Cb), 105.60 (1C, Cj), 105.26 (1C, Cn), 101.04 (1C, Cl), 70.88, 70.77, 69.80, 68.68, 68.22 (7C, all CH2O), 67.63 (1C, Co), 65.35 (1C, OCH2R), 52.31 (1C, CH2N), 39.26 (1C, CH3N), 31.91, 29.65, 29.62, 29.59, 29.39, 29.31, 26.06, 22.65 (10C, other CH2 of the aliphatic chain) 14.03 (1C, CH3 of the aliphatic chain) ppm. MALDITOF: C40H58N4O8 Calcd: 722.91 Found: (m/z = 722.47).
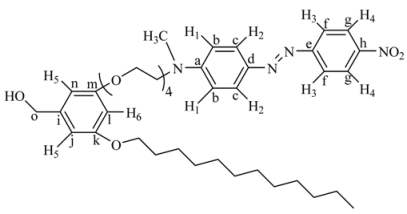
[2-(2-{2-[2-(3-Dodecyloxy-5-iodomethyl-phenoxy)-ethoxy]-ethoxy}-ethoxy)-ethyl]-methyl-[4-(4-nitro-phenylazo)-phenyl]-amine (6G1I). 6G1OH (0.22 g, 0.44 mmol) was treated with imidazole (0.055 g, 0.80 mmol), triphenylphosphine (0.21 g, 0.80 mmol) and iodine (0.20 g, 0.80 mmol) in anhydrous dichloromethane (50 mL) at room temperature. The resulting solution was stirred for 6 h, filtered and concentrated at reduced pressure. The crude product was purified by column chromatography in silica gel using a mixture of ethyl acetate/hexane (5:5 and 6:4) as eluent to give 6G1I. Because of its instability this intermediate was immediately used in the next reaction. Relative yield: 65% (the formation of this compound was confirmed by FTIR spectroscopy).
3,5-Bis-[3-dodecyloxy-5-(2-{2-[2-(2-{methyl-[4-(4-nitro-phenylazo)-phenyl]-amino}-ethoxy)-ethoxy]-ethoxy}-ethoxy)-benzyloxy]-phenyl}-methanol (9G2OH). 3,5-Dihydroxybenzyl alcohol (3, 0.043 g, 0.31 mmol) was reacted with 6G1I (0.13 g, 0.015 mmol), K2CO3 (0.18 g, 1.2 mmol) and a catalytic amount of 18-crown-6 in dry acetone (50 mL). The reaction mixture was heated to reflux for 48 h, cooled to room temperature, filtered and concentrated at reduced pressure. The crude product was purified by column chromatography in silica gel using mixtures of ethyl acetate/hexane 8:2, 9:1 and pure ethyl acetate as eluent, to give 9G2OH. Yield: 45%. 1H-NMR (CDCl3): δ = 8.28 (d, J = 9.10 Hz, 4H, H4), 7.89 (d, J = 9.01 Hz, 4H, H3), 7.87 (d, J = 9.30 Hz, 4H, H2), 6.75 (d, J = 9.30 Hz, 4H, H1), 6.57 (s, 2H, H9), 6.54 (s, 4H, H7-H8), 6.49 (s, 1H, H6), 6.40 (s, 2H, H5), 4.92 (s, 4H, PhOCH2Ph), 4.59 (s, 2H, CH2OH), 4.08 (t, 4H, OCH2), 3.90 (t, 4H, OCH2R), 3.81 (t, 4H, OCH2), 3.70–3.60 (m, 24H, other OCH2 and CH2N), 3.11 (s, 6H, CH3N), 1.74 (m, 4H, OCH2CH2R), 1.41 (m, 4H, OCH2CH2CH2R), 1.33–1.25 (m, 32H, other CH2 of the aliphatic chains), 0.87 (t, 6H, CH3 of the aliphatic chains) ppm. 13C-NMR (CDCl3): δ = 160.51 (2C, Ck), 160.08 (2C, Co), 156.09 (2C, Cq), 152.98 (2C, Ce), 147.29 (2C, Ca), 143.74 (2C, Ch), 143.41 (2C, Cd), 130.12 (2C, Cm), 126.50 (4C, Cg), 124.63 (4C, Cf), 122.34 (4C, Cc), 111.85 (4C, Cb) , 106.03 (4C, Cn-Cr), 105.71 (2C, Cj), 101.31 (2C, Cp), 100.98 (1C, Cl), 70.82 (2C, Cs), 70.73, 70.69, 69.99 (6C, OCH2), 68.57 (1C, Ct), 68.14, 67.63, 67.49, 65.51 (10C, all other OCH2), 52.37 (2C, CH2N), 39.43 (2C, CH3N), 31.88, 30.57, 29.63, 29.59, 29.58, 29.38, 29.30, 29.25, 26.03, 22.64 (22C, CH2 of the aliphatic chain), 14.06 (2C, CH3 of the aliphatic chain) ppm. MALDITOF: C87H120N8O17 Calcd: 1549.93 Found: (m/z = 1549.91).
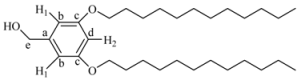
(3,5-Bis-dodecyloxy-phenyl)-methanol (7G1OH). A mixture of 1-bromododecane (1 g, 4.01 mmol), 3,5-dihydroxybenzyl alcohol (3, 0.28 g, 2.0 mmol), K2CO3 (1.10 g, 8.0 mmol) and 18-crown-6 dissolved in 50 mL of acetone was heated to reflux with vigorous stirring for 48 h. The reaction mixture was filtered and concentrated at reduced pressure. The resulting product was purified by flash column chromatography in silica gel using mixtures ethyl acetate/hexane (1:9 and 2:8) as eluent to give 7G1OH. Yield: 75%. 1H-NMR (CDCl3): δ = 6.49 (s, 2H, H1), 6.37 (s, 1H, H2), 4.61 (s, 2H, CH2OH), 3.93 (t, 4H, OCH2), 1.76 (m, 4H, OCH2CH2), 1.26 (s, 36H, other CH2 of the aliphatic chains), 0.88 (t, 6H, CH3) ppm. 13C-NMR (CDCl3): δ = 160.52 (2C, Cc), 143.16 (1C, Ca), 105.02 (2C, Cb), 100.52 (1C, Cd), 68.03 (1C, Ce), 65.44 (2C, OCH2), 63.08 (2C, OCH2CH2), 32.79, 31.90, 29.58, 29.36, 29.25, 26.02, 25.7, 22.87, (18C, all other CH2), 14.09 (2C, CH3) ppm.
1,3-Bis-dodecyloxy-5-iodomethyl-benzene (7G1Br). 7G1OH was treated with carbon tetrabromide (1.33 g, 4 mmol) and triphenylphosphine (1.07 g, 4 mmol) in dry THF (50 mL) at 0 °C and the resulting solution was stirred for 6 h at room temperature. The crude product was purified by column chromatography in silica gel using mixtures acetate/hexane 1:9 and 2:8 as eluent to give 7G1Br. Because of its instability, this intermediate was used in the next step. Relative yield: 60% (the formation of this compound was confirmed by FTIR spectroscopy).
3-(3,5-Bis-dodecyloxy-benzyloxy)-5-hydroxymethyl-phenol (7G1.5OH). 7G1Br (0.235 g, 0.43 mmol), 3,5-dihydroxybenzyl alcohol (3, 0.122 g, 0.87 mmol), K2CO3 (0.602 g, 4.3 mmol) and 18-crown-6 dissolved in 500 mL of acetone were heated to reflux with vigorous stirring for 48 h. The reaction mixture was filtered and concentrated at reduced pressure. The resulting product was purified by flash column chromatography in silica gel using mixtures ethyl acetate/hexane 3:7 and 4:6 as eluent to give 7G1.5OH. Yield: 60%. 1H-NMR (CDCl3): δ = 6.39 (s, 1H, H2), 6.28 (s, 2H, H3), 6.26 (s, 2H, H1), 6.22 (s, 1H, H4), 5.17 (s, 2H, OCH2Ph), 4.70 (s, 2H, CH2OH), 4.35 (s, 4H, PhOCH2R), 3.77 (t, 4H, PhOCH2CH2R), 1.16 (m, 4H, CH2 of the aliphatic chain), 1.28 (m, 4H, CH2 of the aliphatic chain), 1.14 (m, 28H, other CH2 of the aliphatic chain), 0.76 (t, 6H, CH3) ppm. 13C-NMR (CDCl3): δ = 160.43 (1C, Ch), 160.11 (1C, Ci), 157.30 (2C,Cc), 143.03 (1C, Ce), 139.04 (1C, Ca), 106.76 (1C, Cf), 105.81 (1C, Cg), 105.66 (2C, Cb), 101.65 (1C, Cj), 100.79 (1C, Cd), 70.01 (1C, Ck), 68.16 (2C, OCH2R), 64.96 (1C, Cl), 53.43, 31.96, 29.72, 29.68, 29.64, 29.47, 29.39, 29.30, 26.09, 22.72 (20C, other CH2 of the aliphatic chains), 14.14 (2C, CH3) ppm.
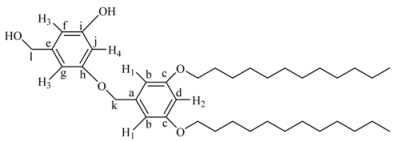
[3,5-Bis-(2-{2-[2-(2-{methyl-[4-(4-nitro-phenylazo)-phenyl]-amino}-ethoxy)-ethoxy]-ethoxy}-ethoxy)-phenyl]-methanol (4G1OH). A mixture of 2 (1.3 g, 2.3 mmol), 3,5-dihydroxybenzyl alcohol (3, 0.11 g, 0.79 mmol), K2CO3 (0.44 g, 3.1 mmol) and 18-crown-6 dissolved in 50 mL of anhydrous DMF was heated at 80 °C with vigorous stirring for 48 h. Then, the reaction mixture was filtered and concentrated under vacuum. The crude product was purified by flash column chromatography in silica gel eluting first with a mixture ethyl acetate/hexane 9:1, and then with ethyl acetate 100% to give the first generation dendron 4G1OH. Yield: 45%. 1H-NMR (CDCl3): δ = 8.31 (d, 4H, J = 9.06 Hz, H4), 7.93 (d, 4H, J = 9.06 Hz, H3), 7.88 (d, 4H, J = 9.32 Hz, H2), 6.77 (d, 4H, J = 9.32 Hz, H1), 6.51 (s, 2H, H5), 6.37 (s, 1H, H6), 4.59 (s, 2H, CH2OH), 4.06 (t, 4H, OCH2), 3.82 (t, 4H, NCH2), 3.68 (m, 24H, all OCH2), 3.06 (s, 6H, CH3N) ppm. 13C RMN (CDCl3): δ = 160.27(2C, Ck), 157.44 (2C, Ce), 152.70 (2C, Ca), 147.55 (2C, Ch), 143.91 (2C, Cd), 143.65 (1C, Ci), 126.05 (4C, Cc), 124.56 (4C, Cg), 122.52 (4C, Cf), 111.61 (4C, Cb), 106.74 (2C, Cj), 101.10 (1C, Cl), 70.90, 70.77, 70.53, 69.74, 68.57 (14C, all OCH2), 67.56 (1C, Cm), 52.19 (2C, CH2N), 39.13 (2C, CH3N) ppm. MALDITOF: C49H60N8O13 Calcd: 969.05 Found: (m/z = 969.5).
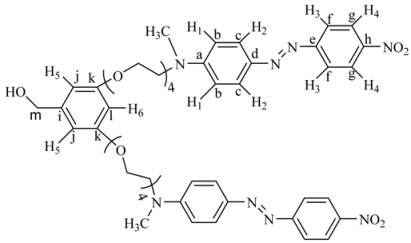
(E)-N,N'-(2,2'-(2,2'-(2,2'-(2,2'-(5-(iodomethyl)-1,3-phenylene)bis(oxy) bis(ethane-2,1-diyl))bis(oxy)bis(ethane-2,1-diyl))bis(oxy)bis(ethane-2,1-diyl))bis(oxy) bis(ethane-2,1-diyl))bis(N-methyl-4-((E)-(4-nitrophenyl)diazenyl)aniline) (4G1I). 4G1OH (0.22 g, 0.44 mmol), imidazole (0.055 g, 0.80 mmol), triphenylphosphine (0.21 g, 0.80 mmol) and iodine (0.20 g, 0.80 mmol) were dissolved in 50 mL of anhydrous dichloromethane at room temperature. The resulting solution was stirred for 6 h, filtered and concentrated at reduced pressure. The crude product was purified by column chromatography in silica gel using a mixture of ethyl acetate/hexane 8:2 as eluent to give 4G1I. Because of its instability this intermediate was immediately used in the next reaction. Relative yield: 60% (the formation of this compound was confirmed by FTIR spectroscopy).
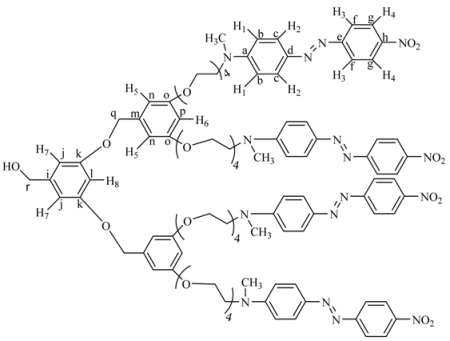
{3-(3,5-Bis-dodecyloxy-benzyloxy)-5-[3,5-bis-(2-{2-[2-(2-{methyl-[4-(4-nitro-phenylazo)-phenyl]-amino}-ethoxy)-ethoxy]-ethoxy}-ethoxy)-benzyloxy]-phenyl}-methanol (10G2OH). 7G1.5OH (0.123 g, 0.02 mmol), 4G1I (0.224 g, 0.022 mmol), K2CO3 (0.11 g, 0.082 mmol) and 18-crown-6 were dissolved in 50 mL of acetone and heated to reflux for 48 h. The reaction mixture was filtered and concentrated at reduced pressure. The crude product was purified by column chromatography in silica gel first eluting with a mixture ethyl acetate/hexane 8:2 and then with ethyl acetate 100%, to yield 10G2OH. Yield: 70%. 1H-NMR (CDCl3): δ = 8.17 (d, J = 8.94 Hz, 4H, H4), 7.77 (d, J = 7.02 Hz, 4H, H3), 7.75 (d, J = 7.08 Hz, 4H, H2), 6.63 (d, J = 9.2 Hz, 4H, H1), 6.44 (s, 4H, H8-H10), 6.41 (s, 2H, H5-H6), 6.37 (s, 1H, H7), 6.27 (s, 2H, H9-H11), 4.80 (s, 4H, PhOCH2Ph), 4.47 (s, 2H, CH2OH), 3.95 (t, 4H, OCH2), 3.79 (t, 4H, OCH2R), 3.68 (t, 4H, NCH2), 3.57–3.49 (m, 24H, OCH2), 2.99 (s, 6H, CH3N), 1.63 (m, 4H, OCH2CH2R), 1.30 (m, 4H, CH2), 1.13 (m, 32H, other CH2 of the aliphatic chains), 0.75 (t, 6H, CH3) ppm. 13C-NMR (CDCl3): δ = 160.52 (2C, Ck-Cm), 160.08 (2C, Cw), 160.01 (2C, Cq), 152.65 (2c, Ce), 147.34 (1C, Ca), 143.68 (2C, Ch), 143.54 (1C, Cs), 139.27 (1C, Co), 138.94 (2C, Cd), 126.22 (4C, Cg), 124.66 (4C, Cc), 122.54 (4C, Cf), 111.59 (4C, Cb), 106.00 (1C, Cj), 105.76 (1C, Cn), 105.67 (2C, Ct), 101.33 (2C, Cp), 101.12 (1C, Cu), 100.76 (1C, Cr), 100.00 (1C, Cl), 70.85 (1C, Cv), 70.74 (1C, Cx), 70.70, 70.12, 69.89, 69.67 (8C, OCH2), 68.59(1C, Cy), 68.10, 67.50, 65.21 (8C, all other OCH2), 52.25 (2C, CH2N), 39.38 (2C, CH3N), 31.93, 29.68, 29.65, 29.42, 29.36, 29.29, 26.08, 22.70 (20C, all other CH2 of the aliphatic chains), 14.13 (2C, CH3 of the aliphatic chains) ppm. MALDITOF: C87H120N8O17 Calcd: 1549.93 Found: (m/z = 1549.91).
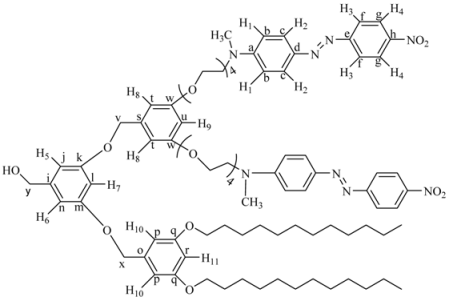
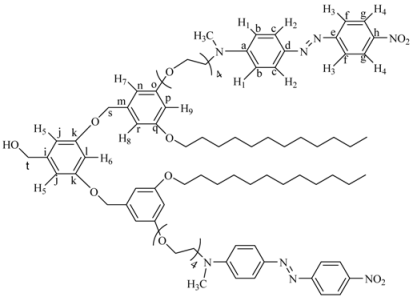
(3,5-bis(3,5-bis(2-(4-((E)-(4-nitropheyl)diazenyl)phenyl)-5,8,11,14,17-pentaoxa-2-azanonadecan-19-yloxy)benzyloxy)phenyl)methanol (8G2OH). 3 (0.0704 g, 0.503 mmol) was reacted in the presence of 4G1I (1.091 g, 1.106 mmol), K2CO3 (0.556 g, 4.02 mmol), DMF (10 mL) and 18-crown-6 as catalyst. The reaction was heated at 100 °C with vigorous stirring for 4 days. The crude product was dried under vacuum and purified by column chromatography in silica gel using chloroform 100% as eluent, increasing gradually the polarity until chloroform: acetone 97:3 to give 8G2OH as a red powder. Yield: 15%. 1H-NMR (CDCl3): δ = 8.34 (d, 8H, J = 8.81 Hz, H4), 7.95 (d, 8H, J = 8.81 Hz, H3), 7.89 (d, 8H, J = 9.32 Hz, H2), 6.87 (d, 8H, J = 9.32 Hz, H1), 6.54 (s, 6H, H5-H7), 6.41 (s, 3H, H6-H8), 5.14 (s, 2H, CH2OH), 4.59 (s, 4H, PhCH2OPh), 4.18 (t, 8H, CH2O), 3.81 (t, 8H, CH2N), 3.64 (m, 48H, OCH2), 3.16 (s, 12H, CH3N) ppm. 13C-NMR (CDCl3): δ = 160.15 (4C, Co), 160.12 (2C, Ck), 156.89 (4C, Ce), 152.63 (4C, Ca), 147.48 (4C, Ch), 143.95 (4C, Cd), 143.46 (1C, Ci), 143.43 (2C, Cm), 126.05 (8C, Cc), 124.89 (8C, Cg), 122.79 (8C, Cf), 111.98 (8C, Cb), 105.98 (2C, Cj), 105.90 (4C, Cn), 101.11 (1C, Cl), 100.02 (2C, Cp), 71.01 (2C, Cq), 70.99 (1C, Cr), 69.98, 68.82, 67.87, 65.98 (28C, all OCH2), 52.18 (4C, CH2N), 39.98 (4C, CH3N) ppm. MALDITOF: Calcd (C105H124N16O27): 2042.20 Found (m/z = 2042.20).
3.3. Computational Details
Density functional theory [49,50,51] as implemented in Gaussian09 [52] was used for all calculations. Full geometry optimizations of the obtained compounds without symmetry constraints and frequency analysis were carried out for all the stationary points, using the BPW91 density functional [53,54,55], and the LANL2DZ basis set [56,57,58]. Local minima were identified by the number of imaginary frequencies (NIMAG = 0). The absorption spectra of the dendritic molecules have been computed with time-dependent density functional theory (TD-DFT) using M062X functional [59] and the same basis sets. Theoretically, the intensity of the bands was expressed in terms of the oscillator strengths (f). Stationary points were first modeled in gas phase (vacuum), and solvent effects were included a posteriori, applying single point calculations at the same level of theory, using a polarizable continuum model, specifically the integral-equation-formalism (IEF-PCM) [60,61,62] with chloroform as solvent, in order to make a comparison with available experimental results. Vertical Ionization Energy (I) was calculated as the difference between the energy of the cation and the neutral molecule, assuming that both of these have the ground-state nuclear configuration of the neutral molecule. Vertical Electron Affinity (A) represents the energy difference between the neutral molecule and the anion, calculated with the ground-state nuclear configuration of the former.
3.4. Preparation of Langmuir and Langmuir-Blodgett Films
Solutions of the dendritic molecules were prepared by dissolving these compounds in chloroform, using concentrations of 1 mg/mL. These solutions were spread on the water surface with a microsyringe, and the film was left to equilibrate for 15–20 min before the compression started. Data were collected with a KSV 5000 system 3 using a Teflon trough and barriers in a dust-free environment, the isotherms were recorded at 20 °C and temperature was controlled to ± 0.1 °C. Ultrapure water (ρ = 18.2 MΩ•cm) obtained from a Milli-DIPAK/ Milli-Q185 ultrapurification system from Millipore was used as subphase. The monolayers were compressed at 5 mm/min. Langmuir-Blodgett films were obtained by transfer on quartz slides. The vertical dipping method with a dipping speed of 5 mm/min was used to obtain 2, 10, 14 and 24 multilayer films. Film transfers were preformed at surface pressures close to the collapse point, corresponding to the most condensed phase in the monolayer. The phase transitions of the spread monolayer were monitored using a Nanofilm Technologie Brewster Angle Microscope (BAM) fitted with a Teflon trough. Along the compression process, some images on the air-solution interface were taken with a CCD camera. The image contrast consisted of 256 gray levels.
4. Conclusions
Two novel series of first and second generation Frechet type dendritic compounds bearing amino-nitro-substituted azobenzene units and tetra(ethylene glycol) spacers were synthesized and characterized. These materials exhibited a good thermal stability with T10 values between 124–302 °C Showing drastical degradation between 270 and 470 °C All compounds bearing pseudostilbene type azobenzene units in CHCl3 solution exhibited maxima absorption wavelengths in the range of λ = 476–482 nm and no aggregation was observed, which is in agreement with the results and the optimized geometries obtained by molecular modelling. However, in cast film these molecules showed an additional absorption band at λ = 400 nm that indicates the formation of H-aggregates. Differential Scanning Calorimetry jointly with Light Polarized Microscopy studies revealed that dendron 4G1OH exhibited a liquid crystalline behaviour, with the formation of a mesophase. Besides, 4G1OH and 6G1OH are able to form Langmuir films and Langmuir Blodgett multilayers due to their amphiphilic character and good compressibility.
Acknowledgments
We are grateful to Miguel Angel Canseco for his assistance with UV-vis spectroscopy and Gerardo Cedillo for his help with 1H and 13C-NMR spectroscopy. We thank Esteban Fregoso for his help with TGA and DSC measurements. We acknowledge José Reyes Gasga and Pedro Mexía Hernández for their assistance recording microscopy images. This project was financially supported by PAPIIT-DGAPA (Projects IN-105610, IN-118808), CONACYT (Project 128788) and resources provided by the Instituto de Investigaciones en Materiales IIM. JOP, ERA and MA acknowledge CONACYT for scholarships. Theoretical work was carried out using a KanBalam supercomputer, provided by DGTIC, UNAM. We also thank DGTIC of Universidad Nacional Autónoma de México for his excellent and free supercomputing services. The authors acknowledge Oralia L Jiménez A. and María Teresa Vázquez for their technical support.
- Sample Availability: Samples of the compounds 4G1OH and 6G1OH are available from the authors.
References
- Fréchet, J.M.J.; Tomalia, D. Dendrimers and Other Dendritric Polymers; Wiley: New York, NY, USA, 2002. [Google Scholar]
- Newkome, G.R.; Vögtle, F.; Moorefield, C.N. Dendrimers and Dendrons; Wiley-VCH: Weinheim, Germany, 2001. [Google Scholar]
- Newkome, G.R.; Shreiner, C.D. Poly(amidoamine), polypropylenimine, and related dendrimers and dendrons possessing different 1/2 branching motifs: An overview of the divergent procedures. Polymer 2008, 49, 1–173. [Google Scholar] [CrossRef]
- Majoral, J.P.; Caminade, A.M. Dendrimers Containing Heteroatoms (Si, P, B, Ge, or Bi). Chem. Rev. 1999, 99, 845–880. [Google Scholar] [CrossRef]
- Vögtle, F.; Richardt, G.; Werner, N. Dendrimer Chemistry; Wiley-VCH: Weinheim, Germany, 2009. [Google Scholar]
- Momotake, A.; Arai, T. Synthesis, excited state properties, and dynamic structural change of photoresponsive dendrimers. Polymer 2004, 45, 5369–5390. [Google Scholar] [CrossRef]
- Momotake, A.; Arai, T. Photochemistry and photophysics of stilbene dendrimers and related compounds. J. Photochem. Photobiol. C. Photochem. Rev. 2004, 5, 1–25. [Google Scholar] [CrossRef]
- Shibaev, V.; Bobrovsky, A.; Boiko, N. Photoactive liquid crystalline polymer systems with light-controllable structure and optical properties. Prog. Polym. Sci. 2003, 28, 729–836. [Google Scholar] [CrossRef]
- Villavicencio, O.; McGrath, D.V. Advances in dendritic. Macromolecules 2002, 5, 1–44. [Google Scholar]
- Deloncle, R.; Caminade, A.M. Stimuli-responsive dendritic structures: The case of light-driven azobenzene-containing dendrimers and dendrons. J. Photochem. Photobiol. C. Photochem. Rev. 2010, 11, 25–45. [Google Scholar] [CrossRef]
- Mekelburger, H.B.; Rissanen, K.; Vögtle, F. Repetitive-Synthesis of Bulky Dendrimers A reversibly Photoactive Dendrimers with six Azobenzene Side Chains. Chem. Ber. 1993, 126, 1161–1169. [Google Scholar] [CrossRef]
- Buhleier, E.W.; Wehner, W.; Vögtle, F. Cascade and Nonskid-Chain-like Syntheses of molecular Cavity Topologies. Synthesis 1978, 2, 155–158. [Google Scholar]
- Wörner, C.; Mülhaupt, R. Polynitrile and polyamine funtional poly(trimethylene imine) dendrimers. Angew. Chem. Int. Ed. Engl. 1993, 32, 1306–1308. [Google Scholar] [CrossRef]
- De Brabander-van den Berg, E.M.M.; Meijer, E.W. Poly(propylene imine) dendrimers: Large-Scale synthesis by Hetereogeneously catalyzed Hydrogenations. Angew. Chem. Int. Ed. Engl. 1993, 32, 1308–1311. [Google Scholar] [CrossRef]
- Archut, A.; Vögtle, F.; De Cola, L.; Azzellini, G.C.; Balzani, V.; Ramanujam, P.S.; Berg, R.H. Azobenzene-Functionalized Cascade Molecules: Photoswitchable Supramolecular Systems. Chem. Eur. J. 1998, 4, 699–706. [Google Scholar] [CrossRef]
- Dirksen, A.; Zuidema, E.; Williams, R.M.; De Cola, L.; Kauffmann, C.; Vögtle, F.; Roque, A.; Pina, F. Photoactivity and pH Sensitivity of Methyl Orange Functionalized Poly(Propyleneamine) Dendrimers. Macromolecules 2002, 35, 2743–2747. [Google Scholar] [CrossRef]
- Schenning, A.P.; Elissen-Roman, C.; Weener, J.W.; Baars, M.W.; Van der Gaast, S.J.; Meijer, E.W. Amphiphilic Dendrimers as Building Blocks in Supramolecular Assemblies. J. Am. Chem. Soc. 1998, 120, 8199–8208. [Google Scholar] [CrossRef]
- Alcala, R.; Gimenez, R.; Oriol, L.; Pinol, M.; Serrano, J.L.; Villacampa, B.; Vinuales, A.I. Synthesis, Characterization, and Induction of Stable Anisotropy inLiquid Crystalline Photo-addressable PPI Dendrimers. Chem. Mater. 2007, 19, 235–246. [Google Scholar] [CrossRef]
- Tomalia, D.A.; Baker, H.; Dewald, J.; Hall, M.; Kallos, G.; Martin, S.; Roeck, J.; Ryder, J.; Smith, P. A New Class of Polymers: Starburts-Dendritic Macromolecules. Polym. J. 1985, 17, 117–132. [Google Scholar] [CrossRef]
- Hawker, C.J.; Fréchet, J.M.J. Preparation of Polymers with Controlled Molecular Architecture. A New Convergent Approach to Dendritic Macromolecules. J. Am. Chem. Soc. 1990, 112, 7638–7647. [Google Scholar] [CrossRef]
- Li, S.; McGrath, D.V. Macromolecular isomers of azobenzene-containing photochromic dendrimers. Polym. Prep. 2000, 41, 861–864. [Google Scholar]
- Li, S.; McGrath, D.V. Effect of Macromolecular Isomerism on the Photomodulation of Dendrimer Properties. J. Am. Chem. Soc. 2000, 122, 6795–6796. [Google Scholar] [CrossRef]
- Nithyanandhan, J.; Jayaraman, N.; Davis, R.; Das, S. Synthesis, Fluorescence and Photoisomerization Studies of Azobenzene-Functionalized Poly(alkyl aryl ether) Dendrimers. Chem. Eur. J. 2004, 10, 689–698. [Google Scholar] [CrossRef]
- Kay, K.Y.; Han, K.J.; Yu, Y.J.; Park, Y.D. Dendritic fullerenes (C60) with photoresponsive azobenzene groups. Tetrahedron Lett. 2002, 43, 5053–5056. [Google Scholar] [CrossRef]
- Rau, H. Photochemistry and Photophysics; Rabek, J.K., Ed.; CRC Press: Boca Raton, FL, USA, 1990; Volume 2, p. 119. [Google Scholar]
- Natansohn, A.; Rochon, P. Macromolecular Science and Engineering Award lecture The Versatility of azobenzene Polymers. Can. J. Chem. 2001, 79, 1093–1100. [Google Scholar]
- Todorov, T.; Nikalova, L.; Tomova, N. Polarization Holography. 1: A new high-efficiency organic material with reversible photoinduced birefringence. Appl. Opt. 1984, 23, 4309–4312. [Google Scholar] [CrossRef]
- Xie, S.; Natansohn, A.; Rochon, P. Recent Developments in Aromatic Azo Polymers Research. Chem. Mater. 1993, 5, 403–411. [Google Scholar] [CrossRef]
- Viswanathan, N.K.; Kim, D.Y.; Bian, S.; Williams, J.; Liu, W.; Li, L.; Samuelson, L.; Kumar, J.; Tripathy, S.K. Surface relief structures on azo polymer films. J. Mater. Chem. 1999, 9, 1941–1955. [Google Scholar] [CrossRef]
- Ichimura, K. Photoalignment of Liquid-Crystal Systems. Chem. Rev. 2000, 100, 1847–1874. [Google Scholar]
- Delaire, J.A.; Nakatani, K. Linear and Nonlinear Optical Properties of Photochromic Molecules and Materials. Chem. Rev. 2000, 100, 1817–1846. [Google Scholar] [CrossRef]
- Natansohn, A.; Rochon, P. Photoinduced Motions in Azo Containing Polymers. Chem. Rev. 2002, 102, 4139–4175. [Google Scholar] [CrossRef]
- Kasha, M. Energy Transfer Mechanisms and the Molecular Exciton Model for Molecular Aggregates. Radiat. Res. 1963, 20, 55–71. [Google Scholar] [CrossRef]
- He, X.; Zhang, H.L.; Yan, D.L.; Wang, X. Synthesis of Side-Chain Liquid-Crystalline Homopolymers and Triblock Copolymers with p-Methoxyazobenzene Moieties and Poly(ethylene glycol) as Coil Segments by Atom Transfer Radical Polymerization and Their Thermotropic Phase Behavior. J. Polym. Sci. Part A Polym. Chem. 2003, 41, 2854–2864. [Google Scholar] [CrossRef]
- Tian, Y.Q.; Watanabe, K.; Kong, X.X.; Abe, J.; Iyoda, T. Synthesis, Nanostructures, and Functionality of Amphiphilic Liquid Crystalline Block Copolymers with Azobenzene Moieties. Macromolecules 2002, 35, 3739–3747. [Google Scholar] [CrossRef]
- Saito, M.; Shimomura, T.; Okumura, Y.; Ito, K.; Hayakawa, R. Temperature dependence of inclusion-dissociation behavior between molecular nanotubes and linear polymers. J. Chem. Phys. 2001, 114, 1–3. [Google Scholar] [CrossRef]
- Shimomura, T.; Funaki, T.; Ito, K. Circular Dichroism Study of the Inclusion-Dissociation Behavior of Complexes between a Molecular Nanotube and Azobenzene Substituted Linear Polymers. J. Incl. Phenom. Macroc. Chem. 2002, 44, 275–278. [Google Scholar] [CrossRef]
- Zheng, P.J.; Wang, C.; Hu, X.; Tam, K.C.; Li, L. Supramolecular Complexes of Azocellulose and R-Cyclodextrin: Isothermal Titration Calorimetric and Spectroscopic Studies. Macromolecules 2005, 38, 2859–2864. [Google Scholar] [CrossRef]
- Hu, X.; Zheng, P.J.; Zhao, X.Y.; Li, L.; Tam, K.C.; Gan, L.H. Preparation, characterization and novel photoregulated rheological properties of azobenzene functionalized cellulose derivatives and their α-CD complexes. Polymer 2004, 45, 6219–6225. [Google Scholar] [CrossRef]
- Takashima, Y.; Nakayama, T.; Miyauchi, M.; Kawaguchi, Y. Complex Formation and Gelation between Copolymers Containing Pendant Azobenzene Groups and Cyclodextrin Polymers. Chem. Lett. 2004, 33, 890–891. [Google Scholar] [CrossRef]
- Ikeda, T.; Ooya, T.; Yui, N. Regulation of Pseudo-Polyrotaxane Formation between α-Cyclodetrins and Azobenzene-terminated poly(ethylene glycol). Polym. J. 1999, 31, 658–663. [Google Scholar] [CrossRef]
- Tung, C.H.; Wu, L.Z.; Zhang, L.P.; Chen, B. Supramolecular Systems as Microreactors: Control of Product Selectivity in Organic Phototransformation. Acc. Chem. Res. 2003, 36, 39–47. [Google Scholar] [CrossRef]
- Rivera, E.; Belletête, M.; Natansohn, A.; Durocher, G. Synthesis characterization and optical properties of a novel azo-dye bearing an oligo(ethylene glycol) methyl ether side chain in solution and in the solid state. Can. J. Chem. 2003, 81, 1076–1082. [Google Scholar] [CrossRef]
- Rivera, E.; Carreón-Castro, M.P.; Buendía, I.; Cedillo, G. Optical properties and aggregation of novel azo-dyes bearing an end-capped oligo(ethylene glycol) side chain in solution, solid state and LangmuireBlodgett films. Dyes Pigm. 2006, 68, 217–226. [Google Scholar] [CrossRef]
- Rivera, E.; Carreón-Castro, M.P.; Salazar, R.; Huerta, G.; Becerril, C.; Rivera, L. Preparation and characterization of novel grafted polyethylene based azo-polymers bearing oligo(ethylene glycol) spacers. Polymer 2007, 48, 3420–3428. [Google Scholar] [CrossRef]
- García, T.; Carreón-Castro, M.P.; Gelover-Santiago, A.; Ponce, P.; Romero, M.; Rivera, E. Synthesis and Characterization of Novel Amphiphilic Azo-Polymers Bearing Well-Defined Oligo(Ethylene Glycol) Spacers. Des. Monomers Polym. 2012, 15, 159–174. [Google Scholar] [CrossRef]
- Caicedo, C.; Rivera, E.; Valdez-Hernández, Y.; Carreón-Castro, M.P. Synthesis and characterization of novel liquid-crystalline azo-dyes bearing two amino-nitro substituted azobenzene units and a well-defined, oligo(ethylene glycol) spacer. Mater. Chem. Phys. 2011, 130, 471–480. [Google Scholar] [CrossRef]
- Alvarez-Venicio, V.; Jiménez-Nava, B.; Carreón-Castro, M.P.; Rivera, E.; Méndez, I.A.; Huerta, A.A.; Gutiérrez-Nava, M. Synthesis and incorporation in Langmuir films of oligophenylenevinylene dendrimers bearing a polar head group and different dendritic poly(benzyl ether) branches. Polymer 2008, 49, 3911–3922. [Google Scholar] [CrossRef]
- Kohn, W.; Becke, A.D.; Parr, R.G. Density Functional Theory of Electronic Structure. J. Phys. Chem. 1996, 100, 12974–12980. [Google Scholar] [CrossRef]
- Hohenberg, P.; Kohn, W. homogeneous electron gas. Phys. Rev. 1964, 136, B864–B871. [Google Scholar] [CrossRef]
- Kohn, W.; Sham, L. self-consistent equations including exchange and correlations effects. Phys. Rev. 1965, 140, A1133–A1138. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision A.02. Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef]
- Perdew, J.P. Electronic Structure of Solids; Ziesche, P., Eschrig, H., Eds.; Akademie Verlag: Berlin, Germany, 1991; p. 11. [Google Scholar]
- Perdew, J.P.; Chevary, J.A.; Vosko, S.H.; Jackson, K.A.; Pederson, M.R.; Singh, D.J.; Fiolhais, C. Atoms, molecules, solids, and surfaces: Applications of the generalized gradient approximation for exchange and correlation. Phys. Rev. B 1992, 46, 6671–6687. [Google Scholar]
- Hay, P.J.; Wadt, W.R. Ab Initio effective core potentials for molecular calculations. Potentials for the transition metal atoms Sc to Hg. J. Chem. Phys. 1985, 82, 270–283. [Google Scholar] [CrossRef]
- Hay, P.J.; Wadt, W.R. Ab initio effective core potentials for molecular calculations. Potentials for K to Au including the outermost core orbitals. J. Chem. Phys. 1985, 82, 299–310. [Google Scholar]
- Wadt, W.R.; Hay, P.J. Ab initio effective core potentials for molecular calculations. Potentials for main group elements Na to Bi. J. Chem. Phys. 1985, 82, 284–298. [Google Scholar] [CrossRef]
- Dunning, T.H.; Hay, P.J. Modern Theoretical Chemistry; Schaefer, H.F., III, Ed.; Plenum: New York, NY, USA, 1976; pp. 1–28. [Google Scholar]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar] [CrossRef]
- Cances, M.T.; Mennucci, B.; Tomasi, J. A new integral equation formalism for the polarizable continuum model: Theoretical background and applications to isotropic and anisotropic Dielectrics. J. Chem. Phys. 1997, 107, 3032–3037. [Google Scholar] [CrossRef]
- Mennucci, B.; Tomasi, J. Continuum solvation models: A new approach to the problem of solute’s charge distribution and cavity boundaries. J. Chem. Phys. 1997, 106, 5151–5158. [Google Scholar] [CrossRef]
- Del Barrio, J.; Oriol, L.; Alcalá, R.; Sánchez, C. Azobenzene-Containing Linear-Dendritic Diblock Copolymers by Click Chemistry: Synthesis, Characterization, Morphological Study, and Photoinduction of Optical Anisotropy. Macromolecules 2009, 42, 5752–5760. [Google Scholar] [CrossRef]
- Iftime, G.; Lagugné-Labarthet, F.; Natansohn, A.; Rochon, P. Control of Chirality of an Azobenzene Liquid Crystalline Polymer with Circularly Polarized Light. J. Am. Chem. Soc. 2000, 122, 12646–12650. [Google Scholar] [CrossRef]
- Lagugne-labarthet, F.; Freiberg, S.; Pellerin, C.; Pezolet, M.; Natansohn, A.; Rochon, P. Spectroscopic and Optical Characterization of a Series of Azobenzene-Containing Side-Chain Liquid Crystalline Polymers. Macromolecules 2000, 33, 6815–6823. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).