Abstract
A new ursane-type triterpenoid, 3β-hydroxy-urs-30-p-Z-hydroxycinnamoyloxy-12-en-28-oic-acid (1), together with three known triterpenoids, 3β-hydroxy-urs-30-p-E-hydroxycinnamoyloxy-12-en-28-oic-acid (2), 2α,3β,19α-trihydroxy-urs-12-en-28-oic-acid (3), and ursolic acid (4), four known lignans, pinoresinol (5), 9α-hydroxypinoresinol (6), (+)-medioresinol (7), and (+)-kobusin (8), and two steroids, β-sitosterol (9), and daucosterol (10), were isolated from the whole parts of Teucrium viscidum. Their structures were established by a combination of spectroscopic data analysis, besides comparison with literature data. Compounds 1–4 were evaluated for their inhibitory activities against 11β-hydroxysteroid dehydrogenase 1 (11β-HSD1).
1. Introduction
Teucrium viscidum Blume (Lamiaceae), known as Shan-Huo-Xiang in China, is an annual herb, whose whole parts are used as a traditional Chinese medicine to treat hemoptysis, hematemesis, pulmonary abscesses, traumatic injuries, and bites of rabies-stricken dogs or venomous snakes [1]. Previous phytochemical studies on T. viscidum have resulted in the isolation of several neoclerodane diterpenoids [2,3,4,5]. In the course of search for novel natural products from traditional Chinese medicines, a new triterpenoid 1, together with nine known compounds 2–10, were isolated from the whole parts of T. viscidum. In this paper, we report the isolation and structural elucidation of compounds 1–10 (Figure 1), as well as the inhibitory activities against 11β-HSD1 of compounds 1–4.
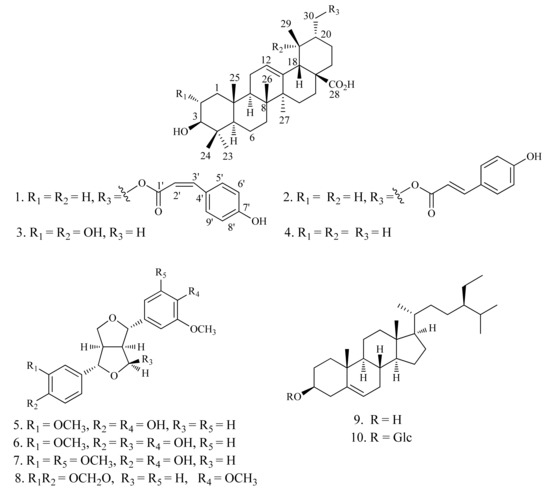
Figure 1.
Structures of compounds 1–10.
2. Results and Discussion
The acetone extract of the air-dried whole parts of T. viscidum was suspended in water and successively partitioned with petroleum ether, CHCl3, and EtOAc. The CHCl3 fraction was subjected to column chromatography to afford one new triterpenoid 1, along with nine known compounds, 3β-hydroxy-urs-30-p-E-hydroxycinnamoyloxy-12-en-28-oic-acid (2) [6], 2α,3β,19α-trihydroxy-urs-12-en-28-oic-acid (3) [7], ursolic acid (4) [7], pinoresinol (5) [8], 9α-hydroxypinoresinol (6) [9], (+)-medioresinol (7) [10], (+)-kobusin (8) [11], β-sitosterol (9) [12], and daucosterol (10) [12]. The known compounds were identified on the basis of NMR spectroscopic analyses and comparison with those reported in the literature (Figure 1).
Compound 1 was obtained as an amorphous powder. Its molecular formula, C39H54O6, could be deduced from the (+)-HRESIMS peak at m/z 641.3799 [M+Na]+ (calcd. for C39H54O6Na+, 641.3818). The IR spectrum showed absorption bands for hydroxyl (3729 cm–1), carbonyl (1685 cm–1) and benzene ring (1602 cm–1) functions. The 1H-NMR spectrum of 1 (Table 1) showed five tertiary methyl singlets at δH 0.92 (3H, s), 1.05 (3H, s), 1.08 (3H, s), 1.23 (3H, s), and 1.26 (3H, s), one secondary methyl doublet protons at δH 1.09 (3H, d, J = 6.0 Hz), three protons attached to oxygenated carbons at δH 4.50 (1H, dd, J = 3.2 Hz, 11.2 Hz), 4.27 (1H, dd, J = 7.2 Hz, 11.2 Hz), 3.48 (1H, dd, J = 6.8 Hz, 9.2 Hz), three olefinic protons at δH 7.03 (1H, d, J = 12.8 Hz), 6.09 (1H, d, J = 12.8 Hz), 5.52 (1H, t, J = 3.2 Hz), and a p-disubstituted benzene ring at δH 8.09 (d, J = 8.4 Hz, 2H) and 7.22 (d, J = 8.4 Hz, 2H). The 13C-NMR, DEPT, and HSQC spectra for 1 exhibited thirty nine carbon signals differentiated as six methyls, ten methylenes (including an oxygenated), thirteen methines (including seven olefins and an oxygenated), and ten quaternary carbons (including two carbonyls, an olefin, and an oxygenated).

Table 1.
1H-NMR (400 MHz) and 13C-NMR (100 MHz) Spectral Data of Compound 1 in C5D5N (δ in ppm, J in Hz).
The NMR data of 1 were quite similar to those of 3β-hydroxy-urs-12-en-28-oic-acid (3), except for signals for a nine-carbon chain [7,13] which was composed of one carbonyl (δC 167.5), one double bond (δH 6.09, d, J = 12.8 Hz, H-2'; δH 7.03, d, J = 12.8 Hz, H-3'; δC 116.4, C-2'; δC 144.5, C-3') and p-substituted phenol (δH 8.09, d, J = 8.4 Hz, H-5', H-9'; δH 7.22, d, J = 8.4 Hz, H-6', H-8'; δC 134.0, C-5', C-9'; δC 116.5, C-6', C-8', δC 161.7, C-7'). In the HMBC spectrum, the cross peaks of these two newly olefinic protons H-2' and H-3' to the conjugated carbonyl C-1' (δC 167.5) and the aromatic carbon C-4' (δC 127.1) of the p-substituted phenol, as well as the aromatic protons H-5'/H-9' of the p-substituted phenol to the newly olefinic carbon C-3', revealed that this double bond was fixed between the carbonyl and the p-substituted phenol, suggested the presence of a p-hydroxycinnamoyl group in 1. The coupling constant JH-2',H-3' = 12.4 Hz of H-2' and H-3', and the NOESY correlation between H-2' and H-3', indicated the Z-geometry of the double bond in the p-hydroxycinnamoyl group. The HMBC correlation of H-30 (δH 4.50, dd, J = 3.2 Hz, 11.2 Hz, H-30a; δH 4.27, dd, J = 7.2 Hz, 11.2 Hz, H-30b) to the carbonyl C-1' of the p-Z-hydroxycinnamoyl group suggested the p-Z-hydroxycinnamoyl group was connected to 30-OH. HSQC, 1H–1H COSY, HMBC, and NOESY analysis (Figure 2) allowed us to assign compound 1 as 3β-hydroxy-urs-30-p-Z-hydroxycinnamoyloxy-12-en-28-oic-acid.
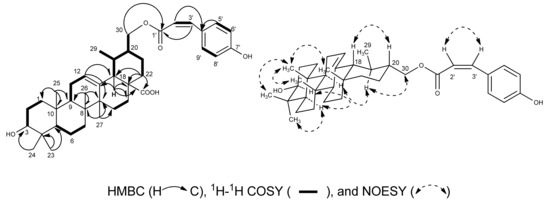
Figure 2.
1H-1H COSY, Key HMBC, and NOESY correlations of compound 1.
The 1 type of 11β-hydroxysteroid dehydrogenase (11β-HSD 1) has been recognized to be an attractive target in metabolic disease or type 2 diabetes, and some distinct 11β-HSD 1 inhibitors have entered clinic trials [14]. Since pentacyclic triterpenoids were reported to be potential inhibitors of 11β-HSD 1 [15], compounds 1–4 were tested for their inhibitory activities against human 11β-HSD 1 in vitro. Ursolic acid (4) showed stronger inhibitory activity on 11β-HSD1, with IC50 values of 1.5 μM, which was in accordance with the reported value [15]. Compound 3 displayed exceedingly weak inhibitory activity on 11β-HSD1 by comparison of the other triterpenoids in our assay. Compounds 1 and 2 did not show obvious activity against 11β-HSD 1, which suggested that the variation of CH3-30 could reduce 11β-HSD 1 inhibitory activity. Structure-activity relationships of pentacyclic triterpenoid inhibitors of 11β-HSD 1 showed that the hydroxyl group at position C-19 could decrease 11β-HSD 1 inhibitory activity [15]. Our bioassay not only confirmed this conclusion, but also indicated that the variation of CH3-30 could also reduce the 11β-HSD 1 inhibitory activity. These results significantly extend our knowledge of the structure-activity relationship of ursolic acid derivatives as 11β-HSD 1 inhibitors.
3. Experimental
3.1. General Procedures
Optical rotations were measured on a Perkin Elmer PE-341LC polarimeter. IR spectra were recorded as KBr disks on a Bruker Vertex 70 FT-IR spectrophotometer. HRESI-MS data were measured on an API QSTAR Pulsar spectrometer. NMR spectra were recorded using a Bruker AM-400 spectrometer, and the 1H-NMR and 13C-NMR chemical shifts were referenced to the solvent peaks for C5D5N at δH 8.74, 7.58, 7.22, and δC 150.35, 135.91, 123.87. Silica gel (200–300 mesh, Qingdao Marine Chemical Inc., Qingdao, China), Amberchrom CG161M (75 μm, Rohm and Haas Company, Philadelphia, PA, USA), ODS (50 μm, YMC, Kyoto, Japan), and Sephadex LH-20 (Pharmacia Biotech AB, Stockholm, Sweden) were used for column chromatography. HPLC separation was performed on an instrument consisting of an Agilent 1100 controller, an Agilent 1100 pump, and an Agilent UV detector with an YMC (250 × 10 mm, 5 μm) preparative column. TLC was carried out on precoated silica gel GF254 plates. Spots were visualized under UV light (254 or 356 nm) or by spraying with 5% H2SO4 in 95% EtOH followed by heating.
3.2. Plant Material
The whole parts of T. viscidum were collected at Shiyan, Hubei Province, China, in June 2010, and identified by Prof. Changgong Zhang of School of Pharmacy, Tongji Medical College, Huazhong University of Science and Technology. The voucher specimen (No. 2010-0616) was deposited in the herbarium of Hubei Key Laboratory of Natural Medicinal Chemistry and Resource Evaluation, School of Pharmacy, Tongji Medical College, Huazhong University of Science and Technology.
3.3. Extraction and Isolation
The dried whole parts of T. viscidum (50 Kg) were extracted with 70% acetone (100 L × 3 times) at the room temperature. After removal of the solvent, the crude extraction (2.3 kg) was suspended in H2O (5.0 L) and partitioned successively with petroleum ether (60–90 °C), CHCl3, and EtOAc to give petroleum ether, CHCl3, and EtOAc portions. The CHCl3 portion (200 g) was subjected to MCI gel column eluted with MeOH/H2O (9:1, v/v), and then chromatographed on a silica gel column eluted with petroleum–acetone (50:1 to 0:1, v/v) to obtain seven fractions (A–G). Compound 9 (40 mg) was recrystallized from fraction A using EtOAc. Compound 4 (100 mg) was obtained by recrystallization in EtOH from fraction B. Fraction C was subjected to a Sephadex LH-20 column eluted with MeOH to give four subfractions (C1–C4). Subfraction C2 was purified by semi-preparative HPLC (70% MeCN in H2O, flow rate 1.5 mL/min, wavelength 254 nm) to obtain compounds 2 (5.0 mg, retention time 30 min) and 1 (6 mg, retention time 33 min). Subfraction C3 was subjected to ODS column chromatography eluted with 80% MeOH in H2O, and then purified by semi-preparative HPLC (85% MeOH in H2O, flow rate 1.5 mL/min, wavelength 254 nm) to yield compound 3 (7 mg, retention time 45 min). Fraction D was applied to RP-C18 gel column eluted with MeOH/H2O (1:5 to 1:0, v/v), followed by chromatography over repeated silica gel column (CHCl3/MeOH, 60:1, v/v) to afford compound 5 (8 mg). Fraction E was subjected to RP-C18 gel column eluted with MeOH/H2O (1:5 to 1:0, v/v), followed by chromatography over silica gel and finally purified by semi-preparative HPLC (40% MeOH in H2O, flow rate 1.5 mL/min, wavelength 205 nm) to get compounds 6 (6 mg, retention time 43 min ) and 7 (9 mg, retention time 46 min). Fraction F was subjected to a Sephadex LH-20 column eluted with MeOH, followed by chromatography over silica gel eluted with petroleum/acetone (15:1, v/v) to get compound 8 (10 mg). Compound 10 (100 mg) was directly crystallized from fraction F.
3β-hydroxy-urs-30-p-Z-hydroxycinnamoyloxy-12-en-28-oic-acid (1). Amorphous powder. [α: + 27 (c = 0.85, THF); UV (THF) λmax (logε) nm: 312 (3.33), 208 (3.39); IR (KBr) νmax 3729, 2924. 1685, 1601, 1512, 1457, 1384, 1260, 1024, and 772 cm–1; 1H-NMR (C5D5N, 400 MHz) and 13C-NMR (C5D5N, 100 MHz) see Table 1; HRESIMS m/z: 641.3799 [M+Na]+ (calcd. for C39H54O6Na+, 641.3818).
3.4. 11β-HSD1 Inhibitory Assays
The scintillation proximity assay (SPA) was used to analyze the inhibitory activities of compounds 1–4 on human 11β-HSD1. Microsomes containing 11β-HSD1 were used according to the reported studies [16]. The full-length cDNAs of human 11β-HSD1 were isolated from the cDNA libraries provided by NIH Mammalian Gene Collection and cloned in pcDNA3 expression plasmid. HEK-293 cells were transformed with the pcDNA3-derived expression plasmid. Transformed cells were screened by 700 μg/mL G418. The microsomal fraction, which stably expressed 11β-HSD1, was prepared from the HEK-293 cells, and then was used for SPA. Briefly, microsomes were incubated with NADPH and [3H] cortisone. The product, [3H] cortisol, was specifically captured by monoclonal antibody coupling to protein A-coated SPA beads. IC50 (X ± SD, n = 3) values were calculated by using Prism Version 4 (GraphPad Software, San Diego, CA, USA).
4. Conclusions
A new ursane-type triterpenoid, 3β-hydroxy-urs-30-p-Z-hydroxycinnamoyloxy-12-en-28-oic-acid (1), was isolated from the whole parts of T. viscidum together with three known triterpenoids, 3β-hydroxy-urs-30-p-E-hydroxycinnamoyloxy-12-en-28-oic-acid (2), 2α,3β,19α-trihydroxy-urs-12-en-28-oic-acid (3), ursolic acid (4), four known lignans, pinoresinol (5), 9α-hydroxypinoresinol (6), (+)-medioresinol (7), (+)-kobusin (8), and two steroids, β-sitosterol (9), daucosterol (10). Their structures were established by a combination of spectroscopic data analysis, and comparison with literature data. Compounds 2–10 were isolated from T. viscidum for the first time. Compounds 1–4 were tested for 11β-HSD1 inhibition activities in vitro, whereby compound 4 showed stronger inhibitory activity, while compounds 1–3 did not show obvious 11β-HSD1 inhibitory activities.
Supplementary Materials
Supplementary materials can be accessed at: http://www.mdpi.com/1420-3049/18/1/1262/s1.
Acknowledgments
This work was financially supported by National Natural Science Foundation of China (No. 81202423), Science Foundation for the Excellent Youth Scholars, Health Department of Hubei Province, China (QJX2010-2), Scientific Research Foundation for the Returned Oversea Chinese Scholars, State Education Ministry of China (2010-1561-40th), Program for New Century Excellent Talents in University (NCET-2008-0224), and National Innovation Experiment Program for University Students, Huazhong University of Science and Technology (to Y.S.).
References
- State Administration of Traditional Chinese Medicine. Zhong Hua Ben Cao; Shanghai Science and Technology Publishing Company: Shanghai, China, 1999; pp. 6239–6240. [Google Scholar]
- Chen, Y.P.; Li, C.M.; Sun, H.D. The diterpenoid from Teucrium viscidum. Acta Botanica Yunnanica 1990, 12, 110. [Google Scholar]
- Fujita, E.; Uchida, I.; Fujita, T.; Masaki, N.; Osaki, K. Teucvin, a novel furanoid norditerpene from Teucrium uiscidum var. miquelianum. J. Chem. Soc. Chem. Commun. 1973, 20, 793–794. [Google Scholar] [CrossRef]
- Node, M.; Sai, M.; Fujita, E. Isolation of the diterpenoid teuflin (6-epiteucvin) from Teucrium viscidum var. miquelianum. Phytochemistry 1981, 20, 757–760. [Google Scholar] [CrossRef]
- Uchida, I.; Fujita, T.; Fujita, E. Terpenoids-XXXIV: Teucvidin, a minor norditerpene from Teucrium viscidum var. miquelianum. Tetrahedron 1975, 31, 841–848. [Google Scholar] [CrossRef]
- Siddiqui, B.S.; Begum, S. Two triterpenoids from the leaves of Plumeria obtusa. Phytochemistry 1999, 52, 1111–1115. [Google Scholar] [CrossRef]
- Jang, D.S.; Kim, J.M.; Lee, G.Y.; Kim, J.; Kim, J.S. Ursane-type triterpenoids from the aerial parts of Potentilla discolor. Agric. Chem. Biotechnol. 2006, 49, 48–50. [Google Scholar]
- Cowan, S.; Stewart, M.; Abbiw, D.K.; Latif, Z.; Sarker, S.D.; Nash, R.J. Lignans from Strophanthus gratus. Fitoterapia 2001, 72, 80–82. [Google Scholar] [CrossRef]
- Li, Y.S.; Wang, Z.T.; Zhang, M.; Luo, S.D.; Chen, J.J. A new pinoresinol-type lignan from Ligularia kanaitizensis. Nat. Prod. Res. 2005, 19, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.A.; Shoeb, A. A lignan from Lonicera hypoleuca. Phytochemistry 1985, 24, 628–630. [Google Scholar] [CrossRef]
- Latip, J.; Hartley, T.G.; Waterman, P.G. Lignans and coumarins metabolites from Melicopehayesii. Phytochemistry 1999, 51, 107–110. [Google Scholar] [CrossRef]
- Al-Qudah, M.A.; Zarga, M.H.A. Chemical constituents of Sisymbrium irio L. from Jordan. Nat. Prod. Res. 2010, 24, 448–456. [Google Scholar] [CrossRef] [PubMed]
- Thuong, P.T.; Lee, C.H.; Dao, T.T.; Nguyen, P.H.; Kim, W.G.; Lee, S.J.; Oh, W.K. Triterpenoids from the leaves of Diospyros kaki (Persimmon) and their inhibitory effects on protein tyrosine phosphatase 1B. J. Nat. Prod. 2008, 71, 1775–1778. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Huang, S.L.; Dou, W.; Zhang, S.; Chen, J.H.; Shen, Y.; Shen, J.H.; Leng, Y. Emodin, a natural product, selectively inhibits 11β-hydroxysteroid dehydrogenase type 1 and ameliorates metabolic disorder in diet-induced obese mice. Br. J. Pharmacol. 2010, 161, 113–126. [Google Scholar] [CrossRef] [PubMed]
- Rollinger, J.M.; Kratschmar, D.V.; Schuster, D.; Pfisterer, P.H.; Gumy, C.; Aubry, E.M.; Brandstoter, S.; Stuppner, H.; Wolber, G.; Odermatt, A. 11β-Hydroxysteroid dehydrogenase 1 inhibiting constituents from Eriobotrya japonica revealed by bioactivity-guided isolation and computational approaches. Bioorg. Med. Chem. 2010, 18, 1507–1515. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Dou, W.; Lou, J.; Leng, Y.; Shen, J. Discovery of novel inhibitors of 11β-hydroxysteroid dehydrogenase type 1 by docking and pharmacophore modeling. Bioorg. Med. Chem. Lett. 2008, 18, 1340–1345. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |
© 2013 by the authors. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).