Platyconic Acid A, a Genuine Triterpenoid Saponin from the Roots of Platycodon grandiflorum
Abstract
:Introduction
 3)-β-d-xylopyranosyl-(1
3)-β-d-xylopyranosyl-(1  4)-α-l-rhamnopyranosyl-(1
4)-α-l-rhamnopyranosyl-(1  2)-α-l-arabinopyranosyl] ester. It had been reported that two artifacts potentially derived from 1, a methylester 1a and a lactonized product, platyconate A lactone (1b), were isolated from the extract treated with excess amount of diazomethane [13]. From this result, the authors suggested the presence in the plant extract of the corresponding genuine saponin, 1, even though they had failed to isolate it. In this paper, we briefly describe an isolation of the genuine
2)-α-l-arabinopyranosyl] ester. It had been reported that two artifacts potentially derived from 1, a methylester 1a and a lactonized product, platyconate A lactone (1b), were isolated from the extract treated with excess amount of diazomethane [13]. From this result, the authors suggested the presence in the plant extract of the corresponding genuine saponin, 1, even though they had failed to isolate it. In this paper, we briefly describe an isolation of the genuine
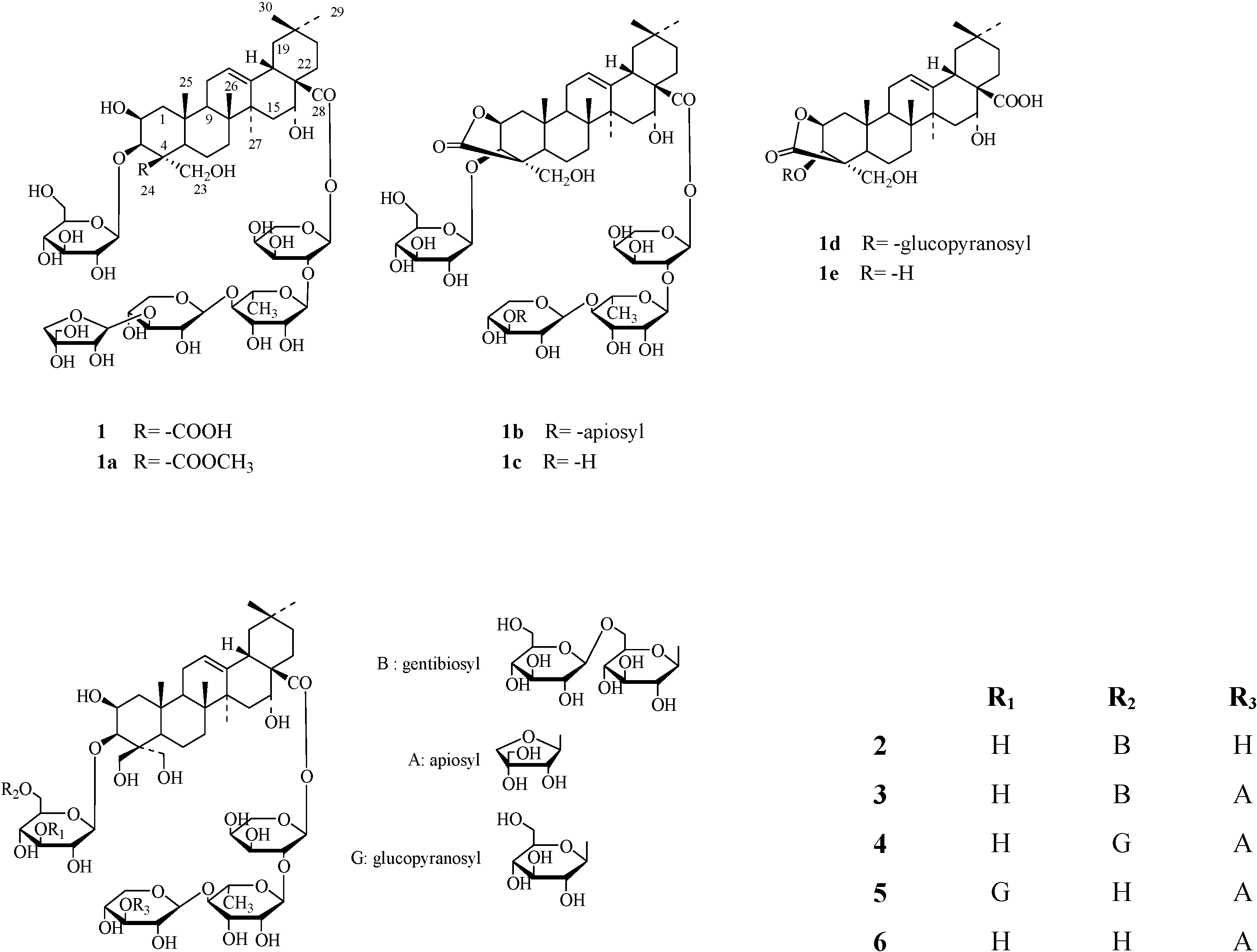
Results and Discussion
| 1 | 1a | 1b | 1c | 1d | 1e | |
|---|---|---|---|---|---|---|
| C-1 | 46.7 | 45.8 | 41.6 | 41.7 | 41.4 | 42.0 |
| C-2 | 69.6 | 69.8 | 82.8 | 83.6 | 83.5 | 84.6 |
| C-3 | 83.3 | 84.4 | 89.8 | 89.5 | 89.5 | 81.8 |
| C-4 | 56.3 | 56.1 | 53.9 | 54.5 | 54.5 | 55.2 |
| C-5 | 49.6 | 50.1 | 52.5 | 52.3 | 52.2 | 52.1 |
| C-6 | 20.3 | 20.5 | 19.5 | 19.8 | 19.7 | 20.1 |
| C-7 | 33.7 | 33.7 | 33.6 | 33.9 | 33.8 | 34.0 |
| C-8 | 40.0 | 40.3 | 40.7 | 40.8 | 40.6 | 38.2 |
| C-9 | 47.2 | 47.6 | 48.5 | 48.5 | 48.5 | 49.1 |
| C-10 | 37.2 | 37.4 | 37.9 | 38.0 | 38.0 | 36.9 |
| C-11 | 24.4 | 24.4 | 24.7 | 25.0 | 25.0 | 25.2 |
| C-12 | 122.9 | 123.0 | 122.3 | 122.6 | 122.2 | 121.3 |
| C-13 | 144.2 | 144.4 | 145.0 | 145.6 | 146.2 | 147.3 |
| C-14 | 42.2 | 42.4 | 42.5 | 42.7 | 42.7 | 43.1 |
| C-15 | 36.2 | 36.1 | 36.0 | 36.5 | 36.9 | 36.7 |
| C-16 | 73.8 | 74.1 | 73.9 | 74.4 | 75.0 | 75.9 |
| C-17 | 49.5 | 50.1 | 50.0 | 50.2 | 49.3 | 50.8 |
| C-18 | 41.3 | 41.6 | 41.6 | 41.6 | 41.9 | 40.9 |
| C-19 | 47.1 | 47.2 | 47.2 | 47.6 | 47.7 | 48.1 |
| C-20 | 30.6 | 30.8 | 30.7 | 30.8 | 30.3 | 28.3 |
| C-21 | 35.9 | 36.1 | 36.0 | 36.4 | 36.8 | 34.2 |
| C-22 | 31.7 | 31.4 | 31.3 | 31.4 | 31.5 | 31.5 |
| C-23 | 63.5 | 64.5 | 57.5 | 57.6 | 57.5 | 58.3 |
| C-24 | 181.4 | 175.5 | 177.7 | 178.6 | 178.6 | 179.5 |
| C-25 | 16.1 | 15.8 | 17.4 | 17.8 | 17.7 | 18.3 |
| C-26 | 17.4 | 17.6 | 18.1 | 18.5 | 18.4 | 19.1 |
| C-27 | 27.0 | 27.2 | 27.4 | 27.7 | 27.7 | 26.9 |
| C-28 | 175.8 | 175.8 | 175.8 | 176.3 | 180.4 | 185.0 |
| C-29 | 33.3 | 33.1 | 33.0 | 33.7 | 33.3 | 31.7 |
| C-30 | 24.8 | 25.2 | 25.2 | 25.2 | 25.2 | 25.7 |
| 24-OCH3 | 51.3 | |||||
| 1 | 1a | 1b | 1c | 1d | ||
| glucose | ||||||
| C-1 | 106.0 | 106.3 | 105.1 | 105.7 | 105.7 | |
| C-2 | 74.8 | 75.3 | 75.0 | 75.6 | 75.7 | |
| C-3 | 78.2 | 78.5 | 78.2 | 79.0 | 79.2 | |
| C-4 | 71.8 | 72.0 | 71.8 | 71.5 | 71.8 | |
| C-5 | 78.2 | 78.1 | 78.2 | 78.8 | 78.8 | |
| C-6 | 61.8 | 63.0 | 62.9 | 63.0 | 63.0 | |
| arabinose | ||||||
| C-1 | 93.4 | 93.7 | 93.7 | 94.1 | ||
| C-2 | 75.5 | 75.7 | 75.7 | 75.8 | ||
| C-3 | 70.3 | 70.1 | 70.2 | 70.8 | ||
| C-4 | 66.2 | 65.8 | 65.9 | 66.8 | ||
| C-5 | 61.8 | 63.0 | 62.9 | 63.8 | ||
| rhamnose | ||||||
| C-1 | 101.2 | 101.1 | 101.1 | 101.7 | ||
| C-2 | 71.8 | 72.0 | 72.0 | 72.4 | ||
| C-3 | 72.3 | 72.4 | 72.4 | 73.2 | ||
| C-4 | 83.3 | 83.6 | 83.6 | 84.3 | ||
| C-5 | 68.4 | 68.7 | 68.6 | 69.1 | ||
| C-6 | 18.4 | 18.1 | 18.3 | 18.8 | ||
| xylose | ||||||
| C-1 | 106.2 | 106.5 | 106.5 | 107.4 | ||
| C-2 | 74.7 | 75.0 | 75.0 | 76.2 | ||
| C-3 | 85.2 | 85.5 | 85.6 | 79.2 | ||
| C-4 | 68.9 | 69.5 | 69.5 | 71.8 | ||
| C-5 | 66.5 | 66.8 | 66.8 | 67.9 | ||
| apiose | ||||||
| C-1 | 110.8 | 111.2 | 111.2 | |||
| C-2 | 77.8 | 77.9 | 77.9 | |||
| C-3 | 80.1 | 80.0 | 80.0 | |||
| C-4 | 74.3 | 75.0 | 75.0 | |||
| C-5 | 65.1 | 65.8 | 65.7 |
 3)-β-d-xylopyranosyl-(1
3)-β-d-xylopyranosyl-(1  4)-α-l-rhamno- pyranosyl-(1
4)-α-l-rhamno- pyranosyl-(1  2)-α-l-arabinopyranoside. This is the first report on the isolation of compound 1 from natural sources.
2)-α-l-arabinopyranoside. This is the first report on the isolation of compound 1 from natural sources.
Conclusions
Experimental
General
Plant Material
Extraction and Isolation
Characterization of platyconic acid A (1)
 = - 8.89 (c 1, MeOH); IR νmax: 3402, 2927, 1726, 1076 and 1033 cm-1; MALDI-TOF/MS m/z: 1284 [M+2Na]; C57H90O29; 1H-NMR (pyridine-d5, 800 MHz): δ 0.98, 1.10, 1.52, 1.69, 1.75 (each 3H, H-25, 26, 27, 29, 30), 2.75 (1H, d, J = 13.3Hz, H-18), 5.61 (1H, brs, H-12); 13C-NMR (pyridine-d5, 200 MHz): see Table 1.
= - 8.89 (c 1, MeOH); IR νmax: 3402, 2927, 1726, 1076 and 1033 cm-1; MALDI-TOF/MS m/z: 1284 [M+2Na]; C57H90O29; 1H-NMR (pyridine-d5, 800 MHz): δ 0.98, 1.10, 1.52, 1.69, 1.75 (each 3H, H-25, 26, 27, 29, 30), 2.75 (1H, d, J = 13.3Hz, H-18), 5.61 (1H, brs, H-12); 13C-NMR (pyridine-d5, 200 MHz): see Table 1. Preparation of 1a by treatment of 1 with diazomethane
Preparation of 1c and 1d by partial hydrolysis of 1
Preparation of 1e by acid hydrolysis of 1
HPLC analysis of the roots extract of P. grandiflorum
Acknowledgments
References
- Kim, Y.S.; Kim, J.S.; Choi, S.U.; Kim, J.S.; Lee, H.S.; Roh, S.H.; Jeong, Y.C.; Kim, Y.K.; Ryu, S.Y. Isolation of a new saponin and cytotoxic effect of saponins from the root of Platycodon grandiflorum on human tumor cell lines. Planta Med. 2005, 71, 566–568. [Google Scholar]
- Xu, B.J.; Han, L.K.; Zheng, Y.N.; Lee, J.H.; Sung, C.K. In vitro inhibitory effect of triterpenoidal saponins from Platycodi Radix on pancreatic lipase. Arch. Pharm. Res. 2005, 28, 180–185. [Google Scholar] [CrossRef]
- Ahn, K.S.; Noh, E.J.; Zhao, H.L.; Jung, S.H.; Kang, S.S.; Kim, Y.S. Inhibition of inducible nitric oxide synthase and cyclooxygenase II by Platycodon grandiflorum saponins via suppression of nuclear factor-kappaB activation in RAW 264.7 cells. Life Sci. 2005, 76, 2315–2328. [Google Scholar] [CrossRef]
- Lee, K.J.; Choi, C.Y.; Chung, Y.C.; Kim, Y.S.; Ryu, S.Y.; Roh, S.H.; Jeong, H.G. Protective effect of saponins derived from roots of Platycodon grandiflorum on tert-butyl hydroperoxide-induced oxidative hepatotoxicity. Toxicol. Lett. 2004, 147, 271–282. [Google Scholar] [CrossRef]
- Kim, Y.S.; Kang, J.S.; Kim, J.S.; Choi, Y.H.; Seo, J.H.; Lee, J.W.; Kim, S.K.; Lee, H.S.; Cho, Y.S.; Roh, S.H.; Jeong, Y.C.; Shim, K.H.; Ryu, S.Y. Ameliorating effect of the root extract from Platycodon grandiflorum on the ethanol-induced cognitive impairment in mice. Kor. J. Pharmacogn. 2004, 35, 239–243. [Google Scholar]
- Choi, Y.H.; Kim, Y.S.; Yeo, S.J.; Roh, S.H.; Jeong, Y.C.; Kang, J.S.; Ryu, S.Y. Ameliorating effect of balloon flower saponin on the ethanol-induced memory impairment in mice. Phytotherapy. Res. 2008, 22, 973–976. [Google Scholar] [CrossRef]
- He, Z.; Qiao, C.; Han, Q.; Wang, Y.; Ye, W.; Xu, H. New triterpenoid saponins from the roots of Platycodon grandiflorum. Tetrahedron 2005, 61, 2211–2215. [Google Scholar] [CrossRef]
- Fu, W.W.; Shimizu, N.; Takeda, T.; Dou, D.Q.; Chen, B; Pei, Y.H.; Chen, Y.J. New A-ring lactone triterpenoid saponins from the roots of Platycodon grandiflorum. Chem. Pharm. Bull. 2006, 54, 1285–1287. [Google Scholar] [CrossRef]
- Fu, W.W.; Shimizu, N.; Dou, D.Q.; Takeda, T.; Fu, R.; Pei, Y.H.; Chen, Y.J. Five new triterpenoid saponins from the roots of Platycodon grandiflorum. Chem. Pharm. Bull. 2006, 54, 557–560. [Google Scholar] [CrossRef]
- Ishii, H.; Tori, K.; Tozyo, T.; Yoshimura, Y. Structures of polygalacin-D and -D2, platycodin-D and -D2, and their monoacetates, saponins isolated from Platycodon grandiflorum A. DC. determined by carbon-13 nuclear magnetic resonance spectroscopy. Chem. Pharm. Bull. 1978, 26, 674–677. [Google Scholar]
- Fu, W.W.; Dou, D.Q.; Shimizu, N.; Takeda, T.; Pei, Y.H.; Chen, Y.J. Studies on the chemical constituents from the roots of Platycodon grandiflorum. J. Nat. Med. 2006, 60, 68–72. [Google Scholar] [CrossRef]
- Li, W.; Xiang, L.; Zhang, J.; Zheng, Y.N.; Han, L.K.; Saoto, M. A new triterpenoid saponin from the roots of Platycodon grandiflorum. Chin. Chem. Lett. 2007, 18, 306–308. [Google Scholar] [CrossRef]
- Ishii, H.; Tori, K.; Tozyo, T.; Yoshimura, Y. Saponins from roots of Platycodon grandiflorum. Part 2. isolation and structure of new triterpene glycosides. J. Chem. Soc. Perkin. Trans. I 1984, 661–668. [Google Scholar]
- Nikaido, T.; Koike, K.; Mitsunaga, K.; Saeki, T. Two new triterpenoid saponins from Platycodon grandiflorum. Chem. Pharm. Bull. 1999, 47, 903–904. [Google Scholar] [CrossRef]
- Ishii, H.; Tori, K.; Tozyo, T.; Yoshimura, Y. Saponins from roots of Platycodon grandiflorum. part 1. structure of prosapogenin. J. Chem. Soc. Perkin. Trans. I 1981, 1928–1933. [Google Scholar]
- Sample Availability: Samples of the compounds 1-6 are available from the authors
© 2008 by the authors. Licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Choi, Y.H.; Yoo, D.S.; Choi, C.W.; Cha, M.-R.; Kim, Y.S.; Lee, H.S.; Lee, K.R.; Ryu, S.Y. Platyconic Acid A, a Genuine Triterpenoid Saponin from the Roots of Platycodon grandiflorum. Molecules 2008, 13, 2871-2879. https://doi.org/10.3390/molecules13112871
Choi YH, Yoo DS, Choi CW, Cha M-R, Kim YS, Lee HS, Lee KR, Ryu SY. Platyconic Acid A, a Genuine Triterpenoid Saponin from the Roots of Platycodon grandiflorum. Molecules. 2008; 13(11):2871-2879. https://doi.org/10.3390/molecules13112871
Chicago/Turabian StyleChoi, Yeon Hee, Dae Seok Yoo, Chun Whan Choi, Mi-Ran Cha, Young Sup Kim, Hyun Sun Lee, Kang Ro Lee, and Shi Yong Ryu. 2008. "Platyconic Acid A, a Genuine Triterpenoid Saponin from the Roots of Platycodon grandiflorum" Molecules 13, no. 11: 2871-2879. https://doi.org/10.3390/molecules13112871
APA StyleChoi, Y. H., Yoo, D. S., Choi, C. W., Cha, M.-R., Kim, Y. S., Lee, H. S., Lee, K. R., & Ryu, S. Y. (2008). Platyconic Acid A, a Genuine Triterpenoid Saponin from the Roots of Platycodon grandiflorum. Molecules, 13(11), 2871-2879. https://doi.org/10.3390/molecules13112871




