Abstract
An improved synthetic method affording 4-chlorocoumarin-3-sulfonyl chloride (4) in very good yield (ca. 85 %) is reported. This compound was reacted with various bidentate nucleophiles such as 2-aminopyridines and 2-aminothiazoles in order to obtain substituted pyrido- and thiazino-1,2,4-thiadiazino-benzopyranone dioxides (potential anticancer and anti-HIV agents). These reactions occurred rapidly at room temperature giving yellowish precipitates, which are insoluble in common organic solvents, making the purification process challenging. Further investigation has shown that these fused heterocycles are not stable and decompose with opening of the 1,2,4-thiadiazine ring.
Introduction
Heterocyclic chemistry is one of the largest areas of research in organic chemistry and it is growing rapidly. Of all published organic chemistry literature, papers on heterocyclic synthesis accounted for around 60 % in 1998 [1], but nowadays the fraction is much larger considering that novel heterocyclic compounds are published in different fields such as biochemistry, pharmaceuticals, materials and others. A similar trend is seen for coumarin, a heterocyclic system with a very large number of different derivatives. Coumarin is a compound with varied biological activities and in 1954 it was classified as a carcinogenic substance [2,3]. Main representatives of the class are its hydroxy derivatives 4-hydroxycoumarin (1) and 7-hydroxycoumarin (umbeliferone), also biologically active and very important for synthesis of other coumarin derivatives. Until now, an enormous number of compounds with coumarin systems in their structure have been synthesized. Those derivatives have shown a remarkably broad spectrum of pharmacological and physiological activities and they are used as anticoagulant [4,5,6], antibacterial [7,8], antiviral [9,10], antitumor [11,12,13,14], bactericidal [15], fungicidal [16], and anti-inflammatory agents [17]. Also, in recent times there are references to derivatives with anti-HIV activity [18,19,20,21].
On the other hand, the nitrogen and sulfur heterocyclic system families are very interesting because of their physicochemical properties with relevance to the design of new drugs and new materials, especially those relating to molecular conductors and magnets [22]. 1,2,4-Thiadizines are also used for treatment of HIV infection [23,24], as cardiovascular agents [25], for their antimicrobial effects [26], and as diuretics and antihypertensive drugs [27,28]. Based on these facts we wanted to combine the coumarinic system with 1,2,4-thiadiazines in the hope that the resulting novel heterocycles would be biologically active, especially as anticancer and anti-HIV agents. Also, we designated positions 3 and 4 of coumarin for annellation, because these positions are mainly attacked by electrophiles and nucleophiles, respectively [29].
Results and Discussion
Checchi et al. had previously reported one of the target compounds, pyrido[1’,2’:2,3]-1,2,4-thiadiazino[6,5-c]benzopyran-6-one 7,7-dioxide (7a), as one of two possible structures [30]. It was obtained by reaction of 4-chlorocoumarin-3-sulfonyl chloride (4) with 2-aminopyridine (5a) in dry benzene. In our attempts to obtain the same compound 7a and its derivatives, unsatisfactory results were obtained and difficulties followed the synthesis of 4 as the key substrate. These problems were avoided by modification of the reaction route used, simplifying the preparation of the sodium salt 3 of 4-hydroxy-coumarin-3-sulfonic acid (2). Thus, considering that 2 is very soluble and stable in aqueous solution, 3 was obtained by simple mixing aqueous solutions of 2 and sodium chloride. Contrary to the abundant literature mentioning the good solubility of alkali-metal salts of sulfonic acids [31], the salts we prepared are not soluble in water and can be easily isolated in nearly quantitative yields from aqueous solutions by simple filtration (Scheme 1). Compound 3 was reported once by Huebner and Link [32], but the product was only characterized by its elemental analysis for sodium. This paper presents the full characterization of 3. In the next step 3 was chlorinated by refluxing with POCl3 for approximately three hours. Afterwards, instead of removing POCl3 by vacuum distillation, the suspension was poured onto a mixture of crushed ice and water to afford a nice precipitate of 4 in 85 % yield (lit. [30]: ca. 25 %) (Scheme 2). Modification of this step was based on the report that the chlorine atom at position 4 cannot be attacked by water [29]. Also, it was found that in general the sulfonyl chlorides are insoluble and stable in water [31].
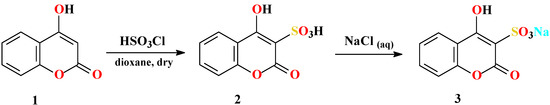
Scheme 1.
Structural assignment of 4 was based on 1H- and 13C-NMR, IR and MS spectral analysis. The signals of four aromatic protons at 7.34-7.85 ppm could be observed in the 1H-NMR spectrum, as well as nine signals in the expected regions of the 13C-NMR spectrum. In the mass spectrum (ESI, +ve mode) there was a peak at m/z 302 corresponding to the [M+Na]+ species. These data confirmed that the product isolated from water is identical with that isolated by vacuum distillation. Substrate 4 is very soluble in organic solvents and it was used without further purification.
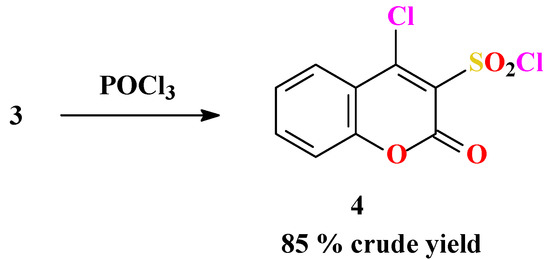
Scheme 2.
Reactions of 4 with 2-aminopyridines 5a-h and 2-aminothiazoles 6a,b were performed in acetonitrile as a polar and aprotic solvent. Reactions were very fast at room temperature and after a few minutes crude yellowish precipitates of 7a-f and 8a,b were formed, respectively (Scheme 3).
Using single crystal X-ray diffraction the structure of 7a has been determined and the dilemma about its configuration was eliminated (Figure 1). The compound crystallizes in monoclinic space group P21/n with cell dimensions a=16.3541(5) Å, b=9.2246(2) Å, c=16.7064(5) Å, α=90.00°, β=110.802(4)°, γ=90.00°, V=2356.04(11) Å3, Z=8 [33]. All atoms in the molecule are in one plane, except for the two oxygen atoms of the SO2 group, which are arranged symmetrically out of plane.
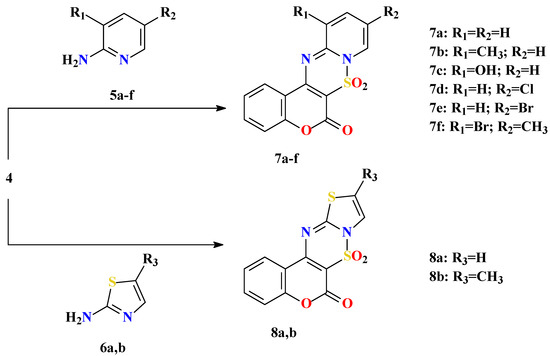
Scheme 3.
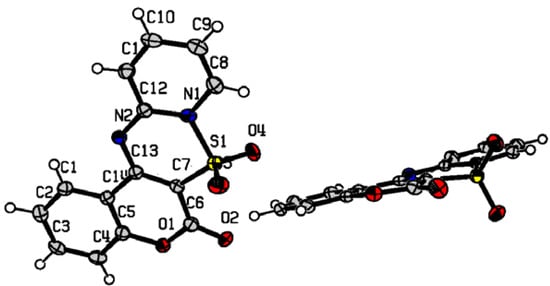
Figure 1.
Diagram of compound 7a with the crystallographic numbering system.
All products 7a-f and 8a,b are very insoluble in common organic solvents, with the exception of DMSO, which caused difficulties when purifying them. Purifications were performed on chromatographic columns with slow gradient elution using dry solvents. Further investigation confirmed that compounds 7a-f and 8a,b are not stable. Analysis carried out on 7d proved that those systems annulated on coumarin decompose with opening of the 1,2,4-thiadiazine ring. Decomposition in the solid state was slow, but when crystals were dissolved in DMSO, where complete elimination of traces of water is not possible, decomposition was rapid (within a few hours). Therefore, it was assumed that these heterocycles are sensitive to moisture. The reaction with water releases sulfuric acid. Since the decomposition is slow in the beginning it is possible that the reaction is autocatalytic, so when the amount of sulfuric acid increases decomposition is more rapid (Scheme 4).
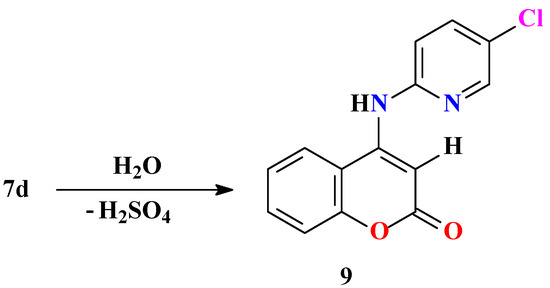
Scheme 4.
4-(5-Chloropyridin-2-yl-amino)-benzopyran-2-one (9) was isolated in pure form as white crystals after decomposition of 7d. In the IR spectrum one sharp band at 3328 cm-1, corresponding to an NH vibrational mode and a C=O band at 1672 cm-1, which is evidently lower than the band of the annulated heterocycles at 1710 cm-1, were noticed. The 1H-NMR spectrum showed a singlet at 9.5 ppm corresponding to the NH proton, seven aromatic protons ranging from 7.4 to 7.9 ppm and one singlet at 7.3 ppm, corresponding to the proton at the 3-position of the coumarin ring. In the 13C-NMR spectrum there were 14 signals, as expected, and confirming our assumptions the signal of the coumarin ring C-3 at 92.8 ppm is a doublet. Finally, in the mass spectrum signals at m/z 273 (ESI pos) for the [M+H]+ species and m/z 272 (EI), corresponding to the molecular peak [M]+, were noticed.
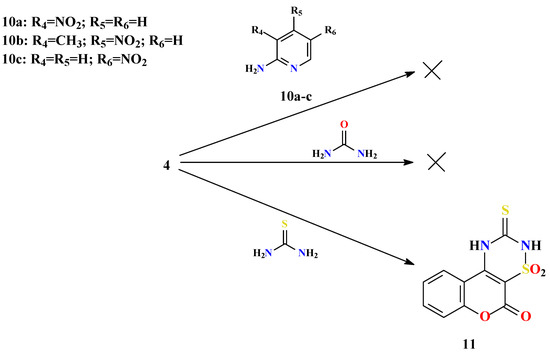
Scheme 5.
It was also observed that the substituent in the 2-aminopyridine moiety is very important because of its influence on the reaction, rate and the stability of derivatives. Substrate 4 did not react with any 2-aminopyridines possessing a nitro group in positions 3, 4 or 5 (10a-c, Scheme 5). This is understandable considering that the nitro group has a strongly negative inductive effect (-I), as well as a negative resonance effect (-M). As a result of both effects, the electron density on the nitrogen atoms is much lower, which consequently weakens the nucleophilicity [34]. In view of these facts it was concluded that weak bidentate nucleophiles cannot substitute the chlorine atoms in 4, to form a 1,2,4-thiadiazine ring, and that only strong nucleophiles are able to do so. To prove this, comparative reactions of 4 were performed with urea as a weaker and thiourea as a stronger bidentate nuclophile. A yellowish precipitate of 8H,10H-1,2,4-thiadiazino[6,5-c]benzopyran-9-thioxo-6-one 7,7-dioxide (11, Scheme 5) was formed within a few minutes after mixing an acetonitrile solution of 4 and thiourea crystals at room temperature, while all attempts to obtain a product from the reaction with urea failed.
Experimental
General
Melting points were determined on a Reichert heating plate and are uncorrected. C, H elemental analysis was carried out on a Coleman Model 33 carbon-hydrogen analyzer. N elemental analysis was carried out by the Dümas method. NMR spectra were recorded on a Bruker 400 MHz instrument using DMSO-d6 as solvent and tetramethylsilane as internal standard. Infrared spectra (KBr pellets) were measured on a Perkin-Elmer System 2000 FT IR. ESI-TOF mass spectra were measured using an LCT mass spectrometer (Waters) equipped with a lockspray dual-electrospray ion source combined with Waters Alliance 2695 HPLC unit. The X-ray structure of 7a was measured on a CCD Xcalibur S diffractometer. All non-hydrogen atoms were refined anisotropically and all hydrogen atoms were placed in ideal positions. All the reagents and solvents were obtained from commercial sources and were used without further purification. 4-Hydroxycoumarin-3-sulfonic acid (2) was synthesized following a literature method [30,35], as described below in more detail.
Synthesis of 4-hydroxycoumarin-3-sulfonic acid (2)
Monochlorosulfonic acid (HSO3Cl, 8 mL) was added dropwise to dry dioxane (85 mL). When the temperature of the solution reached 50 ºC 4-hydroxycoumarin (1, 10 g, 0.062 mol) was added in one portion. After short vigorous mixing a very voluminous yellowish precipitate formed from the bright yellow solution. This precipitate was filtered on a glass funnel and washed first with dioxane (20 mL) and then with ether (2 x 20 mL). After washing we obtained a white powder (92 % yield), with m.p. 98-100 ºC (lit. [34] 92 ºC). White crystals were obtained after recrystallization from acetone. Anal. Calc. for C9H6O6S (242.20): 44.63% C, 2.50% H; found 44.58% C, 2.47% H; IR (KBr, cm-1): 3400-2500 (both OH, stretching vibrations, very broad, indicating hydrogen bonding), 1705 (CO), 1326 (SO2, antisymmetric vibrations), 1157 (SO2, symmetric vibrations), 1621, 1608 and 1556 (aromatic vibrations); 1H-NMR δ: 14.1 (br s, 2H, OH), 7.87 (d, 1H, 5-ArH), 7.67 (t, 1H, 7-ArH) and 7.33-7.36 (m, 2H, 6- and 8-ArH); 13C-NMR δ: 162.42, 157.10, 152.68, 133.66, 124.46, 124.29, 116.32, 114.93 and 107.68; MS (ESI neg) m/z: 241 [M-H]-.
Synthesis of sodium 4-hydroxycoumarin-3-sulfonate (3)
4-Hydroxycoumarin-3-sulfonic acid (2, 10 g, 0.041 mol) was dissolved in a small quantity of water and with vigorous mixing a saturated aqueous solution of sodium chloride (35 g NaCl/100 mL) was added. The rapidly formed white precipitate was filtered on a Buchner funnel and washed with cold water. Recrystallization from acetonitrile gave a white solid (98 % yield) with m.p. > 300. Anal. Calc. for C9H5NaO6S (264.18): 40.92% C, 1.91% H; found 40.18% C, 1.27% H; IR (KBr, cm-1): 3614-2500 (OH, stretching vibrations, very broad, indicating hydrogen bonding), 1713 (CO), 1329 (SO2, anti-symmetric vibrations), 1147 (SO2, symmetric vibrations), 1623, 1610 and 1563 (aromatic vibrations); 1H-NMR δ: 13.9 (br s, H, OH), 7.88 (d, 1H, 5-ArH), 7.68 (t, 1H, 7-ArH) and 7.36-7.40 (m, 2H, 6- and 8-ArH); 13C-NMR δ: 162.36, 157.38, 152.51, 133.64, 124.34, 124.27, 116.26, 114.77 and 107.48; MS (ESI pos) m/z: 287 [M+Na]+.
Synthesis of 4-chlorocoumarin-3-sulfonyl chloride (4)
Compound 3 (10 g, 0.036 mol) was mixed with POCl3 (50 mL). The resulting suspension was refluxed for approx. 3 hours, then cooled to room temperature and slowly poured onto crushed ice mixed with water. The yellowish solid formed was collected by filtration, washed with ice-water and recrystallized from cyclohexane. Yield 85 %; m.p. 148-150 ºC; Anal. Calc. for C9H4Cl2O4S (279.09): 38.73% C, 1.44% H; found 38.07% C, 1.12% H; IR (KBr, cm-1): 3465 (CO, overtone), 1752 and 1742 (CO), 1300 (SO2, antisymmetric vibrations), 1173 (SO2, symmetric vibrations), 1601, 1587 and 1516 (aromatic vibrations); 1H-NMR δ: 7.85 (d, 1H, 5-ArH), 7.67 (t, 1H, 7-ArH) and 7.34-7.40 (m, 2H, 6- and 8-ArH); 13C-NMR δ: 162.15, 157.48, 150.9, 133.97, 125.92, 125.43, 116.33, 114.58 and 110.45; MS (ESI pos) m/z: 302 [M+Na]+.
General procedure for preparation of substitutedpyrido[1’,2’:2,3]-1,2,4-thiadiazino[6,5-c]benzo-pyran-6-one 7,7-dioxides 7a-f and thiazolo[3’,2’:2,3]-1,2,4-thiadiazino[6,5-c]benzopyran-6-one 7,7-dioxides 8a,b
Compound 4 (1 g, 3.6 mmol) was dissolved in acetonitrile (25 mL). To this solution was added the base (5a-f, 6a,b) in equimolar ratio and the mixture was stirred with a magnetic stirrer at room temperature for approx. 1 hour. The reaction was followed by TLC, usually using as eluent an 80:20 mixture of toluene/ethanol. A yellowish precipitate was formed, which was collected on a Buchner funnel. The precipitate was washed with a small amount of acetonitrile and dried in a dessicator. This precipitate was applied to a chromatographic column filled with silica gel 60 and it was eluted with a 100:0, 90:10, 80:20 and 70:30 gradient of toluene/ethanol. The main fraction was evaporated on a rotovapor and the crystals were collected and analyzed.
Pyrido[1’,2’:2,3]-1,2,4-thiadiazino[6,5-c]benzopyran-6-one 7,7-dioxide (7a). Yellow crystals recrystallized from ethyl acetate. Yield 89 %; m.p. 228-230 ºC (lit. [30] 226-228 ºC); Anal. Calc. for C14H8N2O4S (300.29): 56.00% C, 2.69% H, 9.33% N; found 55.26% C, 1.93% H, 8.79% N; IR (KBr, cm-1): 3435 (CO, overtone), 1710 (CO), 1352 (SO2, antisymmetric vibrations), 1158 (SO2, symmetric vibrations), 1633, 1609 and 1554 (aromatic vibrations); 1H-NMR δ: 8.85 (d, 1H, 8-ArH), 8.35 (d, 1H, 1-ArH), 8.14 (t, 1H, 10-ArH) 7.75 (t, 1H, 3-ArH), 7.59 (d, 1H, 11-ArH) and 7.40-7.46 (m, 3H, 2-, 4- and 9-ArH); 13C-NMR δ: 161.63, 153.50, 153.20, 148.23, 146.57, 138.99, 132.20, 123.72, 123.03, 118.62, 117.13, 115.40, 114.74 and 92.55; MS (ESI pos) m/z: 301 [M+H]+, 364 [M+Na+CH3CN]+, 623 [2M+Na]+.
11-Methylpyrido[1’,2’:2,3]-1,2,4-thiadiazino[6,5-c]benzopyran-6-one 7,7-dioxide (7b). Yellow crystals recrystallized from ethanol. Yield 90 %; m.p. 198-200 ºC; Anal. Calc. for C15H10N2O4S (314.32): 57.32% C, 3.21% H, 8.91% N; found 57.12% C, 3.09% H, 8.19% N; IR (KBr, cm-1): 3401 (CO, overtone), 3092 (ArH), 2924 (CH3, antisymmetric), 2853 (CH3, symmetric), 1710 (CO), 1359 (SO2, antisymmetric vibrations), 1155 (SO2, symmetric vibrations), 1626, 1610 and 1568 (aromatic vibrations); 1H-NMR δ: 8.72 (d, 1H, 8-ArH), 8.39 (d, 1H, 1-ArH), 8.05 (d, 1H, 10-ArH), 7.74 (t, 1H, 3-ArH), 7.40-7.45 (m, 2H, 2- and 4-ArH), 7.33 (t, 1H, 9-ArH) and 2.52 (s, 3H, CH3); 13C-NMR δ: 155.10, 153.74, 153.21, 151.83, 141.68, 134.63, 134.47, 126.02, 125.65, 124.93, 117.44, 117.33, 116.97, 97.60 and 17.60;
11-Hydroxypyrido[1’,2’:2,3]-1,2,4-thiadiazino[6,5-c]benzopyran-6-one 7,7-dioxide (7c). Yellow crystals purified by recrystallization from DMF. Yield 78 %; m.p. > 300 ºC (dec); Anal. Calc. for C14H8N2O5S (316.29): 53.16% C, 2.55% H, 8.86% N; found 52.81% C, 1.93% H, 8.71% N; IR (KBr, cm-1): 3429-2650 (OH), 1716 (CO), 1353 (SO2, antisymmetric vibrations), 1156 (SO2, symmetric vibrations), 1660, 1611 and 1571 (aromatic vibrations); 1H-NMR δ: 10.06 (s, 1H, OH), 8.89 (d, 1H, 8-ArH), 8.33 (d, 1H, 1-ArH), 7.71 (t, 1H, 3-ArH), 7.46 (d, 1H, 10-ArH) 7.34-7.48 (m, 2H, 2- and 4-ArH) and 7.27 (t, 1H, 9-ArH); Because of the very low solubility it was not possible to record the corresponding 13C-NMR spectra.
9-Chloropyrido[1’,2’:2,3]-1,2,4-thiadiazino[6,5-c]benzopyran-6-one 7,7-dioxide (7d). Yellow crystals recrystallized from ethanol. Yield 65 %; m.p. > 300 ºC (dec); Anal. Calc. for C14H7ClN2O4S (334.73): 50.24% C, 2.11% H, 8.37% N; found 50.12% C, 1.98% H, 7.89% N; IR (KBr, cm-1): 3080 (ArH), 1715 (CO), 1345 (SO2, antisymmetric vibrations), 1161 (SO2, symmetric vibrations), 1620, 1609 and 1547 (aromatic vibrations); 1H-NMR δ: 8.95 (s, 1H, 8-ArH), 8.37 (d, 1H, 1-ArH), 8.24 (d, 1H, 10-ArH), 7.72 (t, 1H, 3-ArH), 7.50 (d, 1H, 11-ArH) and 7.39-7.48 (m, 2H, 2- and 4-ArH); 13C-NMR δ: 161.52, 153.89, 153.15, 147.99, 147.52, 140.60, 132.27, 123.46, 123.04, 117.15, 116.64, 115.43, 114.27 and 92.61.
9-Bromopyrido[1’,2’:2,3]-1,2,4-thiadiazino[6,5-c]benzopyran-6-one 7,7-dioxide (7e). Yellow crystals recrystallized from benzene. Yield 69 %; m.p. > 300 ºC (dec); Anal. Calc. for C14H7BrN2O4S (379.18): 44.35% C, 1.86% H, 7.39% N; found 45.69% C, 2.08% H, 6.98% N; IR (KBr, cm-1): 3440 (CO, overtone), 3074 (ArH), 1712 (CO), 1344 (SO2, antisymmetric vibrations), 1161 (SO2, symmetric vibrations), 1672, 1608 and 1593 (aromatic vibrations); 1H-NMR δ: 9.10 (s, 1H, 8-ArH), 8.39 (d, 1H, 1-ArH), 8.27 (d, 1H, 10-ArH), 7.79 (t, 1H, 3-ArH), 7.56 (d, 1H, 11-ArH) and 7.46-7.52 (m, 2H, 2- and 4-ArH); 13C-NMR δ: 161.52, 153.99, 153.10, 148.21, 147.54, 140.62, 132.17, 123.66, 122.94, 117.15, 116.85, 115.41, 114.59 and 92.62.
9-Methyl-11-bromopyrido[1’,2’:2,3]-1,2,4-thiadiazino[6,5-c]benzopyran-6-one 7,7-dioxide (7f). Yellow crystals recrystallized from ethanol. Yield 72 %; m.p. > 300 ºC (dec); Anal. Calc. for C15H9BrN2O4S (393.21): 45.82% C, 2.31% H, 7.12% N; found 45.61% C, 2.12% H, 6.99% N; IR (KBr, cm-1): 3429 (CO, overtone), 3101 (ArH), 2962 (CH3, stretching), 1717 (CO), 1345 (SO2, antisymmetric vibrations), 1153 (SO2, symmetric vibrations), 1645, 1610 and 1546 (aromatic vibrations); 1H-NMR δ: 8.78 (s, 1H, 8-ArH), 8.55 (s, 1H, 10-ArH), 8.44 (d, 1H, 1-ArH), 7.81 (t, 1H, 3-ArH), 7.45-7.52 (m, 2H, 2- and 4-ArH) and 2.2 (s, 3H, CH3); 13C-NMR δ: 161.50, 153.85, 153.17, 148.29, 148.0154, 141.51, 132.23, 123.69, 122.43, 117.14, 116.96, 115.31, 114.29, 92.60 and 12.05.
Thiazolo[3’,2’:2,3]-1,2,4-thiadiazino[6,5-c]benzopyran-6-one 7,7-dioxide (8a). Yellowish crystals recrystallized from methanol. Yield 68 %; m.p. > 300 ºC (dec); Anal. Calc. for C12H6N2O4S2 (306.31): 47.05% C, 1.97% H, 9.15% N; found 46.68% C, 1.93% H, 8.72% N; IR (KBr, cm-1): 1707 (CO), 1321 (SO2, antisymmetric vibrations), 1154 (SO2, symmetric vibrations), 1627, 1612 and 1568 (aromatic vibrations); 1H-NMR δ: 8.36 (d, 1H, 8-ArH), 8.30 (d, 1H, 1-ArH), 7.79 (t, 1H, 3-ArH), 7.63 (d, 1H, 9-ArH) and 7.43-7.50 (m, 2H, 2- and 4-ArH); 13C-NMR δ: 169.14, 156.22, 154.38, 153.63, 135.42, 126.75, 126.28, 125.58, 122.18, 117.46, 116.82 and 115.09; MS (ESI pos) m/z: 307 [M+H]+, 370 [M+Na+CH3CN]+ and 635 [2M+Na]+; (EI) m/z: 306 [M]+ and 242 [M-SO2]+.
9-Methylthiazolo[3’,2’:2,3]-1,2,4-thiadiazino[6,5-c]benzopyran-6-one 7,7-dioxide (8b). Yellowish crystals recrystallized from acetonitrile. Yield 71 %; m.p. 240-245 ºC (dec); Anal. Calc. for C13H8N2O4S2 (320.34): 48.74% C, 2.52% H, 8.74% N; found 48.11% C, 2.20% H, 8.34% N; IR (KBr, cm-1): 1709 (CO), 1323 (SO2, antisymmetric vibrations), 1156 (SO2, symmetric vibrations), 1625, 1612 and 1569 (aromatic vibrations); 1H-NMR δ: 8.28 (d, 1H, 8-ArH), 8.17 (d, 1H, 1-ArH), 7.79 (t, 1H, 3-ArH), 7.43-7.49 (m, 2H, 2- and 4-ArH) and 2.20 (s, 3H, CH3); 13C-NMR δ: 169.16, 153.92, 153.23, 135.06, 126.90, 126.37, 125.25, 122.33, 119.84, 117.95, 117.10, 113.46 and 11.75; MS (ESI pos) m/z: 321 [M+H]+, 384 [M+Na+CH3CN]+ and 663 [2M+Na]+; (EI) m/z: 320 [M]+ and 256 [M-SO2]+.
4-(5-Chloropyridin-2-ylamino)-benzopyran-2-one (9). This compound was obtained as white crystals after two recrystallizations of 7d from ethanol; m.p. > 300 ºC (dec); Anal. Calc. for C14H9ClN2O2 (272.69): 61.66% C, 3.33% H, 10.27% N; found 61.01% C, 2.98% H, 9.85% N; IR (KBr, cm-1): 3328 (NH), 1672 (CO), 1626, 1614 and 1561 (aromatic vibrations); 1H-NMR δ: 9.53 (s, 1H, NH), 8.43 (s, 1H, 6-PyH), 8.31 (d, 1H, 4-PyH), 7.89 (d, 1H, 5-CumH), 7.65 (t, 1H, 7-CumH), 7.36-7.50 (m, 3H, 6-CumH, 8-CumH and 3-PyH) and 7.34 (s, 1H, 3-CumH); 13C-NMR δ: 161.96, 153.49, 152.96, 148.21, 145.79, 138.35, 132.54, 124.99, 124.03, 123.26, 117.54, 116.73, 114.97 and 92.87; MS (ESI pos) m/z: 273 [M+H]+; (EI) m/z: 272 [M]+ and 244 [M-CO]+.
8H,10H-1,2,4-thiadiazino[6,5-c]benzopyran-9-thioxo-6-one 7,7-dioxide (11). Yellowish crystals purified with column chromatography. Yield 49.5 %; m.p. 278-280 ºC (dec); Anal. Calc. for C10H6N2O4S2 (282.29): 42.55% C, 2.14% H, 9.92% N; found 42.12% C, 1.96% H, 9.08% N; IR (KBr, cm–1): 3206 (NH), 3027 (NH), 1720 (CO), 1416 (SO2, antisymmetric vibrations), 1187 (SO2, symmetric vibrations); 1H-NMR δ: 9.27 (br s, 2H, NH); 7.75-7.90 (m, 2H, ArH); 7.49 (m, 2H, ArH). 13C-NMR δ: 167.33; 155.79; 153.20; 152.07; 135.43; 125.95; 124.82; 117.78; 117.58; 115.54; MS (EI) m/z: 282 [M]+.
Acknowledgements
We are grateful to Deutscher Akademischer Austausch Dienst (DAAD) for financial support. We also appreciate the technical support and hospitality from the Faculty of Chemistry and Mineralogy at the University of Leipzig, especially to the PhD student Jens Baldamus at the same Faculty who helped with the crystal structure determination.
References and Notes
- Gupta, R. R.; Kumar, M.; Gupta, V. Heterocyclic Chemistry; Springer-Verlag: Berlin, 1998. [Google Scholar]
- Fed. Reg. 19 1239 (Mar. 5, 1954)
- Sax, N. I. Dangerous Properties of Industrial Materials (4th Ed.); Van Nostrand Co.: New York, 1975. [Google Scholar]
- Manolov, I.; Danchev, N.D. Synthesis, toxicological and pharmacological assessment of some 4-hydroxycoumarins. Eur. J. Med. Chem. Chim. Ther. 1995, 30, 531–536. [Google Scholar] [CrossRef]
- Arora, R. B.; Mathur, C. N. Relationship between structure and anticoagulant activity of coumarin derivatives. Brit. J. Pharmacol. 1963, 20, 29–35. [Google Scholar]
- Ziegler, E.; Rossmann, U. Chemistry of 4-hydroxycoumarins. VIII. Synthesis of anticoagulants. Monatsh. Chem. 1957, 88, 25–34. [Google Scholar] [CrossRef]
- Al-Haiza, M. A.; Mostafa, M. S.; El-Kady, M. Y. Synthesis and Biological Evaluation of Some New Coumarin Derivatives. Molecules 2003, 8, 275–286. [Google Scholar]
- Dutton, C. J.; Sutcliffe, J.; Yang, B. 4-Hydroxy coumarin derivatives with antibacterial activity. PCT Int. Appl. US1993/006308 1994. [Google Scholar]
- Parmar, V. S.; Bisht, K. S.; Jain, R.; Singh, S.; Sharma, S. K.; Gupta, S.; Malhotra, S.; Tyagi, O. D.; Vardhan, A.; Pati, H. N.; Berghe, D. V.; Vlietinck, A. J. Synthesis, Antimicrobial and Antiviral Activities of Novel Polyphenolic Compounds. Ind. J. Chem. Sec. B 1996, 35, 220–232. [Google Scholar]
- Ishikawa, T.; Kotake, K.I.; Ishii, H. Synthesis of Toddacoumaquinone, a Coumarin-Naphtho-quinone Dimer, and Its Antiviral Activities. Chem. Pharm. Bull. 1995, 43, 1039–1041. [Google Scholar] [CrossRef] [PubMed]
- Nofal, Z. M.; El-Zahar, M. I.; Abd El-Karim, S. S. Novel Coumarin Derivatives with Expected Biological Activity. Molecules 2000, 5, 99–113. [Google Scholar]
- Raev, L.; Voinova, E.; Ivanov, I.; Popov, D. Antitumor activity of some coumarin derivatives. Pharmazie 1990, 45, 696–702, [Chem. Abstr. 1990, 114, 74711 B]. [Google Scholar]
- Valenti, P.; Rampa, A.; Recanatini, M.; Bisi, A.; Belluti, F.; Da Re, P.; Carrara, M.; Cima, L. Synthesis, cytotoxicity and SAR of simple geiparvarin analogues. Anticancer Drug Des. 1997, 12, 443–451. [Google Scholar] [PubMed]
- Shah, A.; Naliapara, Y.; Sureja, D.; Motohashi, N.; Kawase, M.; Miskolci, C.; Szabo, D.; Molnar, J. 6,12-Dihydro-1-Benzopyrano[3,4-b][1,4]Benzothiazin-6-ones Synthesis and MDR Reversal in Tumor Cells. Anticancer Res. 1998, 18, 3001–3004. [Google Scholar]
- El-Sayed, A. M.; Abd-Allah, O. A. Synthetic and Biological Studies on Coumarin Hydrazone Derivatives. Phosporus, Sulfur Silicon Relat. Elem. 2001, 170, 75–86. [Google Scholar]
- El-Agrody, A. M.; Abd El-Latif, M. S.; El-Hady, N. A.; Fakery, A. H.; Bedair, A. H. Hetero-aromatization with 4-hydroxycoumarin. Part II. Molecules 2001, 6, 519–527. [Google Scholar]
- Emmanuel-Giota, A. A.; Fylaktakidou, K. C.; Hadjipavlou-Litina, D. J.; Litinas, K. E.; Nicolaides, D. N. Synthesis and biological evaluation of several 3-(coumarin-4-yl)-tetrahydroisoxazole and 3-(coumarin-4-yl)-dihydropyrazole derivatives. J. Heterocycl. Chem. 2001, 38, 717–722. [Google Scholar]
- Spino, C.; Dodier, M.; Sotheeswaran, S. Anti-HIV Coumarins from Calophyllum Seed Oil. Bioorg. Med. Chem. Lett. 1998, 8, 3475–3478. [Google Scholar] [CrossRef] [PubMed]
- Thaisrivongs, S.; Watenpaugh, K. D.; Howe, W. J.; Tomich, P. K.; Dolak, L. A.; Chong, K.-T.; Tomich, C.-S. C.; Tomasselli, A. G.; Turner, S. R.; Strohbach, J. W.; Mulichak, A. M.; Janakiraman, M. N.; Moon, J. B.; Lynn, J. C.; Horng, M.-M.; Hinshaw, R. R.; Curry, K. A.; Rothrock, D. J. Structure-Based Design of Novel HIV Protease Inhibitors: Carboxamide-Containing 4-Hydroxycoumarins and 4-Hydroxy-2-pyrones as Potent Nonpeptidic Inhibitors. J. Med. Chem. 1995, 38, 3624–3637. [Google Scholar] [CrossRef] [PubMed]
- Gaddam, S.; Khilevich, A.; Filer., C.; Rizzo, J. D.; Giltner, J.; Flavin, M. T.; Xu, Z. Q. Synthesis of Dual C-14-Labeled (+)-Calanolide-A, a Naturally-Occurring Anti-HIV Agent. J. Label. Comp. Radiopharm. 1997, 39, 901–906. [Google Scholar]
- Zhao, H.; Neamati, N.; Hong, H.; Mazumder, A.; Wang, S.; Sunder, S.; Milner, G. W. A.; Pommier, Y.; Burke, T.R., Jr. Coumarin-Based Inhibitors of HIV Integrase. J. Med. Chem. 1997, 40, 242–249. [Google Scholar] [CrossRef] [PubMed]
- García-Valverde, M.; Tomás, T. Sulfur-Nitrogen Heterocycles. Molecules 2005, 10, 318–320. [Google Scholar]
- Vega, S.; Diaz, J. A.; Arranz, E. Thieno- and pyrazolo-1,2,4-thiadiazino-1,1-dioxides with anti-HIV activity. ES2136013 1999. [Google Scholar]
- Arranz, M. E.; Diaz, J. A.; Ingate, S. T.; Witvrouw, M.; Pannecouque, C.; Balzarini, J.; De Clerq, E.; Vega, S. Synthesis and anti-HIV activity of 1,1,3-trioxo-2H,4H-thieno[3,4-e][1,2,4]-thiadiazines (TTDs): a new family of HIV-1 specific non-nucleozide reversetranscriptase inhibitors. Bioorg. Med. Chem. 1999, 7, 2811–2822. [Google Scholar] [CrossRef] [PubMed]
- Vega, S.; Arranz, M. E. 4H-Thieno[3,4-e]- and 4H-pyrazolo[4,3-e]-1,2,4-thiadiazine 1,1-dioxides. Synthesis, chemical properties and evaluation of their potential cardiovascular activity. J. Heterocycl. Chem. 2004, 41, 45–50. [Google Scholar]
- Di Bella, M.; Monzani, A.; Andrisano, M. G.; Fabio, U.; Quaglio, G. P. Antimicrobial effect of derivatives of 1,2,4-benzothiadiazine-1,1-dioxide. Part VII. Ediz. Scient. 1979, 34, 81–88. [Google Scholar]
- Robertson, D. W.; Steinberg, M. I. Potassium channel modulators: scientific applications and therapeutic promise. J. Med. Chem. 1990, 33, 1529–1541. [Google Scholar] [CrossRef] [PubMed]
- Longman, S. D; Hamilton, T. C. Potassium channel activator drugs: mechanism of action, pharmacological properties, and therapeutical potential. Med. Res. Rev. 1992, 12, 73–148. [Google Scholar]
- Tabakovic, K.; Tabakovic, I.; Trkovnik, M.; Trinajstic, N. Chemistry of Coumarin.-Nucleophilic Substitutions of 4-Chloro-3-nitrocoumarin with Hard and Soft Nucleophiles. Liebigs Ann. Chem. 1983, 11, 1901–1909. [Google Scholar]
- Checchi, S.; Pecori, V. L.; Bambagiotti, A. M. 4-Hydroxycoumarins. VII. Reactivity of 4-chloro- and 4-hydroxycoumarin-3-sulfonyl chlorides. Gazz. Chim. Ital. 1967, 97, 1749–1761. [Google Scholar]
- Vogel, A. I. Vogel’s Text-book of Practical Organic Chemistry, 5th ed.; Longman: London, 1989; pp. 873-874; 874-878. [Google Scholar]
- Huebner, C. F.; Link, K. P. Studies on 4-hydroxycoumarin. VII. Reactions of 4-hydroxycoumarin with cationoid reagents. J. Am. Chem. Soc. 1945, 67, 99–102. [Google Scholar]
- CCDC 646550 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif, or by emailing data_request@ccdc.cam.ac.uk, or by contacting The Cambridge Crystallographic Data Centre, 12, Union Road, Cambridge CB2 1EZ, UK; fax: +44 1223 336033
- Smith, M. B. March’s Advanced Organic Chemistry; Reactions, Mechanism and Structure, 5th ed.; John Wiley & Sons, Inc.: New York, 2001; pp. 363–364. [Google Scholar]
- Kovác, M.; Sabatié, A.; Floch, L. Synthesis of coumarin sulfonamides and sulfonylurea. ARKIVOC 2001, 100–108. [Google Scholar]
- Sample Availability: Samples of compounds 2, 3, 4, 7a-f, 8a,b, 9 and 11 are available from the authors.
© 2007 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.