Abstract
Two furostanol saponins were obtained from the rhizomes of Tupistra chinensis Bak. Their structures were determined as 5β-furost-Δ25(27)-en-1β,2β,3β,4β,5β,7α,22ξ,26-octaol-6-one-26-O-β-D-glucopyranoside (1) and 5β-furost-Δ25(27)-en-1β,2β,3β,4β,5β,6β,7α,22ξ,26-nonaol-26-O-β-D-glucopyranoside (2), on the basis of chemical and spectroscopic evidence. Both compounds displayed marked inhibitory action against NO production in rat abdomen macrophages induced by lipopolysaccharide (LPS) at 40 μg/mL.
Introduction
Kai-Kou-Jian, the Chinese name for rhizomes of Tupistra chinensis Bak., is reputed in Shennongjia Forest District, a National Natural Protection Region of China, as a folk medicine for its anti-inflammatory effects [1,2]. NO acts as an inter-and intracellular messenger molecule in many cell types and overproduction of NO plays a central role in inflammatory responses [3,4]. After exposure to endogenous and exogenous stimulators, overproduction of NO can be induced quantitatively in macrophages. Lipopolysaccharide (LPS) is a component of Gram-negative bacteria, and the induction of NO overproduction by LPS has been demonstrated [5,6]. Therefore, the development of agents capable of blocking LPS-induced NO overproduction can be regarded as a potential therapeutic target for the treatment of inflammation. In the course of our search for anti-inflammatory agents from the native folk medicines of Shennongjia Forest District [7,8,9,10,11,12,13,14,15,16,17], two new furostanol saponins were isolated and their structures were elucidated by means of chemical and spectroscopic methods. Although studies on the lipophilic constituents, including the sapogenins of this plant, were reported in several papers [18,19,20,21,22], the steroidal saponins, especially those showing anti-inflammatory activities, were hardly elucidated yet [10]. This paper reports the structural elucidation of two steroidal saponins from this plant and their inhibition of NO production.
Results and Discussion
Compound 1 was obtained as a white amorphous powder, mp 170~172°C and 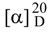 : -60.7° (CH3OH; c 0.94). Positive coloration reactions were observed when 1 was subjected to Ehrlich, Molish and Liebermann-Buchard tests, which suggested that 1 had a furostanol saponin skeleton. Its molecular formula was established as C33H52O15 by its HR-FAB-MS spectrum in positive ion mode, which showed a quasi-molecular ion peak at m/z 711.3208 [M+Na]+ (calcd. 711.3204 for C33H52O15Na). The molecular formula was further confirmed by the broad band and DEPT 13C-NMR spectra, which showed 33 signals comprising three methyl, eight methylene, sixteen methine and six quaternary carbons (Table 1). The downfield signal at δ 210.02 could be assigned as a carbonyl carbon and the presence of an olefinic bond could be deduced from a quaternary signal at δ 144.12 and a methylene signal at δ 112.73. The hemiketal carbon in the aglycone could be inferred by the signal at δ 110.79 [23]. A downfield signal at δ 80.78 could be assigned to the carbon bearing hydroxyl group located at furan ring of aglycone [24]. A methylene signal at δ 71.60 is a typical glycosidation carbon neighboring to an olefinic bond [25], ascribable to C-26 of the side chain. A methine carbon signal at δ 60.70 could be assigned to C-17 of aglycone. The presence of three methyl groups could be inferred by signals at δ 14.90, 10.87 and 14.00, respectively. The 1H-NMR data of 1 contained a methenyl proton at δ 4.68 (m), a doublet methyl group at δ 1.07 (d, J = 6.5 Hz) and two singlet methyl groups at δ 0.88 and 1.08 (each s), two typical protons bonded to ending olefinic carbon at δ 5.20 and 5.11 (each 1H, br s), attributable to a steroidal aglycone moiety [26]. Furthermore, the furostanol glycosidic nature of 1 was confirmed by the strong absorption bands at 3410 and 1050 cm-1 in the IR spectrum, and the signal due to semiketal carbon at δ110.79 in the 13C-NMR spectrum [23]. Upon acid hydrolysis of 1 with 2.0 mol/L HCl, only glucose was detected on thin layer chromatography and paper chromatography in the hydrolyzed product. Besides the spectral data due to aglycone, a group of downfield proton signals due to a hexose moiety were observed at δ 4.51 (1H, d, J = 8.0 Hz), 3.43 (1H, d, J = 8.0 Hz), 3.48 (1H, m), 3.53 (1H, t, J = 9.0 Hz), 3.35 (1H, dd, J = 9.0, 8.0 Hz), 3.87 (1H, d, J = 12.5 Hz) and 3.76 (1H, dd, J = 12.5, 8.0 Hz) in the 1H-NMR spectrum, and the corresponding carbon signals were observed at δ 100.56, 74.01,75.34, 69.24, 75.41 and 60.33 in the HMQC spectrum. The signal due to the anomeric carbon at δ 100.56 showed a correlation with the signal due to H-26 at δ 4.41, and the signal due to the anomeric proton at δ 4.51 showed a correlation with the signal due to C-26 at δ 71.60 in the HMBC spectrum, which indicated that the sugar moiety was attached to C-26 of aglycone. Comparison of the 13C-NMR spectral data of 1 with those of 5β-furost-Δ25(27)-en-22-methoxyl-1β,2β,3β,4β,5β,7α,26-octaol-6-one 26-O-β-D-glucopyranoside [3] suggested that their chemical shifts were in good agreement, except for those due to C-20, C-22 and C-23, which resulted from the methylation of 22-hydroxyl group. Accordingly, 1 was identified as 5β-furost-Δ25(27)-en-1β,2β,3β,4β,5β,7α,22ξ,26-octaol-6-one 26-O-β-D-glucopyranoside. This structure was additionally confirmed by 2D NMR experiments, including 1H-1H COSY, TOCSY, NOESY, HMQC and HMBC.
: -60.7° (CH3OH; c 0.94). Positive coloration reactions were observed when 1 was subjected to Ehrlich, Molish and Liebermann-Buchard tests, which suggested that 1 had a furostanol saponin skeleton. Its molecular formula was established as C33H52O15 by its HR-FAB-MS spectrum in positive ion mode, which showed a quasi-molecular ion peak at m/z 711.3208 [M+Na]+ (calcd. 711.3204 for C33H52O15Na). The molecular formula was further confirmed by the broad band and DEPT 13C-NMR spectra, which showed 33 signals comprising three methyl, eight methylene, sixteen methine and six quaternary carbons (Table 1). The downfield signal at δ 210.02 could be assigned as a carbonyl carbon and the presence of an olefinic bond could be deduced from a quaternary signal at δ 144.12 and a methylene signal at δ 112.73. The hemiketal carbon in the aglycone could be inferred by the signal at δ 110.79 [23]. A downfield signal at δ 80.78 could be assigned to the carbon bearing hydroxyl group located at furan ring of aglycone [24]. A methylene signal at δ 71.60 is a typical glycosidation carbon neighboring to an olefinic bond [25], ascribable to C-26 of the side chain. A methine carbon signal at δ 60.70 could be assigned to C-17 of aglycone. The presence of three methyl groups could be inferred by signals at δ 14.90, 10.87 and 14.00, respectively. The 1H-NMR data of 1 contained a methenyl proton at δ 4.68 (m), a doublet methyl group at δ 1.07 (d, J = 6.5 Hz) and two singlet methyl groups at δ 0.88 and 1.08 (each s), two typical protons bonded to ending olefinic carbon at δ 5.20 and 5.11 (each 1H, br s), attributable to a steroidal aglycone moiety [26]. Furthermore, the furostanol glycosidic nature of 1 was confirmed by the strong absorption bands at 3410 and 1050 cm-1 in the IR spectrum, and the signal due to semiketal carbon at δ110.79 in the 13C-NMR spectrum [23]. Upon acid hydrolysis of 1 with 2.0 mol/L HCl, only glucose was detected on thin layer chromatography and paper chromatography in the hydrolyzed product. Besides the spectral data due to aglycone, a group of downfield proton signals due to a hexose moiety were observed at δ 4.51 (1H, d, J = 8.0 Hz), 3.43 (1H, d, J = 8.0 Hz), 3.48 (1H, m), 3.53 (1H, t, J = 9.0 Hz), 3.35 (1H, dd, J = 9.0, 8.0 Hz), 3.87 (1H, d, J = 12.5 Hz) and 3.76 (1H, dd, J = 12.5, 8.0 Hz) in the 1H-NMR spectrum, and the corresponding carbon signals were observed at δ 100.56, 74.01,75.34, 69.24, 75.41 and 60.33 in the HMQC spectrum. The signal due to the anomeric carbon at δ 100.56 showed a correlation with the signal due to H-26 at δ 4.41, and the signal due to the anomeric proton at δ 4.51 showed a correlation with the signal due to C-26 at δ 71.60 in the HMBC spectrum, which indicated that the sugar moiety was attached to C-26 of aglycone. Comparison of the 13C-NMR spectral data of 1 with those of 5β-furost-Δ25(27)-en-22-methoxyl-1β,2β,3β,4β,5β,7α,26-octaol-6-one 26-O-β-D-glucopyranoside [3] suggested that their chemical shifts were in good agreement, except for those due to C-20, C-22 and C-23, which resulted from the methylation of 22-hydroxyl group. Accordingly, 1 was identified as 5β-furost-Δ25(27)-en-1β,2β,3β,4β,5β,7α,22ξ,26-octaol-6-one 26-O-β-D-glucopyranoside. This structure was additionally confirmed by 2D NMR experiments, including 1H-1H COSY, TOCSY, NOESY, HMQC and HMBC.
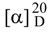 : -60.7° (CH3OH; c 0.94). Positive coloration reactions were observed when 1 was subjected to Ehrlich, Molish and Liebermann-Buchard tests, which suggested that 1 had a furostanol saponin skeleton. Its molecular formula was established as C33H52O15 by its HR-FAB-MS spectrum in positive ion mode, which showed a quasi-molecular ion peak at m/z 711.3208 [M+Na]+ (calcd. 711.3204 for C33H52O15Na). The molecular formula was further confirmed by the broad band and DEPT 13C-NMR spectra, which showed 33 signals comprising three methyl, eight methylene, sixteen methine and six quaternary carbons (Table 1). The downfield signal at δ 210.02 could be assigned as a carbonyl carbon and the presence of an olefinic bond could be deduced from a quaternary signal at δ 144.12 and a methylene signal at δ 112.73. The hemiketal carbon in the aglycone could be inferred by the signal at δ 110.79 [23]. A downfield signal at δ 80.78 could be assigned to the carbon bearing hydroxyl group located at furan ring of aglycone [24]. A methylene signal at δ 71.60 is a typical glycosidation carbon neighboring to an olefinic bond [25], ascribable to C-26 of the side chain. A methine carbon signal at δ 60.70 could be assigned to C-17 of aglycone. The presence of three methyl groups could be inferred by signals at δ 14.90, 10.87 and 14.00, respectively. The 1H-NMR data of 1 contained a methenyl proton at δ 4.68 (m), a doublet methyl group at δ 1.07 (d, J = 6.5 Hz) and two singlet methyl groups at δ 0.88 and 1.08 (each s), two typical protons bonded to ending olefinic carbon at δ 5.20 and 5.11 (each 1H, br s), attributable to a steroidal aglycone moiety [26]. Furthermore, the furostanol glycosidic nature of 1 was confirmed by the strong absorption bands at 3410 and 1050 cm-1 in the IR spectrum, and the signal due to semiketal carbon at δ110.79 in the 13C-NMR spectrum [23]. Upon acid hydrolysis of 1 with 2.0 mol/L HCl, only glucose was detected on thin layer chromatography and paper chromatography in the hydrolyzed product. Besides the spectral data due to aglycone, a group of downfield proton signals due to a hexose moiety were observed at δ 4.51 (1H, d, J = 8.0 Hz), 3.43 (1H, d, J = 8.0 Hz), 3.48 (1H, m), 3.53 (1H, t, J = 9.0 Hz), 3.35 (1H, dd, J = 9.0, 8.0 Hz), 3.87 (1H, d, J = 12.5 Hz) and 3.76 (1H, dd, J = 12.5, 8.0 Hz) in the 1H-NMR spectrum, and the corresponding carbon signals were observed at δ 100.56, 74.01,75.34, 69.24, 75.41 and 60.33 in the HMQC spectrum. The signal due to the anomeric carbon at δ 100.56 showed a correlation with the signal due to H-26 at δ 4.41, and the signal due to the anomeric proton at δ 4.51 showed a correlation with the signal due to C-26 at δ 71.60 in the HMBC spectrum, which indicated that the sugar moiety was attached to C-26 of aglycone. Comparison of the 13C-NMR spectral data of 1 with those of 5β-furost-Δ25(27)-en-22-methoxyl-1β,2β,3β,4β,5β,7α,26-octaol-6-one 26-O-β-D-glucopyranoside [3] suggested that their chemical shifts were in good agreement, except for those due to C-20, C-22 and C-23, which resulted from the methylation of 22-hydroxyl group. Accordingly, 1 was identified as 5β-furost-Δ25(27)-en-1β,2β,3β,4β,5β,7α,22ξ,26-octaol-6-one 26-O-β-D-glucopyranoside. This structure was additionally confirmed by 2D NMR experiments, including 1H-1H COSY, TOCSY, NOESY, HMQC and HMBC.
: -60.7° (CH3OH; c 0.94). Positive coloration reactions were observed when 1 was subjected to Ehrlich, Molish and Liebermann-Buchard tests, which suggested that 1 had a furostanol saponin skeleton. Its molecular formula was established as C33H52O15 by its HR-FAB-MS spectrum in positive ion mode, which showed a quasi-molecular ion peak at m/z 711.3208 [M+Na]+ (calcd. 711.3204 for C33H52O15Na). The molecular formula was further confirmed by the broad band and DEPT 13C-NMR spectra, which showed 33 signals comprising three methyl, eight methylene, sixteen methine and six quaternary carbons (Table 1). The downfield signal at δ 210.02 could be assigned as a carbonyl carbon and the presence of an olefinic bond could be deduced from a quaternary signal at δ 144.12 and a methylene signal at δ 112.73. The hemiketal carbon in the aglycone could be inferred by the signal at δ 110.79 [23]. A downfield signal at δ 80.78 could be assigned to the carbon bearing hydroxyl group located at furan ring of aglycone [24]. A methylene signal at δ 71.60 is a typical glycosidation carbon neighboring to an olefinic bond [25], ascribable to C-26 of the side chain. A methine carbon signal at δ 60.70 could be assigned to C-17 of aglycone. The presence of three methyl groups could be inferred by signals at δ 14.90, 10.87 and 14.00, respectively. The 1H-NMR data of 1 contained a methenyl proton at δ 4.68 (m), a doublet methyl group at δ 1.07 (d, J = 6.5 Hz) and two singlet methyl groups at δ 0.88 and 1.08 (each s), two typical protons bonded to ending olefinic carbon at δ 5.20 and 5.11 (each 1H, br s), attributable to a steroidal aglycone moiety [26]. Furthermore, the furostanol glycosidic nature of 1 was confirmed by the strong absorption bands at 3410 and 1050 cm-1 in the IR spectrum, and the signal due to semiketal carbon at δ110.79 in the 13C-NMR spectrum [23]. Upon acid hydrolysis of 1 with 2.0 mol/L HCl, only glucose was detected on thin layer chromatography and paper chromatography in the hydrolyzed product. Besides the spectral data due to aglycone, a group of downfield proton signals due to a hexose moiety were observed at δ 4.51 (1H, d, J = 8.0 Hz), 3.43 (1H, d, J = 8.0 Hz), 3.48 (1H, m), 3.53 (1H, t, J = 9.0 Hz), 3.35 (1H, dd, J = 9.0, 8.0 Hz), 3.87 (1H, d, J = 12.5 Hz) and 3.76 (1H, dd, J = 12.5, 8.0 Hz) in the 1H-NMR spectrum, and the corresponding carbon signals were observed at δ 100.56, 74.01,75.34, 69.24, 75.41 and 60.33 in the HMQC spectrum. The signal due to the anomeric carbon at δ 100.56 showed a correlation with the signal due to H-26 at δ 4.41, and the signal due to the anomeric proton at δ 4.51 showed a correlation with the signal due to C-26 at δ 71.60 in the HMBC spectrum, which indicated that the sugar moiety was attached to C-26 of aglycone. Comparison of the 13C-NMR spectral data of 1 with those of 5β-furost-Δ25(27)-en-22-methoxyl-1β,2β,3β,4β,5β,7α,26-octaol-6-one 26-O-β-D-glucopyranoside [3] suggested that their chemical shifts were in good agreement, except for those due to C-20, C-22 and C-23, which resulted from the methylation of 22-hydroxyl group. Accordingly, 1 was identified as 5β-furost-Δ25(27)-en-1β,2β,3β,4β,5β,7α,22ξ,26-octaol-6-one 26-O-β-D-glucopyranoside. This structure was additionally confirmed by 2D NMR experiments, including 1H-1H COSY, TOCSY, NOESY, HMQC and HMBC. 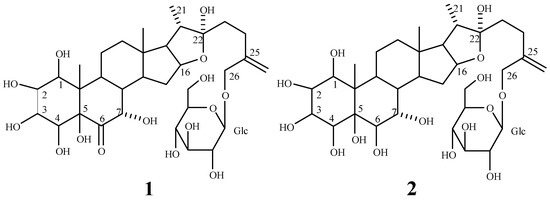
Figure 1.
Structures of compounds 1 and 2.
Compound 2 was obtained as a white powder, mp 178~180°C and 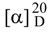 : -27.0° (CH3OH; c 0.59). A skeleton of furostanol saponin was inferred by the positive coloration reactions of 1 with Ehrlich, Molish and Liebermann-Buchard reagents. This skeleton was confirmed by the strong absorption bands at 3406 cm-1 and 1056 cm-1 in the IR spectrum and the signal due to a semiketal carbon (C-22) at δ 111.34 in the 13C-NMR spectrum [23]. Its molecular formula was deduced as C33H54O15 from its HR-FAB-MS spectrum in positive ion mode, which showed a quasi-molecular ion peak at m/z 713.3367 [M+Na]+ (calcd. 713.3360 for C33H54O15Na). This formula was in good agreement with the broad band and DEPT 13C-NMR spectra, which showed 33 signals containing three methyl, eight methylene, seventeen methine and five quaternary carbons (Table 1). A downfield signal due to methine carbon at δ 81.52 showed correlation with a downfield signal at δH 4.66, attributable to H-16, which showed a correlation with semiketal signal at δ 111.34 in the HMBC spectrum. The presence of a terminal olefinic bond was inferred by the signal due to a quaternary carbon at δ 144.67, as well as the signal due to a methylene carbon at δ 113.26, which showed correlations with two olefinic proton signals at δ 5.20 and 5.18 (each 1H, br s) in the HMQC spectrum. A methylene carbon signal at δ 71.96 showed correlation with signals at δH 4.41 (1H, d, J = 13.0 Hz) and 4.25 (1H, d, J =13.0 Hz), ascribable to H-26. H-26 showed connectivity with an anomeric carbon signal at δ 101.10 and the olefinic carbon signal at δ 144.67 in the HMBC spectrum. A β-D-glucopyranosyl moiety was inferred by the facts that a group of proton signals at δ 4.51 (1H, d, J = 8.0 Hz), 3.43 (1H, dd, J = 10.5, 8.0 Hz), 3.47 (1H, m), 3.54 (1H, t, J = 9.0 Hz), 3.35 (1H, dd, J = 9.0, 8.0 Hz), 3.97 (1H, d, J = 12.5 Hz) and 3.77 (1H, dd, J = 12.5, 8.0 Hz) showed correlations with a group of carbon signals at δ 101.10, 73.78, 75.52, 69.29, 75.94 and 60.88, respectively, in the HMQC spectrum. This group of proton signals showed correlations with each other in the TOCSY spectrum.
: -27.0° (CH3OH; c 0.59). A skeleton of furostanol saponin was inferred by the positive coloration reactions of 1 with Ehrlich, Molish and Liebermann-Buchard reagents. This skeleton was confirmed by the strong absorption bands at 3406 cm-1 and 1056 cm-1 in the IR spectrum and the signal due to a semiketal carbon (C-22) at δ 111.34 in the 13C-NMR spectrum [23]. Its molecular formula was deduced as C33H54O15 from its HR-FAB-MS spectrum in positive ion mode, which showed a quasi-molecular ion peak at m/z 713.3367 [M+Na]+ (calcd. 713.3360 for C33H54O15Na). This formula was in good agreement with the broad band and DEPT 13C-NMR spectra, which showed 33 signals containing three methyl, eight methylene, seventeen methine and five quaternary carbons (Table 1). A downfield signal due to methine carbon at δ 81.52 showed correlation with a downfield signal at δH 4.66, attributable to H-16, which showed a correlation with semiketal signal at δ 111.34 in the HMBC spectrum. The presence of a terminal olefinic bond was inferred by the signal due to a quaternary carbon at δ 144.67, as well as the signal due to a methylene carbon at δ 113.26, which showed correlations with two olefinic proton signals at δ 5.20 and 5.18 (each 1H, br s) in the HMQC spectrum. A methylene carbon signal at δ 71.96 showed correlation with signals at δH 4.41 (1H, d, J = 13.0 Hz) and 4.25 (1H, d, J =13.0 Hz), ascribable to H-26. H-26 showed connectivity with an anomeric carbon signal at δ 101.10 and the olefinic carbon signal at δ 144.67 in the HMBC spectrum. A β-D-glucopyranosyl moiety was inferred by the facts that a group of proton signals at δ 4.51 (1H, d, J = 8.0 Hz), 3.43 (1H, dd, J = 10.5, 8.0 Hz), 3.47 (1H, m), 3.54 (1H, t, J = 9.0 Hz), 3.35 (1H, dd, J = 9.0, 8.0 Hz), 3.97 (1H, d, J = 12.5 Hz) and 3.77 (1H, dd, J = 12.5, 8.0 Hz) showed correlations with a group of carbon signals at δ 101.10, 73.78, 75.52, 69.29, 75.94 and 60.88, respectively, in the HMQC spectrum. This group of proton signals showed correlations with each other in the TOCSY spectrum.
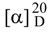 : -27.0° (CH3OH; c 0.59). A skeleton of furostanol saponin was inferred by the positive coloration reactions of 1 with Ehrlich, Molish and Liebermann-Buchard reagents. This skeleton was confirmed by the strong absorption bands at 3406 cm-1 and 1056 cm-1 in the IR spectrum and the signal due to a semiketal carbon (C-22) at δ 111.34 in the 13C-NMR spectrum [23]. Its molecular formula was deduced as C33H54O15 from its HR-FAB-MS spectrum in positive ion mode, which showed a quasi-molecular ion peak at m/z 713.3367 [M+Na]+ (calcd. 713.3360 for C33H54O15Na). This formula was in good agreement with the broad band and DEPT 13C-NMR spectra, which showed 33 signals containing three methyl, eight methylene, seventeen methine and five quaternary carbons (Table 1). A downfield signal due to methine carbon at δ 81.52 showed correlation with a downfield signal at δH 4.66, attributable to H-16, which showed a correlation with semiketal signal at δ 111.34 in the HMBC spectrum. The presence of a terminal olefinic bond was inferred by the signal due to a quaternary carbon at δ 144.67, as well as the signal due to a methylene carbon at δ 113.26, which showed correlations with two olefinic proton signals at δ 5.20 and 5.18 (each 1H, br s) in the HMQC spectrum. A methylene carbon signal at δ 71.96 showed correlation with signals at δH 4.41 (1H, d, J = 13.0 Hz) and 4.25 (1H, d, J =13.0 Hz), ascribable to H-26. H-26 showed connectivity with an anomeric carbon signal at δ 101.10 and the olefinic carbon signal at δ 144.67 in the HMBC spectrum. A β-D-glucopyranosyl moiety was inferred by the facts that a group of proton signals at δ 4.51 (1H, d, J = 8.0 Hz), 3.43 (1H, dd, J = 10.5, 8.0 Hz), 3.47 (1H, m), 3.54 (1H, t, J = 9.0 Hz), 3.35 (1H, dd, J = 9.0, 8.0 Hz), 3.97 (1H, d, J = 12.5 Hz) and 3.77 (1H, dd, J = 12.5, 8.0 Hz) showed correlations with a group of carbon signals at δ 101.10, 73.78, 75.52, 69.29, 75.94 and 60.88, respectively, in the HMQC spectrum. This group of proton signals showed correlations with each other in the TOCSY spectrum.
: -27.0° (CH3OH; c 0.59). A skeleton of furostanol saponin was inferred by the positive coloration reactions of 1 with Ehrlich, Molish and Liebermann-Buchard reagents. This skeleton was confirmed by the strong absorption bands at 3406 cm-1 and 1056 cm-1 in the IR spectrum and the signal due to a semiketal carbon (C-22) at δ 111.34 in the 13C-NMR spectrum [23]. Its molecular formula was deduced as C33H54O15 from its HR-FAB-MS spectrum in positive ion mode, which showed a quasi-molecular ion peak at m/z 713.3367 [M+Na]+ (calcd. 713.3360 for C33H54O15Na). This formula was in good agreement with the broad band and DEPT 13C-NMR spectra, which showed 33 signals containing three methyl, eight methylene, seventeen methine and five quaternary carbons (Table 1). A downfield signal due to methine carbon at δ 81.52 showed correlation with a downfield signal at δH 4.66, attributable to H-16, which showed a correlation with semiketal signal at δ 111.34 in the HMBC spectrum. The presence of a terminal olefinic bond was inferred by the signal due to a quaternary carbon at δ 144.67, as well as the signal due to a methylene carbon at δ 113.26, which showed correlations with two olefinic proton signals at δ 5.20 and 5.18 (each 1H, br s) in the HMQC spectrum. A methylene carbon signal at δ 71.96 showed correlation with signals at δH 4.41 (1H, d, J = 13.0 Hz) and 4.25 (1H, d, J =13.0 Hz), ascribable to H-26. H-26 showed connectivity with an anomeric carbon signal at δ 101.10 and the olefinic carbon signal at δ 144.67 in the HMBC spectrum. A β-D-glucopyranosyl moiety was inferred by the facts that a group of proton signals at δ 4.51 (1H, d, J = 8.0 Hz), 3.43 (1H, dd, J = 10.5, 8.0 Hz), 3.47 (1H, m), 3.54 (1H, t, J = 9.0 Hz), 3.35 (1H, dd, J = 9.0, 8.0 Hz), 3.97 (1H, d, J = 12.5 Hz) and 3.77 (1H, dd, J = 12.5, 8.0 Hz) showed correlations with a group of carbon signals at δ 101.10, 73.78, 75.52, 69.29, 75.94 and 60.88, respectively, in the HMQC spectrum. This group of proton signals showed correlations with each other in the TOCSY spectrum. 
Table 1.
13C- (125 MHz) and 1H- (500 MHz) NMR Data of 1 and 2 (in CD3OD, δ in ppm).
| Position | 1 | 2 | ||||
| 13C | DEPT | 1H (J, Hz) | 13C | DEPT | 1H (J, Hz) | |
| 1 | 74.08 | CH | 3.95, d, (2.0) | 77.01 | CH | 3.88, br s |
| 2 | 65.68 | CH | 3.97, d, (2.0) | 65.24 | CH | 3.90, br s |
| 3 | 72.97 | CH | 4.22, d, (3.5) | 72.15 | CH | 4.18, d, (3.0) |
| 4 | 68.89 | CH | 5.03, d, (3.5) | 68.48 | CH | 4.43, d, (3.0) |
| 5 | 84.86 | C | 77.93 | C | ||
| 6 | 210.02 | C | 73.20 | CH | 4.80, br s | |
| 7 | 72.66 | CH | 3.53, d, (9.0) | 69.78 | CH | 4.12, d, (2.5) |
| 8 | 36.69 | CH | 2.18, br t, (9.0) | 33.03 | CH | 2.16, m |
| 9 | 40.19 | CH | 2.20, br t, (9.0) | 36.81 | CH | 2.31, m |
| 10 | 49.00 | C | 45.41 | C | ||
| 11 | 20.50 | CH2 | 1.38, m | 20.35 | CH2 | 1.36, m |
| 12 | 38.93 | CH2 | 1.60, m; 1.22, m | 38.55 | CH2 | 1.60, td, (13.0, 5.0); 1.21, td, (13.0, 5.0) |
| 13 | 39.77 | C | 39.62 | C | ||
| 14 | 47.76 | CH | 1.96, m | 48.76 | CH | 1.96, m |
| 15 | 29.69 | CH2 | 2.14, m; 1.48, m | 30.61 | CH2 | 2.15, m; 1.45, m |
| 16 | 80.78 | CH | 4.68, m | 81.52 | CH | 4.66, m |
| 17 | 60.70 | CH | 1.66, m | 61.37 | CH | 1.66, m |
| 18 | 14.90 | CH3 | 0.88, s | 15.42 | CH3 | 0.89, s |
| 19 | 10.87 | CH3 | 1.08, s | 14.15 | CH3 | 1.39, s |
| 20 | 40.19 | CH | 1.82, m | 40.34 | CH | 1.83, m |
| 21 | 14.00 | CH3 | 1.07, d, (6.5) | 14.57 | CH3 | 1.08, d, (6.5) |
| 22 | 110.79 | C | 111.34 | C | ||
| 23 | 35.09 | CH2 | 1.87, m; 1.63, m | 35.64 | CH2 | 1.85, m; 1.62, m |
| 24 | 26.29 | CH2 | 2.28, m; 1.98, m | 26.82 | CH2 | 2.30, m; 1.95, m |
| 25 | 144.12 | C | 144.67 | C | ||
| 26 | 71.60 | CH2 | 4.41, d, (12.5, H-ax); 4.25, d, (12.5, H-eq) | 71.96 | CH2 | 4.41, d, (13.0, H-ax); 4.25, d, (13.0, H-eq) |
| 27 | 112.73 | CH2 | 5.20, br s, H-a; 5.11, br s, H-b | 113.26 | CH2 | 5.20, br s, H-a; 5.11, br s, H-b |
| Glc | ||||||
| 1 | 100.56 | CH | 4.51, d, (8.0) | 101.10 | CH | 4.51, d, (8.0) |
| 2 | 74.01 | CH | 3.43, br d, (8.0) | 73.78 | CH | 3.43, dd, (10.5, 8.0) |
| 3 | 75.34 | CH | 3.48, m | 75.52 | CH | 3.47, m |
| 4 | 69.24 | CH | 3.53, t, (9.0) | 69.29 | CH | 3.54, t, (9.0) |
| 5 | 75.41 | CH | 3.35, ddd, (9.0, 5.5, 2.5) | 75.94 | CH | 3.35, ddd, (9.0, 5.5, 2.2) |
| 6 | 60.33 | CH2 | 3.87, dd, (12.5, 2.5); 3.76, dd, (12.5, 5.5) | 60.88 | CH2 | 3.88,dd, (12.5, 2.2); 3.77, dd, (12.5, 5.5) |
Upon acid hydrolysis of 2 with 2.0 mol/L HCl, only glucose was detected on thin layer chromatography and paper chromatography in the hydrolyzed product. The comparison of 1H- and 13C-NMR spectral data of 2 with those of 1 showed that both of chemical shifts were in good agreement, except for those due to A and B rings, especially for C-5, C-6, C-7, C-8, C-9, C-10 and C-19. The carbonyl signal at δC 210.02 in the 13C-NMR spectrum of 1 was not found in the 13C-NMR spectrum of 2, whereas, a methine carbon signal at δC 73.20 was observed in the 13C-NMR spectrum of 2, which suggested that a hydroxyl group be substituted at C-6 of 2. C-6 showed correlation with a downfield proton signal at δ 4.80 in the HMQC spectrum, which could be assigned to H-6. The H-6 showed connectivity with H-8 at δ 2.16 in the NOESY spectrum, which indicated that 6-hyroxyl group was oriented at beta position. The deduction above was confirmed by a good agreement of 1H- and 13C-NMR spectral data due to A-D rings with those of compound 5 [27]. Thus, 2 was identified as 5β-furost-Δ25(27)-en-1β,2β,3β,4β,5β,6β,7α,22ξ,26-nonaol 26-O-β-D-glucopyranoside.
Biological activity
Extracts of Tupistra chinensis rhizomes showed biological effects on tumors [28], phlegm [29], colonitis [30], platelets [31], pains [32], etc. As a folk medicine, it was used to ease pharyngitis and faucitis in Shennongjia Forest District of China [33]. In our experiments, the inhibition of NO production in macrophages of the rat abdomen induced by LPS (lipopolysaccharide) by some extracts and compounds 1 and 2 from Tupistra chinensis rhizomes was observed. Results showed that samples observed exerted marked inhibitory effects on NO production at a doses ranging from 20 to 40 μg/mL (see Table 2).

Table 2.
The inhibitory effects of extracts and compounds 1 and 2 on NO production ( 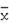 ±S, n= 8).
±S, n= 8).
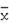 ±S, n= 8).
±S, n= 8).
| Groups | Dosages (μg/mL) | NO content μmol/L | |
|---|---|---|---|
| Blank a) | — | 53.62 ± 7.83 | |
| Model b) | — | 147.35 ± 15.20 | |
| Aspirin c) | 40 | 106.86 ± 4.15 ** g) | |
| n-Butanol soluble fraction d) | 20 | 110.29 ± 58.08 ** | |
| Water soluble fraction e) | 20 | 94.61 ± 26.38 ** | |
| 60% methanolic eluate f) | 20 | 85.78 ± 41.87 ** | |
| Compound 1 | 40 | 87.34 ± 7.33 ** | |
| Compound 2 | 40 | 108.34 ± 11.49 ** | |
a) The blank was the solution without adding cells. b) Control was physiological saline solution. c) Aspirin was used as a positive control. d), e), f) see the experimental section. g) Mean value is significantly different (*p < 0.05, **p < 0.01) from the control.
Conclusions
Two new polyhydroxylated furostanol saponins were characterized as 5β-furost-Δ25(27)-en-1β,2β,3β,4β,5β, 7α,22α,26-octaol-6-one 26-O-β-D-glucopyranoside (1) and 5β-furost-Δ25(27)-en-1β,2β,3β, 4β,5β,6β,7α,22α,26-nonaol 26-O-β-D-glucopyranoside (2). Both compounds displayed marked inhibitory action against NO production in macrophages of the rat abdomen induced by LPS at 40 μg/mL.
Experimental
General
Column chromatography was carried out using silica gel (Qingdao Ocean Chemical Company, 200–300 mesh). TLC was performed with precoated silica gel G-25-UV 254 plates and detection was done at 254 nm, and by spraying with ceric sulphate in 10% H2SO4. The IR spectra were recorded on a Nicolet FT360 spectrometer. Optical rotations were measured on a Perkin-Elmer 241 spectropolarimeter using a 10-cm cell tube. Mass spectra (HRFAB-MS and ESI-MS) were measured in a fast atom bombardment mode using a VG AUTO Spec-300 mass spectrometer and ions are given in m/z. The 1H-, 13C- and 2D-NMR spectra were recorded on a Bruker AM-500 spectrometer in CD3OD. Chemical shifts are given in ppm (δ), relative to tetramethylsilane as an internal standard, and scalar coupling reported in hertz. HPLC was performed using a Varian ProStar 1510 system.
Plant Material
The rhizomes of T. chinensis Baker was collected from Muyu town of Shennongjia Forest District in August 2004 and identified by Dr Fa-Ju Chen, from the Hubei Key Laboratory of Natural Products Research and Development, China Three Gorges University, Yichang, P.R. China, where a voucher specimen has been deposited.
Extraction and Isolation
Air dried whole plant (7.8 kg) was exhaustively extracted with refluxing methanol (20 L × 5). The extract was evaporated in vacuo to yield a residue which was freeze-dried, affording a powder (3,659 g), which was divided into petroleum-ether, ethyl acetate, n-butanol (1,383 g) and water soluble fractions (1,889 g), respectively. A portion of the water soluble extract (150 g out of the total 1,889 g) was dissolved in water (2.0 L), and then subjected to macroporous resin column chromatography eluting with water and 60% methanol, respectively. The methanol part was reduced to dryness, giving rise to a white powder (7.0 g). The white powder was separated by using column chromatography over Sephadex LH-20 eluted with water, affording to 15 fractions. Fraction 7 (2.0 g) was repeatedly separated by means of column chromatograph over reverse phase C18 silica gels in elution with a gradient solvent system of 20% to 25% acetonitrile. The purification was carried out through semi-preparative HPLC eluted with gradient solvent (18%→22% acetonitrile within 35 min, 1.5 ml/min, 203 nm detection), giving rise to compounds 1 (93.9 mg) and 2 (32.6 mg), respectively.
Compound 1. White amorphous powder, mp 170~172°C; 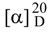 : -60.7° (CH3OH; c 0.94); IR (KBr) cm-1: 3410, 2923, 1708, 1655, 1443, 1050; 1H-NMR and 13C-NMR: see Table 1; HR-FAB-MS (positive ion mode): m/z 711.3208 [M+Na]+, calcd for C33H52O15Na, 711.3204.
: -60.7° (CH3OH; c 0.94); IR (KBr) cm-1: 3410, 2923, 1708, 1655, 1443, 1050; 1H-NMR and 13C-NMR: see Table 1; HR-FAB-MS (positive ion mode): m/z 711.3208 [M+Na]+, calcd for C33H52O15Na, 711.3204.
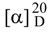 : -60.7° (CH3OH; c 0.94); IR (KBr) cm-1: 3410, 2923, 1708, 1655, 1443, 1050; 1H-NMR and 13C-NMR: see Table 1; HR-FAB-MS (positive ion mode): m/z 711.3208 [M+Na]+, calcd for C33H52O15Na, 711.3204.
: -60.7° (CH3OH; c 0.94); IR (KBr) cm-1: 3410, 2923, 1708, 1655, 1443, 1050; 1H-NMR and 13C-NMR: see Table 1; HR-FAB-MS (positive ion mode): m/z 711.3208 [M+Na]+, calcd for C33H52O15Na, 711.3204.Compound 2 White amorphous powder, mp 178~180°C; 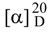 : -27.0° (CH3OH; c 0.59); IR (KBr) cm-1: 3406, 2923, 1655, 1445, 1056; 1H-NMR and 13C-NMR: see Table 1; HR-FAB-MS (positive ion mode): m/z 713.3367 [M+Na]+, calcd for C33H54O15Na, 713.3360.
: -27.0° (CH3OH; c 0.59); IR (KBr) cm-1: 3406, 2923, 1655, 1445, 1056; 1H-NMR and 13C-NMR: see Table 1; HR-FAB-MS (positive ion mode): m/z 713.3367 [M+Na]+, calcd for C33H54O15Na, 713.3360.
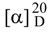 : -27.0° (CH3OH; c 0.59); IR (KBr) cm-1: 3406, 2923, 1655, 1445, 1056; 1H-NMR and 13C-NMR: see Table 1; HR-FAB-MS (positive ion mode): m/z 713.3367 [M+Na]+, calcd for C33H54O15Na, 713.3360.
: -27.0° (CH3OH; c 0.59); IR (KBr) cm-1: 3406, 2923, 1655, 1445, 1056; 1H-NMR and 13C-NMR: see Table 1; HR-FAB-MS (positive ion mode): m/z 713.3367 [M+Na]+, calcd for C33H54O15Na, 713.3360.Acid Hydrolysis
Compounds 1 (10.0 mg) and 2 (6.5 mg) were hydrolyzed with 2.0 mol/L HCl. The hydrolysis and the detection of sugars in hydrolyzed products were carried out according to procedures described in the literature [34].
Bioassay
1 % Soluble starch (1.0 mL/mouse) was injected into the abdominal cavity of C57BL/6 mice. The mice were decapitated after 3 days and Hanker’s solution (8.0 mL/mouse) was injected into their abdominal cavities to obtain a cell solution under aseptic conditions. The cell solution was centrifuged for 8 minutes at 1000 rpm, and the precipitates were purged with Hanker’s solution. The concentration of cells was adjusted to 106 cell/mL using PRMI 1640 culture medium which contains 20% calf serum. Then, the cells were inoculated to a culture plate with 48 pores (0.4 mL for each pore) for cultivation for 3 hrs under 37 °C and 5% CO2. Then, LPS solution (0.1 mL) and samples were added to the plates, respectively, and cultivated for 24 hrs. The supernatant was collected and kept at -20 °C. The content of NO in supernatant was measured by using NO reagent kids (enzymatic method), according to procedures described in the instructions provided.
Acknowledgements
This research was financially supported by the National Natural Science Foundations of China (30670213) and Key Science Program of China Three Gorges University (2005ZD007).
References and Notes
- Zhan, Y.H. China Shennongjia Resources of Medicinal Plants; Hubei Scientific and Technological Press: Wuhan, 1994; p. 418. [Google Scholar]
- Jiangsu New Medical College. Dictionary of Traditional Chinese Medicines; Shanghai Scientific and Technological Press: Shanghai, 1979; p. 907. [Google Scholar]
- Wendehenne, D.; Pugin, A.; Klessig, D.; Durner, J. Nitric oxide: comparative synthesis and signaling in animal and plant cells. Trends Plant Sci. 2001, 6, 177–183. [Google Scholar] [CrossRef]
- Shen, S.C.; Lee, W.R.; Lin, H.Y.; Huang, H.C.; Ko, C.H.; Yang, L.L. In vitro and in vivo inhibitory activities of rutin, wogonin, and quercetin on lipopolysaccharide-induced nitric oxide and prostaglandin E2 productions. Eur. J. Pharmacol. 2002, 446, 187–194. [Google Scholar] [CrossRef]
- Chang, Y.C.; Li, P.C.; Chen, B.C.; Chang, M.S.; Wang, J.L.; Chiu, W.T. Lipoteichoic acid-induced nitric oxide synthase expression in RAW 264.7 macrophages is mediated by cyclooxygenase-2, prostaglandin E2, protein kinase A, p38 MAPK, and nuclear factor-kappaB pathways. Cell Signal 2006, 18, 1235–1243. [Google Scholar] [CrossRef]
- Jiang, W.; Pisetsky, D.S. The role of IFN-alpha and nitric oxide in the release of HMGB1 by RAW 264.7 cells stimulated with polyinosinic–polycytidylic acid or lipopolysaccharide. J. Immunol. 2006, 177, 3337–3343. [Google Scholar] [CrossRef]
- Wang, J.Z.; Zou, K.; Zhang, Y.M.; Liu, C.; Wu, J.; Zhou, Y.; Dan, F.J.; Zhang, Y.X. An 18-norspirostanol saponin with inhibitory action against COX-2 production from the underground part of Trillium tschonoskii. Chem. Pharm. Bull. 2007, 55, 679–681. [Google Scholar] [CrossRef]
- Zou, K.; Wu, J.; Du, M.; Liu, C. Diastereoisomeric saponins from Tupistra chinensis rhizomes. Chin. Chem. Lett. 2007, 18, 65–68. [Google Scholar] [CrossRef]
- Tang, Z.C.; Zou, K.; Wang, J.Z.; Yin, C.Z.; Lu, H.H. Antialcoholism of extracts from Tupistra chinensis rhizomes. Lishizhen Med. Materia Med. Res. 2006, 17, 2163–2165. [Google Scholar]
- Wang, Z.J.; Zou, K.; Xu, H.W. Anti-inflammatory effects of Tupistra chinensis rhizomes. Lishizhen Med. Materia Med. Res. 2006, 17, 1970–1971. [Google Scholar]
- Yang, C.Y.; Zou, K.; Pan, J.R. Volatile constituents from Tupistra chinensis rhizomes. J. Chin. Three Gorges Univ. (Nat. Sci. Ed.) 2006, 28, 360–362. [Google Scholar]
- Zou, K.; Tu, G.Z.; Yang, C.Y.; Wang, J.Z. A furostanol saponin from the cytotoxic extract of Tupistra chinensis rhizomes. Chin. Chem. Lett. 2006, 17, 1335–1338. [Google Scholar]
- Zou, K.; Wang, J.Z.; Wu, J.; Liu, C.; Xiao, Z.H. A pair of diastereoisomeric saponins from the cytotoxic extract of Tupistra chinensis rhizomes. Chem. Pharm. Bull. 2006, 54, 1782–1785. [Google Scholar]
- Liu, Z.X.; Zou, K.; Yang, X.H.; Zhou, Y. Anti-inflammatory and analgesic effects of ethanol extracts from roots of Panax japonicus. Lishizhen Med. Materia Med. Res. 2004, 15, 465–466. [Google Scholar]
- Liu, Z.X.; Zou, K. A survey on the study of Panax plants in Hubei Province. Lishizhen Med. Materia Med. Res. 2003, 14, 571–573. [Google Scholar]
- Huang, L.; Liao, Q.B.; Zou, K.; Yuan, H.; Hu, C.L.; Hu, Y.L. Content determination of steroidal sapogenins in Tupistra chinensis rhizomes. J. Chin. Three Gorges Univ. (Nat. Sci. Ed.) 2003, 25, 562–564. [Google Scholar]
- Gao, Z.; Zou, K.; Liao, Q.B. Scavenging effects of Dipsacus asper Wall on DPPH radical group. J. Chin. Three Gorges Univ. (Nat. Sci. Ed.) 2002, 24, 366–368. [Google Scholar]
- Pan, W.B.; Wei, L.M.; Wei, L.L.; Wu, C.Y. Chemical Constituents of Tupistra chinensis Rhizomes. Chem. Pharm. Bull. 2006, 54, 954–958. [Google Scholar] [CrossRef]
- Wu, G.X.; Wei, X.Y.; Chen, W.X. Spirostane steroidal saponins from the underground parts of Tupistra chinensis. Chin. Chem. Lett. 2005, 16, 911–914. [Google Scholar]
- Pan, W.B.; Chang, F.R.; Wu, Y.C. Tupichigenin A, a new steroidal sapogenin from Tupistra chinensis. J. Nat. Prod. 2000, 63, 861–863. [Google Scholar] [CrossRef]
- Pan, W.B.; Chang, F.R.; Wei, L.M.; Wu, Y.C. New flavans, spirostanol sapogenins, and a pregnane genin from Tupistra chinensis. J. Nat. Prod. 2003, 66, 161–168. [Google Scholar] [CrossRef]
- Pan, W.B.; Chang, F.R.; Wu, Y.C. Spirostanol sapogenins from the underground parts of Tupistra chinensis. Chem. Pharm. Bull. 2000, 48, 1350–1353. [Google Scholar] [CrossRef]
- Dini, I.; Tenore, G.C.; Trimarco, E.; Dini, A. Furostanol saponins in Allium caepa L. Var. tropeana seeds. Food Chem. 2005, 93, 205–214. [Google Scholar] [CrossRef]
- Agrawal, P.K.; Jain, D.C.; Gupta, R.K.; Thakur, R.S. Carbon-13 NMR spectroscopy of steroidal sapogenins and steroidal saponins. Phytochemistry 1985, 24, 2479–2496. [Google Scholar] [CrossRef]
- Agrawal, P.K.; Jain, D.C.; Pathak, A.K. NMR spectroscopy of steroidal sapogenins and steroidal saponins: an update. Magn. Reson. Chem. 1995, 33, 923–953. [Google Scholar] [CrossRef]
- Mimaki, Y.; Kuroda, M.; Takashi, Y.; Sashida, Y. Steroidal saponins from the leaves of Cordyline stricta. Phytochemistry 1998, 47, 79–85. [Google Scholar] [CrossRef]
- Shen, P. Biological constituents from Dioscorea deltoidea Wall var. orbiculata and Tupistra wattii Hook.f. Doctoral Thesis, Shengyang Pharmaceutical University, 2002; pp. 39–43. [Google Scholar]
- Chen, Y.; Liu, J.; Xie, Z. Progress in studies on Tupistra species. Lishizhen J. Med. Materia Med. Res. 2004, 15, 860–861. [Google Scholar]
- Yang, C.Y.; Yang, X.H.; Zou, K.; Liu, Y.; Yue, C.Y. Effects of Tupistra chinensis rhizomes on phlegm, inflammation and bacteria. Chin. J. Folk Med. Materia Med. Res. 2005, 73, 103–106. [Google Scholar]
- Qiu, J.; Dong, W.G. Inhibitory mechanism of extracts from Tupistra chinensis rhizomes on colonitis. J. Shangdong Med. Materia Med. Res. 2005, 45, 4–6. [Google Scholar]
- Qiu, J.; Dong, W.G.; Yu, J.P. Effects of extracts from Tupistra chinensis rhizomes on activity of platelet in rats suffering from colocitis. Chin. J. Comb. Chin. Med. West. Med. Digest. 2005, 13, 363–365. [Google Scholar]
- Li, X.L.; Zhang, Y.Q.; Hong, P.P. Anti-inflammatory and analgesic effects of Tupistra chinensis rhizomes. J. Hubei Chin. Med. Inst. 2005, 7, 28–29. [Google Scholar]
- Chu, S.S. Curative effects of Tupistra chinensis rhizomes on pharyngitis and faucitis. Chin. J. Folk Med. Materia Med. Res. 1999, 38, 140–141. [Google Scholar]
- Zou, K.; Tong, W.Y.; Liang, H.; Cui, J.R.; Tu, G.Z.; Zhao, Y.Y.; Zhang, R.Y. Diastereoisomeric saponins from Albizia julibrissin. Carbohydr. Res. 2005, 340, 1329–1334. [Google Scholar] [CrossRef]
- Sample Availability: available from authors.
© 2007 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.