Abstract
Studies aimed at a comparison of chemical, biomimetic (Gif system GoAggIII) and enzymatic (CHMO) transformations of natural (+)-10β,14-dihydroxy-allo-aromadendrane have led to preparation of an eight-member sesquiterpene lactone.
Introduction
Our research group has been undertaking an effort to perform random transformations in natural compounds with the purpose of obtaining novel, potentially bioactive derivatives. The sesquiterpene (+)-10β,14-dihydroxy-allo-aromadendrane (1) (Figure 1), has already been target of different studies reported by our group. Compound 1 is a natural product isolated from Pulicaria paludosa [1] and Duguetia glabriuscula [2]. Investigations concerning chemical modification [3], microbial transformation [4], electrolysis [5] and NMR assignments [6] of this molecule have demonstrated a great reaction versatility leading to unique carbon frameworks.
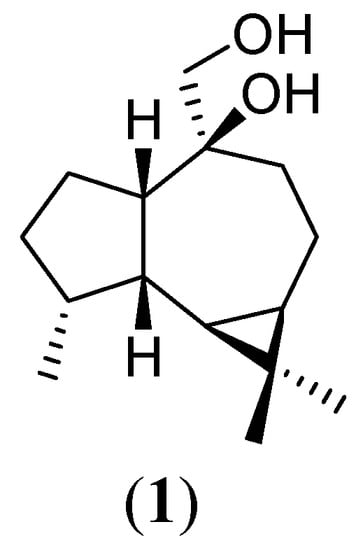
Figure 1.
(+)-10β,14-Dihydroxy-allo-aromadendrane (1)
As part of our research aimed at the oxyfunctionalization of carbon-carbon bonds in natural products, we decided to evaluate the performance of various oxidation systems in the modification of 1 and derivatives. Accordingly, we have applied three different oxidation methodologies to compound 1 and examined the different outcomes of these reactions in the presence of the sesquiterpene substrate. The systems studied were: (a) a biomimetic Gif oxidation; (b) an enzymatic oxidation employing cyclohexanone monooxygenase from Acinetobacter NCIMB 9871 (CHMO; EC 1.14.13.22); and (c) its chemical counterpart process employing m-chloroperbenzoic acid (MCPBA).
The Gif reaction is a model system capable of functionalizing saturated hydrocarbons under mild conditions, simulating the activity of iron-containing enzymes [7,8,9]. The system comprises an Fe(III) salt suspended in a mixture of pyridine/acetic acid in the presence of an extra ligand (generally picolinic acid) and utilizing TBHP or H2O2 as oxidizing agents [10]. Gif system GoAggIII is composed of FeIII-picolinic acid and H2O2 and has already been employed to emulate in vivo oxidation of drug candidates [11]. CHMO is a monomeric NADPH dependent protein, possessing FAD as prosthetic group [12]. Its mechanism of action has been studied and the results reveal that the conversion from ketone to lactone occurs through a classical Baeyer-Villiger rearrangement [12,13,14]. Several examples of the use of this enzyme for the oxidation of organic compounds have been reported [13,14,15,16].
Results and Discussion
We started our endeavor to modify compound 1 by submitting it to the Gif system GoAggIII. We hoped to insert a hydroxyl group somewhere in the molecule in order to eventually generate a new sesquiterpene. However, treatment of 1 under GoAggIII conditions led to formation of a nonhydroxylated, less polar (TLC) product. 1H- and 13C-NMR spectra were consistent with those of the (-)-allo-aromadendrone (2) [4] (Figure 2).
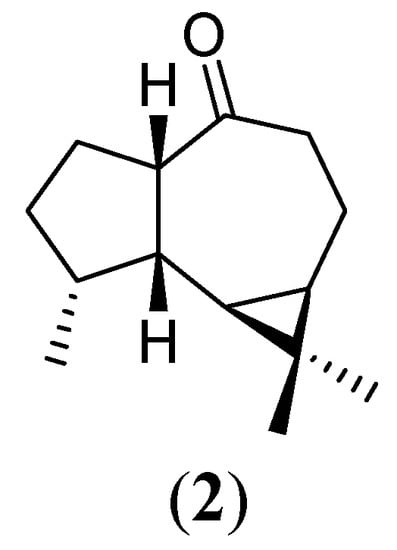
Figure 2.
(-)-Allo-aromadendrone (2)
Physical-chemical data comparison with an authentic sample of 2 conclusively characterized the product as being that ketone. Although it is more usual for the Gif system GoAggIII to functionalize hydrocarbons, there have been reports of this system being related to the oxidation of alcohols to ketones [17].
Since one of our goals was to synthesize novel and unusual lactones by either enzymatic or chemical procedures, and compare eventual differences in regioselectivity, the synthesis of 2 from 1 prompted us to examine the nature of the structural changes that would occur when 2 was subjected to either CHMO or MCPBA, in the context of the Baeyer-Villiger oxidation.
Treatment of 2 with a buffered solution containing CHMO apparently led to no changes in the molecule, as observed on TLC. However, analysis of the NMR data of the isolated product unequivocally indicates that the basic media of the reaction mixture led to the isomerisation of 2 into 3 (Figure 3). The structural assignment was supported by a previous literature report [18].
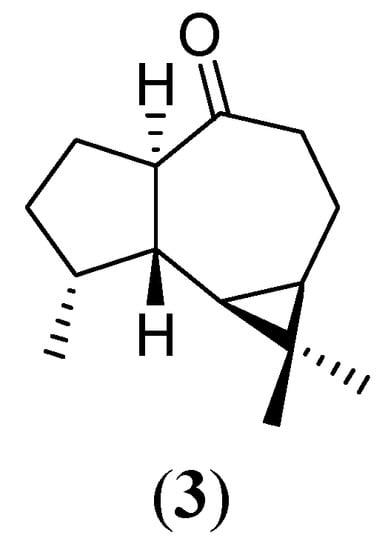
Figure 3.
Apoaromadendrone (3)
Treatment of compound 2 with MCPBA under Bayer-Villiger conditions resulted in the formation of the previously unreported sesquiterpene lactone 3,3,12-trimethyl-8-oxa-tricyclo[7.3.0.02,4]-dodecan-7-one (4) (Figure 4).
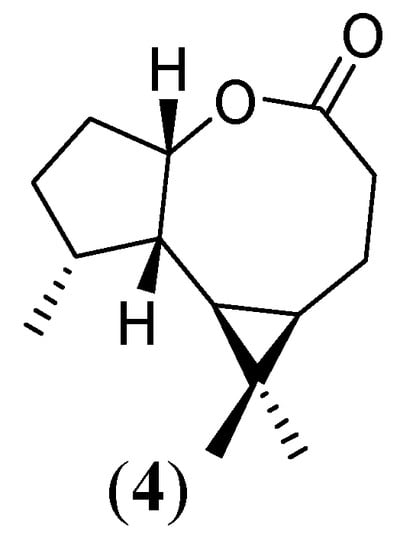
Figure 4.
New sesquiterpene lactone 4
As expected, the Bayer-Villiger procedure occurred with retention of configuration. The IR spectrum showed the characteristic lactone band at 1704 cm-1. The 1H-NMR spectrum displayed one signal at 4.9 due to the hydrogen at C-1. The signal had a dd multiplicity, possibly due to couplings to vicinal hydrogens at C-8 (J= 9.0 Hz) and C-2 (J= 7.5 Hz), suggesting a cis-fusion of the rings as seen in models. Comparison of this data with the previously published spectra [19] of the related eightmember lactone formed from apoaromadendrone supports the assignment. The 13C-NMR spectrum also confirms the proposed structure of 4, as evidenced by the peak at 179.3 ppm, corresponding to a lactone carbonyl, which differs from the starting material ketone carbonyl ( 211.8 ppm).
Experimental
General.
1H- and 13C-NMR spectra were obtained using a Brucker DPX300 NMR and were recorded at 300 and 75 MHz, respectively. IR spectra were obtained using a Perkin Elmer 783. All reagents and chemicals were obtained from Sigma-Aldrich Chemical Company (Brazil) and were used as received unless otherwise noted. (+)-10β,14-dihydroxy-allo-aromadendrane was isolated from Duguetia glabriuscula following the literature procedure [2]. CHMO from Acinetobacter NCIMB 9871 (6.3U/g, Fluka) was a gift from Prof. S. M. Roberts (University of Liverpool – UK).
Typical procedure for the oxidation by the GoAggIII system.
In a 100 mL round bottom flask fitted with a rubber septum and an oxygen-filled balloon, compound 1 (100 mg, 0.42 mmol) was dissolved in a mixture of pyridine (2.0 mL) and acetic acid (2.0 mL), containing FeCl3·6H2O (51 mg, 0.19 mmol) and picolinic acid (2.5 mg, 0.02 mmol). The solution was stirred for 10 min at room temperature, taken to 0 °C, and H2O2 (30% in H2O, 0.5 mL) was then added dropwise. The reaction mixture was allowed to stir overnight at room temperature. After workup, flash chromatography [ethyl acetate-hexane (1:1)] on silica gel afforded the compound (-)-allo-aromadendrone (2, 7.0 mg, 7%), identified by comparison of its physical and spectroscopic data with those of an authentic sample [4] and recovered starting material (42 mg, 42%).
Reaction with CHMO.
Compound 2 (10 mg, 0.04 mmol), NADPH (32.0 mg), CHMO (30.0 mg) and glycine buffer solution (0.2 M, pH 8.0, 10 mL) were placed in a round bottom flask (50 mL). The system was closed and stirred for 48 h. After work-up, flash chromatography (10% ethyl acetate in hexane) on silica gel afforded apoaromadendrone (3, 9.0 mg, 90%), identified by comparison with the literature data for this compound [18].
Reaction with MCPBA.
Compound 2 (10 mg, 0.042mmol), MCPBA (20.0 mg) and chloroform (10 mL) were placed in a 50 mL round bottom flask. The reaction mixture was stirred at room temperature for 4 h. After work-up, flash chromatography (10% ethyl acetate in hexane) afforded 3,3,12-trimethyl-8-oxa-tricyclo-[7.3.0.02,4]dodecan-7-one (4, 7 mg, 64%); IR (thin film) cm-1: 1704, 1162, 1096; 1H-NMR (CDCl3) δ: 4.97 (dd, 1H, J = 7.5, 9.0Hz, H-1), 2.60-2.63 (m, 1H, H-10), 2.31-2.35 (m, 3H, H-2, H-6), 2.10-2.07 (m, 2H, H-8), 1.60-1.65 (m, 2H, H-10), 1.55-1.58 (m, 2H, H-5), 1.25 (s, 6H, H-12, H-13, 2 x CH3), 1.10 [d, J = 7.0Hz, 3H, H-14(CH3)], 0.85-0.87 (m, 2H, H-3, H-4); 13C-NMR (CDCl3) δ: 179.3 (C-7), 76.6 (C-1), 47.9 (C-2), 36.1 (C-10), 32.1 (C-3), 31.9 (C-4, C-6), 30.8 (C-9), 29.6 (C-8), 27.1 (C-12), 26.7 (C-11), 25.5 (C-13), 22.6 (C-5), 18.1 (C-14).
Acknowledgments
The authors thank the Brazilian research agencies CNPq and CAPES for a fellowship to Márcia M. L. Bodas and PROPP-UFMS for financial support. We are also indebted to Prof. S. M. Roberts (University of Liverpool – UK) for providing CHMO.
References
- San Feliciano, A.; Medarde, M.; Gordaliza, M.; Del Olmo, E.; Del Corral, J. M. M. Tetrahedron 1989, 28, 2717–2721.
- De Siqueira, J. M.; De Oliveira, C. D.; Boaventrua, M. A. D. Fitoterapia 1997, LXVIII, 89–90.
- De Lima, D. P.; Beatriz, A.; Ramos, A. A.; De Siqueira, J. M.; De Oliveira, C. C.; Marques, M. R. Quím. Nova 1997, 20, 616–620.
- De Lima, D. P.; Carnell, A. J.; Roberts, S. M. J. Chem. Soc. Res. (S) 1999, 396–397.
- Blanco, M.; De Lima, D. P.; Maia, G. J. Electroanal. Chem. 2001, 512, 49–55.
- Vizzotto, L.; Muzzi, R. M.; De Lima, D. P. Mag. Res. Chem. 2003, 41, 1034–1037.
- Barton, D. H. R.; Hay-Motherwell, R. S.; Motherwell, W. B. Tetrahedron Lett. 1983, 24, 1979–1982.
- Barton, D. H. R.; Doller, D. Acc. Chem. Res. 1992, 25, 504–512.
- Barton, D. H. R.; Hu, B. Tetrahedron 1996, 52, 10313–10326.
- Barton, D. H. R.; Csuhai, E.; Doller, D.; Geletti, Y. V. Tetrahedron 1991, 47, 6561–6570.
- Doller, D.; Chackalamannil, S.; Stamford, A.; McKittrick, B.; Czarniecki, M. Bioorg. Med. Chem. Lett. 1997, 7, 1381–1386.
- Roberts, A. U.; Wan, P. W. H. J. Mol. Cat. B: Enzymatic 1998, 4, 111–136.
- Faber, K. Biotransformations in Organic Chemistry; Springer: Berlin, 1997; pp. 224–225. [Google Scholar]
- Sheng, D.; Ballou, D. P.; Massey, V. Biochemistry 2001, 40, 11156–11167. [PubMed]
- Massey, V. J. Biol. Chem. 1994, 22, 459–462.
- Chi-Huey, W.; Whitesides, G. M. Enzymes in Synthetic Organic Chemistry; Pergamon: Oxford, 1995; p. 169. [Google Scholar]
- Barton, D. H. R.; Bévière, S. D.; Chabot, B. M.; Chavasiri, W.; Taylor, D. K. Tetrahedron Lett. 1994, 35, 4681–4684.
- Lamers, Y. M. A. W. PhD. Thesis, Wageningen University, Wageningen, The Netherlands, 2003.
- Gijsen, H. J. M. PhD. Thesis, Wageningen University, Wageningen, The Netherlands, 1993.
- Sample Availability: Available from the authors.
© 2005 by MDPI (http:www.mdpi.org). Reproduction is permitted for noncommercial purposes.