Microbial Hydroxylation of Sclareol by Rhizopus Stolonifer
Abstract
:Introduction
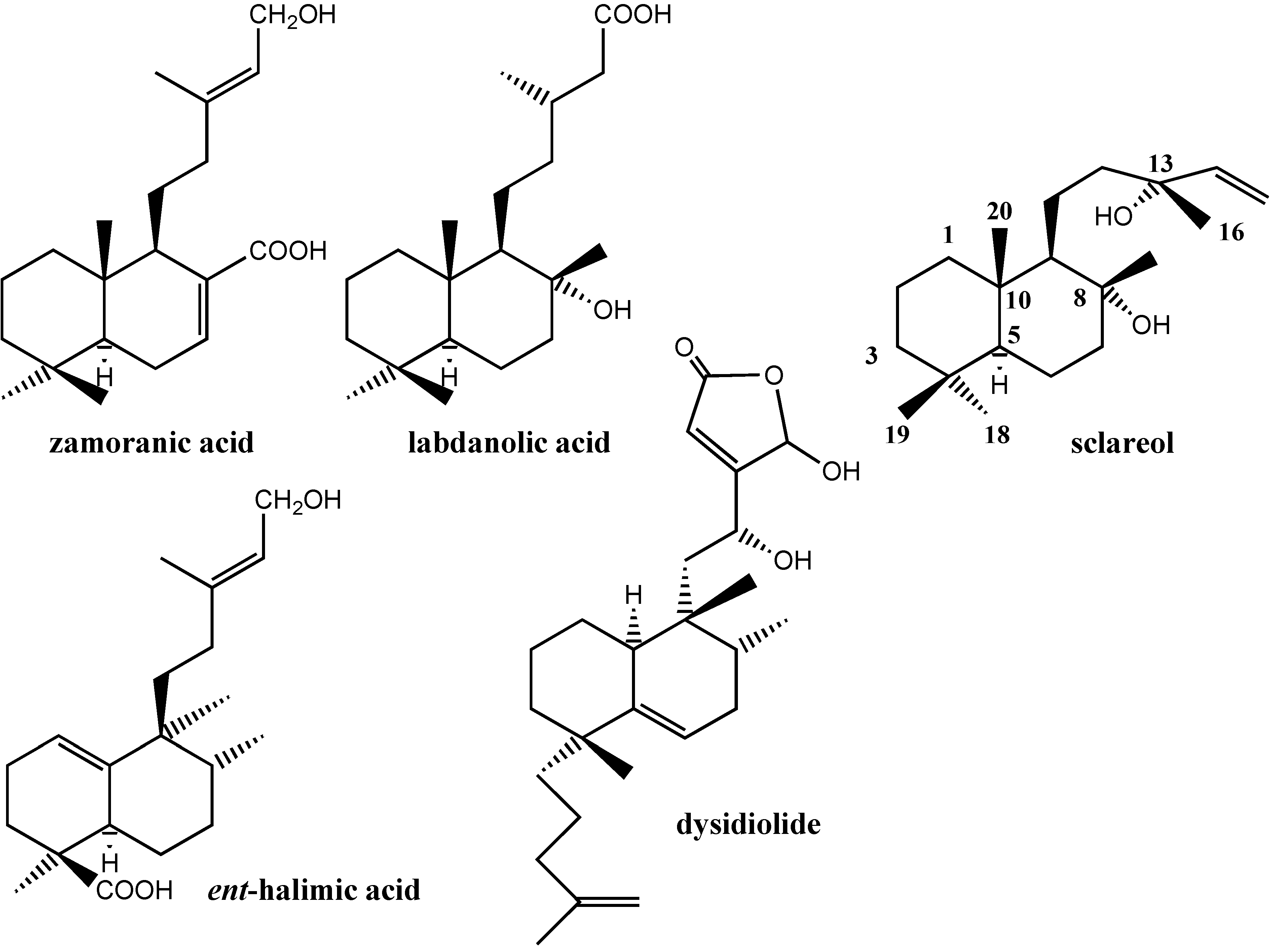
Results and Discussion
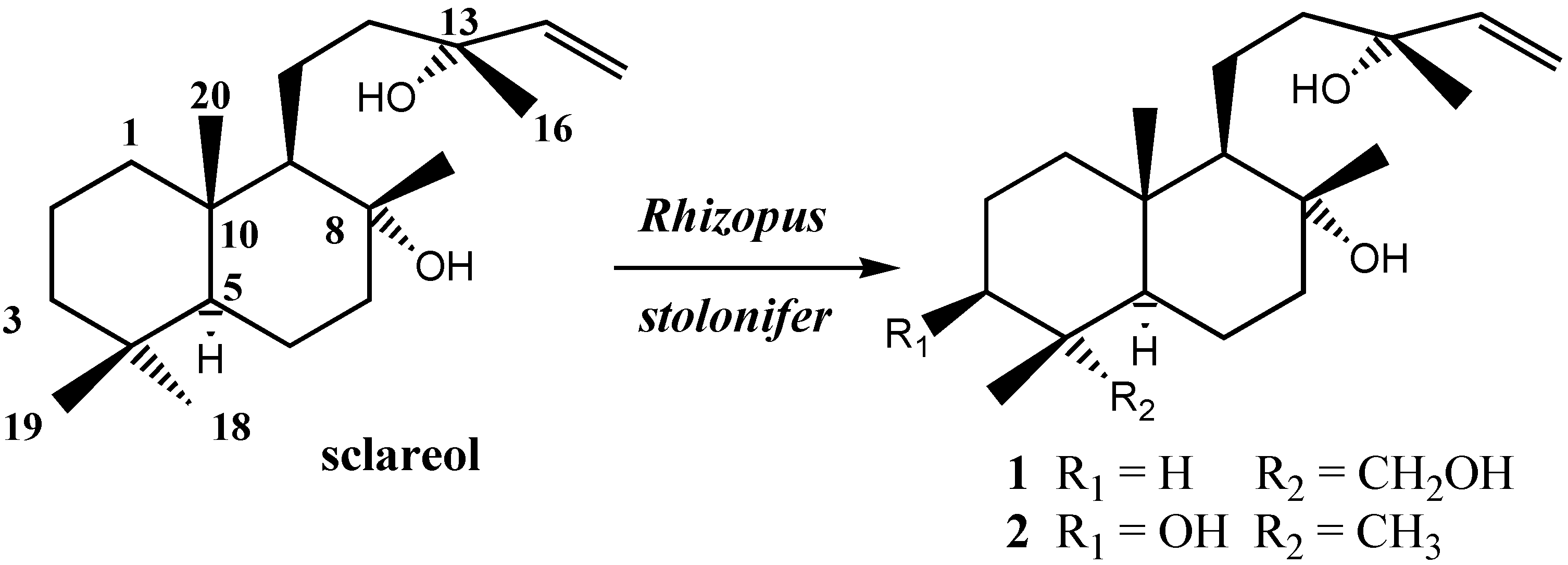
| Conditions* | Time | Transformation of sclareol % | 1 (%) | 2 (%) |
|---|---|---|---|---|
| A | 5 days | 21 | ||
| B | 5 days | 87 | 74 | 9 |
| B | 8 days | 98 | 68 | 20 |
| C | 5 days | 88 | 17 |
Conclusions
Experimental
General
Acknowledgements
References and Notes
- Urones, J. G.; Marcos, I. S.; Basabe, P.; González, J. L.; Sexmero, M. J.; Lithgow, A. M. Ambergris Compounds from Labdanolic acid. Tetrahedron 1992, 48, 9991–9998. [Google Scholar] Lithgow, A. M.; Marcos, I. S.; Basabe, P.; Sexmero, M. J.; Diez, D.; Gomez, A; Estrella, A.; Urones, J. G. Labdanolic acid: synthetic precursor of tricyclic diterpenes. Nat. Prod. Lett. 1995, 6, 291–294, and references cited therein. [Google Scholar]
- Urones, J. G.; Díez, D.; Gomez, P. M.; Marcos, I. S.; Basabe, P.; Moro, R. F. Chemistry of Zamoranic acid. Part 10 Homochiral hemysinthesis of Pereniporin A. J. Chem. Soc. Perkin Trans. 1997, 1815–1818, and references cited therein. [Google Scholar] Marcos, I. S.; Moro, R. F.; Carballares, S.; Urones, J. G. An efficient total synthesis of isodrimeninol from zamoranic acid. Synlett 2000, 541. [Google Scholar]
- Marcos, I. S.; Pedrero, A. B.; Sexmero, M. J.; Díez, D.; Basabe, P.; Hernández, F. A.; Broughton, H. B.; Urones, J. G. Synthesis and absolute Configuration of the supposed Structure of Cladocoran A and B. Synlett 2002, 105–109. [Google Scholar] Marcos, I. S.; Hernández, F. A.; Sexmero, M. J.; Díez, D.; Basabe, P.; Pedrero, A. B.; García, N.; Sanz, F.; Urones, J. G. Synthesis and Absolute Configuration of (-)-Chettaphanin II. Tetrahedron Lett. 2002, 43, 1243–1245. [Google Scholar] [CrossRef]
- Ruzicka, L.; Janot, M.M. Höhere Terpenverbindungen L. Zur Kenntnis des Sclareol. Helv. Chim. Acta 1931, 14, 645. [Google Scholar] [CrossRef]
- Basabe, P.; Diego, A.; Díez, D.; Marcos, I.S.; Urones, J.G. Synthesis and Absolute Configuration of (-)-Hyrtiosal. Synlett 2000, 1807–1809. [Google Scholar] Basabe, P.; Diego, A.; Díez, D.; Marcos, I.S.; Mollinedo, F.; Urones, J.G. Synthesis and Absolute Configuration of (-) Hyrtiosal. Synthesis 2002, 1523–1529. [Google Scholar] [CrossRef]
- Basabe, P.; Estrella, A.; Marcos, I.S.; Díez, D.; Lithgow, A. M.; White, A. J. P.; Williams, D. J.; Urones, J.G. Prehispanolone Analogs: Stereochemistry Control at C-5 in the Preparation of Oxaspiro(4,5)decane Fused Systems and Related Compounds. Synlett 2001, 153–155. [Google Scholar]
- Basabe, P.; Gomez, A.; Marcos, I.S.; Díez, D.; Broughton, H. B.; Urones, J.G. Towards the synthesis of 9,11-secoespongianes. Tetrahedron Lett. 1999, 40, 6857–6860. [Google Scholar] [CrossRef]
- Barrero, A. F.; Cortes, M.; Mazaneda, E. A.; Cabrera, E.; Chahboun, R.; Lara, M.; Rivas, A. R. Synthesis of 11,12-epoxydrim-8,12-en-11-ol, 11,12-diacetoxydrimae and warburganal from (-)-sclareol. J. Nat. Prod. 1999, 62, 1488–1491. [Google Scholar] [CrossRef] [PubMed]Barrero, A. F.; Mazaneda, E. A.; Chahboun, R.; Paiz, M. C. A new enantiospecific route toward monocabocyclic terpenoids: syntheis of (-)-caparrapi oxide. Tetrahedron Lett. 1998, 39, 9543–9544. [Google Scholar] [CrossRef] Muller, M.; Schroder, J.; Magg, C.; Seifert, K. Synthesis of (+)-coronarin E. Tetrahedron Lett. 1998, 39, 4655–4656. [Google Scholar] [CrossRef] Barrero, A. F.; Mazaneda, E. A.; Chahboun, R. Synthesis of the Wiedendiol-A and Wiedendiol-B from Labdane Diterpenes. Tetrahedron 1998, 54, 5635–5650, and references cited therein. [Google Scholar] Jung, M.; Seokjoon, L.; Byunghee, Y. Conversion of Sclareol into (+)-Galanolactone and (+)-Labdienedial. Tetrahedron Lett. 1997, 38, 2871–2874. [Google Scholar] [CrossRef] Barton, D. H. R.; Taylor, D. K.; Tse, C-L. An improved synthesis of (-)-dodecahydro-3a,6,6,9a-tetramethylnaphtol[2,1,-b]furan via ozonolysis of (-)-sclareol. Tetrahedron Lett. 1994, 35, 9505–9508. [Google Scholar] Barton, D. H. R.; Taylor, D. K.; Tse, C-L. An efficient synthesis of (-)-dodecahydro-3a,6,6,9a-tetramethylnaphtol[2,1,-b]furan from (-)-sclareol. Tetrahedron Lett. 1994, 35, 5801–5804. [Google Scholar]
- Kouzi, S. A.; McChesney, J. D. Microbial metabolism of the Diterpene sclareol: oxidation of the A ring by septomyxa affinis. Helv. Chim. Acta 1990, 73, 2157–2164. [Google Scholar] [CrossRef] Kouzi, S. A.; McChesney, J. D. Microbial models of mammalian metabolism: fungal metabolism of the diterpene sclareol by cunninghamella species. J. Nat. Prod. 1991, 54, 483–490. [Google Scholar] Abraham, W-R. Microbial hydroxylation of Sclareol. Phytochemistry 1994, 36, 1421–1424. [Google Scholar] Farooq, A.; Tahara, S. Biotransformation of two cytotoxic terpenes α-santonin and sclareol by botrytis cinerea. J. Biosci. 2000, 55, 713–717. [Google Scholar] Hanson, J. R.; Hitchcock; Nasir, H.; Truneh, A. The Biotransformation of the diterpenoid, Sclareol, by Cephalosporium aphidicola. Phytochemistry 1994, 36, 903–906. [Google Scholar] Aranda, G.; Lallemand, J-Y; Hammoumi, A.; Azerad, R. Microbial hydroxylation of Sclareol by Mucor plumbeus. Tetrahedron Lett. 1991, 32, 1783–1786. [Google Scholar] Aranda, G; El Kortbi, M. S.; Lallemand, J-Y; Neuman, A.; hammoumi, A.; Facon, I.; Azerad, R. Tetrahedron 1991, 39, 8339–8350.
- Sample Availability: Available from the authors.
© 2005 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Díez, D.; Sanchez, J.M.; Rodilla, J.M.; Rocha, P.M.; Mendes, R.S.; Paulino, C.; Marcos, I.S.; Basabe, P.; Urones, J.G. Microbial Hydroxylation of Sclareol by Rhizopus Stolonifer. Molecules 2005, 10, 1005-1009. https://doi.org/10.3390/10081005
Díez D, Sanchez JM, Rodilla JM, Rocha PM, Mendes RS, Paulino C, Marcos IS, Basabe P, Urones JG. Microbial Hydroxylation of Sclareol by Rhizopus Stolonifer. Molecules. 2005; 10(8):1005-1009. https://doi.org/10.3390/10081005
Chicago/Turabian StyleDíez, D., J. M. Sanchez, J. M. Rodilla, P. M. Rocha, R. S. Mendes, C. Paulino, I. S. Marcos, P. Basabe, and J. G. Urones. 2005. "Microbial Hydroxylation of Sclareol by Rhizopus Stolonifer" Molecules 10, no. 8: 1005-1009. https://doi.org/10.3390/10081005
APA StyleDíez, D., Sanchez, J. M., Rodilla, J. M., Rocha, P. M., Mendes, R. S., Paulino, C., Marcos, I. S., Basabe, P., & Urones, J. G. (2005). Microbial Hydroxylation of Sclareol by Rhizopus Stolonifer. Molecules, 10(8), 1005-1009. https://doi.org/10.3390/10081005






