Improved Synthesis of β-D-6-Methylpurine Riboside and Antitumor Effects of the β-D- and α-D-Anomers
Abstract
:Introduction
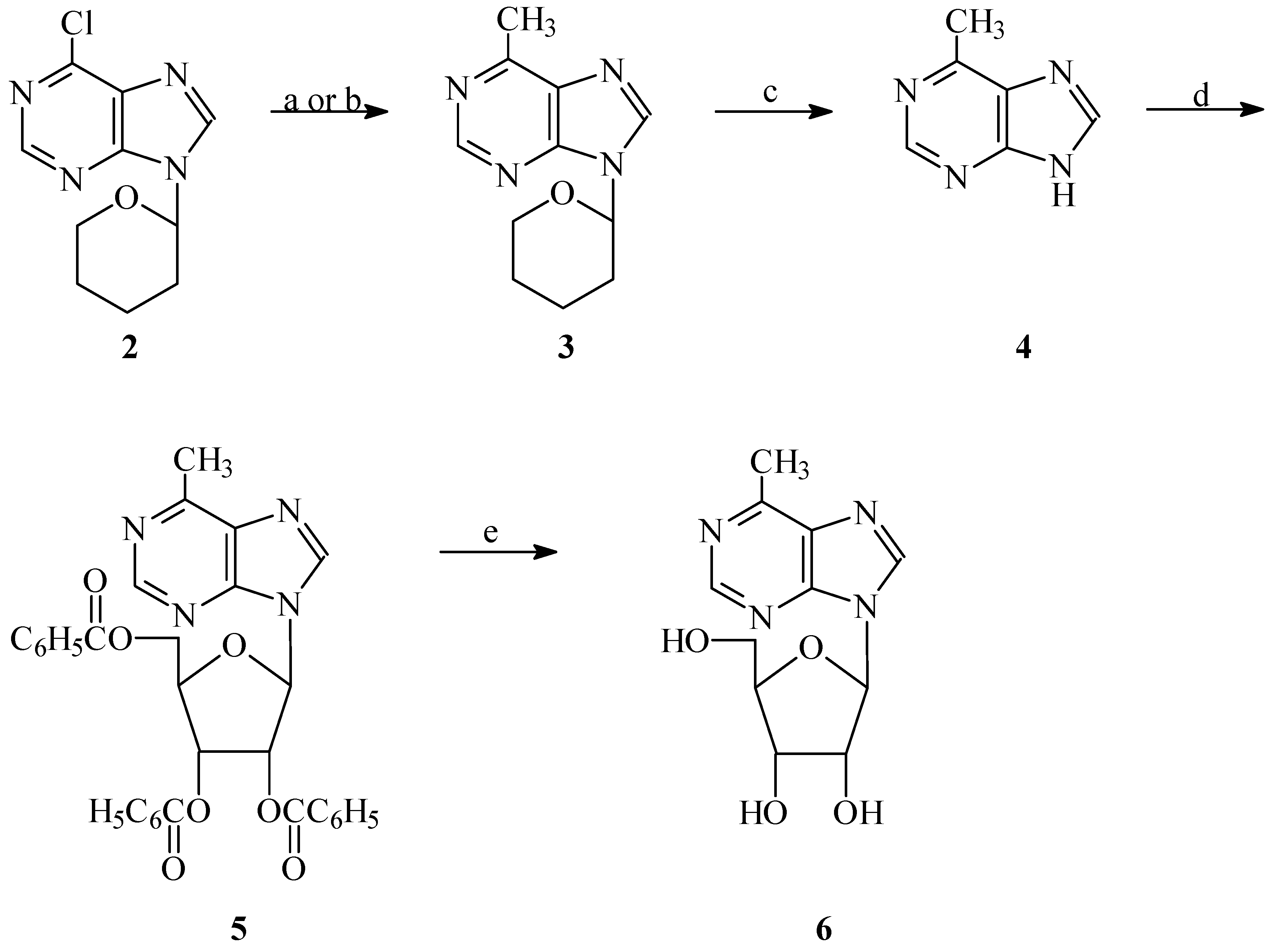
Results and Discussion
Chemistry
Biological Activity
| Cell Line | IC50 (μM) | ||
|---|---|---|---|
| β-D-MPR | α-D-MPR | ||
| A121 (ovarian) | 0.017 | 2.65 | |
| A549 (non-small cell lung) | 0.006 | 1.47 | |
| HT-29 (colon) | 0.034 | 4.83 | |
| MCF7-S (breast) | 0.012 | 1.75 | |
| MCF7-R (breast) | 0.026 | 4.08 | |
Conclusions
Experimental
General.
Biology
Acknowledgements
References
- Leonhardt, K.; Anke, T. Z. Naturforsch 1987, 42c, 420.
- Montgomery, J. A.; Hewson, K. J. Med. Chem 1968, 11, 48. [PubMed]
- Schnebli, H. P.; Hill, D. L.; Bennett, L. L., Jr. J. Biol. Chem 1967, 242, 1997. [PubMed]
- Carson, D. A.; Chang, K.-P. Life Sci 1981, 29, 1617. [PubMed]
- Marr, J. J. Biochemical Protozology; Coombs, G., North, M., Eds.; Taylor & Francis: London, 1991; pp. 524–536. [Google Scholar]
- Ghoda, L. Y.; Savarese, T. M.; Northup, C. H.; Parks, R. E., Jr.; Garofalo, J.; Katz, L.; Ellenbogen, B. B.; Bacchi, C. J. Mol. Biochem. Parasitol 1988, 27, 109. [PubMed]
- Gordon, M. P.; Weliky, V. S.; Brown, G. B. J. Am. Chem. Soc. 1957, 79, 3245.
- Robins, R. K.; Godefroi, E. F.; Taylor, E. C.; Lewis, L. R.; Jackson, A. J. Am. Chem. Soc 1961, 83, 2574.
- Taylor, E. C.; Martin, S. F. J. Am. Chem. Soc 1972, 94, 2874.
- Dvorakova, H.; Dvorak, D.; Holy, A. Tet. Lett 1996, 37, 1285.
- Laursen, R. A.; Grimm, W.; Leonard, N. J. Synthetic Procedures in Nucleic Acid Chemistry; Zorbach, W. W., Tipson, R. S., Eds.; John Wiley & Sons: New York, 1968; Vol. 1, pp. 160–162. [Google Scholar]
- Ojima, I.; Kuduk, S. D.; Pera, P.; Vieth, J. M.; Bernacki, R.J. J. Med. Chem 1997, 40, 279. [PubMed]
- Sufrin, J. R.; Rattendi, D.; Spiess, A. J.; Lane, S.; Marasco, C. J., Jr.; Bacch, C. J. Antimicrob. Agents Chemother 1996, 40, 2567. [PubMed]
- Bennett, L. L., Jr.; Vail, M. H.; Chumley, S.; Montgomery, J. A. Biochem. Pharmacol 1966, 15, 1719.
- Bennett, L.L., Jr.; Allan, P. W.; Hill, D.L; Thomas, H. J.; Carpenter, J. W. Molec. Pharmacol. 1976, 12, 242.
- Sample availability: Not available.
© 2005 by MDPI (http:www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Marasco, C., Jr.; Pera, P.; Spiess, A.; Bernacki, R.; Sufrin, J. Improved Synthesis of β-D-6-Methylpurine Riboside and Antitumor Effects of the β-D- and α-D-Anomers. Molecules 2005, 10, 1015-1020. https://doi.org/10.3390/10081015
Marasco C Jr., Pera P, Spiess A, Bernacki R, Sufrin J. Improved Synthesis of β-D-6-Methylpurine Riboside and Antitumor Effects of the β-D- and α-D-Anomers. Molecules. 2005; 10(8):1015-1020. https://doi.org/10.3390/10081015
Chicago/Turabian StyleMarasco, C., Jr., P. Pera, A. Spiess, R. Bernacki, and J. Sufrin. 2005. "Improved Synthesis of β-D-6-Methylpurine Riboside and Antitumor Effects of the β-D- and α-D-Anomers" Molecules 10, no. 8: 1015-1020. https://doi.org/10.3390/10081015
APA StyleMarasco, C., Jr., Pera, P., Spiess, A., Bernacki, R., & Sufrin, J. (2005). Improved Synthesis of β-D-6-Methylpurine Riboside and Antitumor Effects of the β-D- and α-D-Anomers. Molecules, 10(8), 1015-1020. https://doi.org/10.3390/10081015




