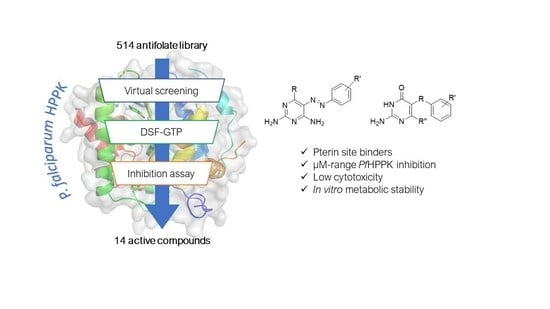
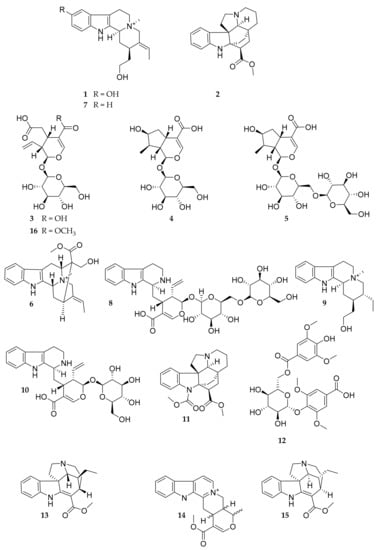
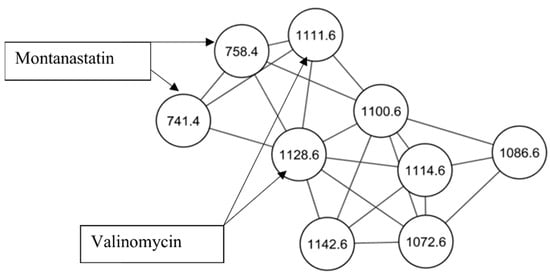
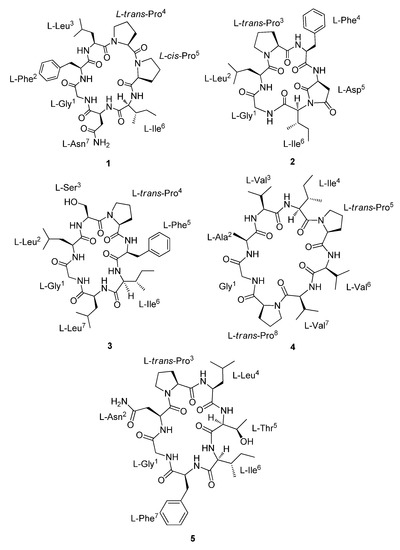
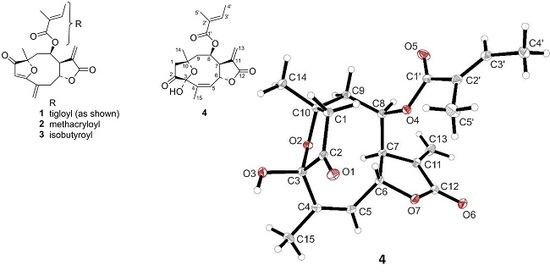
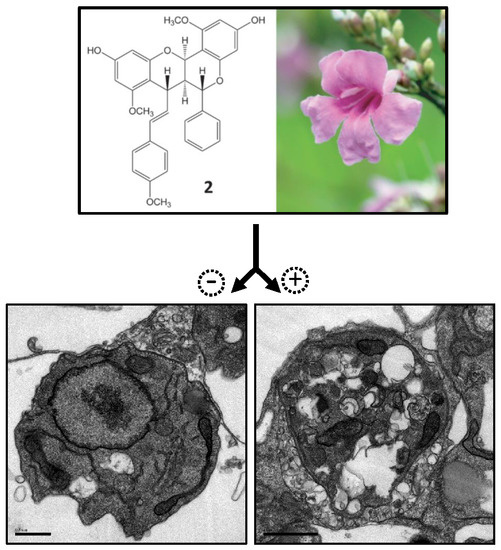
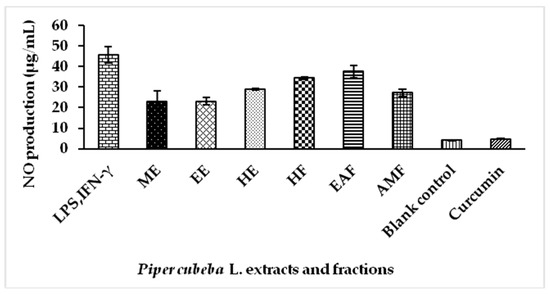
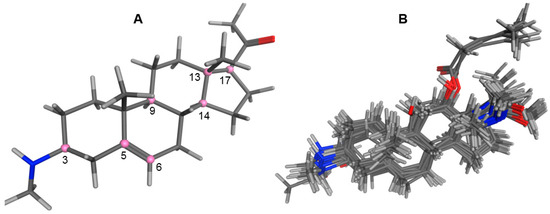
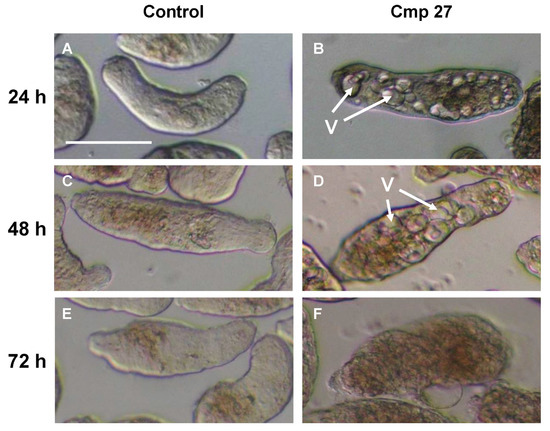
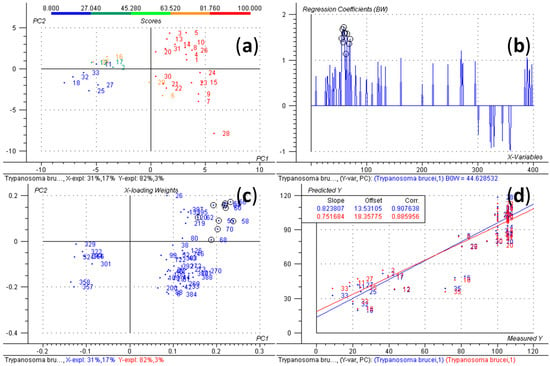
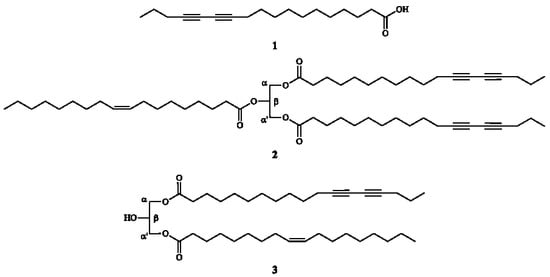
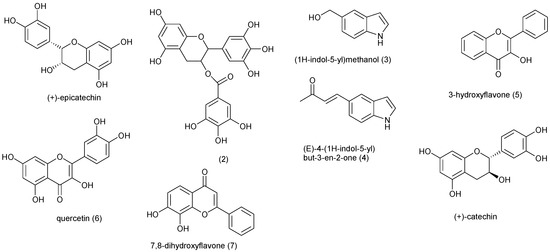
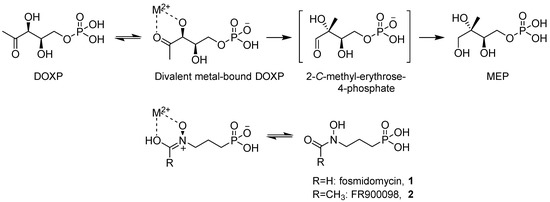
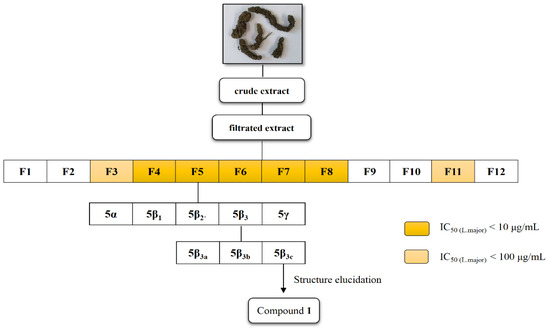
Natural Products as Leads or Drugs against Neglected Tropical Diseases
A topical collection in Molecules (ISSN 1420-3049). This collection belongs to the section "Natural Products Chemistry".
Viewed by 665604
Editors
Interests: natural products; anti-parasitic activity; anti-cancer activity; structure elucidation; spectroscopy; computer-aided structure-activity relationship studies
Special Issues, Collections and Topics in MDPI journals
2. Institute of Chemistry and Metabolism of Drugs (IQUIMEFA), University of Buenos Aires – National Scientific and Technical Research Council, Buenos Aires, Argentina
Interests: neglected diseases; antimicrobial activity; natural compounds; plant extracts; terpenoids; flavonoids; Asteraceae
Special Issues, Collections and Topics in MDPI journals
Interests: isolation of antimicrobial; mosquito larvicidal; insecticidal; acaricidal and anthelmintic secondary metabolites from tropical fungi; plants and their endophytic microbes
Topical Collection Information
Dear Colleagues,
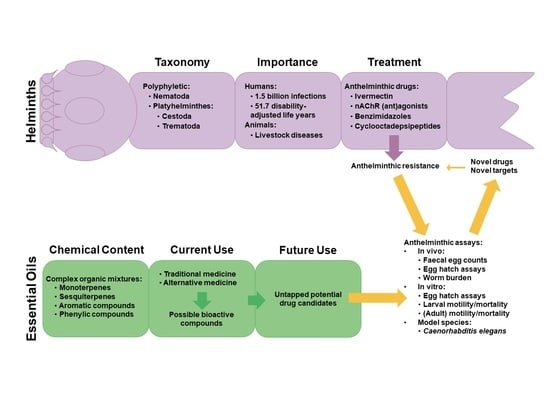
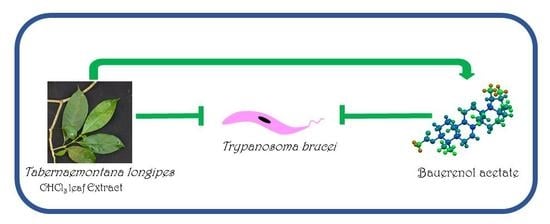
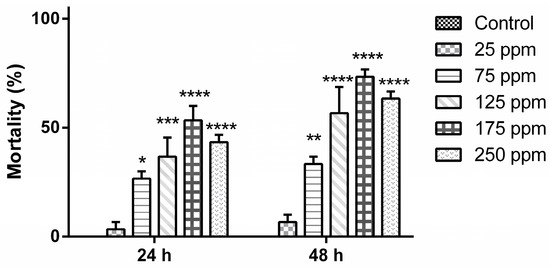
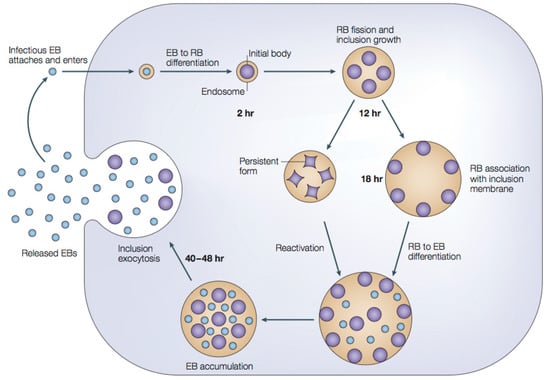
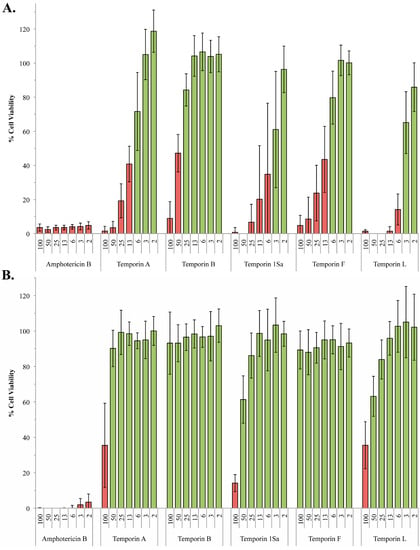
About one billion people world-wide suffer from at least one of the life-threatening diseases currently classified by WHO as Neglected Tropical Diseases (NTDs). These diseases represent a major cause of morbidity, disability and mortality in tropical regions of the world. They are termed “neglected” due to lack of financial investment into research and development of new drugs and almost non-existent public awareness in high-income countries. Being associated with poor socioeconomic and hygienic circumstances, they could also be termed diseases of neglected populations. NTDs comprise infections with pathogens of bacterial (e.g., Leprosy, Trachoma), viral (Dengue fever), helminth (e.g., Schistosomiasis, Filariasis) as well as “protozoan” (African sleeping sickness, Chagas’ disease, Leishmaniasis) origin. In environments where NTDs prevail, Malaria, the most widespread “protozoan” infection—although not currently treated as such by WHO—can also be considered a neglected disease. Notwithstanding recent partial successes in the struggle to eliminate or even eradicate some of these diseases, which have been achieved by WHO’s consequent strategies of disease monitoring, vector control, preventive chemotherapy and others, the development of new, safe and affordable drugs remains an urgent need. Existing pharmacotherapies, especially in case of the “protozoan” parasitoses, suffer from various shortcomings, namely, a high degree of toxicity and unwanted effects, lack of availability and/or problematic application under the life conditions of affected populations, as well as emergence of resistant pathogens, so that the search for new chemical entities showing activity against the NTD-pathogens is a very important field of research.
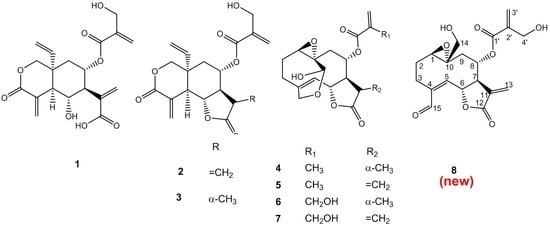
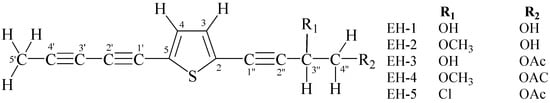
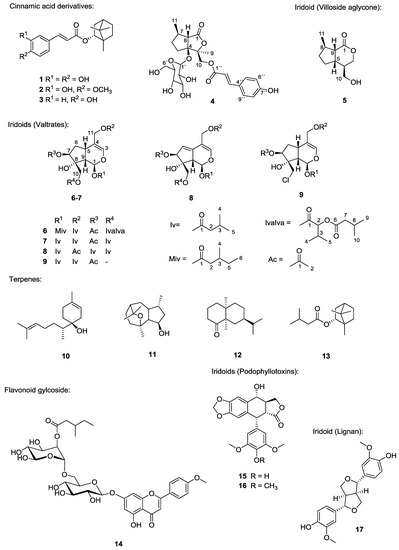
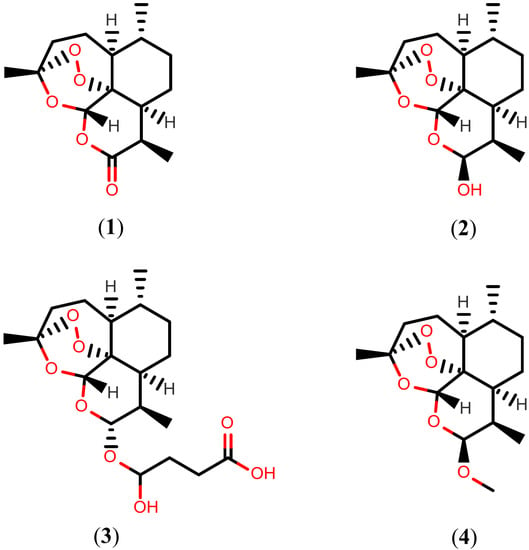
Nature has provided an innumerable wealth of drugs for the treatment of many serious diseases. Among the natural sources for new bioactive chemicals, terrestrial plants, bacteria and fungi have traditionally played the major roles; however, increasingly over the past few decades, many interesting new active molecules are found in marine life forms. Secondary metabolites from an immense diversity of living organisms thus represent a huge repository of chemical structures which has been and will continue to be a source of new drugs, directly in their native form or after optimization by synthetic medicinal chemistry.
The present Topical collection focuses on such molecules of natural origin that show a promising potential to act against the pathogens responsible for neglected tropical diseases, including Malaria. All aspects related to the discovery and further development of natural products against NTDs will be covered by the issue. It is therefore a pleasure to invite high quality studies, as well as timely review papers, on in vitro and in vivo biological activity, isolation and structure elucidation, synthetic optimization, investigations of the pharmacodynamics and -kinetics, as well as structure-activity relationships of natural products acting against NTDs.
Prof. Dr. Thomas J. Schmidt
Dr. Valeria Patricia Sülsen
Prof. Dr. Josphat Matasyoh
Collection Editors
Manuscript Submission Information
Manuscripts for the topical collection can be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. All papers will be peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on this website. The topical collection considers regular research articles, short communications and review articles. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page.
Please visit the Instructions for Authors page before submitting a manuscript. The article processing charge (APC) for publication in this open access journal is 2700 CHF (Swiss francs).
Keywords
- neglected tropical disease
- natural product
- secondary metabolite
- phytochemistry
- medicinal chemistry of natural products
- bioactivity testing/screening
- mechanism of action
- chagas disease
- human african trypanosomiasis (Sleeping sickness)
- leishmaniasis
- malaria
- buruli ulcer (mycobacterium ulcerans infection)
- dengue
- dracunculiasis (guinea-worm disease)
- echinococcosis
- foodborne trematodiases
- leprosy
- lymphatic filariasis
- onchocerciasis (river blindness)
- rabies
- schistosomiasis
- soil transmitted helminthiases
- taeniasis/cysticercosis
- trachoma
- yaws (endemic treponematoses)
- mycetoma
Related Special Issues
- 50th Anniversary Issue of Artemisinin: The Successful Use of Natural Products against Parasitic Agents in Molecules (11 articles - displayed below)
- Drug Discovery for Neglected Diseases in Molecules (104 articles - displayed below)