Preparation of Rotenone Derivatives and in Vitro Analysis of Their Antimalarial, Antileishmanial and Selective Cytotoxic Activities
Abstract
:1. Introduction
2. Results and Discussion
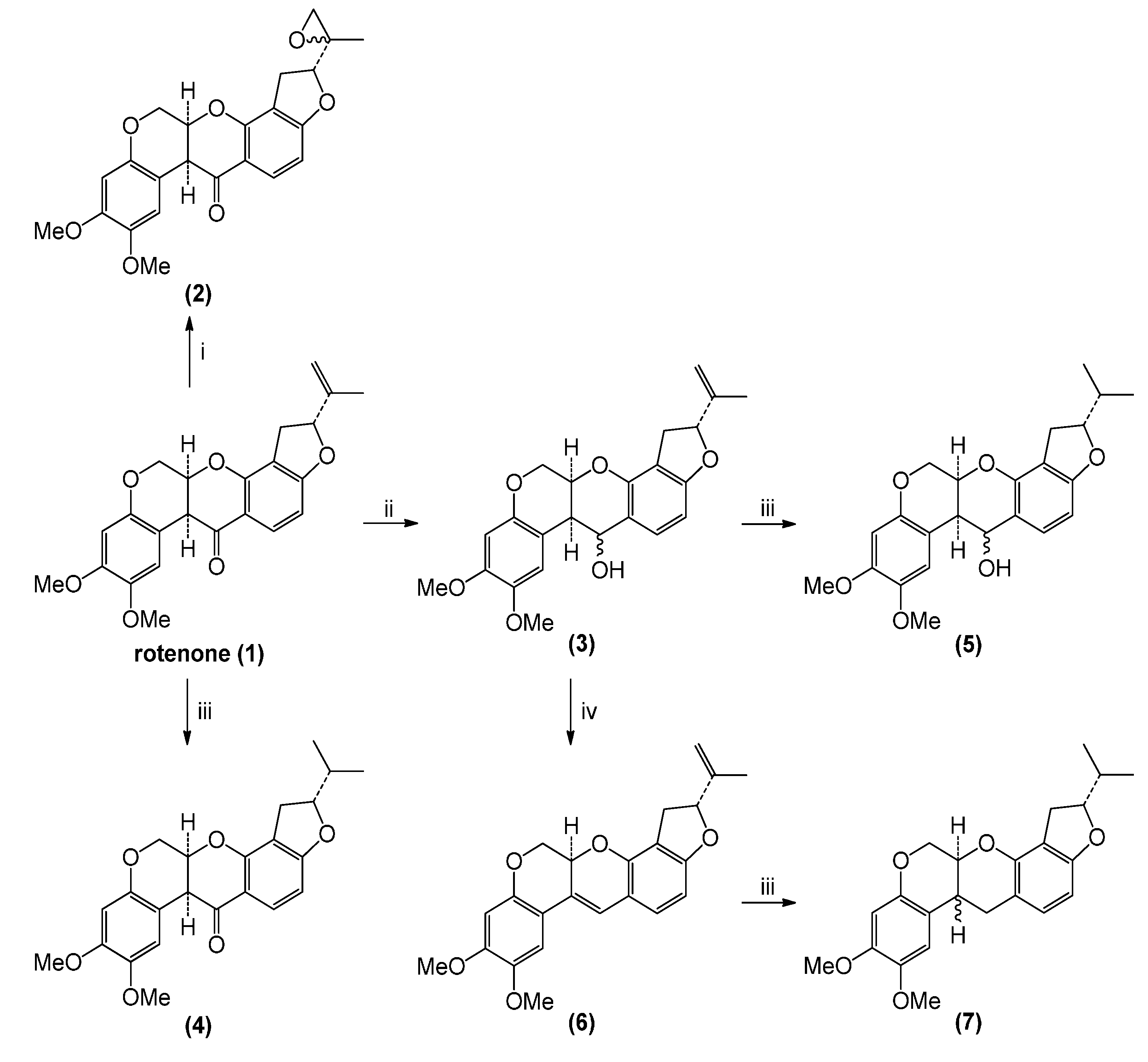
2.1. Rotenone and Synthesized Derivatives
2.2. Cytotoxic and Antiparasitic Activities
| Compound | Cytotoxicity (LC50, μM) | ||||||
|---|---|---|---|---|---|---|---|
| U937 | CAD-R1 | HepG2 | Vero | huMDM | huRBC | ||
| 1 (Rotenone) | 115.1 ± 7.6 | 10.2 ± 0.0 | 1.8 ± 0.3 | >508.0 | 378.8 ± 15.0 | >508.0 | |
| 2 | 0.3 ± 0.1 | 29.0 ± 3.0 | 52.0 ± 6.3 | 81.3 ± 16.9 | 456.8 ± 22.7 | >505.0 | |
| 3 | 0.1 ± 0.03 | 17.0 ± 3.4 | 1 2.0 ± 7.1 | 284.0 ± 23.6 | 518.3 ± 41.4 | >523.0 | |
| 4 | 4.8 ± 2.5 | 185.9 ± 21.2 | 6.6 ± 3.8 | >505.0 | >505.0 | >505.0 | |
| 5 | 35.2 ± 9.3 | 166.3 ± 20.1 | 18.6 ± 4.8 | >503.0 | >503.0 | 421.9 ± 33.2 | |
| 6 | 0.3 ± 0.1 | 50.5 ± 22.5 | 14.8 ± 2.9 | 68.0 ± 14.0 | 397.8 ± 43.4 | >529.0 | |
| 7 | 0.7 ± 0.2 | 1.67 ± 0.9 | 5.0 ± 0.71 | >476.0 | >476.0 | 416.7 ± 76.9 | |
| Amphotericin B | 54.4 ± 0.1 | 54.5 ± 0.3 | 30.0 ± 2.2 | 15.6 ± 0.4 | 108.5 ± 10.0 | 19.4 ± 5.8 | |
| Chloroquine | 475.8 ± 16.3 | 2.2 ± 0.1 | 0.1 ± 0.03 | 26.0 ± 6.6 | 69.5 ± 11.3 | >625.0 | |
| Doxorubicin | 0.1 ± 0.01 | 2.6 ± 0.7 | 0.7 ± 0.4 | 9.36 ± 2.2 | 1.29 ± 0.2 | 42.5 ± 16.9 | |
| Compound | Biological activity CE50 (μM) | |
|---|---|---|
| Leishmanicidal | Antiplasmodial | |
| 1 (Rotenone) | 127.2 ± 17.3 | 19.0 ± 8.6 |
| 2 | >126.0 | 41.7 ± 12.6 |
| 3 | >130.0 | 53.9 ± 2.4 |
| 4 | >126.0 | 41.2 ± 13.4 |
| 5 | >125.0 | >50.0 |
| 6 | 12.7 ± 6.1 | >53.0 |
| 7 | >119.0 | 47.4 ± 0.2 |
| Amphotericin B | 0.8 ± 0.2 | NT |
| Chloroquine | NT | 0.06 ± 0.03 |
| Compound | Index of Selectivity | |
|---|---|---|
| huMDM a | huRBC b | |
| 1 (Rotenone) | 3.1 | >26.1 |
| 2 | <3.6 | >12.1 |
| 3 | <3.9 | >9.6 |
| 4 | <4.0 | >12.2 |
| 5 | <4.0 | <8.4 |
| 6 | 31.3 | <9.9 |
| 7 | <4.0 | 8.7 |
| Amphotericin B | 143.0 | NC c |
| Chloroquine | NC c | >10.416 |
3. Experimental Section
3.1. General Information
3.2. Chemical Transformations of Rotenone
3.3. Biological Activity Assays
3.3.1. Cells and in Vitro Culture
3.3.2. In Vitro Cytotoxic Activity
3.3.3. In Vitro Antiplasmodial and Leishmanicidal Activities
3.4. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Molyneux, D.H.; Malecela, M.N. Neglected tropical diseases and the millennium development goals: Why the “other diseases” matter: Reality versus rhetoric. Parasit Vector. 2011, 4, 234. [Google Scholar]
- Rosenthal, P.J. Antimalarial drug discovery: Old and new approaches. J. Exp. Biol. 2003, 206, 3735–3744. [Google Scholar]
- Lukes, J.; Hashimi, H.; Zíková, A. Unexplained complexity of the mitochondrial genome and transcriptome in kinetoplastid flagellates. Curr. Genet. 2005, 48, 277–299. [Google Scholar]
- Sheiner, L.; Vaidya, A.B.; McFadden, G.I. The metabolic roles of the endosymbiotic organelles of Toxoplasma and Plasmodium spp. Curr. Opin. Microbiol. 2013, 16, 452–458. [Google Scholar]
- Van, T.; Deschacht, M.; da Luz, R.; Maes, L.; Cos, P. Leishmania–macrophage interactions: Insights into the redox biology. Free Radic. Biol. Med. 2011, 51, 337–351. [Google Scholar]
- Pal, C.; Bandyopadhyay, U. Redox-active antiparasiticdrugs. Antioxid. Redox Sign. 2012, 17, 555–582. [Google Scholar]
- Li, N.; Ragheb, K.; Lawler, G.; Sturgis, J.; Rajwa, B.; Melendez, J.A.; Robinson, J.P. Mitochondrial complex I inhibitor rotenone induces apoptosis through enhancing mitochondrial reactive oxygenspecies production. J. Biol. Chem. 2003, 278, 8516–8525. [Google Scholar]
- Vrablic, A.S.; Albright, C.D.; Craciunescu, C.N.; Salganik, R.I.; Zeisel, S.H. Altered mitochondrial function and overgeneration of reactive oxygen species precede the induction of apoptosis by 1-O-octadecyl-2-methyl-rac-glycero-3-phosphocholine in p53-defective hepatocytes. FASEB J. 2001, 15, 1739–1744. [Google Scholar]
- Torres-Roca, J.F.; Tung, J.W.; Greenwald, D.R.; Brown, J.M.; Herzenberg, L.A.; Herzenberg, L.A.; Katsikis, P.D. An early oxygen-dependent step is required for dexamethasone-induced apoptosis of immature mouse thymocytes. J. Immunol. 2000, 165, 4822–4830. [Google Scholar]
- Deshpande, S.S.; Angkeow, P.; Huang, J.; Ozaki, M.; Irani, K. Rac1 inhibits TNF-alpha-induced endothelial cell apoptosis: Dual regulation by reactive oxygen species. FASEB J. 2000, 14, 1705–1714. [Google Scholar]
- Catteau, L.; Lautié, E.; Koné, O.; Coppée, M.; Hell, K.; Pomalegni, C.B.; Quetin-Leclercq, J. Degradation of rotenone in yam bean seeds (Pachyrhizus sp.) through food processing. J. Agric. Food Chem. 2013, 61, 11173–1117. [Google Scholar]
- Sherer, T.B.; Betarbet, R.; Testa, C.M.; Seo, B.B.; Richardson, J.R.; Kim, J.H.; Miller, G.W.; Yagi, T.; Matsuno-Yagi, A.; Greenamyre, J.T. Mechanism of toxicity in rotenone models of Parkinson’s disease. J. Neurosci. 2003, 23, 10756–10764. [Google Scholar]
- Insuasty, B.; Montoya, A.; Becerra, D.; Quiroga, J.; Abonia, R.; Robledo, S.; Vélez, I.; Upegui, Y.; Nogueras, M.; Cobo, J. Synthesis of novel analogs of 2-pyrazoline obtained from [(7-chloroquinolin-4-yl)amino] chalcones and hydrazine as potential antitumor and antimalarial agents. Eur. J. Med. Chem. 2013, 67, 252–262. [Google Scholar]
- Pulido, S.A.; Muñoz, D.L.; Restrepo, A.M.; Mesa, C.V.; Alzate, J.F.; Vélez, I.D.; Robledo, S.M. Improvement of the green fuorescent protein reporter system in Leishmania. spp. for the in vitro and in vivo screening of antileishmanial drugs. Acta Trop. 2012, 122, 36–45. [Google Scholar]
- Taylor, V.M.; Cedeño, D.L.; Muñoz, D.L.; Jones, M.A.; Lash, T.D.; Young, A.M.; Constantino, M.H.; Esposito, N.; Velez, I.D.; Robledo, S.M. In vitro and vivo studies of the utility of dimethyl and diethyl carbaporphyrin ketals in treatment of cutaneous leishmaniasis. Antimicrob. Agents Chemother. 2011, 55, 4755–4764. [Google Scholar]
- Lazaroff, M.; Dunlap, K.; Chikaraishi, D.M. A CNS catecholaminergic cell line expresses voltage-gated currents. J. Membr. Biol. 1996, 151, 279–291. [Google Scholar]
- Robledo, S.; Wozencraft, A.; Valencia, A.Z.; Saravia, N. Human monocyte infection by Leishmania (Viannia) panamensis. Role of complement receptors and correlation of susceptibility in vitro with clinical phenotype. J. Immunol. 1994, 152, 1265–1276. [Google Scholar]
- Mesa, C.; Muñoz, D.L.; Echeverry, M.; Velez, I.D.; Robledo, S.M. Susceptibilidad in vitro a infección por Leishmania y sensibilidad a medicamentos difiere según tipo de macrófagos. Salud UIS (Colombia) 2010, 42, 200–211. [Google Scholar]
- Conceição, K.; Konno, K.; Richardson, M.; Antoniazzi, M.M.; Jared, C.; Daffre, S.; Camargo, A.C.; Pimenta, D.C. Isolation and biochemical characterization of peptides presenting antimicrobial activity from the skin of Phyllomedusa. hypochondrialis. Peptides 2006, 27, 3092–3099. [Google Scholar]
- Trager, W.; Jensen, J.B. Human malaria in continuous culture. Science 1976, 193, 673–675. [Google Scholar]
- Finney, J.D. Probit Analysis: Statistical Treatment of the Sigmoid Response Curve, 3rd ed; Cambridge University Press: Cambridge, UK, 1978; p. 550. [Google Scholar]
- Nepveu, F.; Turrini, F. Targeting the redox metabolism of Plasmodium falciparum. Future Med. Chem. 2013, 5, 1993–2006. [Google Scholar]
- Medina, J.M.; Rodrigues, J.C.; de Souza, W.; Atella, G.C.; Barrabin, H. Tomatidine promotes the inhibition of 24-alkylated sterol biosynthesis and mitochondrial dysfunction in Leishmania amazonensis promastigotes. Parasitology 2012, 139, 1253–1265. [Google Scholar]
- Smirlis, D.; Duszenko, M.; Ruiz, A.J.; Scoulica, E.; Bastien, P.; Fasel, N.; Soteriadou, K. Targeting essential pathways in trypanosomatids gives insights into protozoan mechanisms of cell death. Parasit Vector. 2010, 3, 107. [Google Scholar]
- Debrabant, A.; Lee, N.; Bertholet, S.; Duncan, R.; Nakhasi, H.L. Programmed cell death in trypanosomatids and other unicellular organisms. Int. J. Parasitol. 2003, 33, 257–267. [Google Scholar]
- Wink, M. Medicinal plants: A source of anti-parasitic secondary metabolites. Molecules 2012, 17, 12771–12791. [Google Scholar]
- Sample Availability: Samples of the compounds 1–7 are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Upegui, Y.; Gil, J.F.; Quiñones, W.; Torres, F.; Escobar, G.; Robledo, S.M.; Echeverri, F. Preparation of Rotenone Derivatives and in Vitro Analysis of Their Antimalarial, Antileishmanial and Selective Cytotoxic Activities. Molecules 2014, 19, 18911-18922. https://doi.org/10.3390/molecules191118911
Upegui Y, Gil JF, Quiñones W, Torres F, Escobar G, Robledo SM, Echeverri F. Preparation of Rotenone Derivatives and in Vitro Analysis of Their Antimalarial, Antileishmanial and Selective Cytotoxic Activities. Molecules. 2014; 19(11):18911-18922. https://doi.org/10.3390/molecules191118911
Chicago/Turabian StyleUpegui, Yulieth, Juan F. Gil, Wiston Quiñones, Fernando Torres, Gustavo Escobar, Sara M. Robledo, and Fernando Echeverri. 2014. "Preparation of Rotenone Derivatives and in Vitro Analysis of Their Antimalarial, Antileishmanial and Selective Cytotoxic Activities" Molecules 19, no. 11: 18911-18922. https://doi.org/10.3390/molecules191118911
APA StyleUpegui, Y., Gil, J. F., Quiñones, W., Torres, F., Escobar, G., Robledo, S. M., & Echeverri, F. (2014). Preparation of Rotenone Derivatives and in Vitro Analysis of Their Antimalarial, Antileishmanial and Selective Cytotoxic Activities. Molecules, 19(11), 18911-18922. https://doi.org/10.3390/molecules191118911




