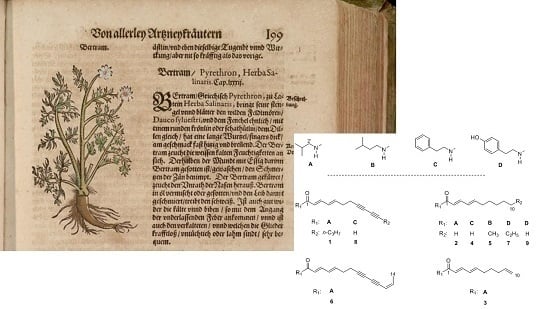
Alkamides from Anacyclus pyrethrum L. and Their in Vitro Antiprotozoal Activity
Abstract
:1. Introduction
2. Results and Discussion
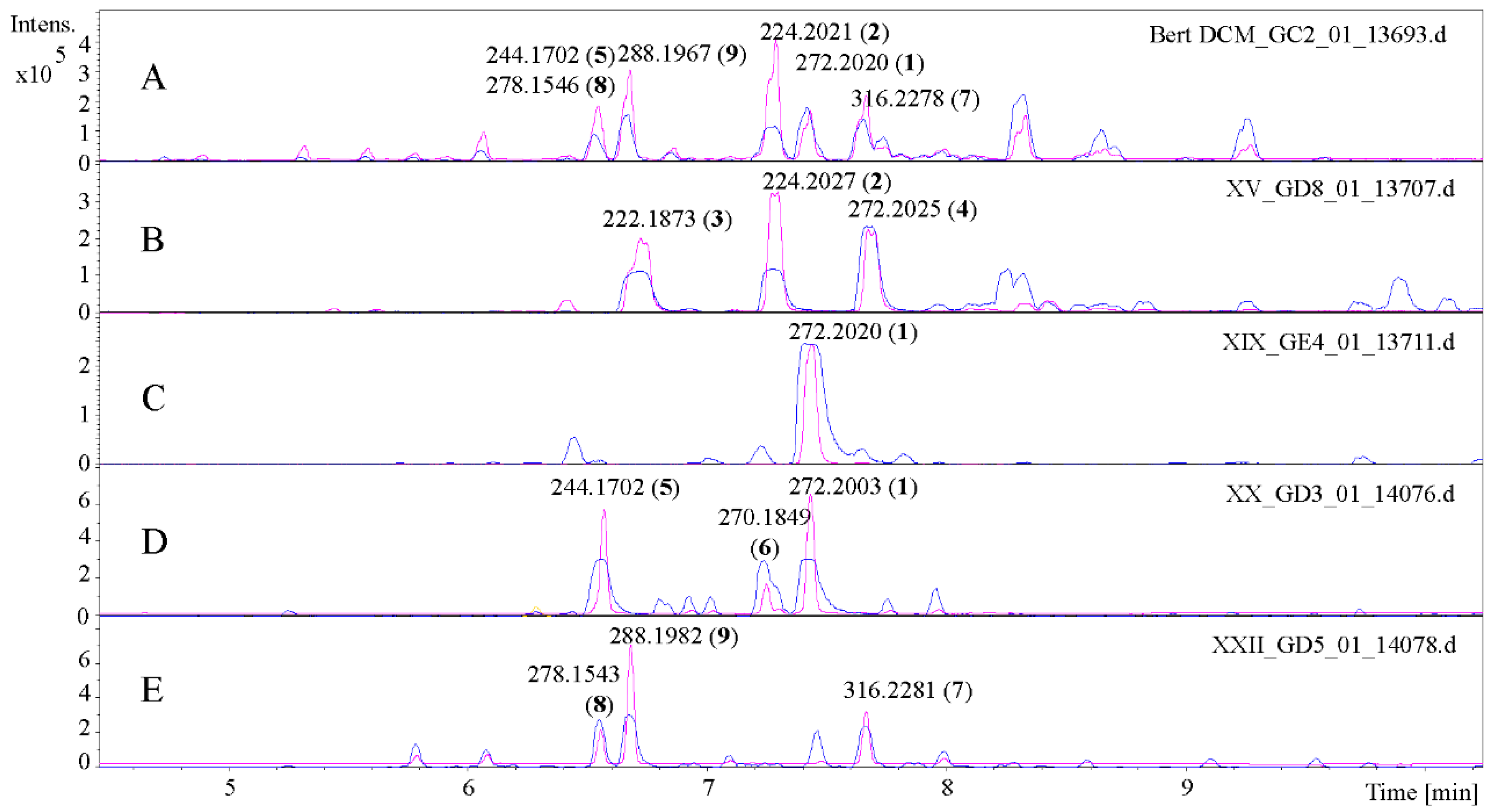
2.1. Isolation and Structural Characterization of the Alkamides
2.2. Antiprotozoal Activity of the Isolated Alkamides
3. Materials and Methods
3.1. Analytical Procedures and Instruments
3.1.1. UHPLC/+ESI-QTOF-MS/MS
3.1.2. NMR Spectroscopy
3.2. Isolation Process
3.2.1. Plant Material
3.2.2. Sohxlet Extraction
3.2.3. Gravity Flow Column Chromatography (CC)
3.2.4. Purification by Preparative High Performance Liquid Chromatography (HPLC/UV-DAD)
3.2.5. Analytical Data
3.3. In Vitro Assays and IC50 Determination
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Greger, H. Alkamides: A critical reconsideration of a multifunctional class of unsaturated fatty acid amides. Phytochem. Rev. 2015. [Google Scholar] [CrossRef]
- Althaus, J.B.; Kaiser, M.; Brun, R.; Schmidt, T.J. Antiprotozoal Activity of Achillea ptarmica (Asteraceae) and Its Main Alkamide Constituents. Molecules 2014, 19, 6428–6438. [Google Scholar] [CrossRef] [PubMed]
- Silveira, N.; Saar, J.; Santos, A.D.C.; Barison, A.; Sandjo, L.P.; Kaiser, M.; Schmidt, T.J.; Biavatti, M.W. A New Alkamide with an Endoperoxide Structure from Acmella ciliata (Asteraceae) and Its in Vitro Antiplasmodial Activity. Molecules 2016, 21, 765. [Google Scholar] [CrossRef] [PubMed]
- Himmelreich, S.; Källersjö, M.; Eldenäs, P.; Oberprieler, C. Phylogeny of southern hemisphere Compositae-Anthemideae based on nrDNA ITS and cpDNA ndhF sequence information. Plant Syst. Evol. 2008, 272, 131–153. [Google Scholar] [CrossRef]
- Oberprieler, C.; Himmelreich, S.; Vogt, R. A new subtribal classification of the tribe Anthemideae (Compositae). Willdenowia 2007, 37, 89–114. [Google Scholar] [CrossRef]
- Watson, L.E.; Evans, T.M.; Boluarte, T. Molecular Phylogeny and Biogeography of Tribe Anthemideae (Asteraceae), Based on Chloroplast Gene ndhF. Mol. Phylogenet. Evol. 2000, 15, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Greger, H. Ungesättigte Säureamide—Ein chemisches Merkmal der Gattung Achillea. Planta Med. 1982, 45, 142–143. [Google Scholar] [CrossRef] [PubMed]
- Dioskurides, P. Kräuterbuch deß Uralten unnd in aller Welt berühmtesten Griechischen Scribenten Pedacii Dioscorides Anazarbaei; Dantz, J.; Uffenbach, P., Translators; Kempfer: Franckfurt am Mayn, Germany, 1614. [Google Scholar]
- Adams, M.; Alther, W.; Kessler, M.; Kluge, M.; Hamburger, M. Malaria in the renaissance: Remedies from European herbals from the 16th and 17th century. J. Ethnopharmacol. 2011, 133, 278–288. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, S.; Thomi, S.; Kaiser, M.; Hamburger, M.; Adams, M. Screening and HPLC-Based Activity Profiling for New Antiprotozoal Leads from European Plants. Sci. Pharm. 2012, 80, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Kuropka, G.; Glombitza, K.W. Further Polyenic and Polyynic Carboxamides and Sesamin from Achillea ptarmica. Planta Med. 1987, 53, 440–442. [Google Scholar] [CrossRef] [PubMed]
- Hofer, O.; Greger, H.; Robien, W. 13C-NMR and 1H lanthanide induced shifts of naturally occurring alkamides with cyclic amide moieties—Amides from Achillea falcata. Tetrahedron 1986, 42, 2707–2716. [Google Scholar] [CrossRef]
- Greger, H.; Hofer, O. Highly Unsaturated Isopentyl Amides from Achillea wilhelmsii. J. Nat. Prod. 1987, 50, 1100–1107. [Google Scholar] [CrossRef]
- Jente, R.; Bonnet, P.H.; Bohlmann, F. Polyacetylenverbindungen, 206. Über die Inhaltsstoffe von Anacyclus pyrethrum DC. Chem. Ber. 1972, 105, 1694–1700. [Google Scholar] [CrossRef]
- Burden, R.S.; Crombie, L. Amides of vegetable origin. Part XII. A new series of Alka-2,4-dienoic Tyramine-amides from Anacyclus pyrethrum D.C. (Compositae). J. Chem. Soc. C 1969, 19, 2477–2481. [Google Scholar] [CrossRef]
- Bohlmannn, F.; Inhoffen, E. Polyacetylenverbindungen (XVI. Mitteil.) Synthese des Anacyclins. Chem. Ber. 1956, 89, 1276–1281. [Google Scholar] [CrossRef]
- Schmidt, T.J.; Nour, A.M.M.; Khalid, S.A.; Kaiser, M.; Brun, R. Quantitative Structure-Antiprotozoal Activity Relationships of Sesquiterpene Lactones. Molecules 2009, 14, 2062–2076. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 1, 5, 6, 7 and the mixture of 8 + 9 are available from the authors. |

| Sample | Tbr 1 | Tc 2 | Ld 3 | Pf 4 | Cytotox 5 | SI Tbr | SI Ld | SI Pf |
|---|---|---|---|---|---|---|---|---|
| Pos. contr. | 0.003 ± 0.001 | 0.439 ± 0.094 | 0.127 ± 0.052 | 0.080 ± 0.003 | 0.008 ± 0.001 | |||
| (0.008) | (1.688) | (0.312) | (0.250) | (0.019) | ||||
| DCM extr. 6 | >10 | 8.83 ± 0.75 | 4.22 ± 1.57 | 3.04 ± 0.07 | 13.4 ± 3.2 | >1.3 | 3.2 | 4.4 |
| 3 | 2.91 ± 0.21 | 39.9 | 4.77 ± 1.02 | 7.13 ± 0.63 | 16.7 ± 0.1 | 5.7 | 3.5 | 2.3 |
| (13.2) | (181) | (21.6) | (32.2) | (75.3) | ||||
| 4 | 4.03 | 5.02 ± 0.05 | 2.97 ± 0.54 | >5 | 7.88 | 2.0 | 2.7 | >1.6 |
| (14.9) | (18.5) | (11.0) | (>18) | (29.1) | ||||
| 5 | 4.59 ± 0.39 | 16.3 ± 0.3 | 4.04 ± 0.71 | 10.3 ± 1.4 | 28.2 ± 6.9 | 6.2 | 7.0 | 2.7 |
| (18.9) | (66.9) | (16.6) | (42.5) | (116) | ||||
| 6 | 6.37 ± 0.94 | 38.8 ± 2.1 | 5.04 ± 1.17 | 7.19 ± 0.49 | 19.4 ± 1.7 | 3.1 | 3.9 | 2.7 |
| (23.7) | (144) | (18.7) | (26.7) | (72.2) | ||||
| 7 | 2.26 ± 0.18 | 1.88 | 4.19 ± 1.64 | 3.18 ± 0.20 | 0.19 ± 0.05 | 0.1 | <0.1 | <0.1 |
| (7.17) | (5.97) | (13.3) | (10.1) | (0.69) | ||||
| 8 + 9 (1:4) | 1.66 ± 0.12 | 3.90 | 5.31 ± 0.53 | 7.64 ± 2.57 | 2.47 ± 0.36 | 1.5 | 0.5 | 0.3 |
| Fractions and Combined Eluates * | Elution Volume (mL) ** | Yield (g) | Isolated Compounds |
|---|---|---|---|
| I-VIII 1–540 | 4455 | 0.71977 | |
| IX 541–550 | 200 | 0.0921 | |
| X 551–565 | 320 | 0.1638 | |
| XI 566–585 | 300 | 0.0801 | |
| XII 586–625 | 670 | 0.3187 | |
| XIII 626–660 | 530 | 0.4814 | |
| XIV 661–690 | 410 | 0.2181 | |
| XV 691–720 | 370 | 0.1548 | 2, 3, 4 |
| XVI 721–819 | 1020 | 0.3595 | |
| XVII 820–890 | 980 | 0.2426 | |
| XVIII 891–909 | 330 | 0.0696 | |
| XIX 910–940 | 510 | 0.1803 | 1 |
| XX 941–1010 | 760 | 0.3141 | 1, 5, 6 |
| XXI 1011–1080 | 840 | 0.5080 | |
| XXII 1081–1140 | 850 | 0.5072 | 8, 9, 10 |
| XXIII 1141–1200 | 710 | 0.1973 | |
| XXIV 1201–1210 | 83 | 0.0244 | |
| XXV 1211–1225 | 120 | 0.0381 | |
| XXVI 1226–1280 | 490 | 0.1175 | |
| XXVII 1281–1319 | 340 | 0.0619 | |
| XXVIII 1320–1335 | 230 | 0.0493 | |
| XXIX 1336–1380 | 780 | 0.1580 | |
| XXX 1381–1410 | 480 | 0.1136 | |
| XXXI 1411–1470 | 950 | 0.1796 | |
| XXXII 1471–1750 | 4420 | 0.5834 | |
| XXXIII 1751–1830 | 1230 | 0.0782 | |
| XXXIV 1831–1875 | 540 | 0.0508 | |
| XXXV 1876–1885 | 80 | 0.7643 | |
| XXXVI 1886–1919 | 520 | 0.3215 | |
| XXXVII 1920–1959 | 800 | 0.1256 | |
| XXXVIII 1960–2023 | 1080 | 0.1762 | |
| Total: | Total: | ||
| 25,398 | 7.4498 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Althaus, J.B.; Malyszek, C.; Kaiser, M.; Brun, R.; Schmidt, T.J. Alkamides from Anacyclus pyrethrum L. and Their in Vitro Antiprotozoal Activity. Molecules 2017, 22, 796. https://doi.org/10.3390/molecules22050796
Althaus JB, Malyszek C, Kaiser M, Brun R, Schmidt TJ. Alkamides from Anacyclus pyrethrum L. and Their in Vitro Antiprotozoal Activity. Molecules. 2017; 22(5):796. https://doi.org/10.3390/molecules22050796
Chicago/Turabian StyleAlthaus, Julia B., Claudine Malyszek, Marcel Kaiser, Reto Brun, and Thomas J. Schmidt. 2017. "Alkamides from Anacyclus pyrethrum L. and Their in Vitro Antiprotozoal Activity" Molecules 22, no. 5: 796. https://doi.org/10.3390/molecules22050796